Adsorption and Diffusion of Hydrogen in Carbon Honeycomb
Abstract
1. Introduction
2. Methodology
3. Results and Discussion
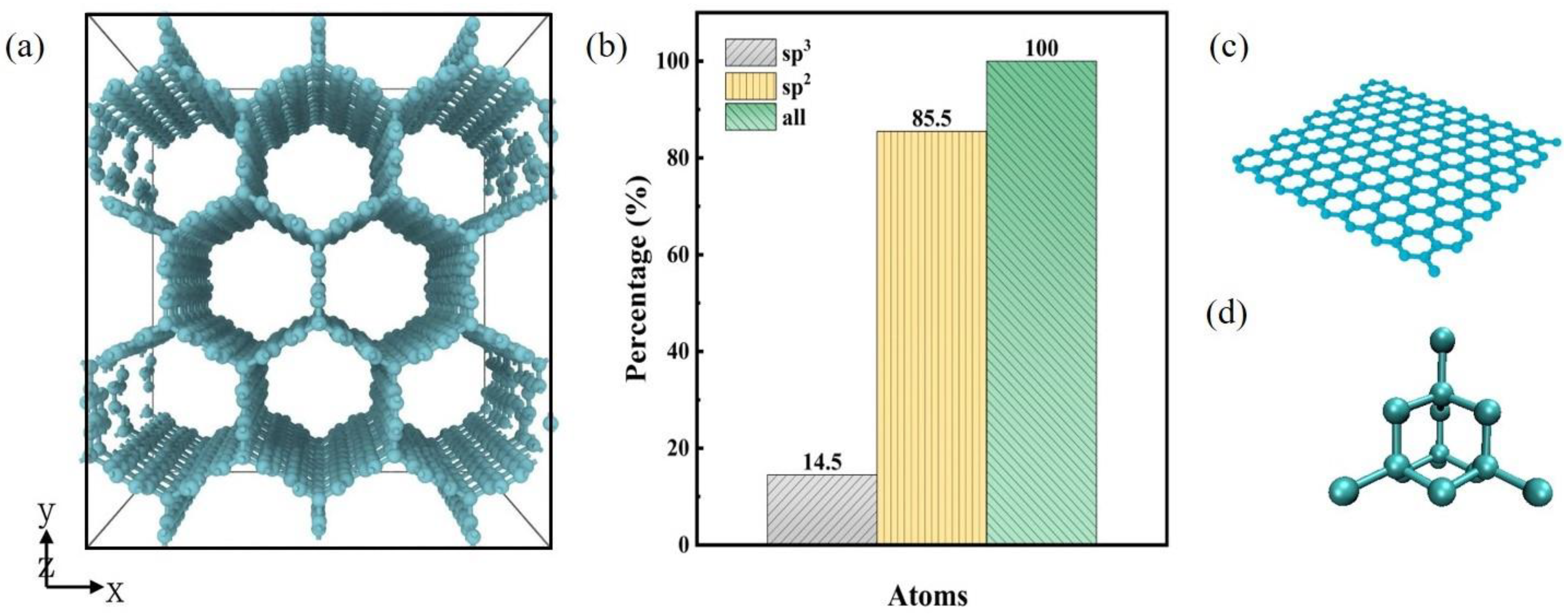
3.1. Mechanical Properties of Carbon Honeycomb
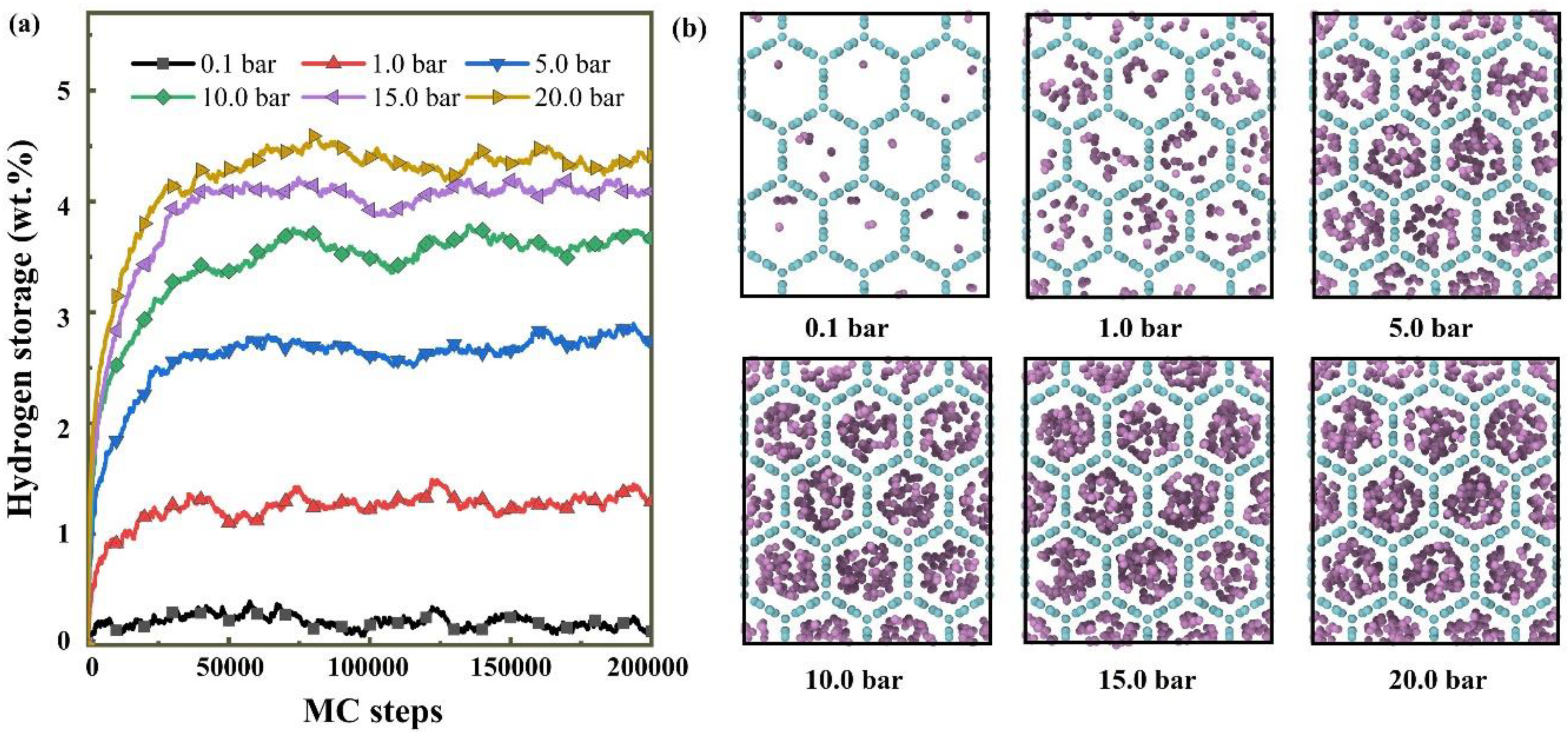
3.2. Pressure Effect on the Hydrogen Adsorption
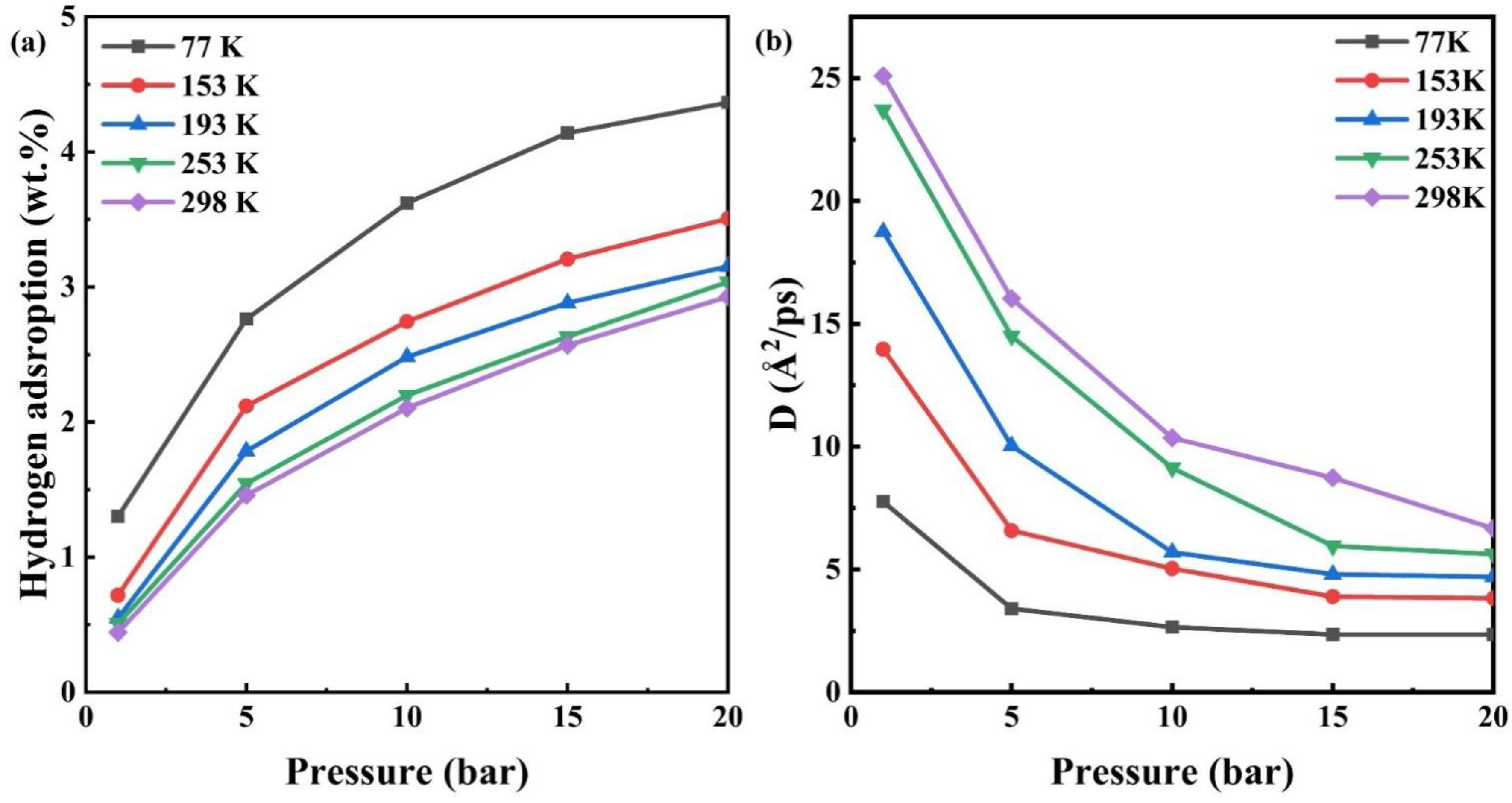
3.3. Temperature Effect on the Hydrogen Adsorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Schlapbach, L.; Züttel, A. Hydrogen-storage materials for mobile applications. In Materials for Sustainable Energy: A Collection of Peer-Reviewed Research and Review Articles from Nature Publishing Group; World Scientific: Singapore, 2011; pp. 265–270. [Google Scholar]
- Züttel, A. Materials for hydrogen storage. Mater. Today 2003, 6, 24–33. [Google Scholar] [CrossRef]
- Dong, J.; Wang, X.; Xu, H.; Zhao, Q.; Li, J. Hydrogen storage in several microporous zeolites. Int. J. Hydrog. Energy 2007, 32, 4998–5004. [Google Scholar] [CrossRef]
- Li, H.; Wang, K.; Sun, Y.; Lollar, C.T.; Li, J.; Zhou, H.-C. Recent advances in gas storage and separation using metal—Organic frameworks. Mater. Today 2018, 21, 108–121. [Google Scholar] [CrossRef]
- Niaz, S.; Manzoor, T.; Pandith, A.H. Hydrogen storage: Materials, methods and perspectives. Renew. Sustain. Energy Rev. 2015, 50, 457–469. [Google Scholar] [CrossRef]
- Ströbel, R.; Garche, J.; Moseley, P.T.; Jörissen, L.; Wolf, G. Hydrogen storage by carbon materials. J. Power Source 2006, 159, 781–801. [Google Scholar] [CrossRef]
- Ma, Y.X.; Li, X.; Shao, W.J.; Kou, Y.L.; Yang, H.P.; Zhang, D.J. Fabrication of 3D Porous Polyvinyl Alcohol/Sodium Alginate/Graphene Oxide Spherical Composites for the Adsorption of Methylene Blue. J. Nanosci. Nanotechnol. 2020, 20, 2205–2213. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhou, S.; Liu, S. Atomistic simulation on mechanical behaviors of Al/SiC nanocomposites. In Proceedings of the 2017 18th International Conference on Electronic Packaging Technology (ICEPT), Harbin, China, 16–19 August 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 357–362. [Google Scholar]
- Sethia, G.; Sayari, A. Activated carbon with optimum pore size distribution for hydrogen storage. Carbon 2016, 99, 289–294. [Google Scholar] [CrossRef]
- Ozturk, Z.; Baykasoglu, C.; Kirca, M. Sandwiched graphene-fullerene composite: A novel 3-D nanostructured material for hydrogen storage. Int. J. Hydrog. Energy 2016, 41, 6403–6411. [Google Scholar] [CrossRef]
- Wu, C.-D.; Fang, T.-H.; Lo, J.-Y. Effects of pressure, temperature, and geometric structure of pillared graphene on hydrogen storage capacity. Int. J. Hydrog. Energy 2012, 37, 14211–14216. [Google Scholar] [CrossRef]
- de la Casa-Lillo, M.A.; Lamari-Darkrim, F.; Cazorla-Amorós, D.; Linares-Solano, A. Hydrogen Storage in Activated Carbons and Activated Carbon Fibers. J. Phys. Chem. B 2002, 106, 10930–10934. [Google Scholar] [CrossRef]
- Rzepka, M.; Lamp, P.; de la Casa-Lillo, M.A. Physisorption of Hydrogen on Microporous Carbon and Carbon Nanotubes. J. Phys. Chem. B 1998, 102, 10894–10898. [Google Scholar] [CrossRef]
- Wu, C.D.; Fang, T.H.; Lo, J.Y.; Feng, Y.L. Molecular dynamics simulations of hydrogen storage capacity of few-layer grapheme. J. Mol. Model. 2013, 19, 3813–3819. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Li, S.H.; Cao, J.X. First-principles study of Pd-decorated carbon nanotube for hydrogen storage. Chem. Phys. Lett. 2009, 483, 111–114. [Google Scholar] [CrossRef]
- Taheri, S.; Shadman, M.; Soltanabadi, A.; Ahadi, Z. Grand canonical Monte Carlo simulation of hydrogen physisorption in Li- and K-doped single-walled silicon carbide nanotube. Int. Nano Lett. 2014, 4, 81–90. [Google Scholar] [CrossRef]
- Gadipelli, S.; Guo, Z.X. Graphene-based materials: Synthesis and gas sorption, storage and separation. Prog. Mater. Sci. 2015, 69, 1–60. [Google Scholar] [CrossRef]
- Ewels, C.P.; Rocquefelte, X.; Kroto, H.W.; Rayson, M.J.; Briddon, P.R.; Heggie, M.I. Predicting experimentally stable allotropes: Instability of penta-graphene. Proc. Natl. Acad. Sci. USA 2015, 112, 15609–15612. [Google Scholar] [CrossRef]
- Braun, E.; Lee, Y.; Moosavi, S.M.; Barthel, S.; Mercado, R.; Baburin, I.A.; Proserpio, D.M.; Smit, B. Generating carbon schwarzites via zeolite-templating. Proc. Natl. Acad. Sci. USA 2018, 115, E8116–E8124. [Google Scholar] [CrossRef]
- Nueangnoraj, K.; Nishihara, H.; Imai, K.; Itoi, H.; Ishii, T.; Kiguchi, M.; Sato, Y.; Terauchi, M.; Kyotani, T. Formation of crosslinked-fullerene-like framework as negative replica of zeolite Y. Carbon 2013, 62, 455–464. [Google Scholar] [CrossRef]
- Roussel, T.; Didion, A.; Pellenq, R.J.M.; Gadiou, R.; Bichara, C.; Vix-Guterl, C. Experimental and Atomistic Simulation Study of the Structural and Adsorption Properties of Faujasite Zeolite−Templated Nanostructured Carbon Materials. J. Phys. Chem. C 2007, 111, 15863–15876. [Google Scholar] [CrossRef]
- Moore, J.D.; Palmer, J.C.; Liu, Y.-C.; Roussel, T.J.; Brennan, J.K.; Gubbins, K.E. Adsorption and diffusion of argon confined in ordered and disordered microporous carbons. Appl. Surf. Sci. 2010, 256, 5131–5136. [Google Scholar] [CrossRef]
- Xie, L.; An, H.; He, C.; Qin, Q.; Peng, Q. Mechanical Properties of Vacancy Tuned Carbon Honeycomb. Nanomaterials 2019, 9, 156. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.; Kolesnikov, A.I.; Rother, G.; Dai, S.; Qiao, Z.-A.; Cole, D. Effects of Confinement and Pressure on the Vibrational Behavior of Nano-Confined Propane. J. Phys. Chem. A 2018, 122, 6736–6745. [Google Scholar] [CrossRef] [PubMed]
- Krainyukova, N.V.; Zubarev, E.N. Carbon Honeycomb High Capacity Storage for Gaseous and Liquid Species. Phys. Rev. Lett. 2016, 116, 055501. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Haojie, A.; Chenwei, H.; Lu, X.; Qing, P. Anisotropic and temperature dependent mechanical properties of carbon honeycomb. Nanotechnology 2019, 30, 325704. [Google Scholar] [CrossRef] [PubMed]
- Kuc, A.; Seifert, G. Hexagon-preserving carbon foams: Properties of hypothetical carbon allotropes. Phys. Rev. B 2006, 74, 214104. [Google Scholar] [CrossRef]
- Blees, M.K.; Barnard, A.W.; Rose, P.A.; Roberts, S.P.; McGill, K.L.; Huang, P.Y.; Ruyack, A.R.; Kevek, J.W.; Kobrin, B.; Muller, D.A.; et al. Graphene kirigami. Nature 2015, 524, 204–207. [Google Scholar] [CrossRef]
- Bonaccorso, F.; Colombo, L.; Yu, G.; Stoller, M.; Tozzini, V.; Ferrari, A.C.; Ruoff, R.S.; Pellegrini, V. 2D materials. Graphene, related two-dimensional crystals, and hybrid systems for energy conversion and storage. Science 2015, 347, 1246501. [Google Scholar] [CrossRef]
- Willems, T.F.; Rycroft, C.H.; Kazi, M.; Meza, J.C.; Haranczyk, M. Algorithms and tools for high-throughput geometry-based analysis of crystalline porous materials. Microporous Mesoporous Mater. 2012, 149, 134–141. [Google Scholar] [CrossRef]
- Martin, R.L.; Smit, B.; Haranczyk, M. Addressing challenges of identifying geometrically diverse sets of crystalline porous materials. J. Chem. Inf. Model. 2012, 52, 308–318. [Google Scholar] [CrossRef]
- Pinheiro, M.; Martin, R.L.; Rycroft, C.H.; Jones, A.; Iglesia, E.; Haranczyk, M. Characterization and comparison of pore landscapes in crystalline porous materials. J. Mol. Graph. Model. 2013, 44, 208–219. [Google Scholar] [CrossRef]
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular Dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Peng, Q.; Meng, F.; Yang, Y.; Lu, C.; Deng, H.; Wang, L.; De, S.; Gao, F. Shockwave generates <100> dislocation loops in bcc iron. Nat. Commun. 2018, 9, 4880. [Google Scholar] [CrossRef] [PubMed]
- Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO—The Open Visualization Tool. Model. Simul. Mater. Sci. Eng. 2010, 18, 015012. [Google Scholar] [CrossRef]
- Xie, L.; An, H.; Peng, Q.; Qin, Q.; Zhang, Y. Sensitive Five-Fold Local Symmetry to Kinetic Energy of Depositing Atoms in Cu-Zr Thin Film Growth. Materials 2018, 11, 2548. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, D.; Smit, B. Understanding Molecular Simulation: From Algorithms to Applications; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Donald, W.B.; Olga, A.S.; Judith, A.H.; Steven, J.S.; Boris, N.; Susan, B.S. A second-generation reactive empirical bond order (REBO) potential energy expression for hydrocarbons. J. Phys. Condens. Matter 2002, 14, 783. [Google Scholar]
- Lamari, F.D.; Levesque, D. Hydrogen adsorption on functionalized grapheme. Carbon 2011, 49, 5196–5200. [Google Scholar] [CrossRef]
- Ye, Y.; Ahn, C.C.; Witham, C.; Fultz, B.; Liu, J.; Rinzler, A.G.; Colbert, D.; Smith, K.A.; Smalley, R.E. Hydrogen adsorption and cohesive energy of single-walled carbon nanotubes. Appl. Phys. Lett. 1999, 74, 2307–2309. [Google Scholar] [CrossRef]
- Darkrim, F.; Levesque, D. High adsorptive property of opened carbon nanotubes at 77 K. J. Phys. Chem. B 2000, 104, 6773–6776. [Google Scholar] [CrossRef]
- Burress, J.W.; Gadipelli, S.; Ford, J.; Simmons, J.M.; Zhou, W.; Yildirim, T. Graphene oxide framework materials: Theoretical predictions and experimental results. Angew. Chem. Int. Ed. Engl. 2010, 49, 8902–8904. [Google Scholar] [CrossRef]
- Srinivas, G.; Burress, J.W.; Ford, J.; Yildirim, T. Porous graphene oxide frameworks: Synthesis and gas sorption properties. J. Mater. Chem. 2011, 21, 11323–11329. [Google Scholar] [CrossRef]
- Langmi, H.W.; Book, D.; Walton, A.; Johnson, S.R.; Al-Mamouri, M.M.; Speight, J.D.; Edwards, P.P.; Harris, I.R.; Anderson, P.A. Hydrogen storage in ion-exchanged zeolites. J. Alloys Compd. 2005, 406, 637–642. [Google Scholar] [CrossRef]
- Wang, L.; Yang, R.T. Hydrogen Storage Properties of Low-Silica Type X Zeolites. Ind. Eng. Chem. Res. 2010, 49, 3634–3641. [Google Scholar] [CrossRef]
- Chung, K.H. High-pressure hydrogen storage on microporous zeolites with varying pore properties. Energy 2010, 35, 2235–2241. [Google Scholar] [CrossRef]
- Zito, P.F.; Caravella, A.; Brunetti, A.; Drioli, E.; Barbieri, G. Light gases saturation loading dependence on temperature in LTA 4A zeolite. Microporous Mesoporous Mater. 2017, 249, 67–77. [Google Scholar] [CrossRef]
- Bär, N.K.; Ernst, H.; Jobic, H.; Kärger, J. Combined quasi-elastic neutron scattering and NMR study of hydrogen diffusion in zeolites. Magn. Reson. Chem. 1999, 37, S79–S83. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, Q.; Sun, T.; Wang, H.; Brault, P.; An, H.; Xie, L.; Peng, Q. Adsorption and Diffusion of Hydrogen in Carbon Honeycomb. Nanomaterials 2020, 10, 344. https://doi.org/10.3390/nano10020344
Qin Q, Sun T, Wang H, Brault P, An H, Xie L, Peng Q. Adsorption and Diffusion of Hydrogen in Carbon Honeycomb. Nanomaterials. 2020; 10(2):344. https://doi.org/10.3390/nano10020344
Chicago/Turabian StyleQin, Qin, Tingwei Sun, Hanxiao Wang, Pascal Brault, Haojie An, Lu Xie, and Qing Peng. 2020. "Adsorption and Diffusion of Hydrogen in Carbon Honeycomb" Nanomaterials 10, no. 2: 344. https://doi.org/10.3390/nano10020344
APA StyleQin, Q., Sun, T., Wang, H., Brault, P., An, H., Xie, L., & Peng, Q. (2020). Adsorption and Diffusion of Hydrogen in Carbon Honeycomb. Nanomaterials, 10(2), 344. https://doi.org/10.3390/nano10020344






