Thermal Transport in Graphene Oxide Films: Theoretical Analysis and Molecular Dynamics Simulation
Abstract
1. Introduction
2. Model and Methodology
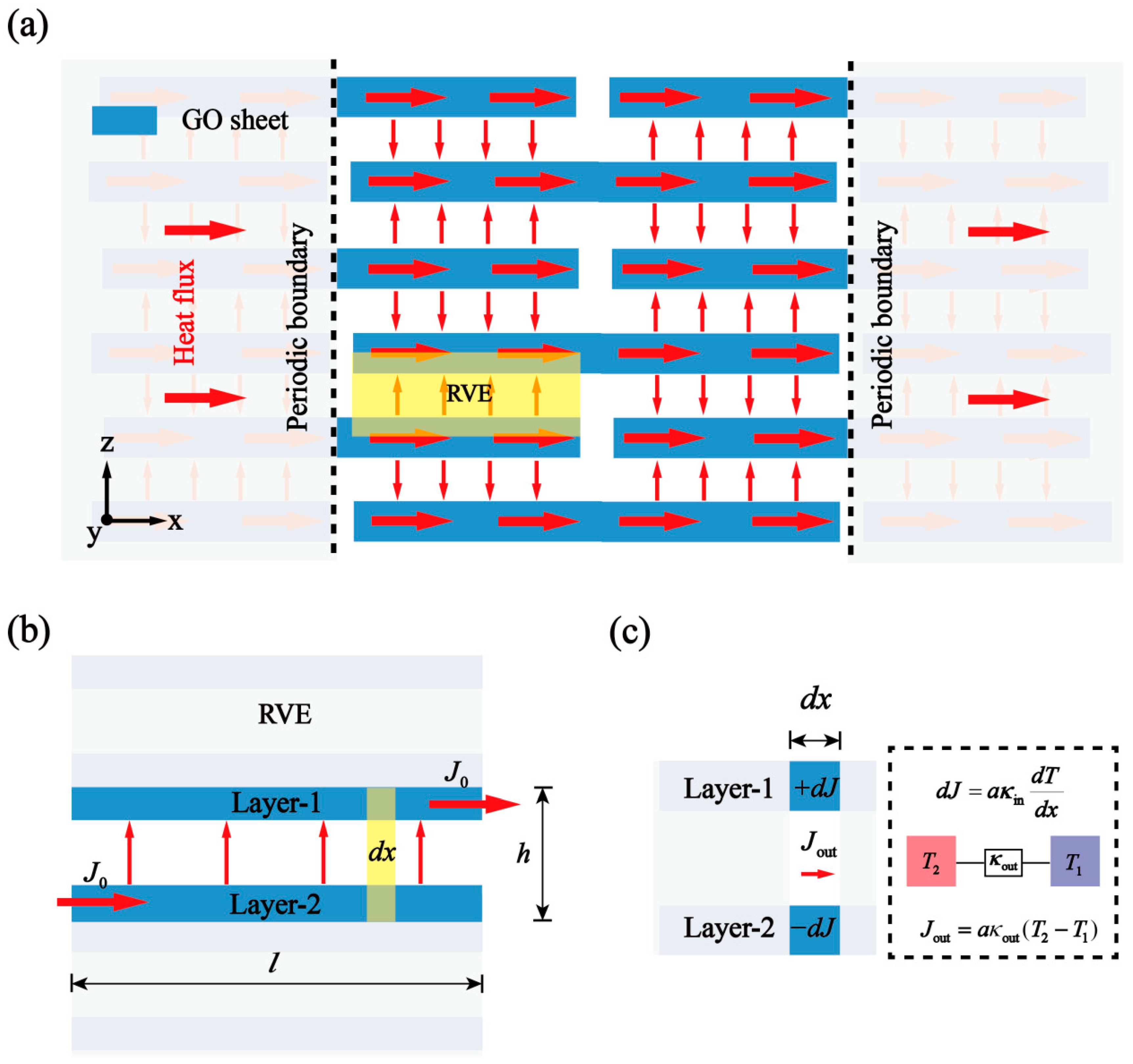
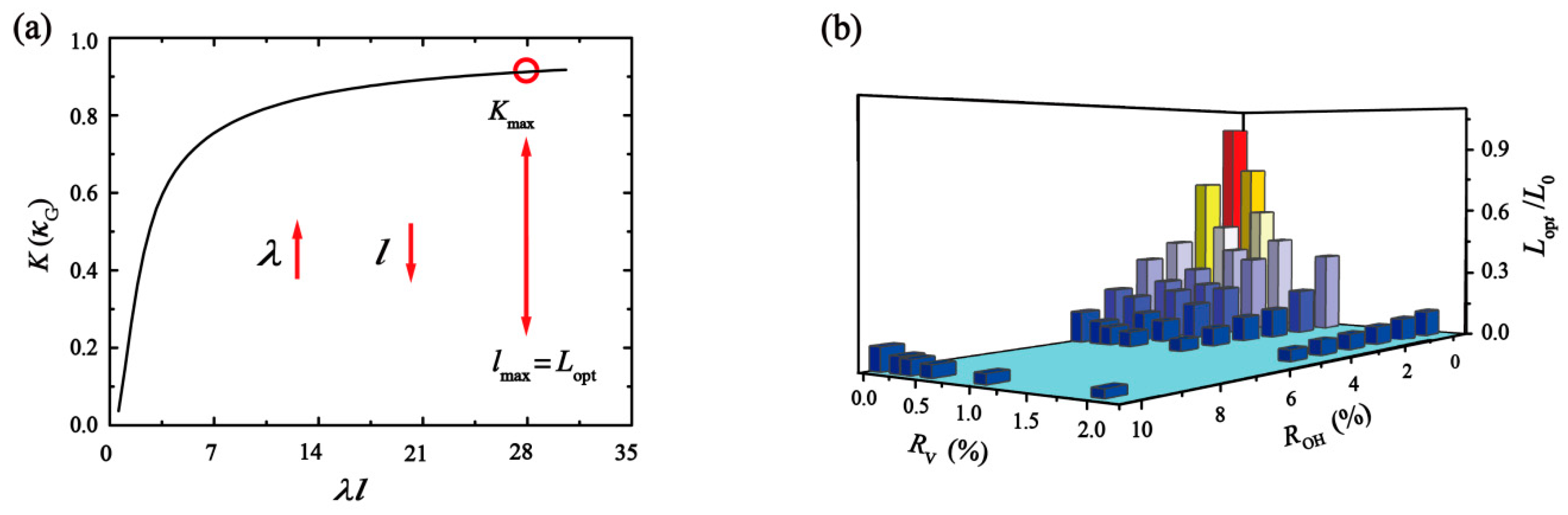
2.1. Theoretical Analysis
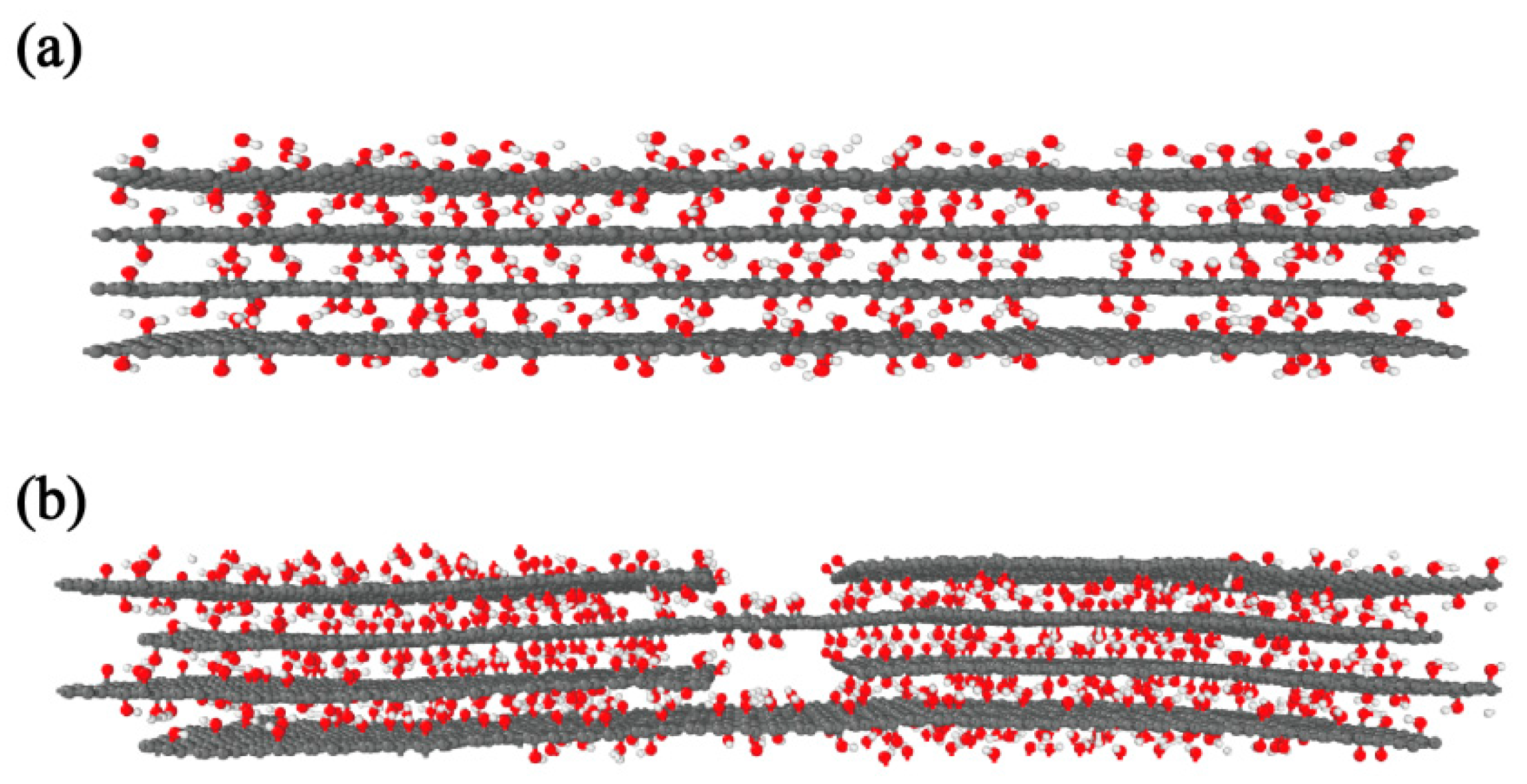
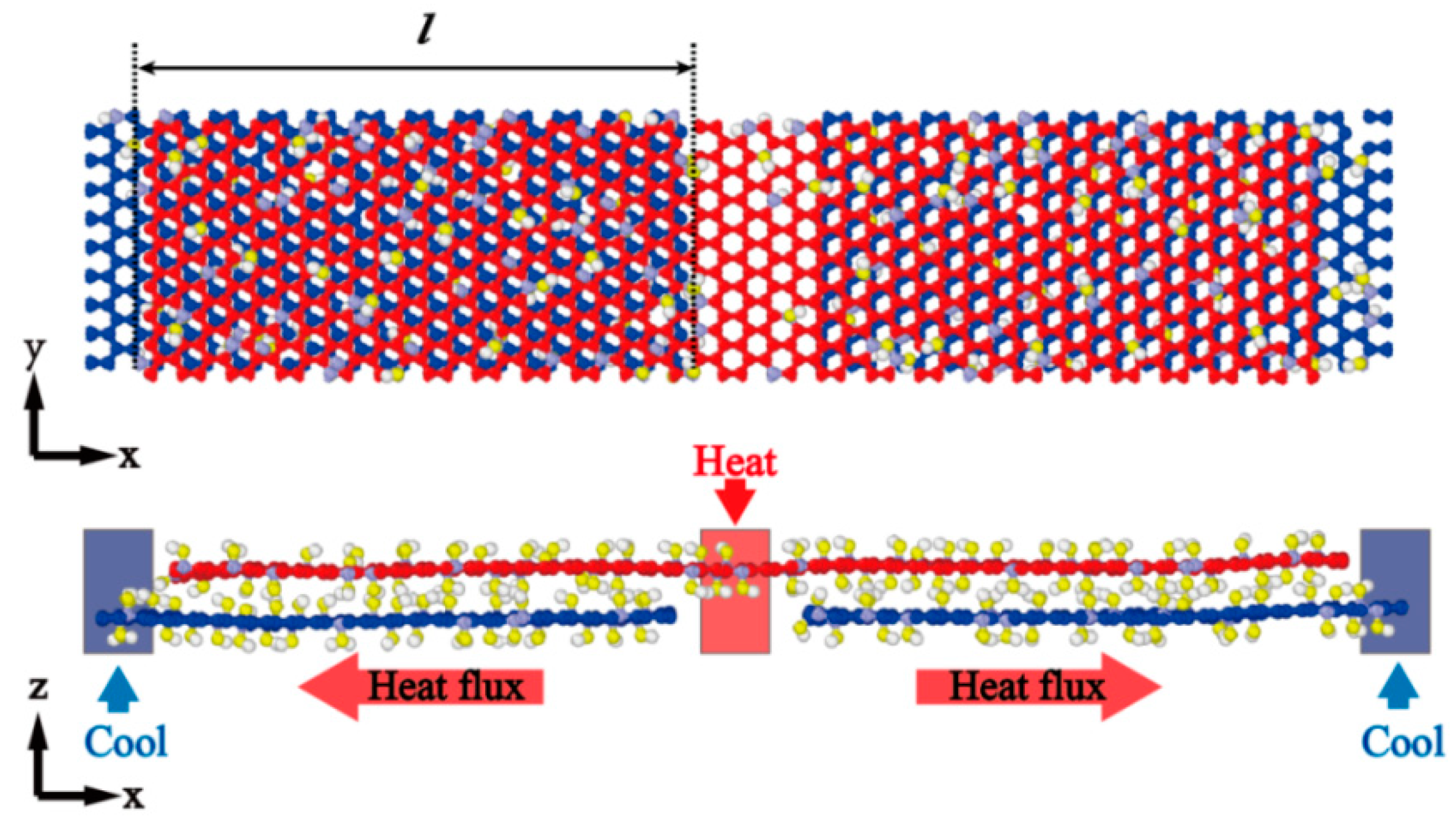
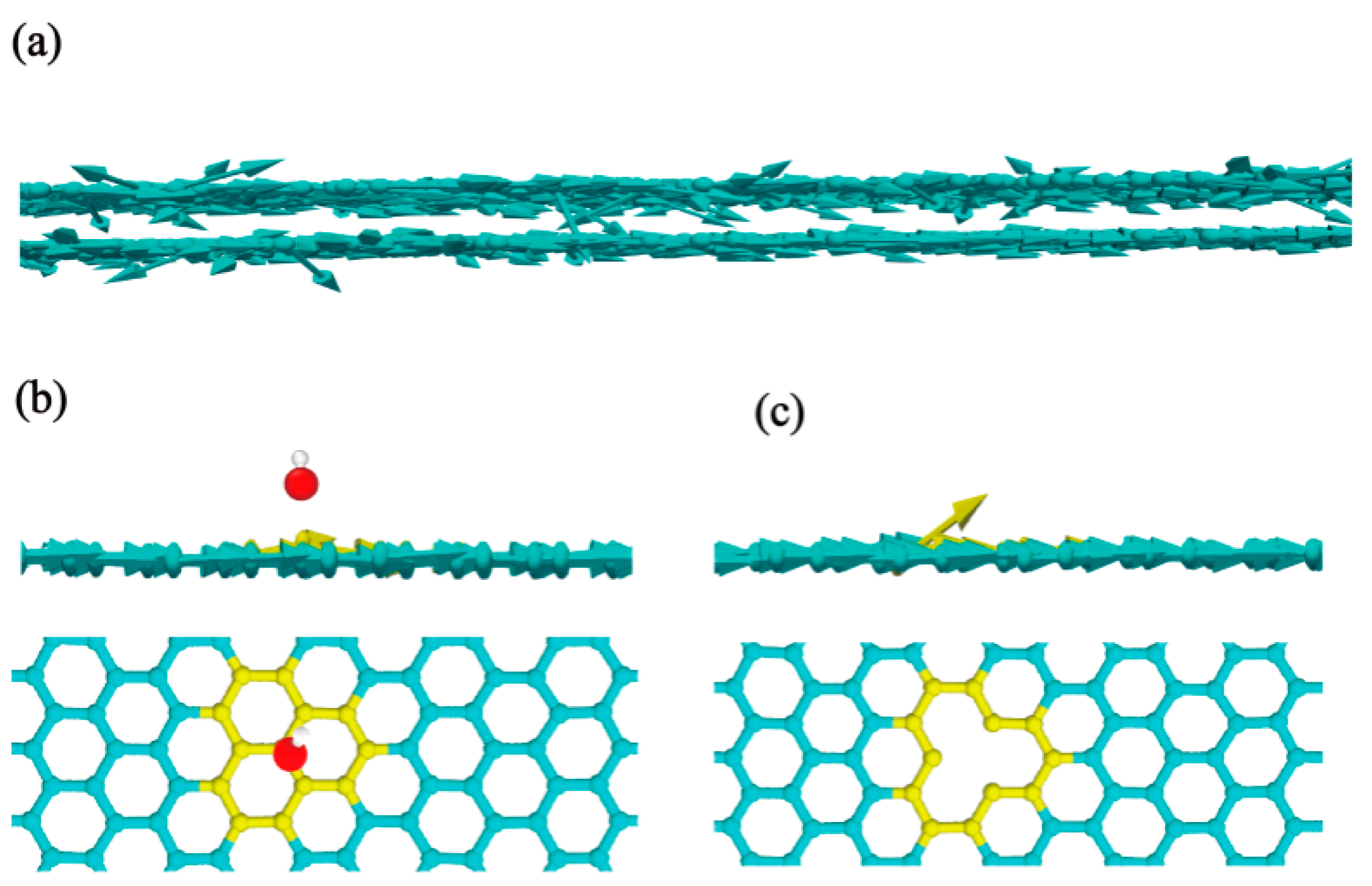
2.2. Simulation Analysis
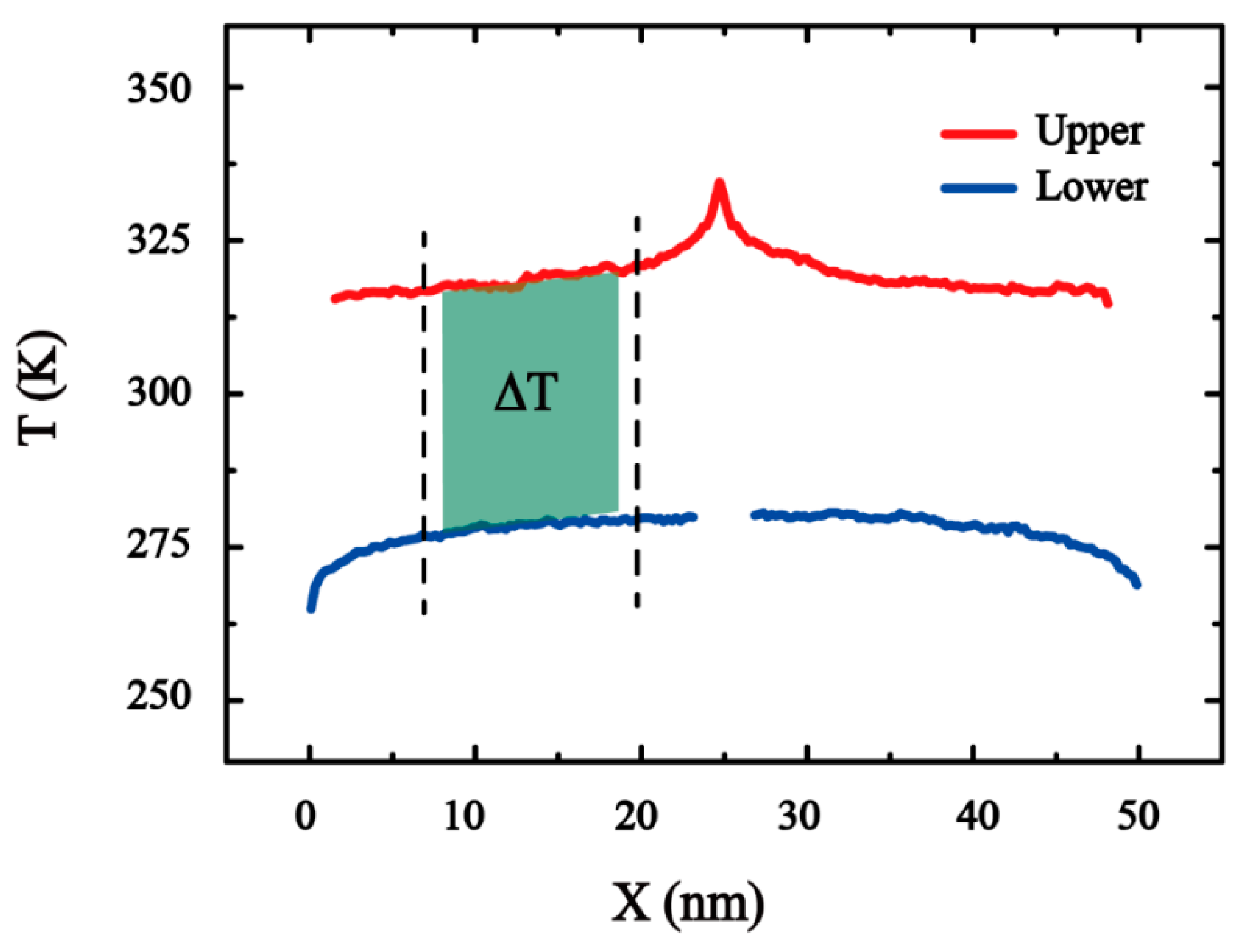
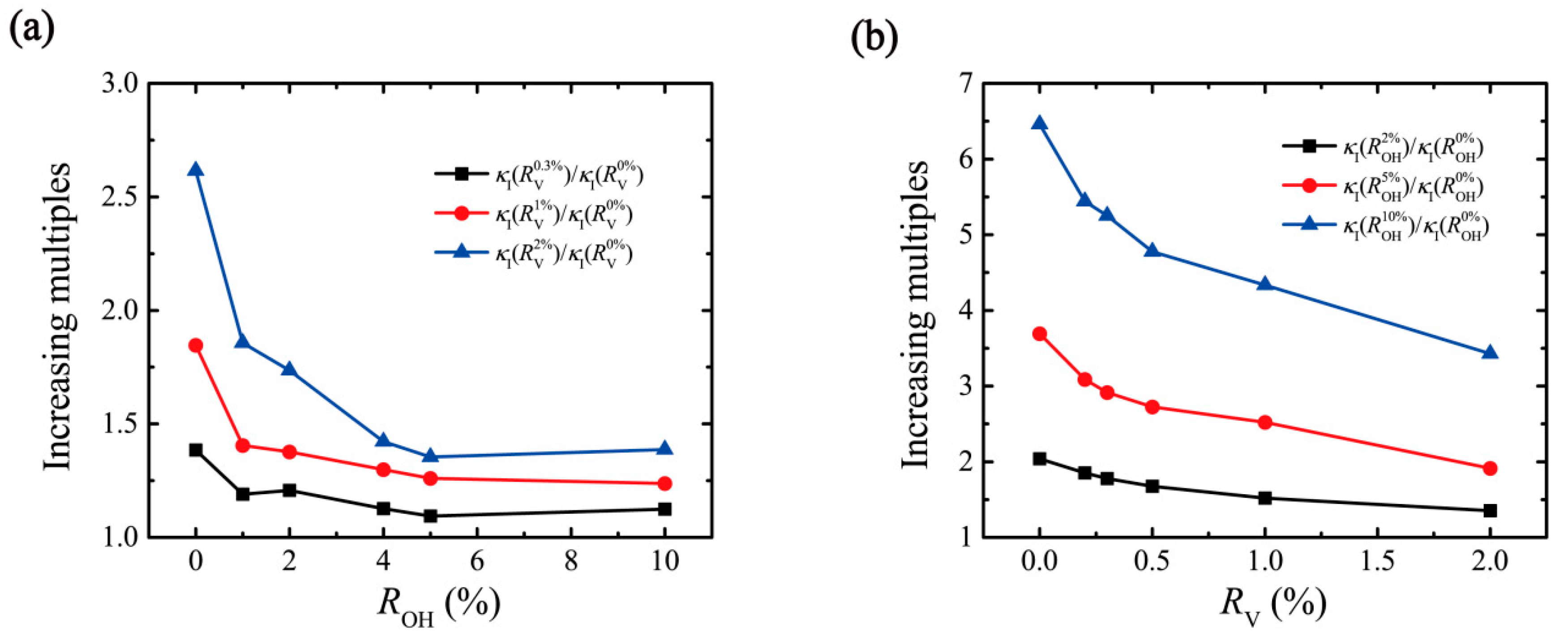
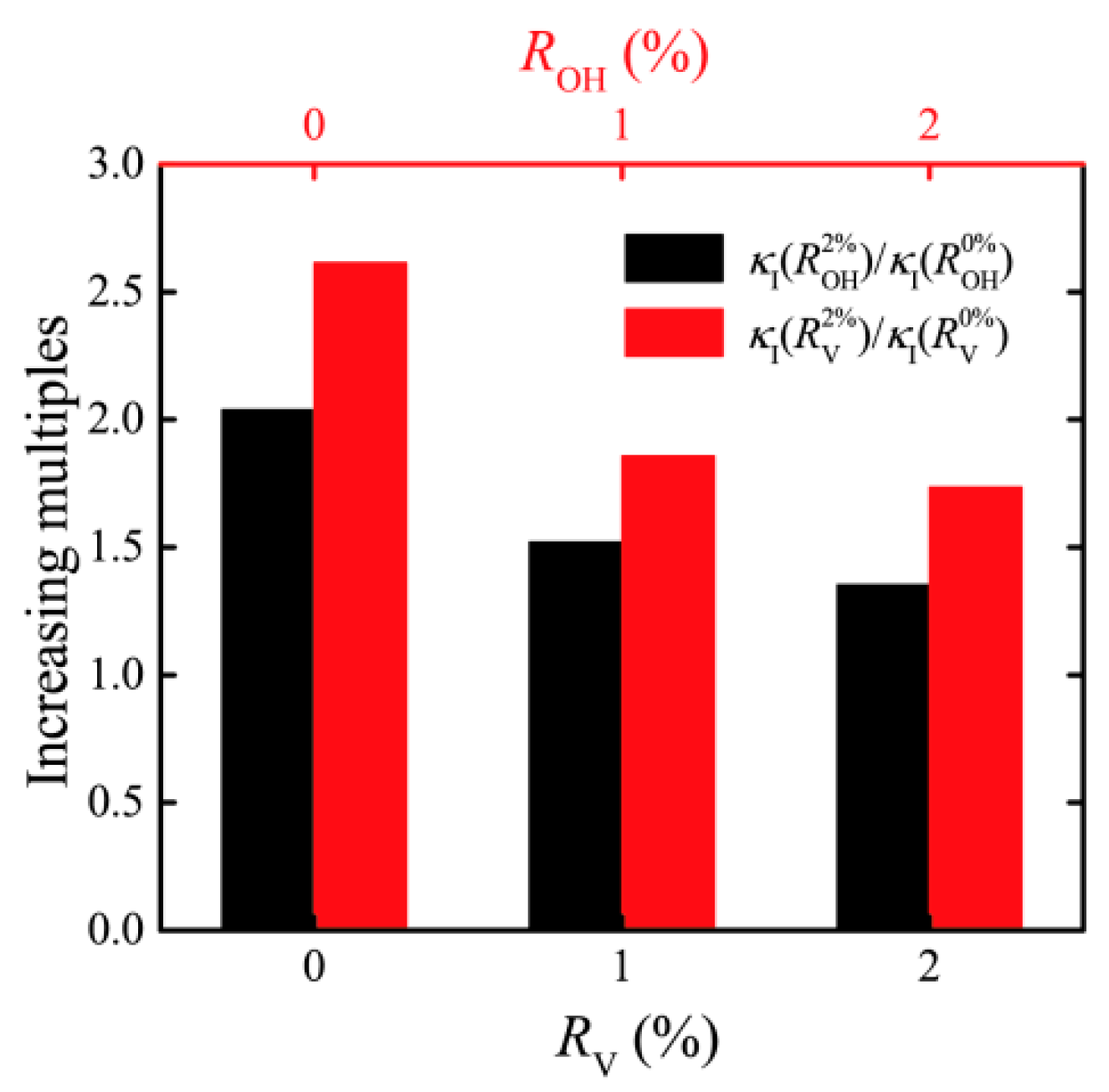
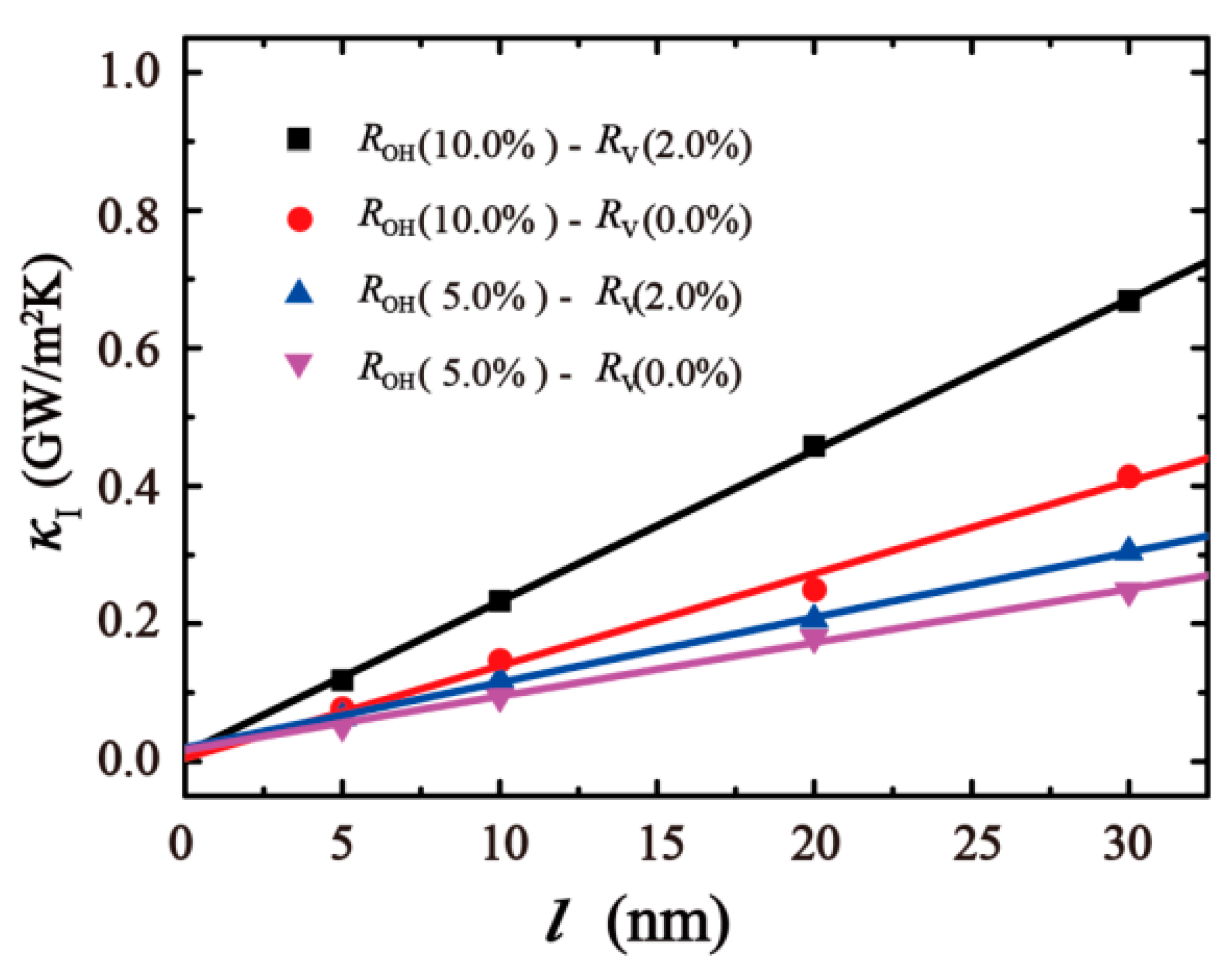
3. Result and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bonaccorso, F.; Colombo, L.; Yu, G.; Stoller, M.; Tozzini, V.; Ferrari, A.C.; Ruoff, R.S.; Pellegrin, V. 2D materials. Graphene, related two-dimensional crystals, and hybrid systems for energy conversion and storage. Science 2015, 347, 1246501. [Google Scholar] [CrossRef]
- Hu, G.; Xu, C.; Sun, Z.; Wang, S.; Cheng, H.M.; Li, F.; Ren, W. 3D graphene—Foam-reduced—Graphene—Oxide hybrid nested hierarchical networks for high—Performance Li-S Batteries. Adv. Mater. 2016, 2828, 1603–1609. [Google Scholar] [CrossRef]
- Dong, X.C.; Xu, H.; Wang, X.W.; Huang, Y.X.; Chanpark, M.B.; Zhang, H.; Zhang, H.; Wang, L.; Huang, W.; Chen, P. 3D Graphene–cobalt oxide electrode for high-performance supercapacitor and enzymeless glucose detection. ACS Nano 2012, 6, 3206. [Google Scholar] [CrossRef]
- Yun, Y.J.; Hong, W.G.; Choi, N.J.; Park, H.J.; Moon, S.E.; Kim, B.H.; Song, K.; Jun, Y.; Lee, H. A 3D scaffold for ultra-sensitive reduced graphene oxide gas sensors. Nanoscale 2014, 6, 6511. [Google Scholar] [CrossRef]
- Loh, K.P.; Bao, Q.; Eda, G.; Chhowalla, M. Graphene oxide as a chemically tunable platform for optical applications. Nat. Chem. 2010, 2, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Shi, G.; Shen, J.; Peng, B.; Zhang, B.; Wang, Y.; Bian, F.; Wang, J.; Li, D.; Qian, Z.; et al. Ion sieving in graphene oxide membranes via cationic control of interlayer spacing. Nature 2017, 550, 380. [Google Scholar] [CrossRef] [PubMed]
- Dikin, D.A.; Stankovich, S.; Zimney, E.J.; Piner, R.D.; Dommett, G.H.B.; Evmenenko, G.; Nguyen, S.T.; Ruoff, R.S. Preparation and characterization of graphene oxide paper. Nature 2007, 448, 457. [Google Scholar] [CrossRef]
- Yan, J.A.; Xian, L.; Chou, M.Y. Structural and electronic properties of oxidized graphene. Phys. Rev. Lett. 2009, 103, 086802. [Google Scholar] [CrossRef] [PubMed]
- Mathkar, A.; Tozier, D.; Cox, P.; Ong, P.; Galande, C.; Balakrishnan, K.; Reddy, A.L.M.; Ajayan, P.M. Controlled, stepwise reduction and band gap manipulation of graphene oxide. J. Phys. Chem. Lett. 2012, 3, 986–991. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Kim, S.; Zhou, S.; Hu, Y.; Acik, M.; Chabal, Y.J.; Berger, C.; de Heer, W.; Bongiorno, A.; Riedo, E. Room-temperature metastability of multilayer graphene oxide films. Nat. Mater. 2012, 11, 544. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Xie, H.; Bao, D. Enhanced thermal conductivities of nanofluids containing graphene oxide nanosheets. Nanotechnology 2010, 21, 055705. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Zhang, H.; Fonseca, A.F.; Cho, K. Tailoring thermal transport property of graphene through oxygen functionalization. J. Phys. Chem. C 2014, 118, 1436–1442. [Google Scholar] [CrossRef]
- Balandin, A.A. Thermal properties of graphene and nanostructured carbon materials. Nat. Mater. 2011, 10, 569–581. [Google Scholar] [CrossRef]
- Nika, D.L.; Balandin, A.A. Phonons and thermal transport in graphene and graphene-based materials. Rep. Phys. 2017, 80, 036502. [Google Scholar] [CrossRef]
- Chen, H.; Ginzburg, V.V.; Yang, J.; Yang, Y.; Liu, W.; Huang, Y.; Du, L.; Chen, B. Thermal conductivity of polymer-based composites: Fundamentals and applications. Prog. Polym. Sci. 2016, 59, 41–85. [Google Scholar] [CrossRef]
- Meziani, M.J.; Song, W.L.; Wang, P.; Lu, F.; Hou, Z.; Anderson, A.; Sun, Y.P. Boron nitride nanomaterials for thermal management applications. Chemphyschem A 2015, 16, 1339–1346. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, J.; Rabczuk, T. The interface strength and delamination of fiber-reinforced composites using a continuum modeling approach. Compos. Part B 2018, 137, 225–234. [Google Scholar] [CrossRef]
- Li, B.; Fang, H.; He, H.; Yang, K.; Chen, C.; Wang, F. Numerical simulation and full-scale test on dynamic response of corroded concrete pipelines under Multi-field coupling. Constr. Build. Mater. 2019, 200, 368–386. [Google Scholar] [CrossRef]
- Losego, M.D.; Grady, M.E.; Sottos, N.R.; Cahill, D.G.; Braun, P.V. Effects of chemical bonding on heat transport across interfaces. Nat. Mater. 2012, 11, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Yoon, H.J. New Approach for large-area thermoelectric junctions with a liquid eutectic gallium–indium electrode. Nano Lett. 2018, 18, 7715–7718. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Miao, R.; Wang, K.; Thompson, D.; Zotti, L.A.; Cuevas, J.; Meyhofer, E.; Reddy, P. Peltier cooling in molecular junctions. Nat. Nanotechnol. 2018, 13, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kang, S.; Yoon, H.J. Power factor of one molecule thick films and length dependence. ACS Cent. Sci. 2019, 5, 1975–1982. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kang, H.; Yoon, H.J. Structure–thermopower relationships in molecular thermoelectrics. J. Mater. Chem. 2019, 7, 14419–14446. [Google Scholar] [CrossRef]
- Cui, L.; Hur, S.; Akbar, Z.A.; Klockner, J.C.; Jeong, W.; Pauly, F.; Jang, S.; Reddy, P.; Meyhofer, E. Thermal conductance of single-molecule junctions. Nature 2019, 572, 628–633. [Google Scholar] [CrossRef]
- Park, S.; Cho, N.; Yoon, H.J. Two different length-dependent regimes in thermoelectriclarge-area junctions of n—Alkanethiolates. Chem. Mater. 2019, 31, 5973–5980. [Google Scholar] [CrossRef]
- Renteria, J.D.; Ramirez, S.; Malekpour, H.; Alonso, B.; Centeno, A.; Zurutuza, A.; Cocemasov, A.I.; Nika, D.L.; Balandin, A.A. Strongly anisotropic thermal conductivity of free—Standing reduced graphene oxide films annealed at high temperature. Adv. Funct. Mater. 2015, 25, 4664–4672. [Google Scholar] [CrossRef]
- Barani, Z.; Mohammadzadeh, A.; Geremew, A.; Huang, C.; Coleman, D.; Mangolini, L.; Kargar, F.; Balandin, A.A. Thermal properties of the binary—Filler hybrid composites with graphene and copper nanoparticles. Adv. Funct. Mater. 2019. [Google Scholar] [CrossRef]
- Malekpour, H.; Ramnani, P.; Srinivasan, S.; Balasubramanian, G.; Nika, D.L.; Mulchandani, A.; Lake, R.K.; Balandin, A.A. Thermal conductivity of graphene with defects induced by electron beam irradiation. Nanoscale 2016, 8, 14608–14616. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, Y.; Wu, Z.; Wang, W.; Bi, K.; Liang, Z.; Yang, J.; Chen, Y.; Xu, Z.; Ni, Z. Defect-engineered heat transport in graphene: A route to high efficient thermal rectification. Sci. Rep. 2015, 5, 11962. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Walther, J.H.; Koumoutsakos, P. Strain engineering of Kapitza resistance in few-layer graphene. Nano Lett. 2014, 14, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Li, S.; Zhang, Y.; Chen, J.; Chen, Y.; Zhao, J. Thermal rectification of graphene on substrates with inhomogeneous stiffness. Carbon 2019, 154, 81–89. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, G.; Zhang, Y.W. Thermal Conduction Across Graphene Cross-Linkers. J. Phys. Chem. C 2014, 118, 12541–12547. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, Y.; Zhao, X.; Peng, L.; Sun, H.; Xu, Y.; Ren, X.; Jin, C.; Xu, P.; Wang, M.; et al. Ultrastiff and strong graphene fibers via full-scale synergetic defect engineering. Adv. Mater. 2016, 28, 6449–6456. [Google Scholar] [CrossRef]
- Xu, Z.; Buehler, M.J. Nanoengineering heat transfer performance at carbon nanotube interfaces. ACS Nano 2009, 3, 2767–2775. [Google Scholar] [CrossRef]
- Qin, H.; Pei, Q.; Liu, Y.; Zhang, Y. Thermal transport in graphene-based layered materials: An analytical model validated with extensive molecular dynamics simulations. Carbon 2019, 155, 114–121. [Google Scholar] [CrossRef]
- Yang, Y.; Cao, J.; Wei, N.; Meng, D.; Wang, L.; Ren, G.; Yan, R.; Zhang, N. Thermal conductivity of defective graphene oxide: A molecular dynamic study. Molecules 2019, 24, 1103. [Google Scholar] [CrossRef]
- Wang, N.; Samani, M.K.; Li, H.; Dong, L.; Zhang, Z.; Su, P.; Chen, S.; Chen, J.; Huang, S.; Yuan, G.; et al. Tailoring the thermal and mechanical properties of graphene film by structural engineering. Small 2018, 14, 1801346. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Wang, Y.; Chai, J.; Shi, J. Nano-peapods from C60-encapsulated CNTs driving self-assembly of phosphorus nanotube: A molecular dynamics study. Comput. Mater. Sci. 2019, 160, 403–410. [Google Scholar] [CrossRef]
- Cao, J.; Cai, K. Thermal expansion producing easier formation of a black phosphorus nanotube from nanoribbon on carbon nanotube. Nanotechnology 2017, 29, 055603. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Lei, J.; Yang, M.; Li, Z. Analysis of GPR Wave Propagation Using CUDA-implemented conformal symplectic partitioned runge-kutta method. Complexity 2019, 2019, 4025878. [Google Scholar] [CrossRef]
- Plimpton, S. Fast parallel algoritlnns for short-range molecular dynamics i Plimpton S. J. Comput. Phys. 1995, 117, 1–16. [Google Scholar] [CrossRef]
- Cao, J.; Wang, Y.; Shi, J.; Chai, J.; Cai, K. Initial relative position influencing self-assembly of a black phosphorus ribbon on a cnt. Int. J. Mol. Sci. 2018, 19, 4085. [Google Scholar] [CrossRef]
- Shih, C.J.; Lin, S.; Sharma, R.; Strano, M.S.; Blankschtein, D. Understanding the pH-dependent behavior of graphene oxide aqueous solutions: A comparative experimental and molecular dynamics simulation study. Langmuir 2012, 28, 235–241. [Google Scholar] [CrossRef]
- Ning, W.; Peng, X.; Xu, Z. Understanding water permeation in graphene oxide membranes. ACS Appl. Mater. Interfaces 2014, 6, 5877–5883. [Google Scholar]
- Werder, T.; Walther, J.H.; Jaffe, R.L.; Halicioglu, T.; Koumoutsakos, P. On the water−carbon interaction for use in molecular dynamics simulations of graphite and carbon nanotubes. J. Phys. Chem. B 2008, 107, 1345–1352. [Google Scholar] [CrossRef]
- Hockney, R.W.; Eastwood, J.W. Computer Simulation Using Particles; Taylor and Francis; Inc.: London, UK, 1988. [Google Scholar]
- Müller-Plathe, F. A simple nonequilibrium molecular dynamics method for calculating the thermal conductivity. J. Chem. Phys. 1998, 106, 6082–6085. [Google Scholar] [CrossRef]
- Yang, S.; Ma, C.M.; Teng, C.; Huang, Y.; Liao, S.; Huang, Y.; Tien, H.; Lee, T.; Chiou, K. Effect of functionalized carbon nanotubes on the thermal conductivity of epoxy composites. Carbon 2010, 48, 592–603. [Google Scholar] [CrossRef]
- Wei, N.; Chen, Y.; Cai, K.; Zhao, J.; Wang, H.-Q.; Zheng, J.-C. Thermal conductivity of graphene kirigami: Ultralow and strain robustness. Carbon 2016, 104, 203–213. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Cai, K.; Jiang, J.; Zheng, J.C.; Zhao, J.; Wei, N. Interfacial thermal conductance in graphene/black phosphorus heterogeneous structures. Carbon 2017, 117, 399–410. [Google Scholar] [CrossRef]
| = 0.0% | 0.26 * | 0.42 * | 0.53 * | 0.75 * | 0.87 * | 0.96 * | 1.68 * |
| = 0.2% | 0.34 * | 0.44 * | 0.63 * | 0.78 * | 0.89 * | 1.05 * | 1.85 * |
| = 0.3% | 0.36 * | 0.50 * | 0.64 * | 0.80 * | 0.98 * | 1.05 * | 1.89 * |
| = 0.5% | 0.40 * | 0.55 * | 0.67 * | 0.84 * | 1.04 * | 1.09 * | 1.91 * |
| = 1.0% | 0.48 * | 0.59 * | 0.73 * | 0.89 * | 1.13 * | 1.21 * | 2.08 * |
| = 2.0% | 0.68 * | 0.78 * | 0.92 * | 1.05 * | 1.23 * | 1.30 * | 2.33 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Zhong, D.; Liu, Y.; Meng, D.; Wang, L.; Wei, N.; Ren, G.; Yan, R.; Kang, Y. Thermal Transport in Graphene Oxide Films: Theoretical Analysis and Molecular Dynamics Simulation. Nanomaterials 2020, 10, 285. https://doi.org/10.3390/nano10020285
Yang Y, Zhong D, Liu Y, Meng D, Wang L, Wei N, Ren G, Yan R, Kang Y. Thermal Transport in Graphene Oxide Films: Theoretical Analysis and Molecular Dynamics Simulation. Nanomaterials. 2020; 10(2):285. https://doi.org/10.3390/nano10020285
Chicago/Turabian StyleYang, Yi, Dan Zhong, Yilun Liu, Donghui Meng, Lina Wang, Ning Wei, Guohua Ren, Rongxin Yan, and Yang Kang. 2020. "Thermal Transport in Graphene Oxide Films: Theoretical Analysis and Molecular Dynamics Simulation" Nanomaterials 10, no. 2: 285. https://doi.org/10.3390/nano10020285
APA StyleYang, Y., Zhong, D., Liu, Y., Meng, D., Wang, L., Wei, N., Ren, G., Yan, R., & Kang, Y. (2020). Thermal Transport in Graphene Oxide Films: Theoretical Analysis and Molecular Dynamics Simulation. Nanomaterials, 10(2), 285. https://doi.org/10.3390/nano10020285






