RETRACTED: Nitrogen Doped Intercalation TiO2/TiN/Ti3C2Tx Nanocomposite Electrodes with Enhanced Pseudocapacitance
Abstract
1. Introduction
2. Materials and Methods
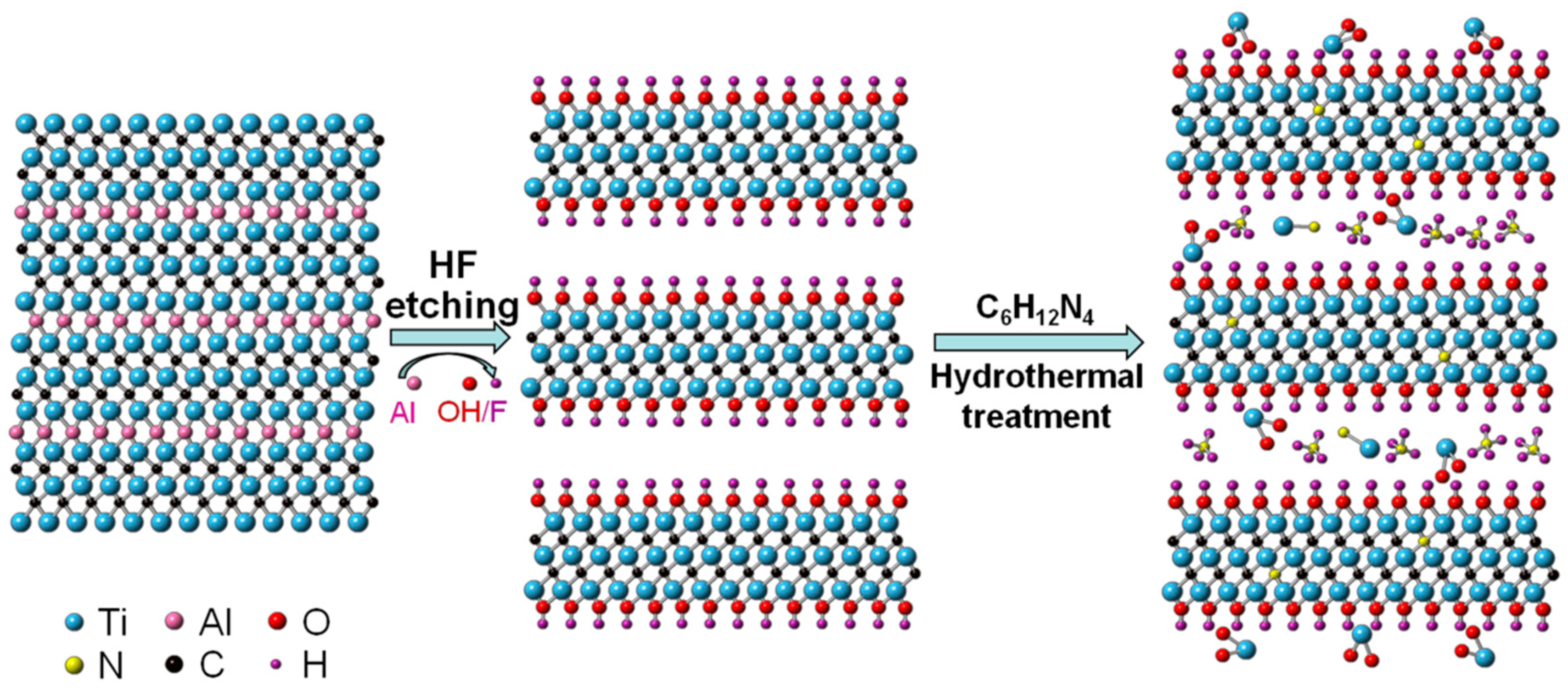
2.1. Preparation of Ti3C2Tx
2.2. Synthesis of N Doped Intercalation TiO2/TiN/Ti3C2Tx
2.3. Material Characterization
2.4. Preparation of Electrodes and Electrochemical Measurements
3. Results and Discussion
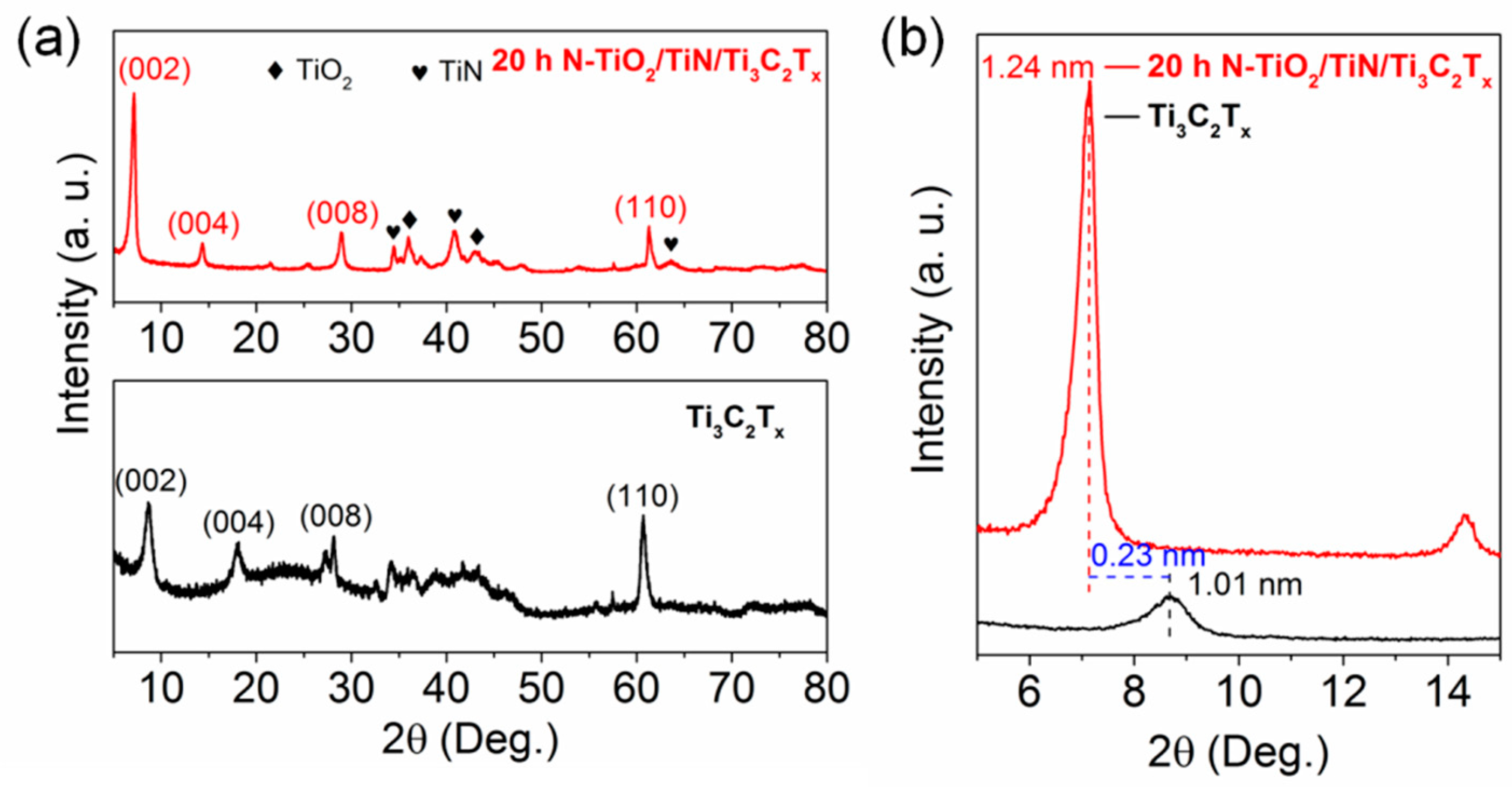
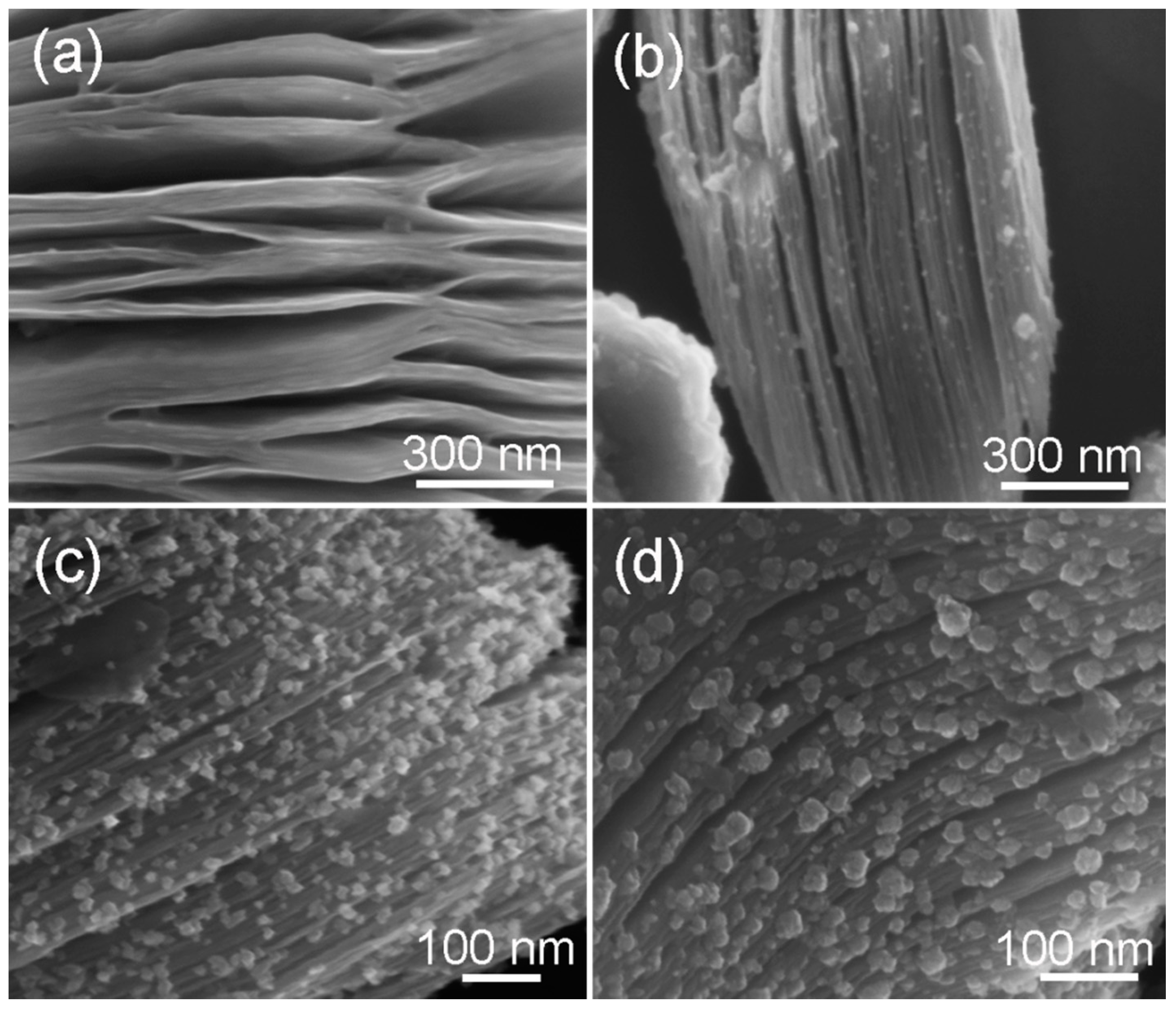
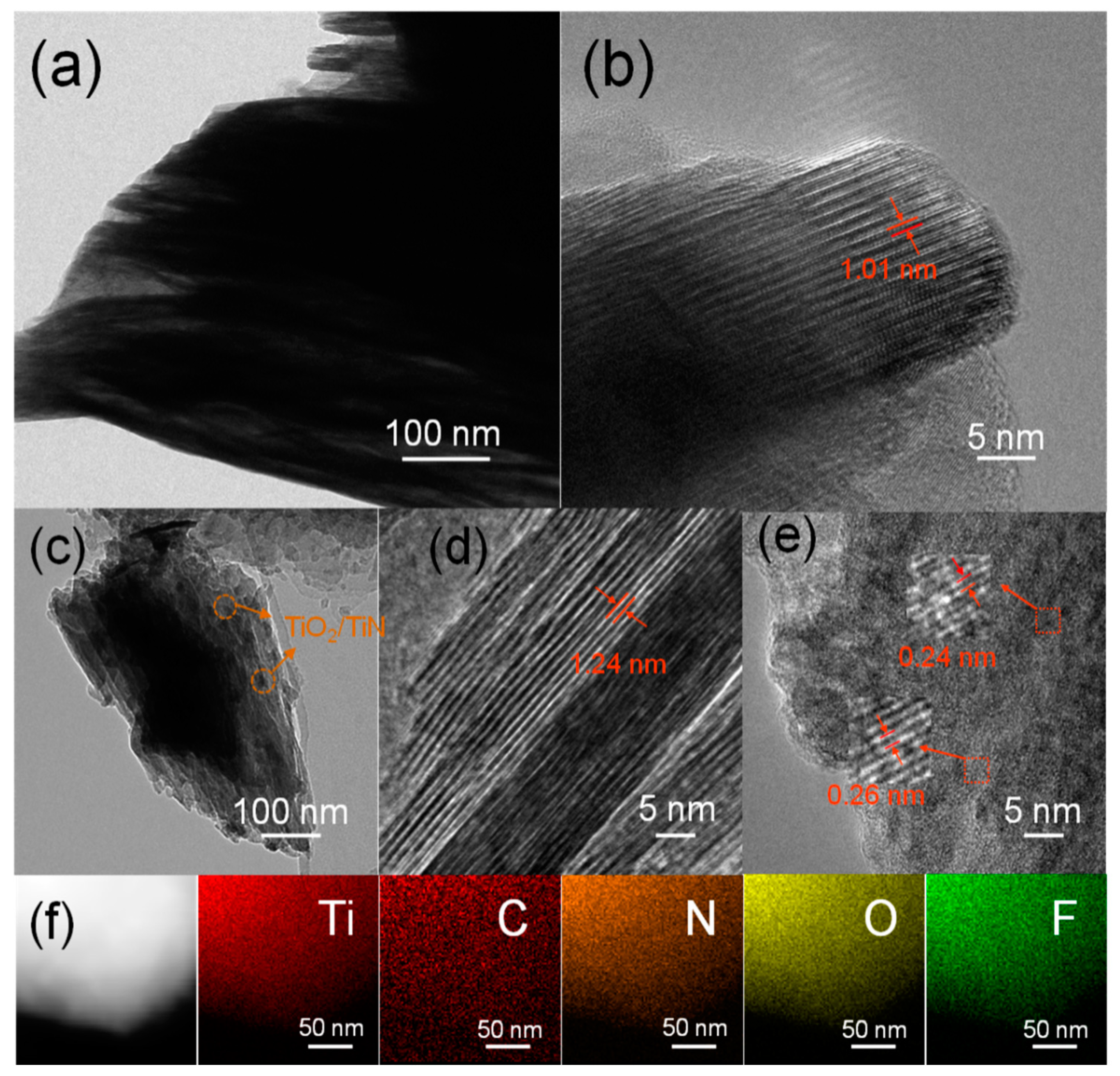
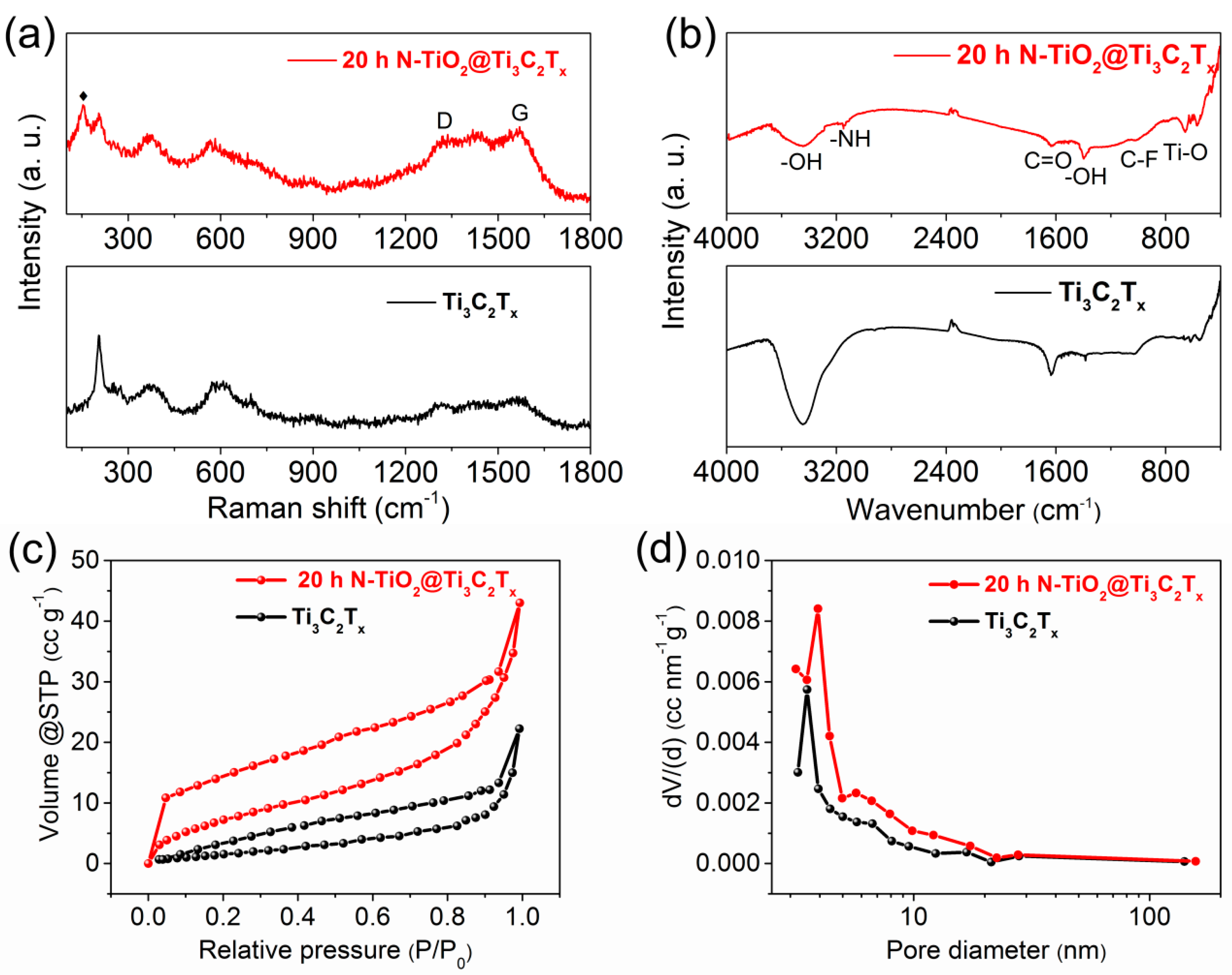
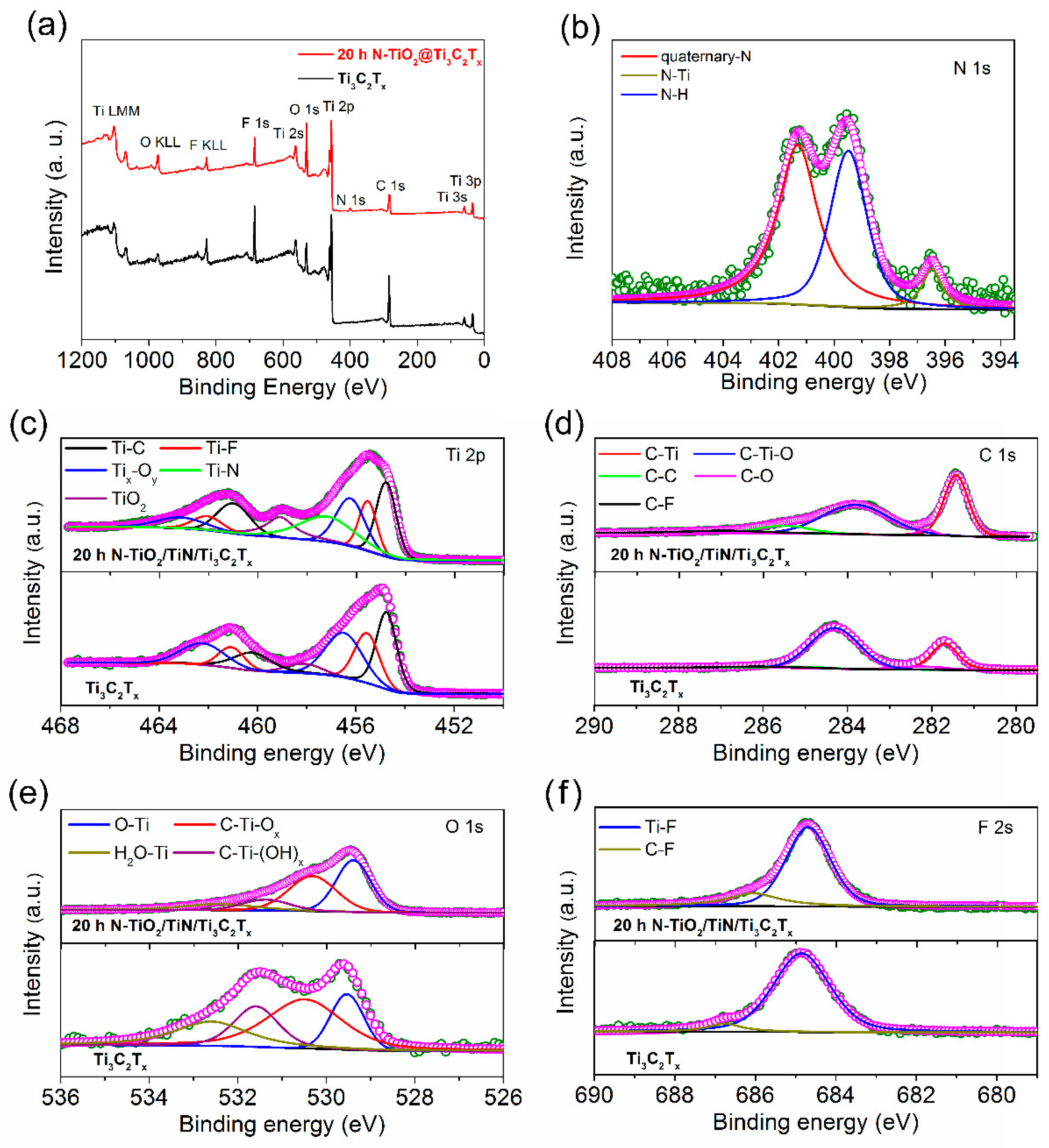
3.1. Material Characterizations and Analysis
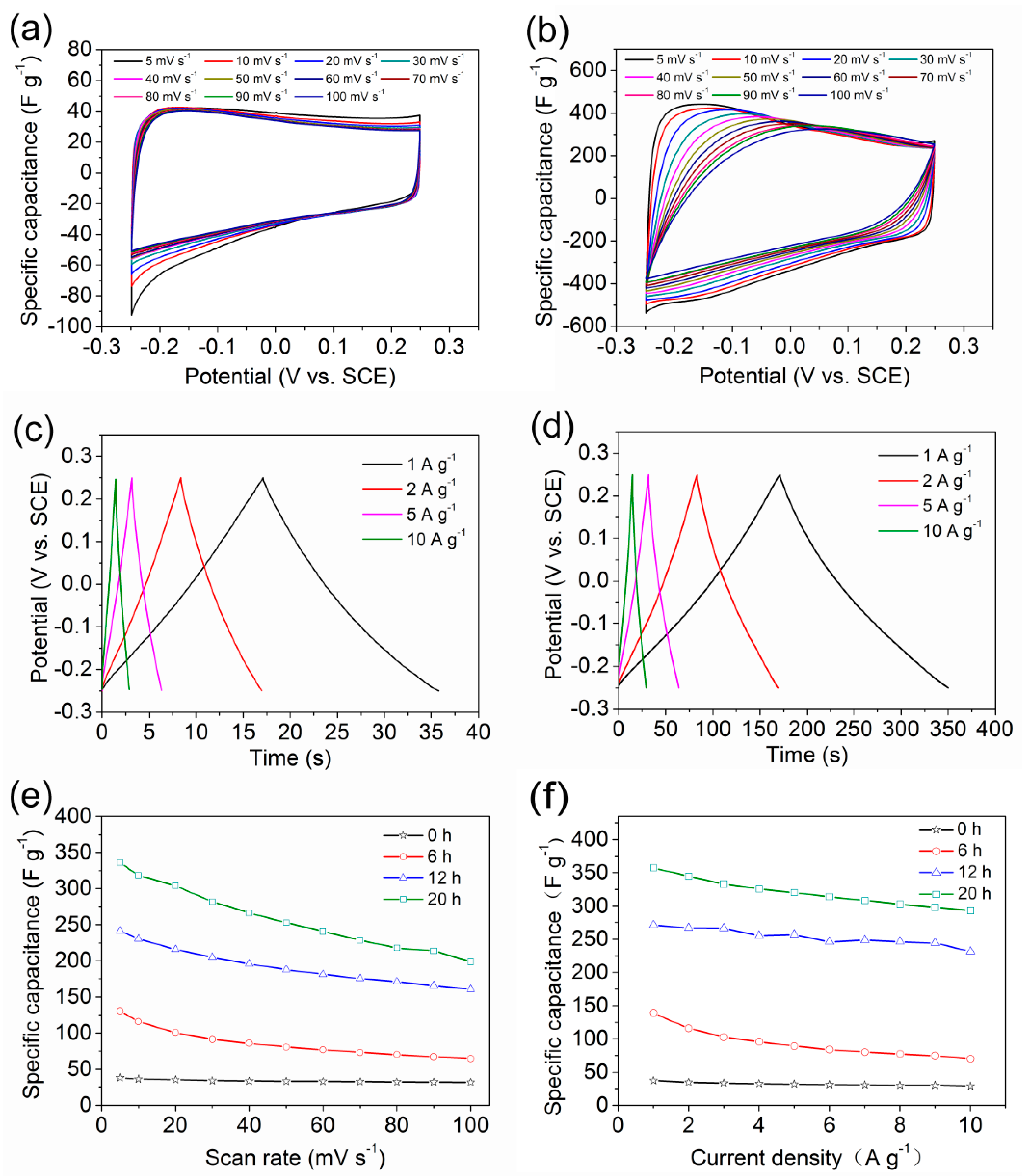
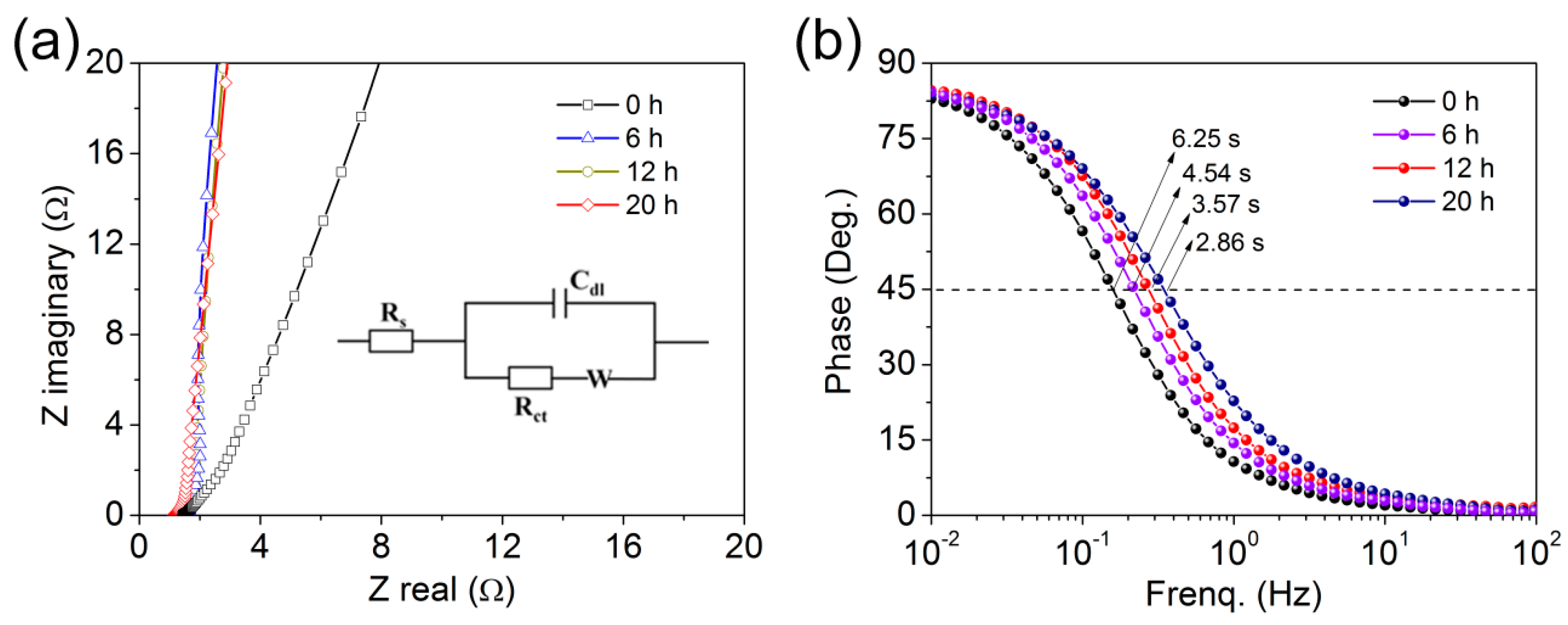
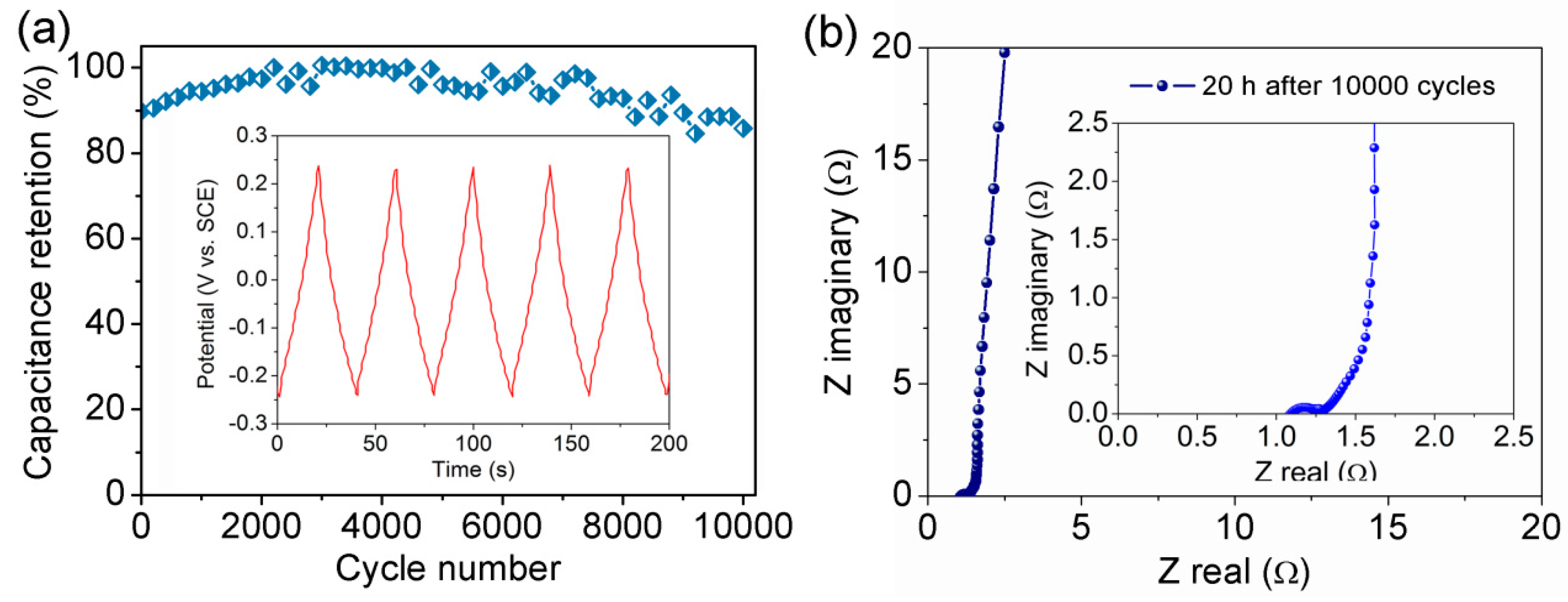
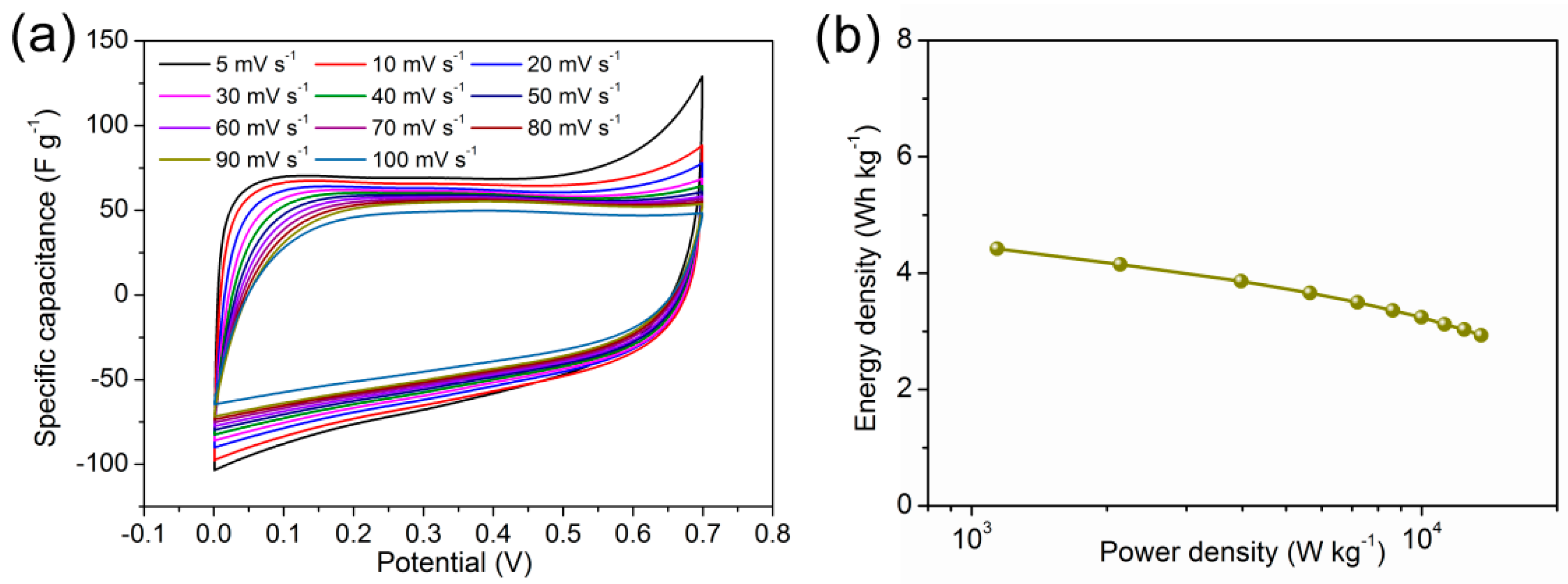
3.2. Electrochemical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dubal, D.P.; Ayyad, O.V.; Ruiz, V.; Gomez-Romero, P. Hybrid Energy Storage: The Merging of Battery and Supercapacitor. Chem. Soc. Rev. 2015, 44, 1777–1790. [Google Scholar] [CrossRef] [PubMed]
- Dunn, B.; Kamath, H.; Tarascon, J.M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Béguin, F.; Presser, V.; Balducci, A.; Frackowiak, E. Carbons and Electrolytes for Advanced Supercapacitors. Adv. Mater. 2014, 26, 2219–2251. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wei, H.; She, Y.; Tang, X.; Zhou, M.; Zang, Z.; Du, J.; Gao, C.; Guo, Y.; Bao, D. Flower-Like Nickel-Zinc-Cobalt Mixed Metal Oxide Nanowire Arrays for Electrochemical Capacitor Applications. J. Alloys Compd. 2017, 708, 146–153. [Google Scholar] [CrossRef]
- Hu, W.; Chen, R.; Xie, W.; Zou, L.; Qin, N.; Bao, D. CoNi2S4 Nanosheet Arrays Supported on Nickel Foams with Ultrahigh Capacitance for Aqueous Asymmetric Supercapacitor Applications. ACS Appl. Mater. Interfaces 2014, 6, 19318–19326. [Google Scholar] [CrossRef]
- Snook, G.A.; Kao, P.; Best, A.S. Conducting-Polymer-Based Supercapacitor Devices and Electrodes. J. Power Source 2011, 196, 1–12. [Google Scholar] [CrossRef]
- Yin, Y.; Zhou, G.; Gao, X.; Chen, J.; Zhang, L.; Xu, J.; Zhao, P.; Gao, F. α- and β-Phase Ni-Mg Hydroxide for High Performance Hybrid Supercapacitors. Nanomaterials 2019, 9, 1686. [Google Scholar] [CrossRef]
- Lu, Q.; Chen, J.G.; Xiao, J.Q. Nanostructured Electrodes for High-Performance Pseudocapacitors. Angew. Chem. Int. Ed. 2013, 44, 1882–1889. [Google Scholar] [CrossRef]
- Wang, C.; Xu, J.; Yuen, M.F.; Zhang, J.; Li, Y.; Chen, X.; Zhang, W. Hierarchical Composite Electrodes of Nickel Oxide Nanoflake 3D Graphene for High-Performance Pseudocapacitors. Adv. Funct. Mater. 2015, 24, 6372–6380. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhou, R.; Zhao, X.S. Graphene-Based Materials as Supercapacitor Electrodes. J. Mater. Chem. 2010, 20, 5983–5992. [Google Scholar] [CrossRef]
- Ng, V.M.H.; Huang, H.; Zhou, K.; Lee, P.S.; Que, W.; Xu, J.Z.; Kong, L.B. Recent Progress in Layered Transition Metal Carbides and/or Nitrides (MXenes) and Their Composites: Synthesis and Applications. J. Mater. Chem. A 2017, 5, 3039–3068. [Google Scholar]
- Xia, Q.X.; Shinde, N.M.; Yun, J.M.; Zhang, T.; Mane, R.S.; Mathur, S.; Kim, K.H. Bismuth Oxychloride/MXene Symmetric Supercapacitor with High Volumetric Energy Density. Electrochim. Acta 2018, 271, 351–360. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Pazhamalai, P.; Sahooa, S.; Kim, S.J. Titanium Carbide Sheet Based High Performance Wire Type Solid State Supercapacitors. J. Mater. Chem. A 2017, 5, 5726–5736. [Google Scholar] [CrossRef]
- Yang, Y.; Umrao, S.; Shen, L.; Lee, S. Large-Area Highly Conductive Transparent Two-Dimensional Ti2CTx Film. J. Phys. Chem. Lett. 2017, 8, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Ren, C.E.; Zhao, M.Q.; Yang, J.; Giammarco, J.M.; Qiu, J.; Barsouma, M.W.; Gogotsi, Y. Flexible and Conductive MXene Films and Nanocomposites with High Capacitance. Proc. Natl. Acad. Sci. USA 2014, 111, 16676–16681. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y.; Yu, X.; Nam, K.W.; Yang, X.Q.; Kolesnikov, A.I.; Kent, P.R.C. Role of Surface Structure on Li-Ion Energy Storage Capacity of Two-Dimensional Transition-Metal Carbides. J. Am. Chem. Soc. 2014, 136, 6385–6394. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, H.B.; Sun, R.; Liu, Y.; Liu, Z.; Zhou, A.; Yu, Z.Z. Hydrophobic, Flexible, and Lightweight MXene Foams for High-Performance Electromagnetic-Interference Shielding. Adv. Mater. 2017, 29, 1702367. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Mashtalir, O.; Ren, C.E.; Agnese, Y.D.; Rozier, P.; Taberna, P.L.; Naguib, M.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. Cation Intercalation and High Volumetric Capacitance of Two-Dimensional Titanium Carbide. Science 2013, 341, 1502–1505. [Google Scholar] [CrossRef]
- Ghidiu, M.; Kota, S.; Halim, J.; Sherwood, A.W.; Nedfors, N.; Rosen, J.; Mochalin, V.N.; Barsoum, M.W. Alkylammonium Cation Intercalation into Ti3C2 (MXene): Effects on Properties and Ion-Exchange Capacity Estimation. Chem. Mater. 2017, 29, 1099–1106. [Google Scholar] [CrossRef]
- Augustyn, V.; Come, J.; Lowe, M.A.; Kim, J.W.; Taberna, P.L.; Tolbert, S.H.; Abruña, H.D.; Simon, P.; Dunn, B. High-Rate Electrochemical Energy Storage through Li+ Intercalation Pseudocapacitance. Nat. Mater. 2013, 12, 518–522. [Google Scholar] [CrossRef]
- Mashtalir, O.; Naguib, M.; Mochalin, V.N.; Dall’Agnese, Y.; Heon, M.; Barsoum, M.W.; Gogotsi, Y. Intercalation and Delamination of Layered Carbides and Carbonitrides. Nat. Commun. 2013, 4, 1716. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Mashtalir, O.; Carle, J.; Presser, V.; Lu, J.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Transition Metal Carbides. ACS Nano 2012, 6, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D Metal Carbides and Nitrides (MXenes) for Energy Storage. Nat. Rev. Mater. 2017, 2, 16098. [Google Scholar] [CrossRef]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. 25th Anniversary Article: MXenes: A New Family of Two-Dimensional Materials. Adv. Mater. 2014, 26, 992–1005. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, L.H.; Birch, J.; Halim, J.; Barsoum, M.W.; Persson, P.O.Å. Atomically Resolved Structural and Chemical Investigation of Single MXene Sheets. Nano Lett. 2015, 15, 4955–4960. [Google Scholar] [CrossRef]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.; Gogotsi, Y.; Barsoum, M.W. Conductive Two-Dimensional Titanium Carbide Clay with High Volumetric Capacitance. Nature 2014, 516, 78–81. [Google Scholar] [CrossRef]
- Yan, J.; Ren, C.E.; Maleski, K.; Hatter, C.B.; Anasori, B.; Urbankowski, P.; Sarycheva, A.; Gogotsi, Y. Flexible MXene/Graphene Films for Ultrafast Supercapacitors with Outstanding Volumetric Capacitance. Adv. Funct. Mater. 2017, 27, 1701264. [Google Scholar] [CrossRef]
- Khazaei, M.; Ranjbar, A.; Ghorbani-Asl, M.; Arai, M.; Sasaki, T.; Liang, Y.; Yunoki, S. Nearly Free Electron States in MXenes. Phys. Rev. B 2016, 93, 205125. [Google Scholar] [CrossRef]
- Li, J.; Yuan, X.; Lin, C.; Yang, Y.; Xu, L.; Du, X.; Xie, J.; Lin, J.; Sun, J. Achieving High Pseudocapacitance of 2D Titanium Carbide (MXene) by Cation Intercalation and Surface Modification. Adv. Energy Mater. 2017, 7, 1602725. [Google Scholar] [CrossRef]
- Lin, T.; Chen, I.W.; Liu, F.; Yang, C.; Bi, H.; Xu, F.; Huang, F. Nitrogen-Doped Mesoporous Carbon of Extraordinary Capacitance for Electrochemical Energy Storage. Science 2015, 350, 1508–1513. [Google Scholar] [CrossRef]
- Bao, W.; Liu, L.; Wang, C.; Choi, S.; Wang, D.; Wang, G. Facile Synthesis of Crumpled Nitrogen-Doped MXene Nanosheets as A New Sulfur Host for Lithium-Sulfur Batteries. Adv. Energy Mater. 2018, 8, 1702485. [Google Scholar] [CrossRef]
- Wen, Y.; Rufford, T.E.; Chen, X.; Li, N.; Lyu, M.; Dai, L.; Wang, L. Nitrogen-Doped Ti3C2Tx MXene Electrodes For High-Performance Supercapacitors. Nano Energy 2017, 38, 368–376. [Google Scholar] [CrossRef]
- Yang, C.; Que, W.; Yin, X.; Tian, Y.; Yang, Y.; Que, M. Improved Capacitance of Nitrogen-Doped Delaminated Two-Dimensional Titanium Carbide by Urea-Assisted Synthesis. Electrochim. Acta 2017, 225, 416–424. [Google Scholar] [CrossRef]
- Yang, C.; Que, W.; Tang, Y.; Tian, Y.; Yin, X. Nitrogen and Sulfur Co-Doped 2D Titanium Carbides for Enhanced Electrochemical Performance. J. Electrochem. Soc. 2017, 164, 1939–1945. [Google Scholar] [CrossRef]
- Rakhi, R.B.; Ahmed, B.; Hedhili, M.N.; Anjum, D.H.; Alshareef, H.N. Effect of Postetch Annealing Gas Composition on The Structural and Electrochemical Properties of Ti2CTx MXene Electrodes for Supercapacitor Applications. Chem. Mater. 2015, 27, 5314–5323. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, J.; Du, Z.; Liu, Y.; Zhou, G.; Wang, J. Dopamine-Derived N-Doped Carbon Decorated Titanium Carbide Composite for Enhanced Supercapacitive Performance. Electrochim. Acta 2017, 254, 308–319. [Google Scholar] [CrossRef]
- Boota, M.; Anasori, B.; Voigt, C.; Zhao, M.; Barsoum, M.W.; Gogotsi, Y. Pseudocapacitive electrodes produced by oxidant-free polymerization of pyrrole between the layers of 2D titanium carbide (MXene). Adv. Mater. 2016, 28, 1517–1522. [Google Scholar] [CrossRef]
- Rakhi, R.B.; Ahmed, B.; Anjum, D.; Alshareef, H.N. Direct Chemical Synthesis of MnO2 Nanowhiskers on Transition-Metal Carbide Surfaces for Supercapacitor Applications. ACS Appl. Mater. Interfaces 2016, 8, 18806–18814. [Google Scholar] [CrossRef]
- Cao, M.; Wang, F.; Wang, L.; Wu, W.; Lv, W.; Zhu, J. Room Temperature Oxidation of Ti3C2 MXene for Supercapacitor Electrodes. J. Electrochem. Soc. 2017, 164, 3933–3942. [Google Scholar] [CrossRef]
- Zhu, M.; Huang, Y.; Deng, Q.; Zhou, J.; Pei, Z.; Xue, Q.; Huang, Y.; Wang, Z.; Li, H.; Huang, Q.; et al. Highly Flexible, Freestanding Supercapacitor Electrode with Enhanced Performance Obtained by Hybridizing Polypyrrole Chains with MXene. Adv. Energy Mater. 2016, 6, 1600969. [Google Scholar] [CrossRef]
- Jiang, Q.; Kurra, N.; Alhabeb, M.; Gogotsi, Y.; Alshareef, H.N. All Pseudocapacitive MXene-RuO2 Asymmetric Supercapacitors. Adv. Energy Mater. 2018, 8, 1703043. [Google Scholar] [CrossRef]
- Huang, J.; Meng, R.; Zu, L.; Wang, Z.; Feng, N.; Yang, Z.; Yu, Y.; Yang, J. Sandwich-Like Na0.23TiO2 Nanobelt/Ti3C2 MXene Composites from a Scalable in Situ Transformation Reaction for Long-Life High-Rate Lithium/Sodium-Ion Batteries. Nano Energy 2018, 46, 20–28. [Google Scholar] [CrossRef]
- Dong, Y.; Wu, Z.; Zheng, S.; Wang, X.; Qin, J.; Wang, S.; Shi, X.; Bao, X. Ti3C2 MXene-Derived Sodium/Potassium Titanate Nanoribbons for High-Performance Sodium/Potassium Ion Batteries with Enhanced Capacities. ACS Nano 2017, 11, 4792–4800. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; We, G.; Li, J.; Ji, Y.; Klyui, N.; Izotov, V.; Han, W. Binder-Free Ti3C2Tx MXene Electrode Film for Supercapacitor Produced. Chem. Eng. J. 2017, 317, 1026–1036. [Google Scholar] [CrossRef]
- Burton, A.W.; Ong, K.; ReaIgnatius, T.; Chan, Y. On the Estimation of Average Crystallite Size of Zeolites from the Scherrer Equation: A Critical Evaluation of its Application to Zeolites with One-Dimensional Pore Systems. Microporous Mesoporous Mater. 2009, 117, 75–90. [Google Scholar] [CrossRef]
- Maldonado, K.L.L.; Presa, P.D.L.; De La Rubia, M.A.; Crespo, P.; Frutos, J.D.; Hernando, A.; Aquino, J.A.M.; Galindo, J.T.E. Effects of Grain Boundary Width and Crystallite Size on Conductivity and Magnetic Properties of Magnetite Nanoparticles. J. Nanopart. Res. 2014, 16, 2482–2493. [Google Scholar] [CrossRef]
- Wang, Z.; Xuan, J.; Zhao, Z.; Li, Q.; Geng, F. Versatile Cutting Method for Producing Fluorescent Ultrasmall MXene Sheets. ACS Nano 2017, 11, 11559–11565. [Google Scholar] [CrossRef]
- Wang, W.R.; Wang, S.; Zhang, Y.; Jin, D.; Tao, X.; Zhang, L. Graphene-Coupled Ti3C2 MXenes-Derived TiO2 Mesostructure Promising Sodium-Ion Capacitor Anode with Fast Ion Storage and Long-Term Cycling. J. Mater. Chem. A 2018, 6, 1017–1027. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Dong, S.; Ye, Z.; Guo, Y. One-Step Hydrothermal Synthesis of a TiO2-Ti3C2Tx Nanocomposite with Small Sized TiO2 Nanoparticles. Ceram. Int. 2017, 43, 11065–11070. [Google Scholar] [CrossRef]
- Xue, Q.; Zhang, H.; Zhu, M.; Pei, Z.; Li, H.; Wang, Z.; Huang, Y.; Huang, Y.; Deng, Q.; Zhou, J.; et al. Photoluminescent Ti3C2 MXene Quantum Dots for Multicolor Cellular Imaging. Adv. Mater. 2017, 29, 1604847. [Google Scholar] [CrossRef]
- Luo, J.; Tao, X.; Zhang, J.; Xia, Y.; Huang, H.; Zhang, L.; Gan, Y.; Liang, C.; Zhang, W. Sn4+ Ions Decorated Highly Conductive Ti3C2 MXene: Promising Lithium-Ion Anodes with Enhanced Volumetric Capacity and Cyclic Performance. ACS Nano 2016, 10, 2491–2499. [Google Scholar] [CrossRef] [PubMed]
- Sedira, S.; Achour, S.; Avci, A.; Eskizeybekb, V. Physical Deposition of Carbon Doped Titanium Nitride Film by DC Magnetron Sputtering for Metallic Implant Coating Use. Appl. Surf. Sci. 2014, 5, 81–85. [Google Scholar] [CrossRef]
- Xie, X.; Zhao, M.; Anasori, B.; Maleski, K.; Ren, C.E.; Li, J.; Byles, B.W.; Pomerantseva, E.; Wang, G.; Gogotsi, Y. Porous Heterostructured MXene/Carbon Nanotube Composite Paper with High Volumetric Capacity for Sodium-Based Energy Storage Devices. Nano Energy 2016, 26, 513–523. [Google Scholar] [CrossRef]
- Peng, C.; Yang, X.; Li, Y.; Yu, H.; Wang, H.; Peng, F. Hybrids of Two-Dimensional Ti3C2 and TiO2 Exposing {001} Facets toward Enhanced Photocatalytic Activity. ACS Appl. Mater. Interfaces 2016, 8, 6051–6060. [Google Scholar] [CrossRef]
- Qian, A.; Hyeon, S.E.; Seo, J.Y.; Chung, C.H. Capacitance Changes Associated with Cation-Transport in Free-Standing Flexible Ti3C2Tx MXene Film Electrodes. Electrochim. Acta 2018, 266, 86–93. [Google Scholar] [CrossRef]
- Soundiraraju, B.; George, B.K. Two-Dimensional Titanium Nitride (Ti2N) MXene Synthesis, Characterization, and Potential Application as Surface-Enhanced Raman Scattering Substrate. ACS Nano 2017, 11, 8892–8900. [Google Scholar] [CrossRef]
- Lu, X.; Zhu, J.; Wu, W.; Zhang, B. Hierarchical Architecture of PANI@TiO2/Ti3C2Tx Ternary Composite Electrode for Enhanced Electrochemical Performance. Electrochim. Acta 2017, 228, 282–289. [Google Scholar] [CrossRef]
- Han, M.; Yin, X.; Wu, H.; Hou, Z.; Song, C.; Li, X.; Zhang, L.; Cheng, L. Ti3C2 MXenes with Modified Surface for High-Performance Electromagnetic Absorption and Shielding in the X-Band. ACS Appl. Mater. Interfaces 2016, 8, 21011–21019. [Google Scholar] [CrossRef]
- Zhang, C.; Anasori, B.; Seral-Ascaso, A.; Park, S.H.; McEvoy, N.; Shmeliov, A.; Duesberg, G.S.; Coleman, J.N.; Gogotsi, Y.; Nicolosi, V. Transparent, Flexible, and Conductive 2D Titanium Carbide (MXene) Films with High Volumetric Capacitance. Adv. Mater. 2017, 29, 1702678. [Google Scholar] [CrossRef]
- Zhu, K.; Zhang, H.; Ye, K.; Zhao, W.; Yan, J.; Cheng, K.; Wang, G.; Yang, B.; Cao, D. Two-Dimensional Titanium Carbide MXene as Capacitor-Type Electrode for Rechargeable Aqueous Li-Ion and Na-Ion Capacitor Batteries. ChemElectroChem 2017, 4, 1–9. [Google Scholar] [CrossRef]
- Ren, C.E.; Hatzell, K.B.; Alhabeb, M.; Ling, Z.; Mahmoud, K.A.; Gogotsi, Y. Charge- and Size-Selective Ion Sieving through Ti3C2Tx MXene Membranes. J. Phys. Chem. Lett. 2015, 6, 4026–4031. [Google Scholar] [CrossRef] [PubMed]
- Lukatskaya, M.R.; Kota, S.; Lin, Z.; Zhao, M.Q.; Shpigel, N.; Levi, M.D.; Halim, J.; Taberna, P.L.; Barsoum, M.W.; Simon, P.; et al. Ultra-High-Rate Pseudocapacitive Energy Storage in Two-Dimensional Transition Metal Carbides. Nat. Energy 2017, 6, 17105. [Google Scholar] [CrossRef]
- Xu, S.; Wei, G.; Li, J.; Han, W.; Gogotsi, Y. Flexible MXene-Graphene Electrodes with High Volumetric Capacitance for Integrated Co-Cathode Energy Conversion/Storage Devices. J. Mater. Chem. A 2017, 5, 17442–17451. [Google Scholar] [CrossRef]
- Lin, Z.; Barbara, D.; Taberna, P.L.; Aken, K.L.V.; Anasori, B.; Gogotsi, Y.; Simon, P. Capacitance of Ti3C2Tx MXene in Ionic Liquid Electrolyte. J. Power Source 2016, 326, 575–579. [Google Scholar] [CrossRef]
- Yoon, Y.; Lee, M.; Kim, S.K.; Bae, G.; Song, W.; Myung, S.; Lim, J.; Lee, S.S.; Zyung, T.; An, K.S. A Strategy for Synthesis of Carbon Nitride Induced Chemically Doped 2D MXene for High-Performance Supercapacitor Electrodes. Adv. Energy Mater. 2018, 8, 1703173. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, Z.; Fang, B.; Huang, T.; Cai, S.; Chen, H.; Liu, Y.; Gopalsamy, K.; Gao, W.; Gao, C. MXene/Graphene Hybrid Fibers for High Performance Flexible Supercapacitors. J. Mater. Chem. A 2017, 5, 22113–22119. [Google Scholar] [CrossRef]

| Materials | Contents | |||||
|---|---|---|---|---|---|---|
| Ti | C | O | F | Al | N | |
| Ti3C2Tx | 32.2 | 28.3 | 20.1 | 15.6 | 3.8 | - |
| 6 h N-TiO2/TiN/Ti3C2Tx | 27.8 | 34.5 | 23.5 | 10.5 | 2.9 | 1.3 |
| 12 h N-TiO2/TiN/Ti3C2Tx | 23.7 | 38.1 | 25.4 | 9.4 | 0.6 | 2.8 |
| 20 h N-TiO2/TiN/Ti3C2Tx | 23.8 | 39.2 | 24.1 | 7.9 | 0.8 | 4.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; She, Y.; Zhang, C.; Kang, S.; Zhou, J.; Hu, W. RETRACTED: Nitrogen Doped Intercalation TiO2/TiN/Ti3C2Tx Nanocomposite Electrodes with Enhanced Pseudocapacitance. Nanomaterials 2020, 10, 345. https://doi.org/10.3390/nano10020345
Yang B, She Y, Zhang C, Kang S, Zhou J, Hu W. RETRACTED: Nitrogen Doped Intercalation TiO2/TiN/Ti3C2Tx Nanocomposite Electrodes with Enhanced Pseudocapacitance. Nanomaterials. 2020; 10(2):345. https://doi.org/10.3390/nano10020345
Chicago/Turabian StyleYang, Ben, Yin She, Changgeng Zhang, Shuai Kang, Jin Zhou, and Wei Hu. 2020. "RETRACTED: Nitrogen Doped Intercalation TiO2/TiN/Ti3C2Tx Nanocomposite Electrodes with Enhanced Pseudocapacitance" Nanomaterials 10, no. 2: 345. https://doi.org/10.3390/nano10020345
APA StyleYang, B., She, Y., Zhang, C., Kang, S., Zhou, J., & Hu, W. (2020). RETRACTED: Nitrogen Doped Intercalation TiO2/TiN/Ti3C2Tx Nanocomposite Electrodes with Enhanced Pseudocapacitance. Nanomaterials, 10(2), 345. https://doi.org/10.3390/nano10020345





