Hierarchically Porous Carbon Derived from Biomass Reed Flowers as Highly Stable Li-Ion Battery Anode
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Characterization
2.3. Electrochemical Measurements
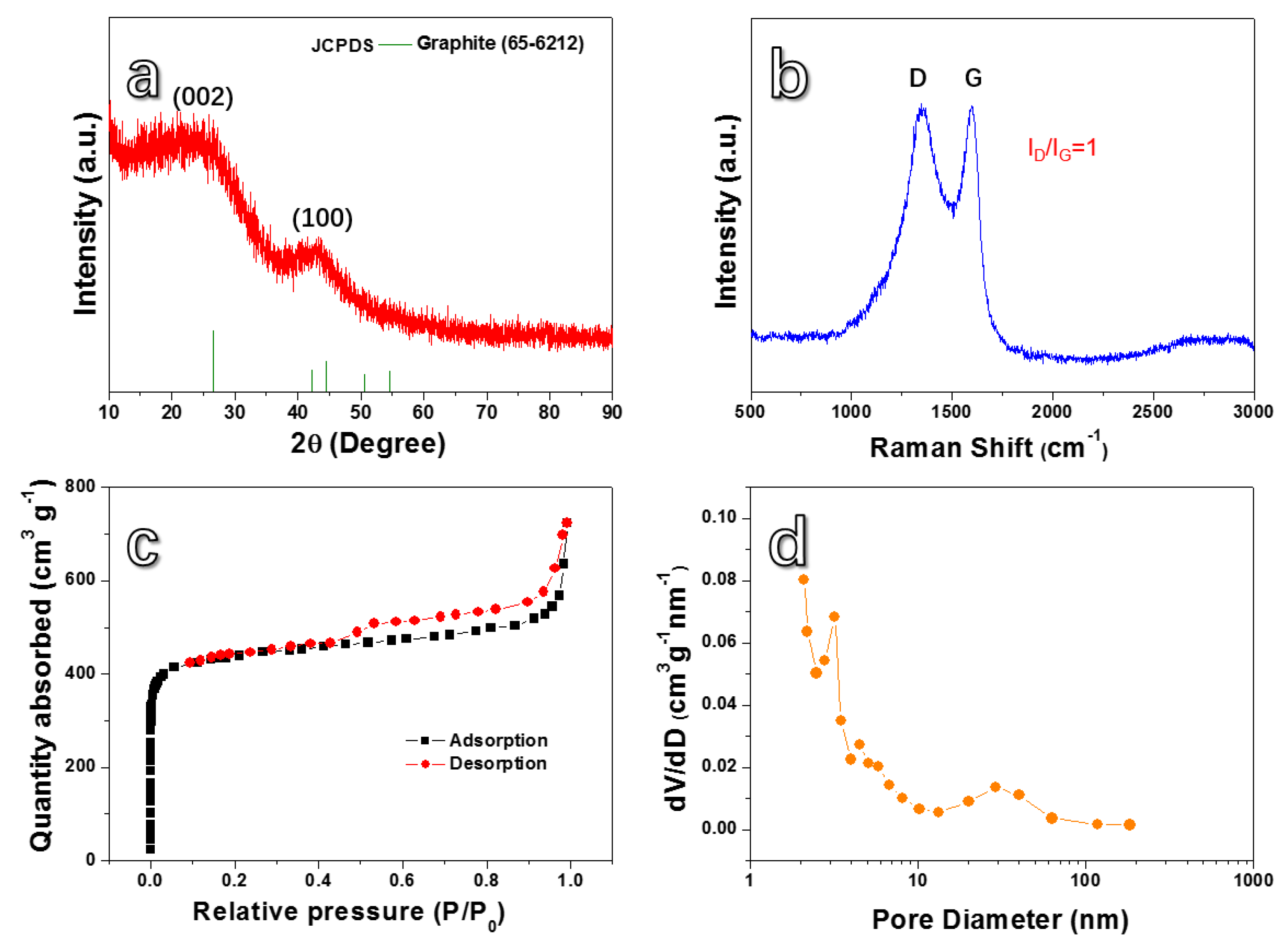
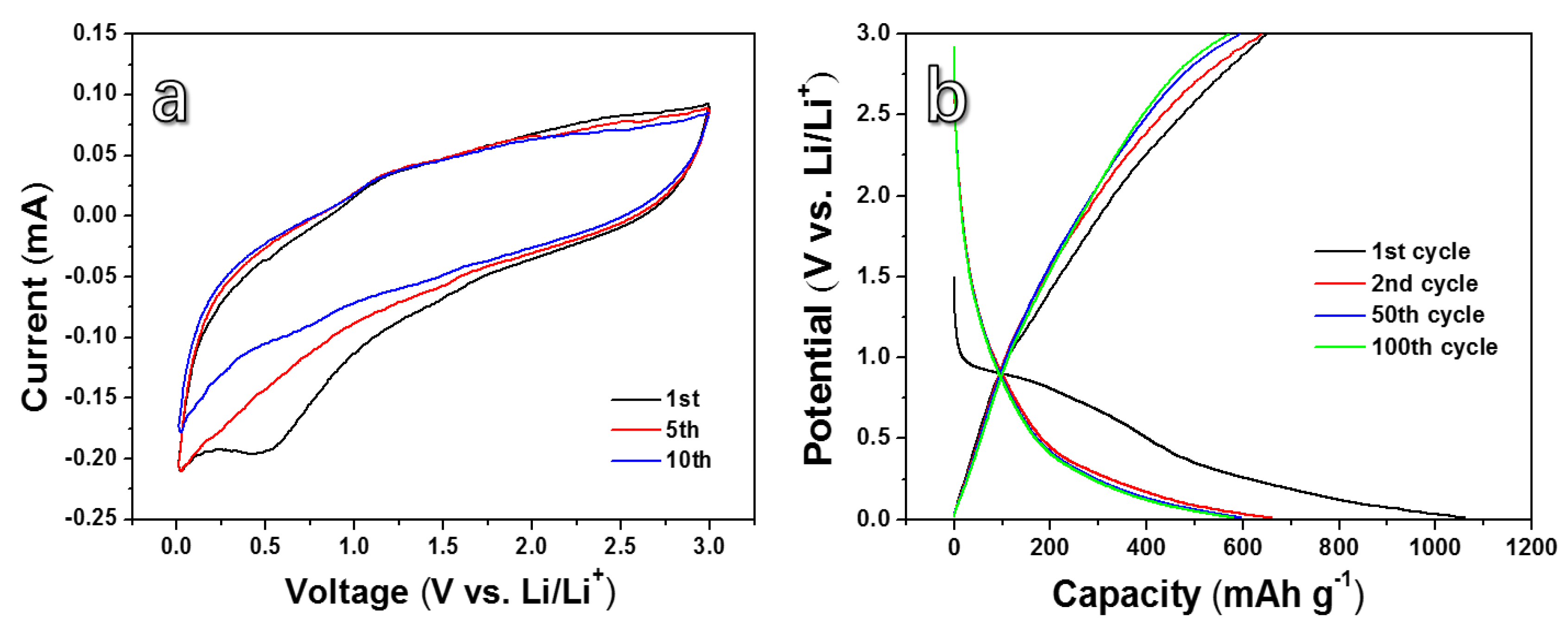
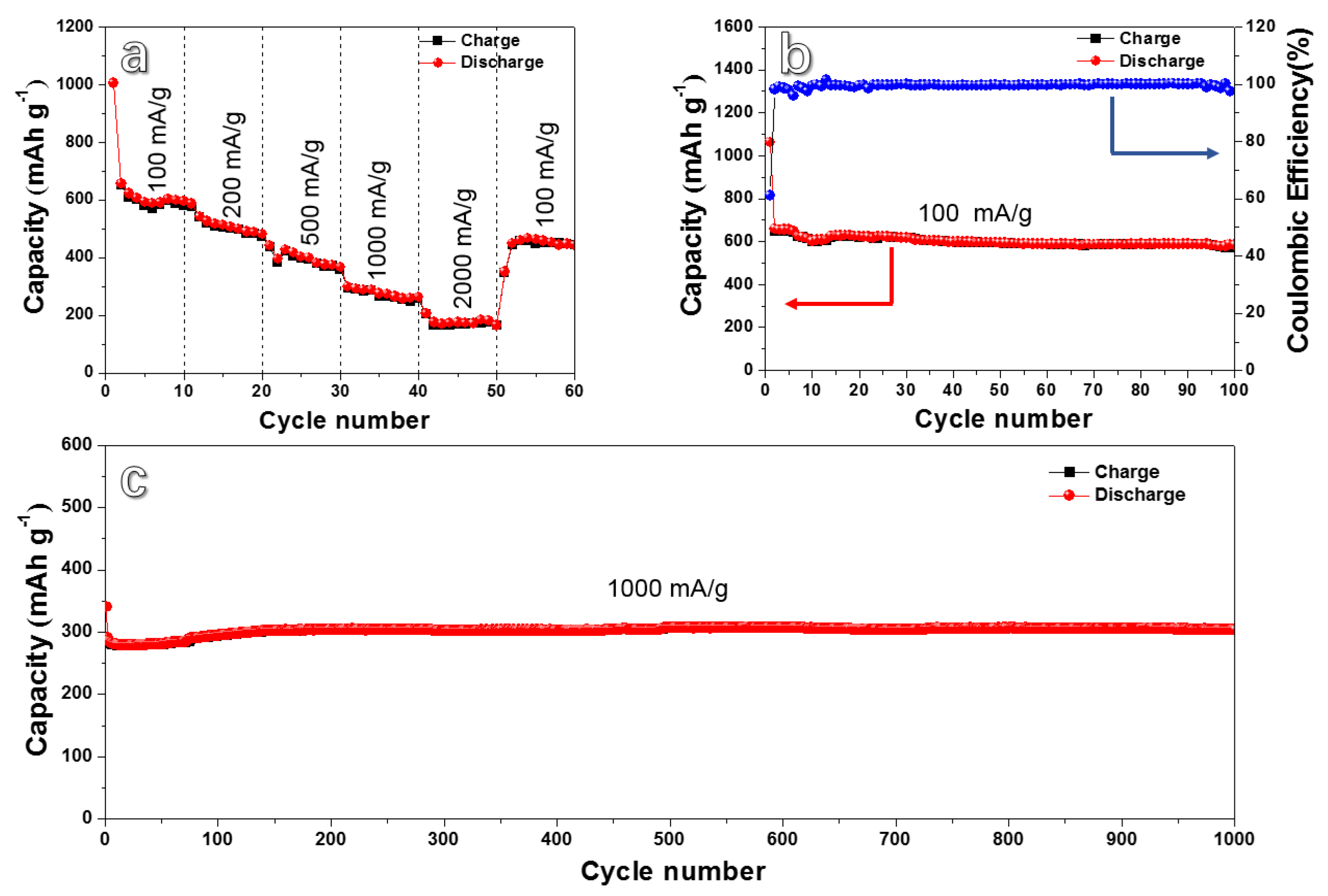
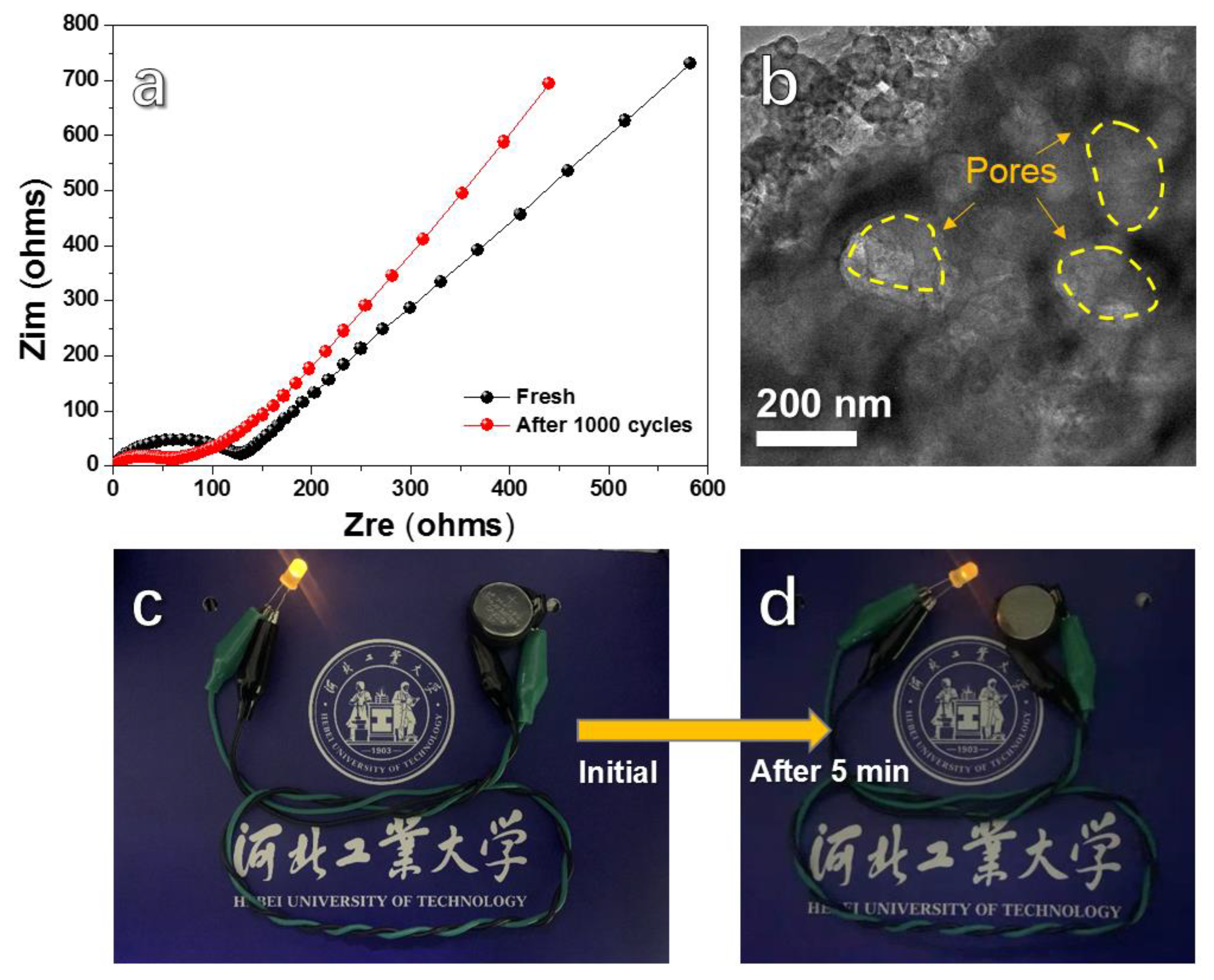
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Qin, C.L.; Zheng, D.H.; Hu, Q.F.; Zhang, X.M.; Wang, Z.F.; Li, Y.Y.; Zhu, J.S.; Ou, J.Z.; Yang, C.H.; Wang, Y.C. Flexible integrated metallic glass-based sandwich electrodes for high-performance wearable all-solid-state supercapacitors. Appl. Mater. Today 2020, 19, 100539. [Google Scholar] [CrossRef]
- Qin, C.L.; Zhang, Y.S.; Wang, Z.F.; Xiong, H.Q.; Yu, H.; Zhao, W.M. One-step synthesis of CuO@brass foil by dealloying method for low-cost flexible supercapacitor electrodes. J. Mater. Sci. Mater. Electron. 2016, 27, 9206–9215. [Google Scholar] [CrossRef]
- Wang, Z.F.; Fei, P.Y.; Xiong, H.Q.; Qin, C.L.; Zhao, W.M.; Liu, X.Z. CoFe2O4 nanoplates synthesized by dealloying method as high performance Li-ion battery anodes. Electrochim. Acta 2017, 252, 295–305. [Google Scholar] [CrossRef]
- Liu, X.Z.; Zhang, R.E.; Yu, W.; Yang, Y.J.; Wang, Z.F.; Zhang, C.; Bando, Y.; Golberg, D.; Wang, X.; Ding, Y. Three-dimensional electrode with conductive Cu framework for stable and fast Li-ion storage. Energy Storage Mater. 2018, 11, 83–90. [Google Scholar] [CrossRef]
- Yu, S.H.; Feng, X.; Zhang, N.; Seok, J.; Abruña, H.D. Understanding conversion-type electrodes for lithium rechargeable batteries. Acc. Chem. Res. 2018, 51, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Zhu, B.; Lu, Z.D.; Liu, N.; Zhu, J. Challenges and recent progress in the development of Si anodes for lithium-ion battery. Adv. Energy Mater. 2017, 7, 1700715. [Google Scholar] [CrossRef]
- Wang, Z.F.; Zhang, X.M.; Yan, Y.H.; Zhang, Y.G.; Wang, Y.C.; Qin, C.L.; Bakenov, Z. Nanoporous GeO2/Cu/Cu2O network synthesized by dealloying method for stable Li-ion storage. Electrochim. Acta 2019, 300, 363–372. [Google Scholar] [CrossRef]
- Wang, Z.F.; Zhang, Y.S.; Xiong, H.Q.; Qin, C.L.; Zhao, W.M.; Liu, X.Z. Yucca fern shaped CuO nanowires on Cu foam for remitting capacity fading of Li-ion battery anodes. Sci. Rep. 2018, 8, 6530. [Google Scholar] [CrossRef]
- Zhou, X.; Yu, L.; Lou, X.W.D. Formation of uniform N-doped carbon-coated SnO2 submicroboxes with enhanced lithium storage properties. Adv. Energy Mater. 2016, 6, 1600451. [Google Scholar] [CrossRef]
- Wang, Z.F.; Zhang, X.M.; Liu, X.L.; Wang, Y.C.; Zhang, Y.G.; Li, Y.Y.; Zhao, W.M.; Qin, C.L.; Mukanova, A.; Bakenov, Z. Bimodal nanoporous NiO@Ni–Si network prepared by dealloying method for stable Li-ion storage. J. Power Sources 2020, 449, 227550. [Google Scholar] [CrossRef]
- Zhao, W.M.; Fei, P.Y.; Zhang, X.M.; Zhang, Y.G.; Qin, C.L.; Wang, Z.F. Porous TiO2/Fe2O3 nanoplate composites prepared by de-alloying method for Li-ion batteries. Mater. Lett. 2018, 211, 254–257. [Google Scholar] [CrossRef]
- Wang, Z.F.; Zhang, X.M.; Liu, X.L.; Zhang, W.Q.; Zhang, Y.G.; Li, Y.Y.; Qin, C.L.; Zhao, W.M.; Bakenov, Z. Dual-network nanoporous NiFe2O4/NiO composites for high performance Li-ion battery anodes. Chem. Eng. J. 2020, 388, 124207. [Google Scholar] [CrossRef]
- Ma, W.Q.; Liu, X.Z.; Wang, X.; Wang, Z.F.; Zhang, R.E.; Yuan, Z.H.; Ding, Y. Crystalline Cu-silicide stabilizes the performance of a high capacity Si-based Li-ion battery anode. J. Mater. Chem. A 2016, 4, 19140–19146. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Peng, Y.; Wang, X.; Wang, N.; Wang, J.; Zhao, J. Nitrogen-doped biomass-based hierarchical porous carbon with large mesoporous volume for application in energy storage. Chem. Eng. J. 2018, 348, 850–859. [Google Scholar] [CrossRef]
- Talapaneni, S.N.; Lee, J.H.; Je, S.H.; Buyukcakir, O.; Kwon, T.W.; Polychronopoulou, K.; Choi, J.W.; Coskun, A. Chemical blowing approach for ultramicroporous carbon nitride frameworks and their applications in gas and energy storage. Adv. Funct. Mater. 2017, 27, 1604658. [Google Scholar] [CrossRef]
- Zhu, M.; Luo, Z.; Pan, A.; Yang, H.; Zhu, T.; Liang, S.; Cao, G. N-doped one-dimensional carbonaceous backbones supported MoSe2 nanosheets as superior electrodes for energy storage and conversion. Chem. Eng. J. 2018, 334, 2190–2200. [Google Scholar] [CrossRef]
- Benzigar, M.R.; Talapaneni, S.N.; Joseph, S.; Ramadass, K.; Singh, G.; Scaranto, J.; Ravon, U.; Al-Bahily, K.; Vinu, A. Recent advances in functionalized micro and mesoporous carbon materials: Synthesis and applications. Chem. Soc. Rev. 2018, 47, 2680–2721. [Google Scholar] [CrossRef]
- Atchudan, R.; Muthuchamy, N.; Perumal, S.; Vinodh, R.; Park, K.H.; Lee, Y.R. An ultrasensitive photoelectrochemical biosensor for glucose based on bio-derived nitrogen-doped carbon sheets wrapped titanium dioxide nanoparticles. Biosens. Bioelectron. 2019, 126, 160–169. [Google Scholar] [CrossRef]
- Thirukumaran, P.; Balasubramanian, R.; Atchudan, R.; Parveen, A.S.; Lee, Y.R.; Kim, S.C. Metal-free nitrogen-rich glassy carbon as an electrocatalyst for hydrogen evolution reaction. Mater. Res. Bull. 2020, 124, 110734. [Google Scholar] [CrossRef]
- Jin, R.; Cui, Y.; Wang, Q.; Li, G. Facile fabrication of CNTs@C@MoSe2@Se hybrids with amorphous structure for high performance anode in lithium-ion batteries. J. Colloid Interface Sci. 2017, 508, 435–442. [Google Scholar] [CrossRef]
- Kang, J.; Su, Q.; Feng, H.; Huang, P.; Du, G.; Xu, B. MoSe2 nanosheets-wrapped flexible carbon cloth as binder-free anodes for high-rate lithium and sodium ion storages. Electrochim. Acta 2019, 301, 29–38. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.; Hyeon, T. Recent progress in the synthesis of porous carbon materials. Adv. Mater. 2006, 18, 2073–2094. [Google Scholar] [CrossRef]
- Wang, H.W.; Yi, H.; Zhu, C.R.; Wang, X.F.; Fan, H.J. Functionalized highly porous graphitic carbon fibers for high-rate supercapacitive electrodes. Nano Energy 2015, 13, 658–669. [Google Scholar] [CrossRef]
- Atchudan, R.; Perumal, S.; Thirukumaran, P.; Vinodh, R.; Lee, Y.R. Green synthesis of nitrogen-doped carbon nanograss for supercapacitors. J. Taiwan Inst. Chem. Eng. 2019, 102, 475–486. [Google Scholar] [CrossRef]
- Atchudan, R.; Perumal, S.; Parveen, A.S.; Lee, Y.R. Electrocatalytic and energy storage performance of bio-derived sulphur-nitrogen-doped carbon. J. Electroanal. Chem. 2019, 833, 357–369. [Google Scholar] [CrossRef]
- Zheng, F.C.; Liu, D.; Xia, G.L.; Yang, Y.; Liu, T.; Wu, M.Z.; Chen, Q.W. Biomass waste inspired nitrogen-doped porous carbon materials as high-performance anode for lithium-ion batteries. J. Alloys Compd. 2017, 693, 1197–1204. [Google Scholar] [CrossRef]
- Mullaivananathan, V.; Sathish, R.; Kalaiselvi, N. Coir pith derived bio-carbon: Demonstration of potential anode behavior in lithium-ion batteries. Electrochim. Acta 2017, 225, 143–150. [Google Scholar] [CrossRef]
- Ru, H.H.; Xiang, K.X.; Zhou, W.; Zhu, Y.R.; Zhao, X.S.; Chen, H. Bean-dreg-derived carbon materials used as superior anode material for lithium-ion batteries. Electrochim. Acta 2016, 222, 551–560. [Google Scholar] [CrossRef]
- Zhu, Y.D.; Huang, Y.; Chen, C.; Wang, M.Y.; Liu, P.B. Phosphorus-doped porous biomass carbon with ultra-stable performance in sodium storage and lithium storage. Electrochim. Acta 2019, 321, 134698. [Google Scholar] [CrossRef]
- Dou, Y.L.; Liu, X.; Yu, K.F.; Wang, X.F.; Liu, W.P.; Liang, J.C.; Liang, C. Biomass porous carbon derived from jute fiber as anode materials for lithium-ion batteries. Diam. Relat. Mater. 2019, 98, 107514. [Google Scholar] [CrossRef]
- Atchudan, R.; Perumal, S.; Lee, Y.K. Green synthesis of nitrogen-doped graphitic carbon sheets with use of prunus persica for supercapacitor applications. Appl. Surf. Sci. 2017, 393, 276–286. [Google Scholar] [CrossRef]
- Purnomo, C.W.; Kesuma, E.P.; Perdana, I.; Aziz, M. Lithium recovery from spent Li-ion batteries using coconut shell activated carbon. Waste. Manage 2018, 79, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.Y.; Luo, Y.Y.; Lei, W.D.; Xiang, Q.; Ren, W.; Song, W.C.; Chen, K.; Sun, J. Hierarchical porous carbon materials derived from waste lentinus edodes by a hybrid hydrothermal and molten salt process for supercapacitor applications. Appl. Surf. Sci. 2018, 462, 862–871. [Google Scholar] [CrossRef]
- Zhong, Y.; Shi, T.L.; Huang, Y.Y.; Cheng, S.Y.; Liao, G.L.; Tang, Z.R. One-step synthesis of porous carbon derived from starch for all-carbon binder-free high-rate supercapacitor. Electrochim. Acta 2018, 269, 676–685. [Google Scholar] [CrossRef]
- Ru, H.H.; Bai, N.B.; Xiang, K.X.; Zhou, W.; Chen, H.; Zhao, X.S. Porous carbons derived from microalgae with enhanced electrochemical performance for lithium-ion batteries. Electrochim. Acta 2016, 194, 10–16. [Google Scholar] [CrossRef]
- Ou, J.K.; Yang, L.; Zhang, Z.; Xi, X.H. Honeysuckle-derived hierarchical porous nitrogen, sulfur, dual-doped carbon for ultra-high rate lithium ion battery anodes. J. Power Sources 2016, 333, 193–202. [Google Scholar] [CrossRef]
- Song, S.; Ma, F.; Wu, G.; Ma, D.; Geng, W.; Wan, J. Facile self-templating large scale preparation of biomass-derived 3D hierarchical porous carbon for advanced supercapacitors. J. Mater. Chem. A 2015, 3, 18154–18162. [Google Scholar] [CrossRef]
- Sekar, S.; Lee, Y.; Kim, D.Y.; Lee, S. Substantial LIB anode performance of graphitic carbon nanoflakes derived from biomass green-tea waste. Nanomaterials 2019, 9, 871. [Google Scholar] [CrossRef]
- Nagalakshmi, M.; Kalaiselvi, N. Mesoporous dominant cashewnut sheath derived bio-carbon anode for LIBs and SIBs. Electrochim. Acta 2019, 304, 175–183. [Google Scholar] [CrossRef]
- Sankar, S.; Lee, H.; Jung, H.; Kim, A.; Ahmed, A.T.A.; Inamdar, A.I.; Kim, H.; Lee, S.; Im, H.; Kim, D.Y. Ultrathin graphene nanosheets derived from rice husks for sustainable supercapacitor electrodes. New J. Chem. 2017, 41, 13792–13797. [Google Scholar] [CrossRef]
- Wang, S.X.; Zou, K.X.; Qian, Y.X.; Deng, Y.F.; Zhang, L.; Chen, G.H. Insight to the synergistic effect of N-doping level and pore structure on improving the electrochemical performance of sulfur/N-doped porous carbon cathode for Li-S batteries. Carbon 2019, 144, 745–755. [Google Scholar] [CrossRef]
- Qie, L.; Chen, W.M.; Wang, Z.H.; Shao, Q.G.; Li, X.; Yuan, L.X.; Hu, X.L.; Zhang, W.X.; Huang, Y.H. Nitrogen-doped porous carbon nanofiber webs as anodes for lithium ion batteries with a superhigh capacity and rate capability. Adv. Mater. 2012, 24, 2047–2050. [Google Scholar] [CrossRef]
- Yu, K.F.; Wang, J.J.; Song, K.X.; Wang, X.F.; Liang, C.; Dou, Y.L. Hydrothermal synthesis of cellulose-derived carbon nanospheres from corn straw as anode materials for lithium ion batteries. Nanomaterials 2019, 9, 93. [Google Scholar] [CrossRef]
- Sekar, S.; Ahmed, A.A.; Inamdar, A.I.; Lee, Y.; Im, H.; Kim, D.Y.; Lee, S. Activated carbon-decorated spherical silicon nanocrystal composites synchronously-derived from rice husks for anodic source of lithium-ion battery. Nanomaterials 2019, 9, 1055. [Google Scholar] [CrossRef]
- Lim, D.G.; Kim, K.; Razdan, M.; Diaz, R.; Osswald, S.; Pol, V.G. Lithium storage in structurally tunable carbon anode derived from sustainable source. Carbon 2017, 121, 134–142. [Google Scholar] [CrossRef]
- Xiang, J.; Lv, W.; Mu, C.; Zhao, J.; Wang, B. Activated hard carbon from orange peel for lithium/sodium ion battery anode with long cycle life. J. Alloy. Compd. 2017, 701, 870–874. [Google Scholar] [CrossRef]
- Cui, J.; Xi, Y.; Chen, S.; Li, D.; She, X.; Sun, J.; Han, W.; Yang, D.; Guo, S. Prolifera-green-tide as sustainable source for carbonaceous aerogels with hierarchical pore to achieve multiple energy storage. Adv. Funct. Mater. 2016, 26, 8487–8495. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, N.; Liu, D.; Xu, J.; Sha, J.; Yin, J.; Tan, X.; Yang, H.; Lu, H.; Lin, H. Direct carbonization of rice husk to prepare porous carbon for supercapacitor applications. Energy 2017, 128, 618–625. [Google Scholar] [CrossRef]
- Kim, J.M.; Guccini, V.; Seong, K.D.; Oh, J.; Salazar-Alvarez, G.; Piao, Y. Extensively interconnected silicon nanoparticles via carbon network derived from ultrathin cellulose nanofibers as high performance lithium ion battery anodes. Carbon 2017, 118, 8–17. [Google Scholar] [CrossRef]
- Liu, L.; Yang, L.; Wang, P.; Wang, C.Y.; Cheng, J.; Zhang, G.; Gu, J.J.; Cao, F.F. Porous nitrogen-doped carbon derived from peanut shell as anode material for lithium ion battery. Int. J. Electrochem. Sci. 2017, 12, 9844–9854. [Google Scholar] [CrossRef]
- Gao, F.; Geng, C.; Xiao, N.; Qu, J.Y.; Qiu, J.S. Hierarchical porous carbon sheets derived from biomass containing an activation agent and in-built template for lithium ion batteries. Carbon 2018, 139, 1085–1092. [Google Scholar] [CrossRef]
- Fan, W.J.; Zhang, H.; Wang, H.L.; Zhao, X.C.; Sun, S.J.; Shi, J.; Huang, M.H.; Liu, W.; Zheng, Y.L.; Li, P. Dual-doped hierarchical porous carbon derived from biomass for advanced supercapacitors and lithium ion batteries. RSC Adv. 2019, 9, 32382–32394. [Google Scholar] [CrossRef]
- Luna-Lama, F.; Rodríguez-Padrón, D.; Puente-Santiago, A.R.; Muñoz-Batista, M.J.; Caballero, A.; Balu, A.M.; Romero, A.A.; Luque, R. Non-porous carbonaceous materials derived from coffee waste grounds as highly sustainable anodes for lithium-ion batteries. J. Clean. Prod. 2019, 207, 411–417. [Google Scholar] [CrossRef]
- Zhang, C.; Xie, Z.H.; Yang, W.F.; Liang, Y.; Meng, D.D.; He, X.; Liang, P.; Zhang, Z.H. NiCo2O4/biomass-derived carbon composites as anode for high-performance lithium ion batteries. J. Power Sources 2020, 451, 227761. [Google Scholar] [CrossRef]
- Zhang, Q.B.; Chen, H.X.; Luo, L.L.; Zhao, B.; Luo, H.; Han, X.; Wang, J.W.; Wang, C.M.; Yang, Y.; Zhu, T.; et al. Harnessing the concurrent reaction dynamics in active Si and Ge to achieve high performance of lithium-ion batteries. Energy Environ. Sci. 2018, 11, 669–681. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Wang, S.; Matsumura, Y.; Yamaguchi, C. Study on irreversible capacity loss in lithium ion rechargeable batteries. Synth. Met. 1997, 85, 1343–1344. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Wang, Y.J.; Meng, Y.; Tan, G.Q.; Guo, Y.; Xiao, D. Porous nitrogen-doped carbon tubes derived from reed catkins as a high-performance anode for lithium ion batteries. RSC Adv. 2016, 6, 98434–98439. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Yu, Y.; Zhang, Y.; Zhu, X.Y.; Yue, H.Y.; Wang, Z.Q.; Zhou, W.H. Synthesis of Si/C composites derived from directly-carbonized reed plants as high-performance anode for lithium ion batteries. J. For. Eng. 2019, 4, 84–91. [Google Scholar]
- Liu, J.; Kopold, P.; Maier, J.; Yu, Y. Energy storage materials from nature through nanotechnology: A sustainable route from reed plants to a silicon anode for lithium-ion batteries. Angew. Chem. Int. Edit. 2015, 54, 9632–9636. [Google Scholar] [CrossRef]
- Wu, Z.R.; Wang, L.P.; Huang, J.; Zou, J.; Chen, S.L.; Cheng, H.; Jiang, C.; Gao, P.; Niu, X.B. Loofah-derived carbon as an anode material for potassium ion and lithium ion batteries. Electrochimi. Acta 2019, 306, 446–453. [Google Scholar] [CrossRef]
- Sankar, S.; Saravanan, S.; Inamdar, A.I.; Im, H.; Lee, S.; Kim, D.Y. Spherical activated-carbon nanoparticles derived from biomass green tea wastes for anode material of lithium-ion battery. Mater. Lett. 2019, 240, 189–192. [Google Scholar] [CrossRef]
- Tao, L.; Huang, Y.B.; Zheng, Y.W.; Yang, X.Q.; Liu, C.; Di, M.W.; Larpkiattaworn, S.; Nimlos, M.R.; Zheng, Z.F. Porous carbon nanofiber derived from a waste biomass as anode material in lithium-ion batteries. J. Taiwan Inst. Chem. Eng. 2019, 95, 217–226. [Google Scholar] [CrossRef]


| Biomasses (Corresponding Carbon Materials) | Current Density (mA g−1) | Cycle Number | Reversible Capacity (mAh g−1) | Ref. |
|---|---|---|---|---|
| Coir pith waste (porous carbon) | 100 | 50 | 837 | [27] |
| Bean-dreg (graphitic sheet) | 100 | 100 | 396 | [28] |
| Waste green tea (graphitic carbon nanoflakes) | 100 | 100 | 400 | [38] |
| Rice husks (carbon-decorated silicon spheres) | 100 | 100 | 429 | [44] |
| Coffee waste (nonporous carbons) | 100 | 100 | 285 | [53] |
| Wheat flour (carbon particles) | 1000 | 100 | 217 | [45] |
| Orange peel (porous carbon) | 1000 | 100 | 301 | [46] |
| Loofah (three-dimensional porous carbon framework) | 100 | 100 | 225 | [60] |
| Waste green tea (nanoparticles) | 100 | 100 | 498 | [61] |
| walnut shell (porous carbon nanofiber) | 100 | 200 | 280 | [62] |
| Reed flowers (hierarchical porous carbon) | 100 1000 | 100 1000 | 581.2 298.5 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; Wen, J.; Zhao, Y.; Wang, Z.; Shi, Y.; Zhao, Y. Hierarchically Porous Carbon Derived from Biomass Reed Flowers as Highly Stable Li-Ion Battery Anode. Nanomaterials 2020, 10, 346. https://doi.org/10.3390/nano10020346
Zhao W, Wen J, Zhao Y, Wang Z, Shi Y, Zhao Y. Hierarchically Porous Carbon Derived from Biomass Reed Flowers as Highly Stable Li-Ion Battery Anode. Nanomaterials. 2020; 10(2):346. https://doi.org/10.3390/nano10020346
Chicago/Turabian StyleZhao, Weimin, Jingjing Wen, Yanming Zhao, Zhifeng Wang, Yaru Shi, and Yan Zhao. 2020. "Hierarchically Porous Carbon Derived from Biomass Reed Flowers as Highly Stable Li-Ion Battery Anode" Nanomaterials 10, no. 2: 346. https://doi.org/10.3390/nano10020346
APA StyleZhao, W., Wen, J., Zhao, Y., Wang, Z., Shi, Y., & Zhao, Y. (2020). Hierarchically Porous Carbon Derived from Biomass Reed Flowers as Highly Stable Li-Ion Battery Anode. Nanomaterials, 10(2), 346. https://doi.org/10.3390/nano10020346






