KxWO Is a Novel Ferroelectric Nanomaterial for Application as a Room Temperature Acetone Sensor
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Characterization
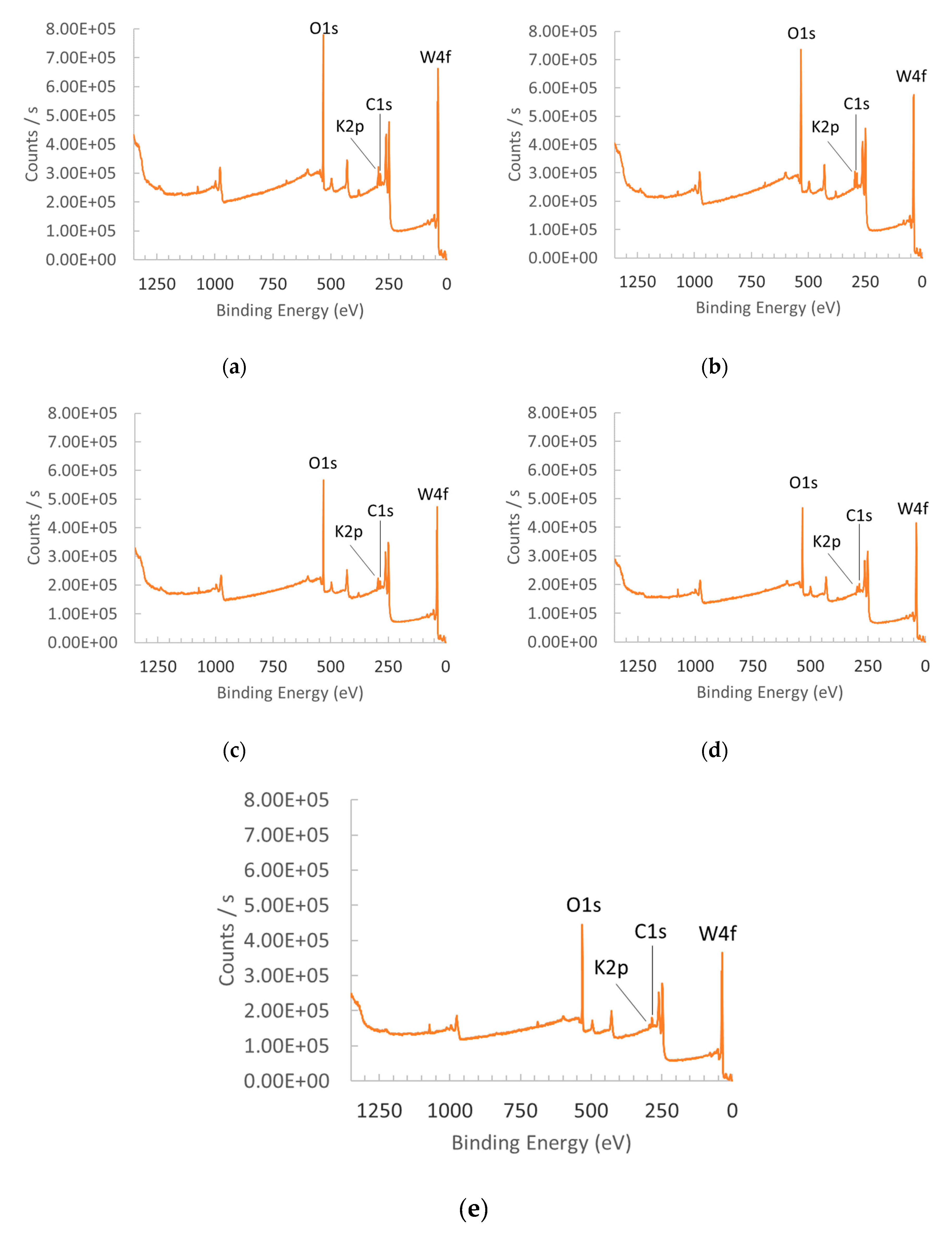
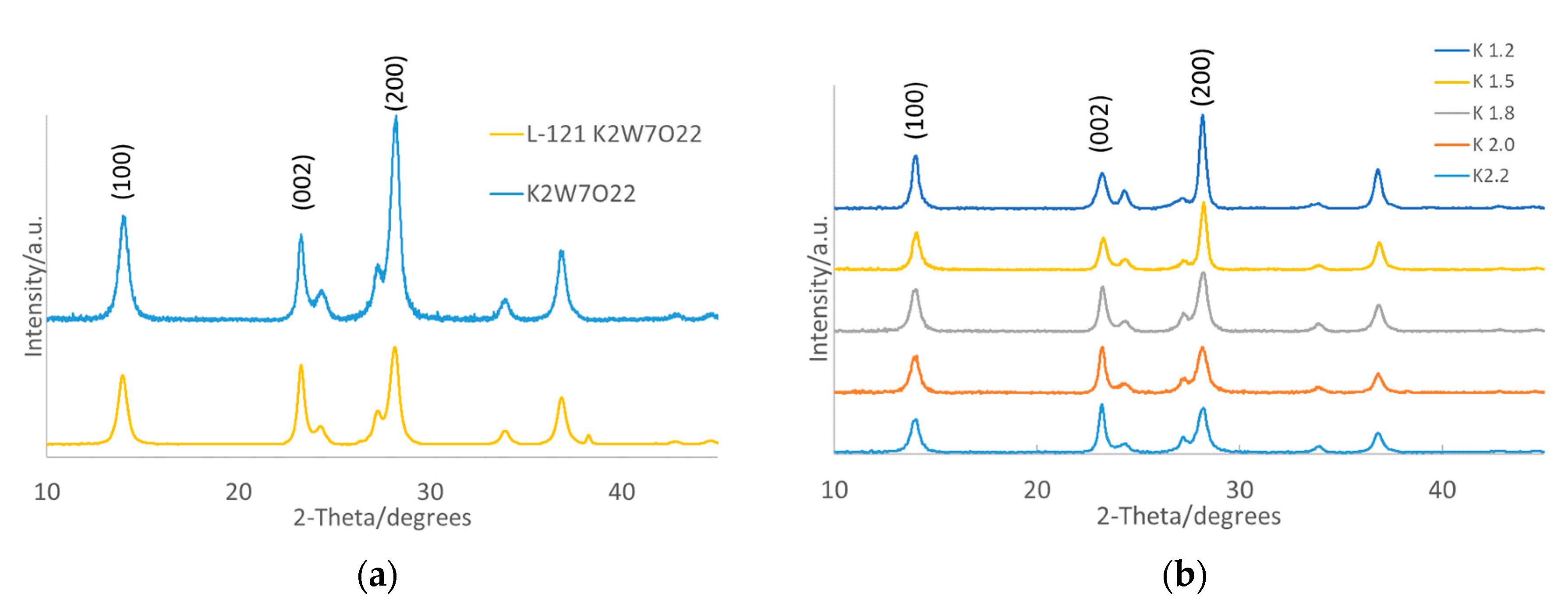
3.1.1. X-ray Diffraction (XRD) and X-ray Photoelectron Spectroscopy (XPS)
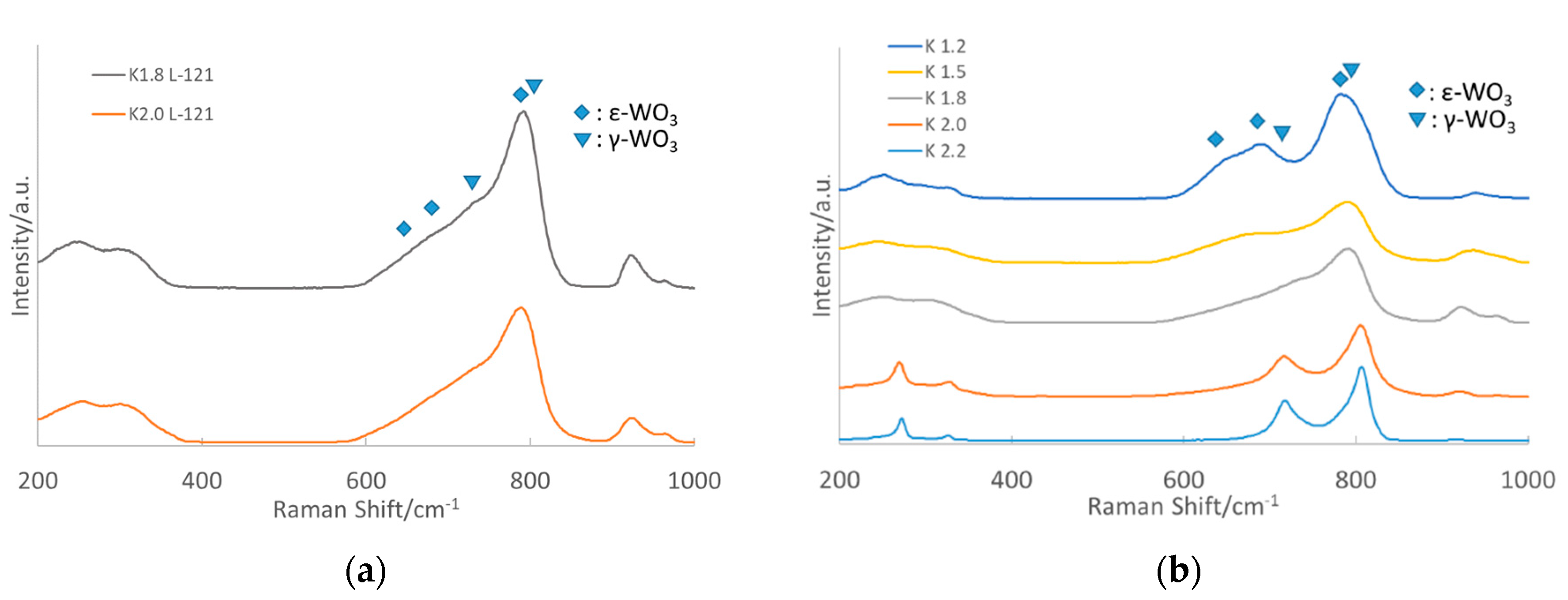
3.1.2. Raman Spectroscopy
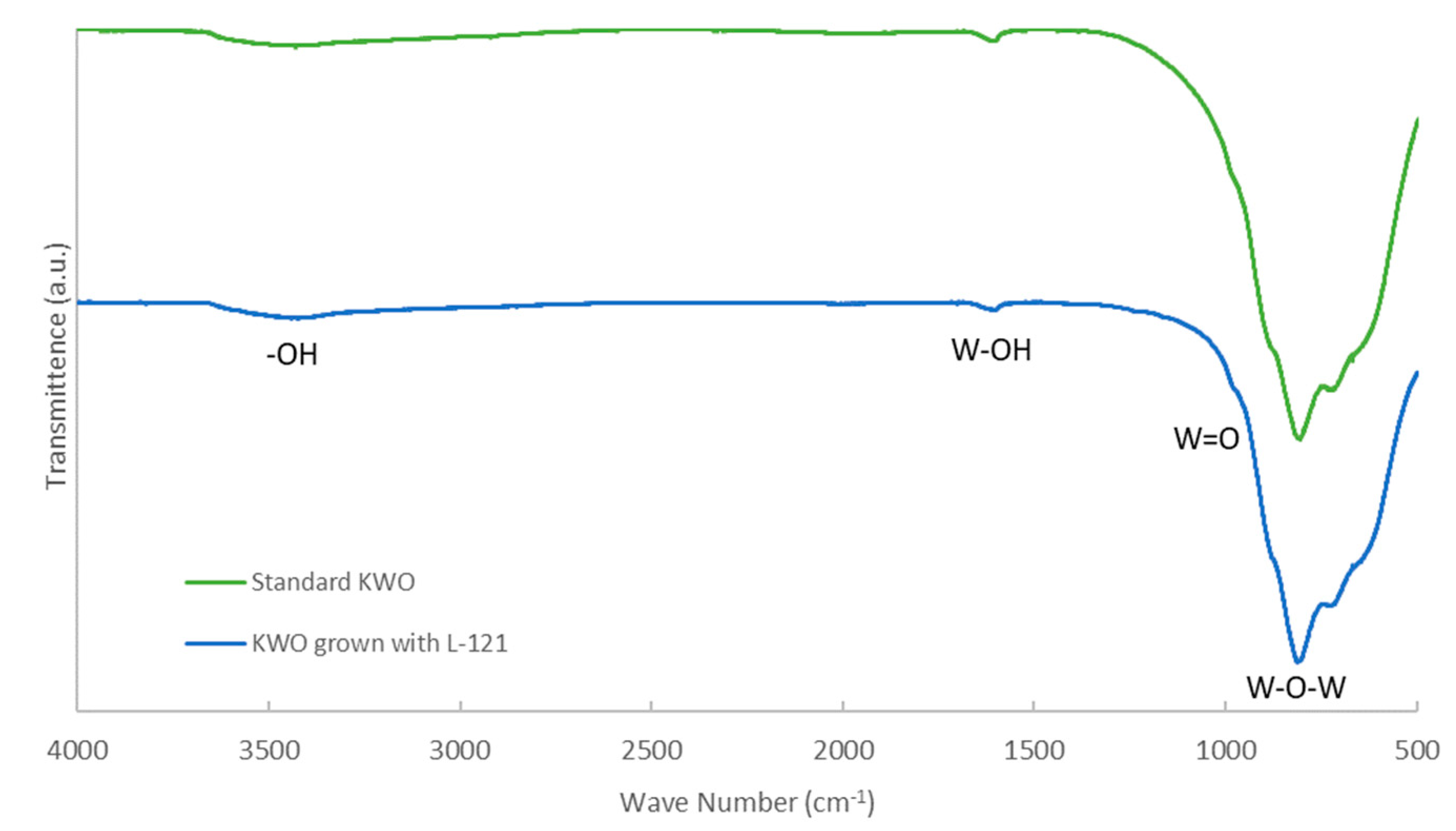
3.1.3. Fourier-Transform Infrared Spectroscopy (FT-IR)
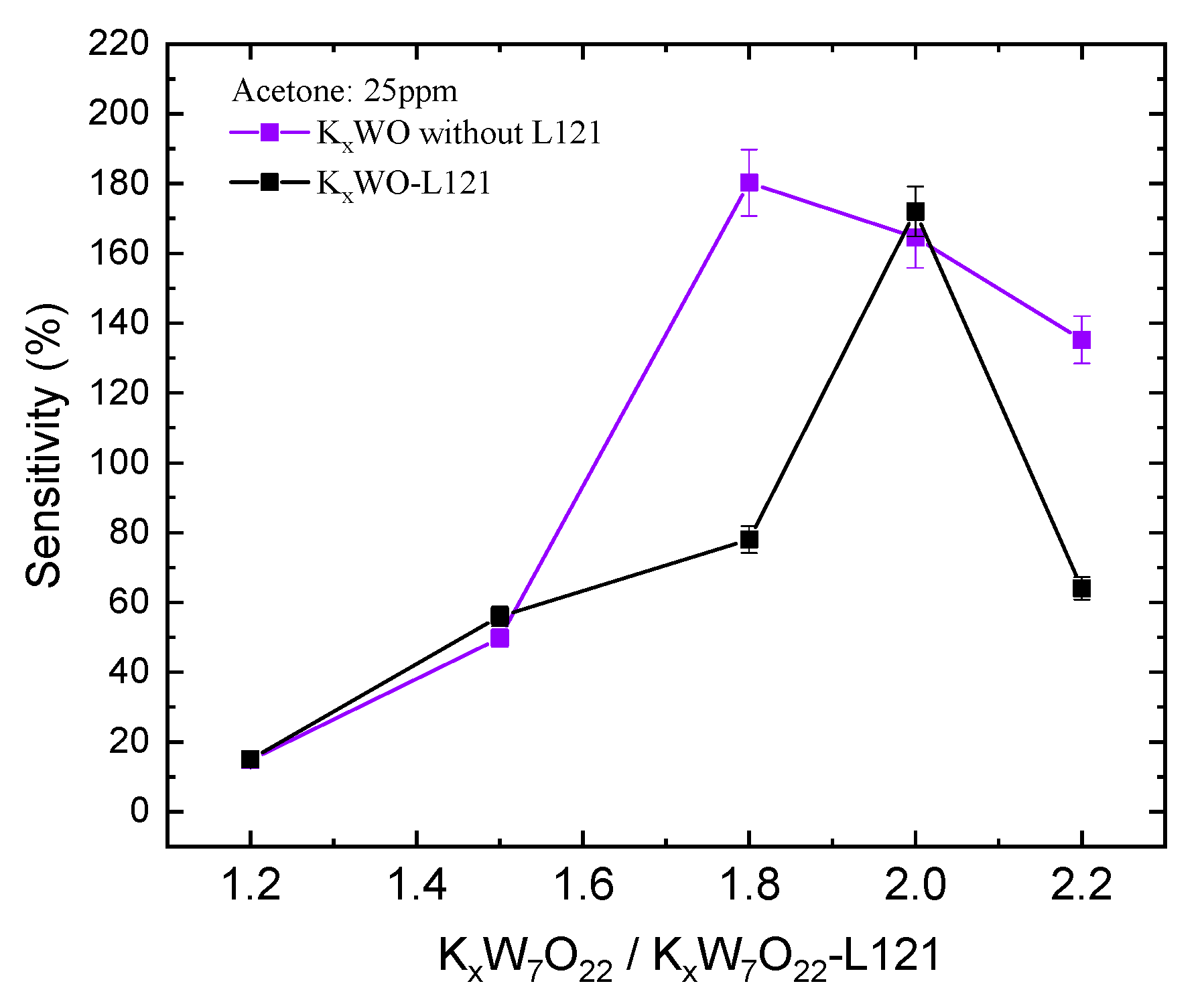
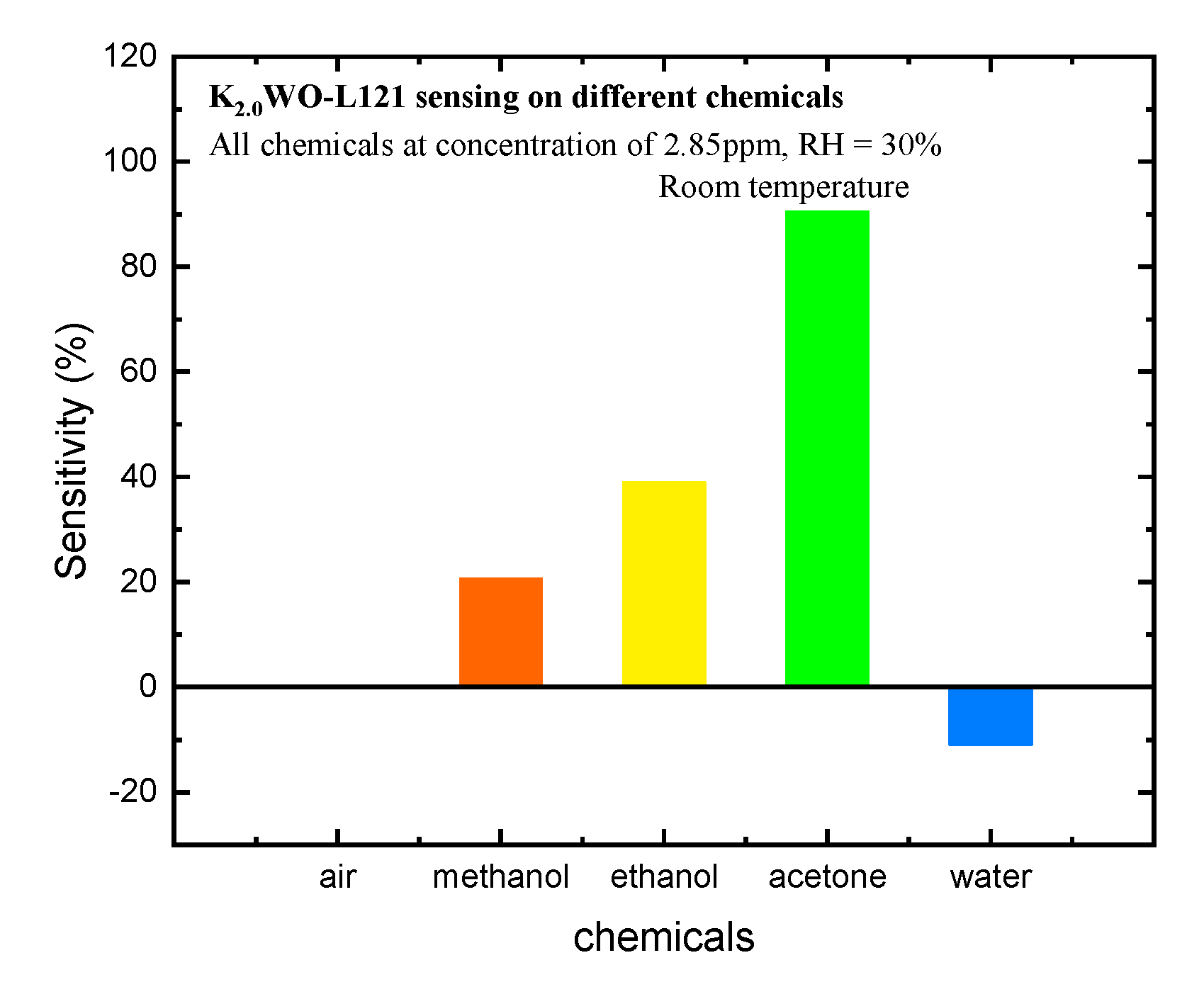
3.2. Sensitivity Testing
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Buszewski, B.; Kesy, M.; Ligor, T.; Amann, A. Human exhaled air analytics: Biomarkers of diseases. Biomed. Chromatogr. 2007, 21, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Konvalina, G.; Haick, H. Sensors for Breath Testing: From Nanomaterials to Comprehensive Disease Detection. Acc. Chem. Res. 2014, 47, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.H.; Zhang, J.; Yu, X.F.; Zhang, W.; Zhang, X.M. Determination of acetone in human breath by gas chromatography-mass spectrometry and solid-phase microextraction with on-fiber derivatization. J. Chromatogr. B-Anal. Technol. Biomed. Life Sci. 2004, 810, 269–275. [Google Scholar] [CrossRef]
- Staerz, A.; Weimar, U.; Barsan, N. Understanding the Potential of WO3 Based Sensors for Breath Analysis. Sensors 2016, 16, 16. [Google Scholar] [CrossRef]
- Tang, B.L.; Jiang, G.H.; Chen, W.X.; Wan, J.M. First-Principles Study on Hexagonal WO3 for HCHO Gas Sensing Application. Acta Metall. Sin. Engl. Lett. 2015, 28, 772–780. [Google Scholar] [CrossRef]
- Supothina, S.; Suwan, M.; Wisitsoraat, A. Hydrothermal synthesis of K2W4O13 nanowire with high H2S gas sensitivity. Microelectron. Eng. 2014, 126, 88–92. [Google Scholar] [CrossRef]
- Righettoni, M.; Tricoli, A.; Pratsinis, S.E. Si:WO3 Sensors for Highly Selective Detection of Acetone for Easy Diagnosis of Diabetes by Breath Analysis. Anal. Chem. 2010, 82, 3581–3587. [Google Scholar] [CrossRef]
- Barsan, N.; Weimar, U. Conduction model of metal oxide gas sensors. J. Electroceramics 2001, 7, 143–167. [Google Scholar] [CrossRef]
- Gurlo, A.; Riedel, R. In situ and operando spectroscopy for assessing mechanisms of gas sensing. Angew. Chem. Int. Ed. 2007, 46, 3826–3848. [Google Scholar] [CrossRef]
- Morrison, S. Mechanism of semiconductor gas sensor operation. Sens. Actuators 1987, 11, 283–287. [Google Scholar] [CrossRef]
- Ma, L.; Ma, S.Y.; Shen, X.F.; Wang, T.T.; Jiang, X.H.; Chen, Q.; Qiang, Z.; Yang, H.M.; Chen, H. PrFeO3 hollow nanofibers as a highly efficient gas sensor for acetone detection. Sens. Actuators B-Chem. 2018, 255, 2546–2554. [Google Scholar] [CrossRef]
- Wang, X.F.; Ma, W.; Jiang, F.; Cao, E.S.; Sun, K.M.; Cheng, L.; Song, X.Z. Prussian Blue analogue derived porous NiFe2O4 nanocubes for low-concentration acetone sensing at low working temperature. Chem. Eng. J. 2018, 338, 504–512. [Google Scholar] [CrossRef]
- Shin, J.; Choi, S.J.; Lee, I.; Youn, D.Y.; Park, C.O.; Lee, J.H.; Tuller, H.L.; Kim, I.D. Thin-Wall Assembled SnO2 Fibers Functionalized by Catalytic Pt Nanoparticles and their Superior Exhaled-Breath-Sensing Properties for the Diagnosis of Diabetes. Adv. Funct. Mater. 2013, 23, 2357–2367. [Google Scholar] [CrossRef]
- Jia, Q.Q.; Ji, H.M.; Wang, D.H.; Bai, X.; Sun, X.H.; Jin, Z.G. Exposed facets induced enhanced acetone selective sensing property of nanostructured tungsten oxide. J. Mater. Chem. A 2014, 2, 13602–13611. [Google Scholar] [CrossRef]
- Wang, L.; Teleki, A.; Pratsinis, S.E.; Gouma, P.I. Ferroelectric WO(3) nanoparticles for acetone selective detection. Chem. Mater. 2008, 20, 4794–4796. [Google Scholar] [CrossRef]
- Wang, D.L.; Zhang, Q.; Hossain, M.R.; Johnson, M. High Sensitive Breath Sensor Based on Nanostructured K2W7O22 for Detection of Type 1 Diabetes. IEEE Sens. J. 2018, 18, 4399–4404. [Google Scholar] [CrossRef]
- Pokhrel, S.; Simion, C.E.; Teodorescu, V.S.; Barsan, N.; Weimar, U. Synthesis, Mechanism, and Gas-Sensing Application of Surfactant Tailored Tungsten Oxide Nanostructures. Adv. Funct. Mater. 2009, 19, 1767–1774. [Google Scholar] [CrossRef]
- Hu, L.H.; Peng, Q.; Li, Y.D. Selective Synthesis of Co3O4 Nanocrystal with Different Shape and Crystal Plane Effect on Catalytic Property for Methane Combustion. J. Am. Chem. Soc. 2008, 130, 16136. [Google Scholar] [CrossRef]
- Han, X.G.; Li, L.; Wang, C. Synthesis of Tin Dioxide Nanooctahedra with Exposed High-Index {332} Facets and Enhanced Selective Gas Sensing Properties. Chem. Asian J. 2012, 7, 1572–1575. [Google Scholar] [CrossRef]
- Boppella, R.; Anjaneyulu, K.; Basak, P.; Manorama, S.V. Facile Synthesis of Face Oriented ZnO Crystals: Tunable Polar Facets and Shape Induced Enhanced Photocatalytic Performance. J. Phys. Chem. C 2013, 117, 4597–4605. [Google Scholar] [CrossRef]
- Zhang, H.B.; Yao, M.S.; Bai, L.Y.; Xiang, W.C.; Jin, H.C.; Li, J.L.; Yuan, F.L. Synthesis of uniform octahedral tungsten trioxide by RF induction thermal plasma and its application in gas sensing. Crystengcomm 2013, 15, 1432–1438. [Google Scholar] [CrossRef]
- Arai, M.; Hayashi, S.; Yamamoto, K.; Kim, S.S. Raman Studies of Phase-transitions in Gas-evaporated WO3 Microcrystals. Solid State Commun. 1990, 75, 613–616. [Google Scholar] [CrossRef]
- Yoshimura, M.; Byrappa, K. Hydrothermal processing of materials: Past, present and future. J. Mater. Sci. 2008, 43, 2085–2103. [Google Scholar] [CrossRef]
- Hossain, M.R.; Zhang, Q.F.; Johnson, M.; Wang, D.L. Highly Sensitive Room-Temperature Sensor Based on Nanostructured K2W7O22 for Application in the Non-Invasive Diagnosis of Diabetes. Sensors 2018, 18, 9. [Google Scholar] [CrossRef]
- Johnson, M.; Zhang, Q.; Wang, D.L. Room-Temperature Ferroelectric K2W7O22 (KWO) Nanorods as a Sensor for Detection of Acetone. Med Devices Sens. 2019, 2, e10044. [Google Scholar] [CrossRef]
- Hossain, M.R.; Zhang, Q.F.; Johnson, M.; Wang, D.L. Investigation of humidity cross-interference effect on acetone breath sensor based on nanostructured K2W7O22. Eng Press 2017, 1, 30–34. [Google Scholar] [CrossRef]
| Sample | Atomic % K | Atomic % W | Atomic % O |
|---|---|---|---|
| K1.2WO | 2.70 | 19.61 | 57.22 |
| K1.5WO | 2.99 | 19.27 | 60.31 |
| K1.8WO | 4.42 | 19.69 | 59.47 |
| K2.0WO | 4.99 | 18.44 | 57.61 |
| K2.2WO | 5.48 | 18.57 | 59.54 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, M.E.; Zhang, Q.; Wang, D. KxWO Is a Novel Ferroelectric Nanomaterial for Application as a Room Temperature Acetone Sensor. Nanomaterials 2020, 10, 225. https://doi.org/10.3390/nano10020225
Johnson ME, Zhang Q, Wang D. KxWO Is a Novel Ferroelectric Nanomaterial for Application as a Room Temperature Acetone Sensor. Nanomaterials. 2020; 10(2):225. https://doi.org/10.3390/nano10020225
Chicago/Turabian StyleJohnson, Michael E., Qifeng Zhang, and Danling Wang. 2020. "KxWO Is a Novel Ferroelectric Nanomaterial for Application as a Room Temperature Acetone Sensor" Nanomaterials 10, no. 2: 225. https://doi.org/10.3390/nano10020225
APA StyleJohnson, M. E., Zhang, Q., & Wang, D. (2020). KxWO Is a Novel Ferroelectric Nanomaterial for Application as a Room Temperature Acetone Sensor. Nanomaterials, 10(2), 225. https://doi.org/10.3390/nano10020225






