1. Introduction
The acoustic biosensor approach is a widespread technology for label-free assessment of molecular interactions at biointerfaces. Among them, the shear bulk acoustic resonators are commonly used for biosensing due to their low in-liquid radiation losses. Quartz crystal microbalance (QCM) is a recognized technology that is used to detect interactions at the surface. When the QCM is excited at a shear bulk vibration mode, any resonant frequency shift reflects a change in the acoustic load at the QCM surface. QCM has been introduced by Sauerbrey, who studied the change of resonant frequency in-air as a function of the mass of a rigid solid material deposited onto the QCM surface [
1], corresponding to the so called gravimetric regime. Sauerbrey’s approximation is basically a relation between the attached mass and the mass of quartz crystal per unit area, typically presented as
where
f0 is the resonant frequency of the QCM;
is the density and
the shear modulus of quartz; Δ
m is the probed mass attached to the active surface
A.
Later work of Kanazawa and Gordon [
2] has shown that a Newtonian liquid in contact with a QCM creates an acoustic load of the resonator whose equivalent mass is proportional to the density of the fluid and to
δ, the penetration depth of the shear evanescent wave in the liquid, expressed as
where
ρL and
ηL are the density and the viscosity of the liquid. The resulting frequency shift is
A typical value of the shear wave penetration depth in water at a fundamental vibration mode around 5 MHz is about 250 nm causing a frequency decrease of roughly 2 kHz. If a more viscous liquid, particularly whole blood, replaces water, the penetration depth of the acoustic wave, as well as the frequency shift, increases.
If a rigid layer at the sensor surface is covered by a liquid, the contribution of the layer Equation (1) and the liquid Equation (3) are additive. However, in biosensor applications, the material at the sensor interface is generally viscoelastic and can no longer be approximated as a rigidly attached mass. Viscoelasticity is a frequency-dependent phenomenon correlated with relaxation times of the biomolecule or its segments [
3]. A viscoelastic layer simultaneously exposes elastic loading and viscous losses that causes significant deviation of frequency shift from Sauerbrey’s approximation. In this case, the QCM is operating in non-gravimetric regime [
4] and responds to the viscoelastic properties of the layer [
5].
Acoustic load of complex layer arrangement have been described by using acoustic impedance concept [
6,
7]. The surface acoustic impedance represents the overall acoustic load at the interface and is given by
where
is the mass factor (
ρ is the density and
h is the thickness of the load layer) and
is the acoustic factor with
ϕ being the phase shift of the acoustic wave in the layer. For
we are operating in the gravimetric regime, but it is worth to note that
even for very thin (bio)films in contact with a liquid and appears as a “missing mass” [
8,
9,
10,
11].
While the QCM frequency shift for monolayer loading is well described, the theory still lacks sufficient background for inhomogeneous loading when both liquid and solid/viscoelastic materials are in contact with the sensor surface. Moreover, the influence of dimensional effects on the overall frequency shift is rarely discussed. Martin et al. [
12] partly addressed the issue considering two-dimensional solid structures and showed that the liquid trapped in the structure contributes with an additional loading.
The assessment of cell deposits with shear bulk acoustic approach should take into an account all the above-mentioned contributions. However, they are often neglected and the QCM frequency shift is commonly attributed to ‘mass loading’ only. Some of the challenges associated with living cell assessment using acoustic biosensor approach were already addressed. It was shown that for a typical mammalian cell with dimensions of 10 μm, only a small part of the cell leads to a frequency shift [
13]. Cells deposited onto the surface without firm attachment via a bio-chemical bond can create a liquid filled region between the cell and the vibrating surface. The sensor response at different stages of the cell–surface interaction reflects the nature of the cell attachment, its structural modification, secretion, and expression of extracellular vesicles and its surface spreading [
14]. The cell-biointerface interaction triggers cellular biophysical mechanisms that in turn affect its material properties and vary the frequency shift [
15]. Actually, QCM frequency shift has been previously related to the spreading of the attached cells [
13]. The QCM dissipation factor was also shown to provide a distinctive response to the cell spreading at extremely low cell coverage, when the frequency shift is no longer detectable [
16]. Cell stiffness and the changes in the cytoskeleton were reported to predominantly contribute to this frequency shift [
17], but viscosity contributions were not clearly identified.
Our work is focused on the study of platelet deposits onto a relevant reactive biointerface for the study of the physiological process of primary hemostasis [
18]. Platelets are small discoid shaped anucleated blood cells of 2–3 µm in largest diameter, which circulate in resting state in blood [
19]. Their primary function is to prevent and stop bleeding at the site of an injured vessel, thanks to a sequence of interactions between the vessel wall, blood proteins, and platelets, culminating in a platelet plug [
20]. The surface coverage with platelets can vary widely from localized (low coverage) to spread (high coverage) deposits, which could affect the acoustic loading conditions. Since most of cell studies are conducted in liquid environment, the acoustic shear vibrations excite evanescent waves that are partially distributed in the viscoelastic biolayer and in the liquid. One finally must consider that some liquid may be trapped between the cells, whereas parts of the cells are separated from the biointerface by a thin liquid layer. The analysis of such platelet deposits with acoustic sensors thus becomes a multifactorial problem [
21].
In the following contribution, we concentrate on the assessment of topologically different platelet deposits. We challenge the idea that the frequency shift is self-sufficient to assess topologically different cell deposits. We show the results of QCM biosensor measurements for different types of platelet deposit topologies. We address how the biosensor frequency shift is impacted by platelet deposits topology at the QCM surface and for which condition the frequency shift is directly related to the amount of deposited cells. The results of our numerical studies explain how the deposit topology at the surface impacts the frequency shift response of the QCM biosensor.
2. Materials and Methods
2.1. Materials
QCM crystals of 14 mm diameter with fundamental frequency 5 MHz (QCM5140CrAu120-050-Q, Quartz Pro AB, Stockholm, Sweden) were utilized for the experimental studies as standard shear bulk acoustic resonant sensors. Each crystal was manually prepared with a collagen biointerface and assembled into a microfluidic cell for blood perfusion tests. Collagen Horm
® (type I) and isotonic glucose solution pH 2.7–2.9 (SKF solution) (Takeda Austria GmbH, Linz, Austria) were used for this procedure. N-(3-dimethylamino-propyl)-N-ethylcarbodiimide (EDC), N-hydroxysulfosuccinimide (sulfo-NHS), phosphate buffered saline (PBS), bovine serum albumin (BSA), ethanolamine HCl 1M, 16-mercaptohexadecanoic acid (16-MHA), and 11-mercapto-1-undecanol (11-MUOH) (Sigma Aldrich, Lyon, France) were used as solutions for the biointerface processing. Fixation of cell deposits after each experiment was done with glutaraldehyde 0.5% concentration solution in deionized water (Sigma Aldrich, France). The grafting of collagen followed a procedure established previously for protein grafting on gold chip in our research group [
22].
Whole blood samples from healthy male donors were provided by the Etablissement Français du Sang (EFS, Besancon, France), in compliance with current ethical regulations in France. Whole blood was collected into 1.6 mL collecting tubes with hirudin as anticoagulant and used for the experiments within two hours.
2.2. Collagen Biointerface
Before engaging experimental investigations in whole blood with rheological conditions that are relevant to microcirculation, it is crucial to control the supramolecular architecture of the biointerface. We used a collagen type I layer to initiate platelet adhesion, activation and plug growth via interaction with additional platelets. The reconstitution of the collagen layer is performed on a self-assembled monolayer of 16-MHA and 11-MUOH onto the gold surface of the chip; this ensures a covalent grafting of collagen and a robustness under the shear rates used in this study. An in-depth characterization of that collagen biointerface included the surface coverage, topological profiles, the thickness, and orientation of the collagen fibers.
AFM image of immobilized collagen on gold surface, its height measurement profile and corresponding frequency shift (including negative control with BSA) are shown in
Figure 1.
Reproducibility of the grafting is a prerequisite for sensing purposes, especially in terms of covering rates which are of 23% ± 2.3% (based on AFM images) in this architecture with a mean collagen fiber height of 86 ± 12 nm. The assessment of the acoustic load imposed by the biointerface itself, in-air condition, was completed using several QCM sensors. Measurements were performed before and after the collagen immobilization revealing an average shift close to 80 Hz. As a negative control, reference QCMs were processed within a similar immobilization protocol omitting the step of collagen immobilization. They presented an average shift close to 20 Hz, likely to represent the loading caused by passivating BSA protein. The results of recorded frequency shift in both cases are shown in
Figure 1c and demonstrated a standard deviation below 10%.
2.3. Experimental Setup and Conditions
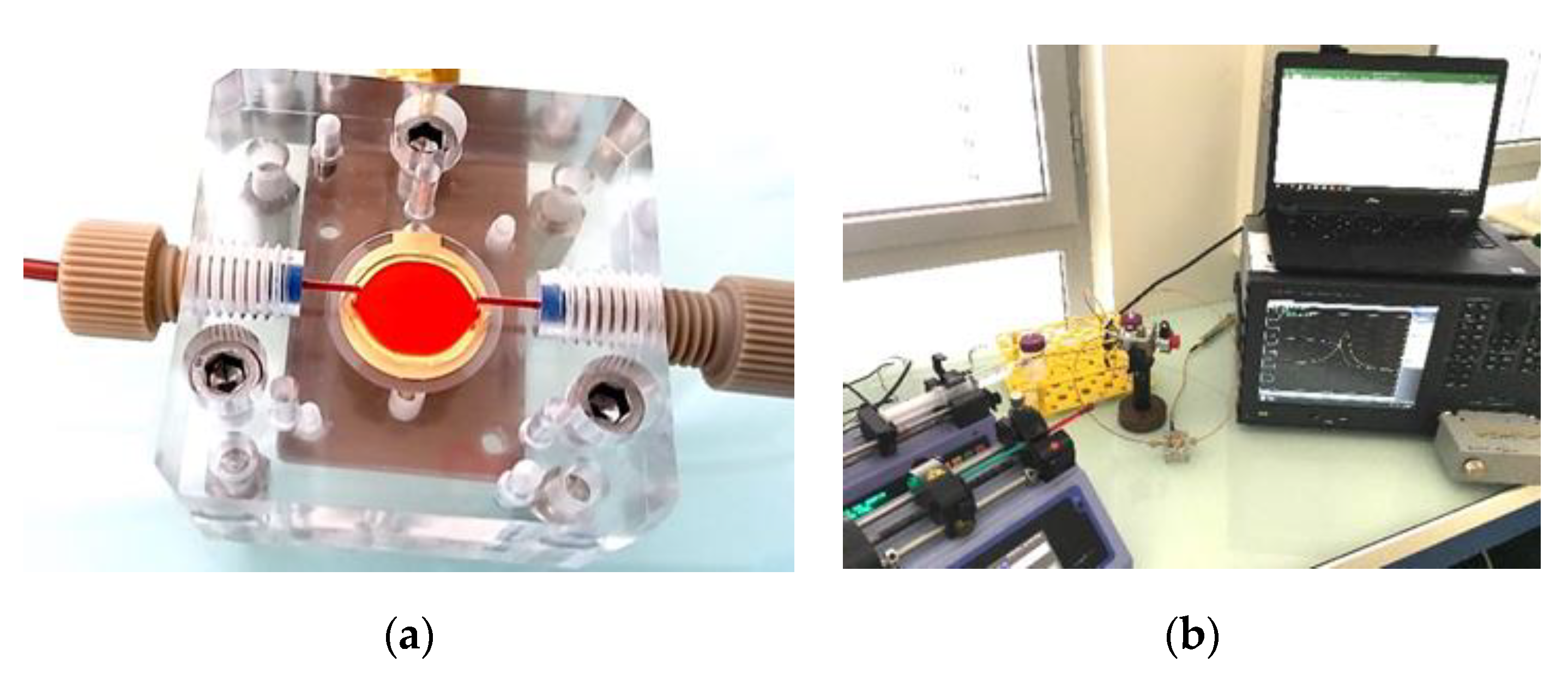
A Poly(methyl methacrylate) transparent microfluidic chamber of 50 µm height with the collagen coated QCM defining the floor plane was fabricated to mimic experimental conditions similar to microcirculation. Perfusion chamber and experimental setup are shown in
Figure 2.
The whole blood perfusion through the chamber was performed for 5 min at room temperature, which was kept stable in the range 23–24 °C. The perfusion tests were made at rheological conditions corresponding to small vessels by using microfluidic setup with separate injection lines [
23]. The QCM admittance spectrum was continuously recorded during the blood perfusion with a Keysight 4990A (Keysight Technologies, Santa Rosa, CA, USA) impedance analyzer using custom made software. This spectrum is used to track the evolution of the QCM resonant frequency. The ‘frequency shift’ used in the experiment is referenced to the resonance frequency obtained when the biointerface is in contact with the PBS buffer (zero frequency shift).
The deposited cells on the QCM were subsequently studied with Nanowizard III AFM (JPK Instruments, Berlin, Germany) in order to characterize the surface topography of the biointerface. Images were taken for the maximal scanning area 100 × 100 µm using Nano World NPS-10C cantilevers (NanoAndMore, Paris, France) made from silicon nitride with a stiffness of 0.32 N/m. The cellular deposits were fixed with 0.5% solution of glutaraldehyde in deionized water. The images were obtained in a contact mode in air at a frequency of 0.5 line/s with a resolution of 512 × 512 pixels. Height trace scans were recorded to evaluate the amount and morphology of platelet deposits. The average thickness of cells deposits was determined by an arithmetic mean value of the height across the 100 × 100 µm scanned area. The coverage was measured as a ratio of the surface covered by platelet deposits to the whole scanned surface when 0 corresponds to free surface and 1 to full surface coverage.
2.4. Platelet Deposits Characterization
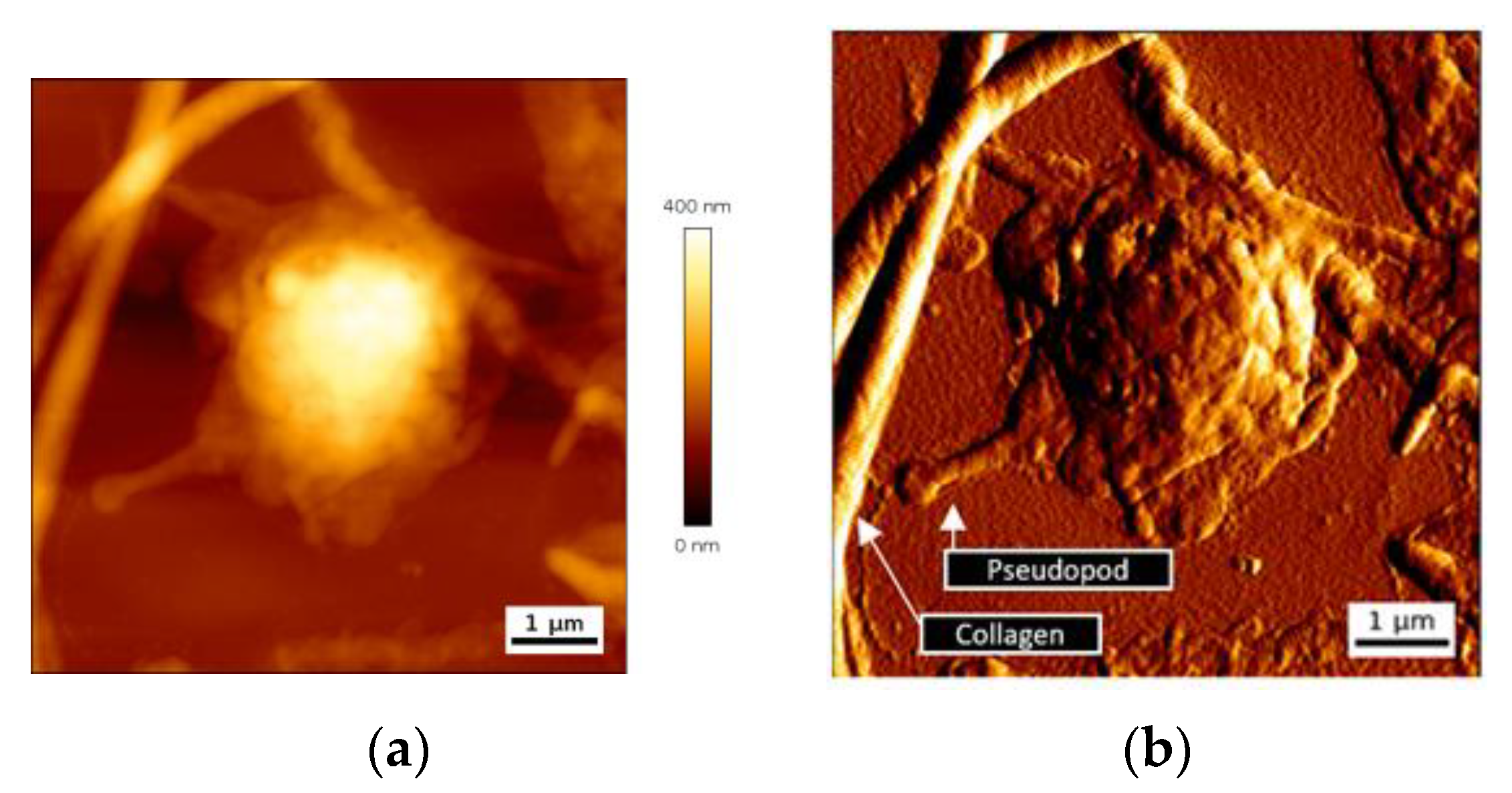
Platelet-collagen interaction leads to firm platelet adhesion. Structural modifications of platelets ensue, with cytoskeleton modifications, release of granule content, and cell spreading. We have first looked at single platelet deposits to assess their dimension and more particularly their thickness. AFM images of height and deflection traces for a representative single adherent platelet on collagen biointerface are shown in
Figure 3.
Adherent single platelets have a semispherical shape with a variable spreading. Deflection trace image reveals pseudopods emitted by cells and the presence of nanoscale vesicles close to the cells, and probably released by them (
Figure 3b). Maximal height of a single deposited platelet was in a range 460 ± 70 nm.
Interaction of platelet GPVI receptor with collagen leads to activation of platelet α
IIbβ
3 integrin, which enables the direct interaction of deposited platelet with circulating ones [
24,
25,
26]. As a result, platelets aggregate and form deposits of varying topology. The deposits are distributed across the biointerface with lateral dimensions that are nearly two orders of magnitude larger than the height of a single adherent platelet [
27,
28]. An example of a deposit of aggregated platelets is shown in
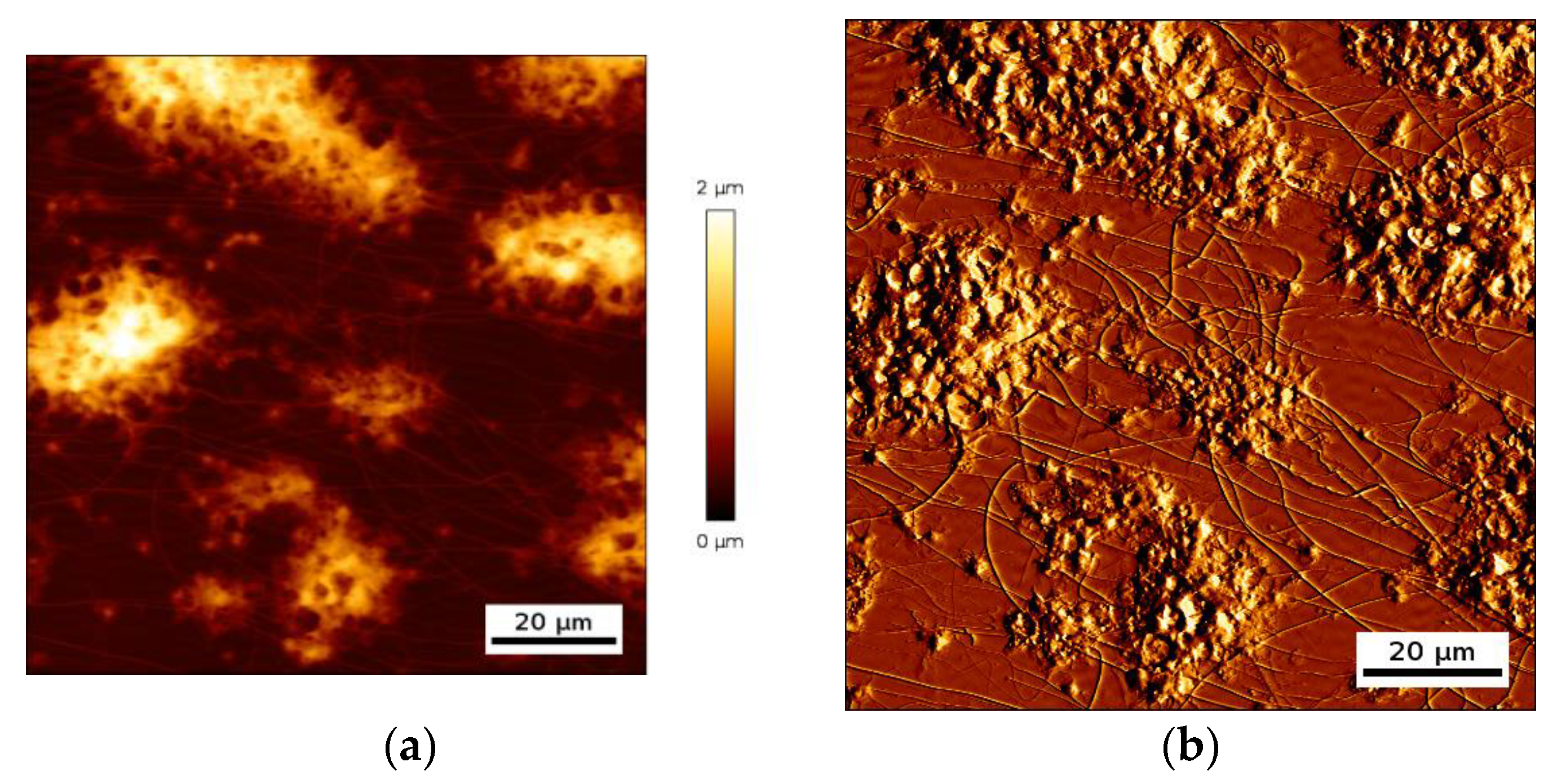
Figure 4.
One can clearly distinguish single platelet deposits from platelet aggregates with a lateral dimension of several tens of micrometers and a maximal height in some cases reaching two micrometer. Those platelet deposits create a complex surface topology, with some areas of the biointerface that are completely covered with platelets and others that are directly in contact with the liquid, which is whole blood during such an experiment. As a result, the acoustic resonator surface sees a distributed cells/liquid load.
2.5. Computational Model
Numerical studies were performed with COMSOL Multiphysics 5.4 software (COMSOL France, Grenoble, France). A three-dimensional computation model with periodic boundary conditions in X and Y directions was developed. The acoustic resonator was described as a piezoelectric domain with AT-cut quartz of 330 μm thickness. The solid layer load was simulated with a structural mechanics model. Solid fluid interactions were computed with the acoustic-structure interaction model. We used two different liquids, the buffer liquid (Liquid1—density 1000 kg/m
3; viscosity 0.001 Pa·s) and the whole blood (Liquid2—density 1060 kg/m
3; viscosity 0.003Pa·s). Although whole blood is a non-Newtonian fluid (shear thinning effect), above a shear rate of 200 s
−1, as used in this study, its viscosity may be considered constant [
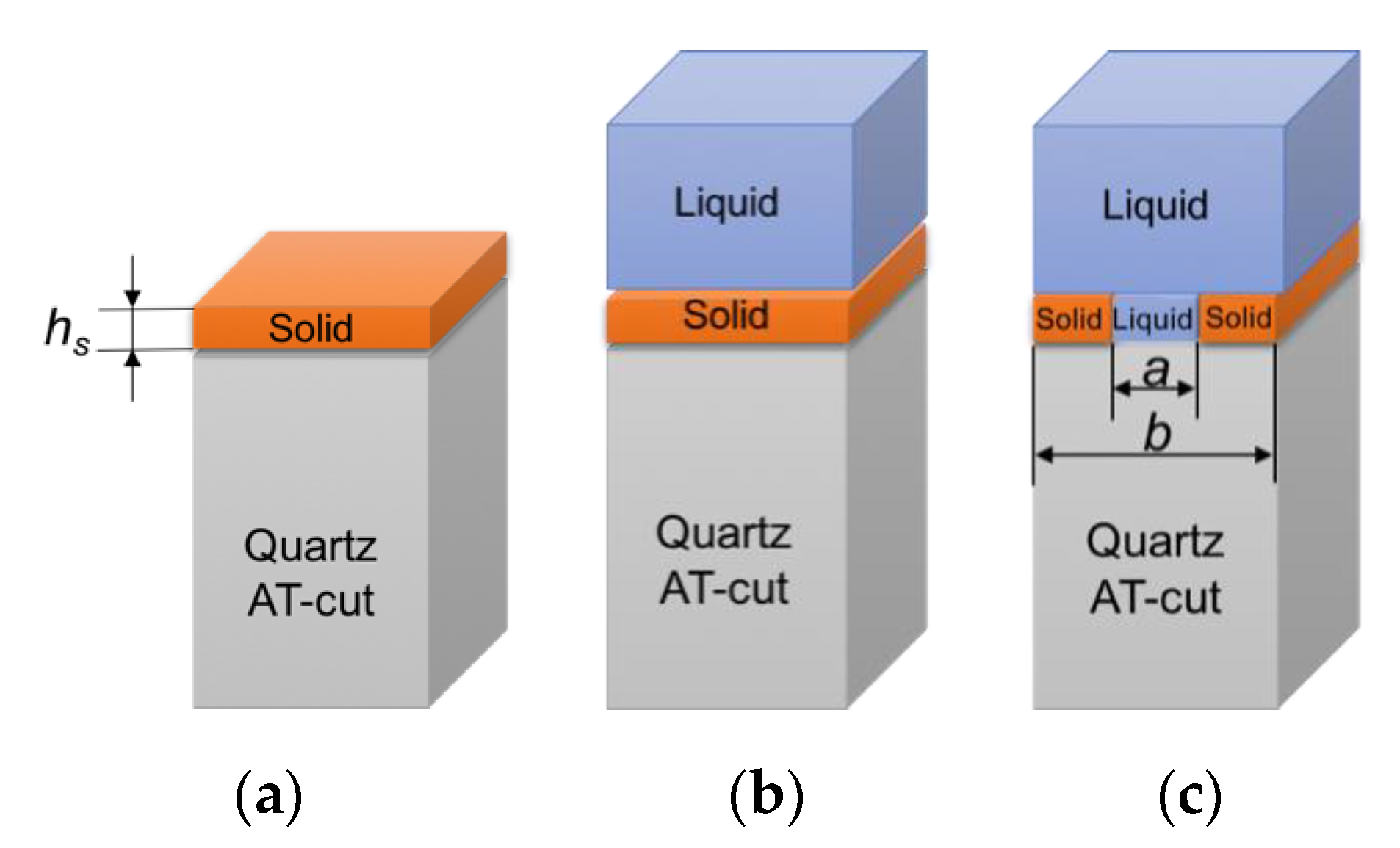
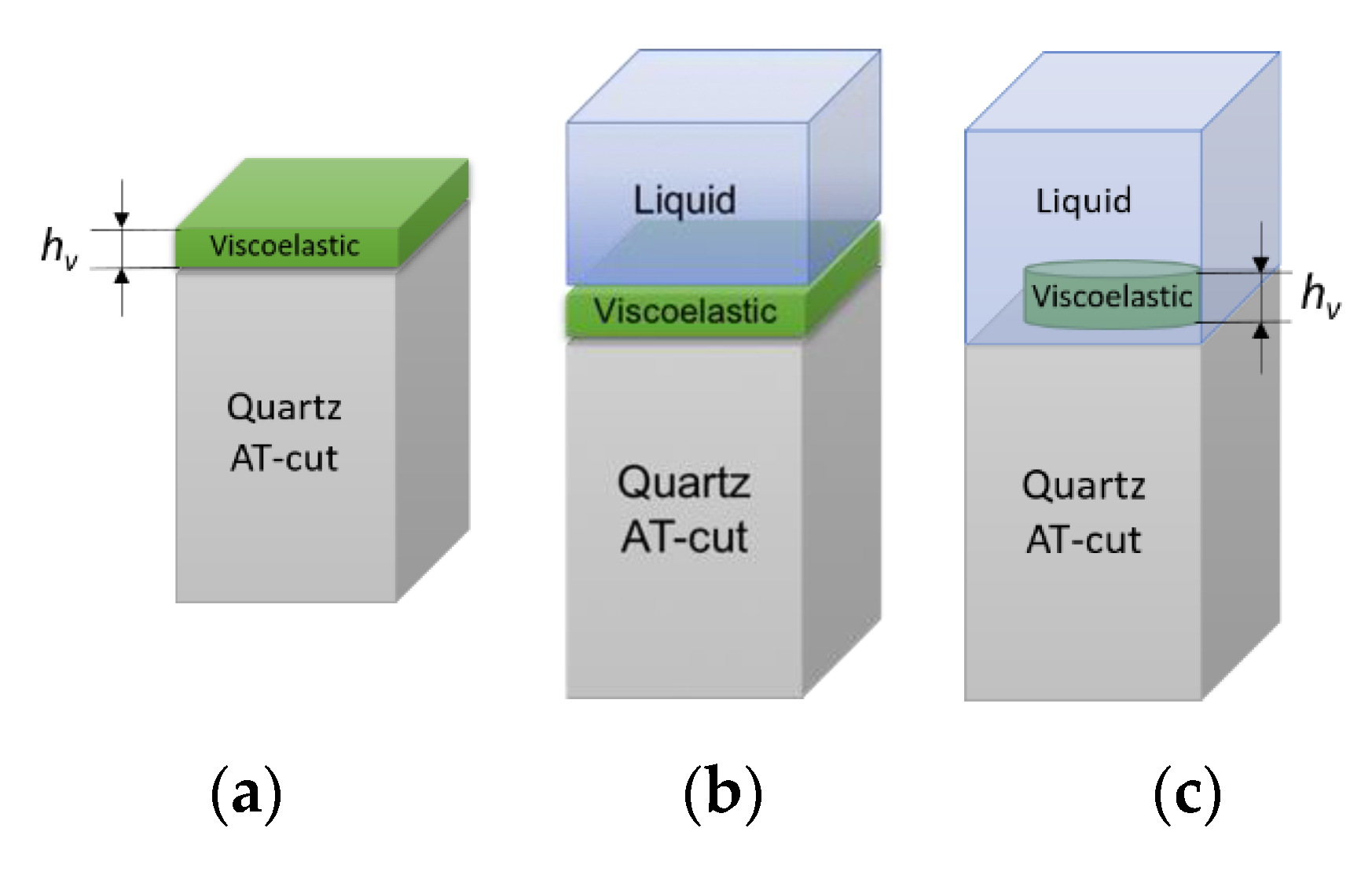
29]. The validation of numerical model was completed for the computational problems that are schematically shown in
Figure 5.
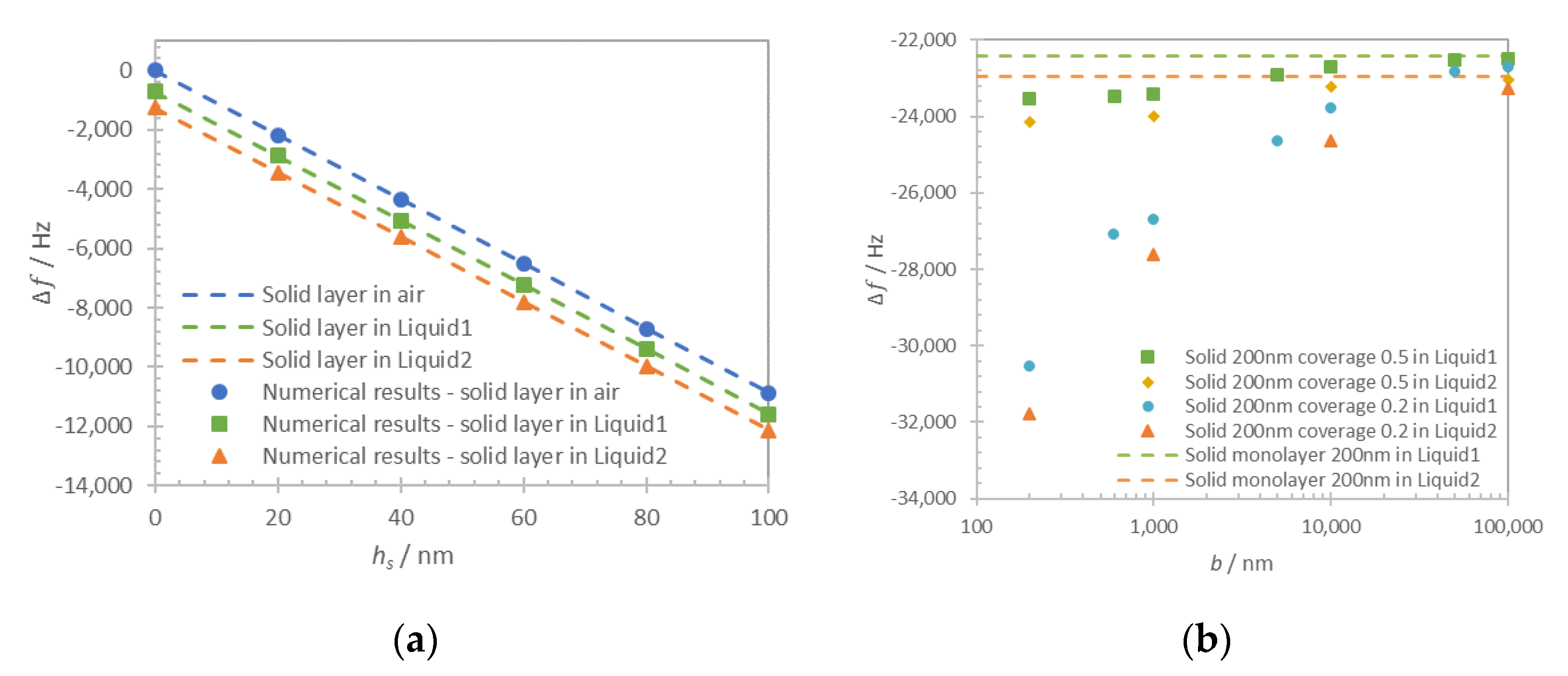
The thickness of the solid layer (
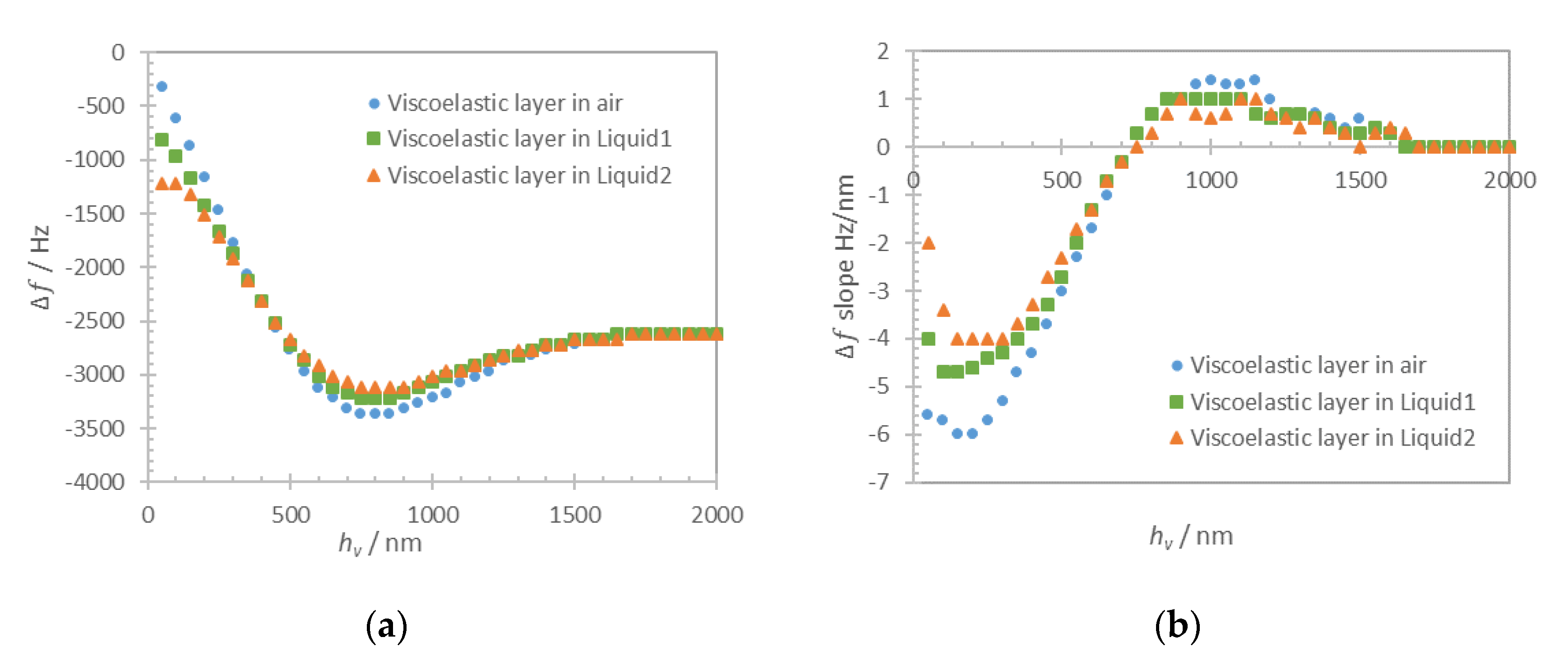
hs) is varied in a range 0–100 nm with a step of 20 nm. Computational results for frequency shift of maximum of admittance for the solid monolayer in-air and in-liquid for Liquid1 and Liquid2 are shown in
Figure 6a (dots). The respective approximations with Equation (1) for a solid layer in-air and the sum of Equations (1) and (3) for the case in-liquid are shown in
Figure 6a as dashed lines. Zero frequency shift corresponds to the unloaded resonator in air.
In contrast to the monolayer type of loading, the structured solid layer presents more complex behavior. In this case, the resonator sensing surface is partially covered with a solid layer and in a partial contact with the liquid. The solid layer was defined as a two-dimensional arrangement of rigidly attached lines with a variable period
b in a range 0.2–100 μm. In fact, the term period is used to simplify the computation problem only with no claimed effects that depend on the existence of a periodicity. The coverage
was defined as the ratio between the surface covered by the solid layer and the total surface:
. Two representative values of coverage 0.2 and 0.5 were used in this computation. The average thickness of the structured solid layer
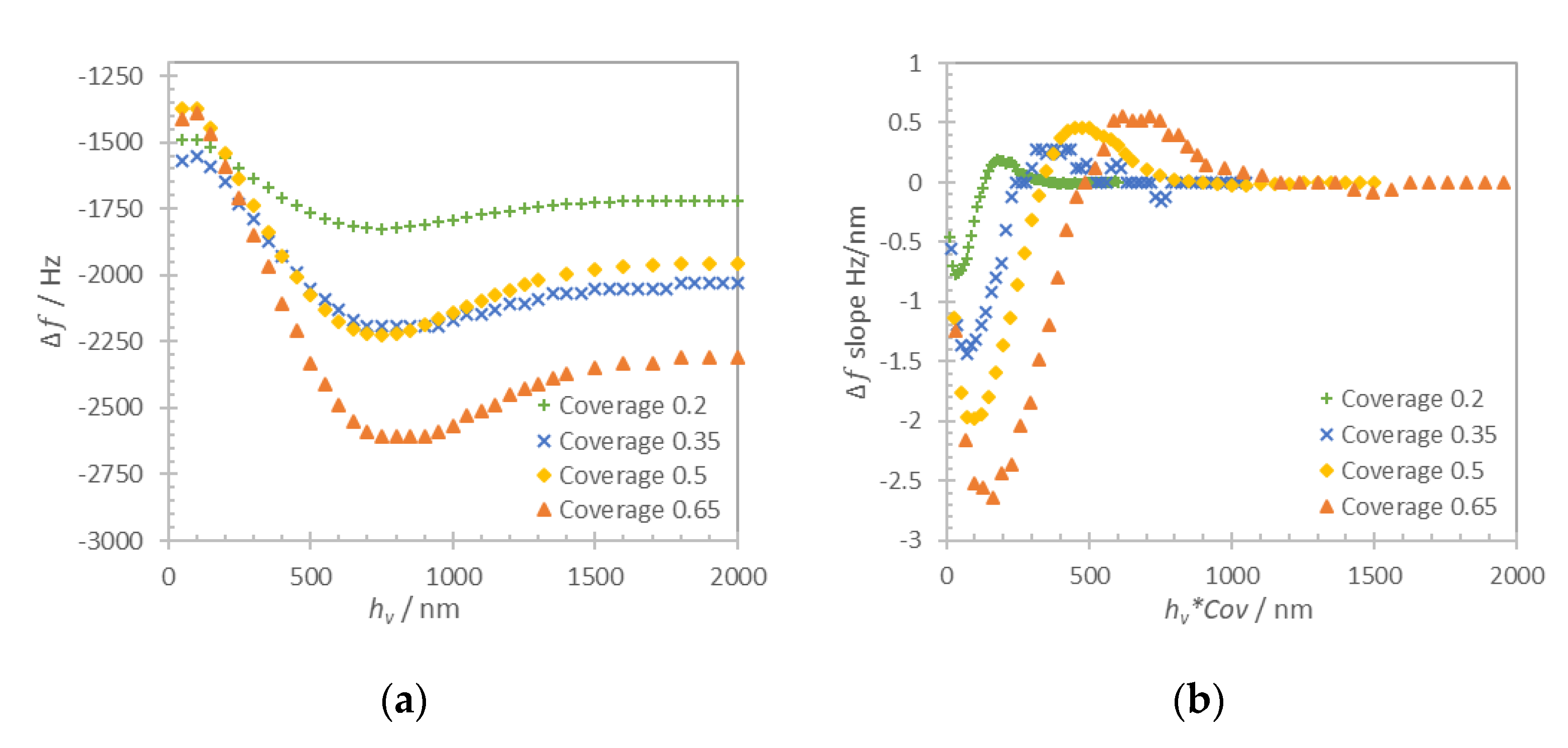
was always kept equal to 200 nm to ensure similar solid mass loading contribution in each case. The thickness of the solid layer was then respectively defined as 400 nm for coverage 0.5 and 1 μm for coverage 0.2. The computation was performed for Liquid1 and Liquid2 and the results are shown in
Figure 6b.
As it can be seen in
Figure 6a, the numerical results and analytical approximations for continuous film loading of quartz resonator are in close agreement (discrepancy lower than 0.2%), proving the validity of the developed computational model. In the case of both liquid and solid monolayer loadings, the additive contribution of rigidly attached solid Equation (1) and liquid Equation (3) clearly cause the shift of the resonant frequency.
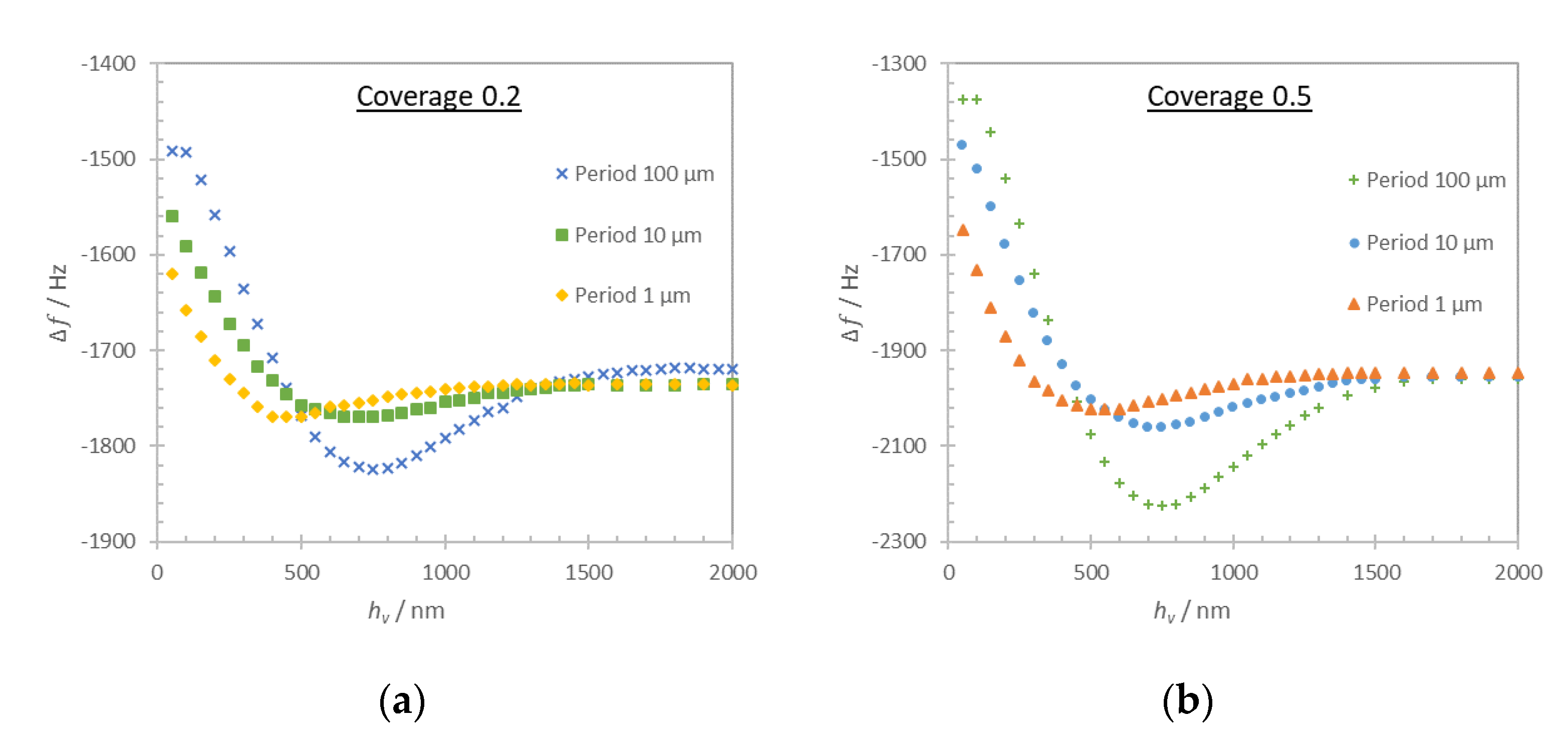
For the discontinuous film in
Figure 6b, we distinguish two different loading regimes that are regulated by the period of the layer structures. When the period is several times larger than the layer thickness, the approximation of the loading can be made using the additive contribution of Equations (1) and (3). When the period is comparable or smaller than the thickness of the layer this approximation is no longer valid, particularly in the low coverage case (
Cov = 0.2). The frequency shift in this case is impacted by the contribution of trapped liquid and can be obtained only numerically.
4. Discussion
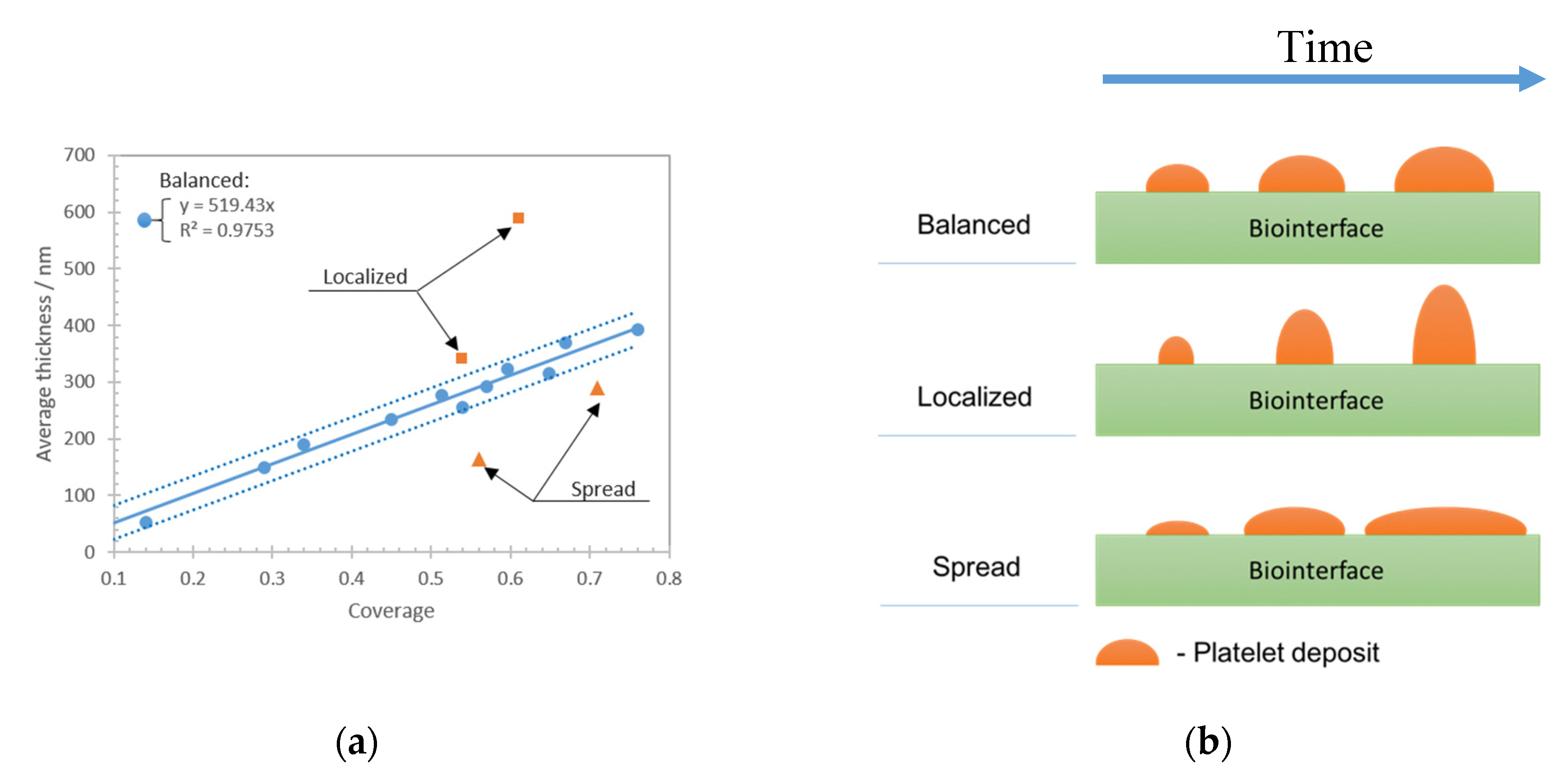
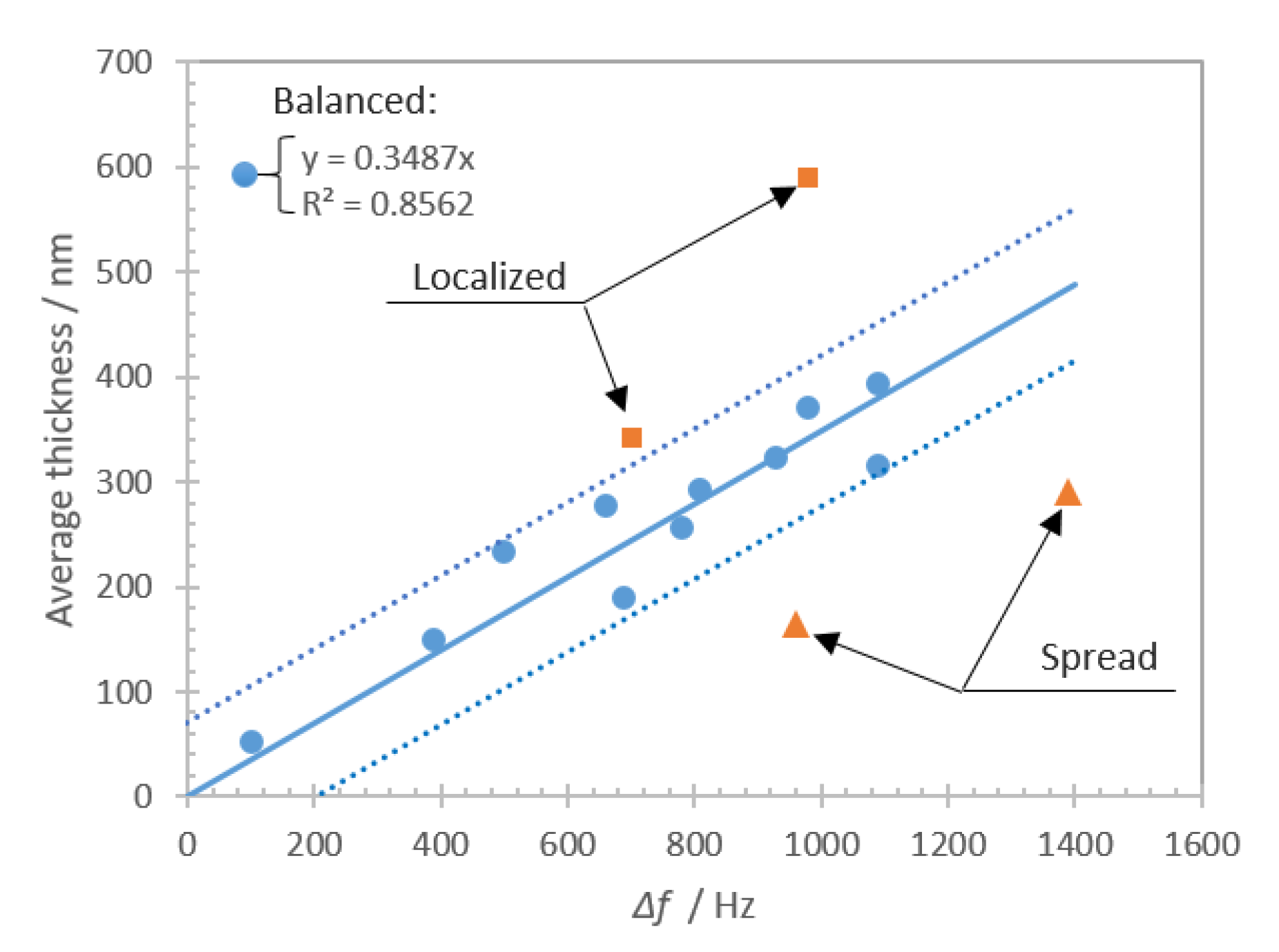
This study was focused on the assessment of living cells, i.e., platelet deposits onto a biologically reactive surface, with an acoustic biosensor. The study consisted of experimental investigations supported with computational results. Before addressing the particular behavior of platelets on the sensing layer, we have deeply characterized the biointerface by various biophysical techniques (QCM, AFM, optical microscopy). Once well established in a reproducible manner, it was possible to extend the investigation to platelets.
The experimental and numerical results show that the effective loading of the shear bulk acoustic resonator with living cells is a challenging problem, as the topology of cell deposits plays a significant role in the overall sensor response. Platelet–biointerface interaction is a dynamic process with a certain equilibrium between the amount of deposited cells on a given area (defined as an average thickness) and overall coverage. We show theoretically that the topology of the viscoelastic layer impacts the sensitivity and the maximum range of the biosensor. This hypothesis can explain the experimental observations for ‘localized’ and ‘spread’ deposits that are clearly distinct from ‘balanced’ cases. It was shown that, for the ‘balanced’ deposit, the biosensor frequency shift approximates accurately the thickness of the platelet deposits. On the other hand, it was found that for “localized’ and ‘spread’ deposits the frequency shift significantly deviates from expected values. The ‘spread’ cells deposits affect the sensor as a ‘monolayer’ type of loading, which theoretically shows the largest frequency shift range and slope. Thus, the ‘spread’ platelet deposits have larger frequency shift for the same average thickness compared to the balanced case. On the other hand, ‘localized’ cells deposits have lower coverage comparing to a monolayer, that leads to a reduction of the frequency shift range and slope. Moreover, the computational results clearly demonstrate the contribution of trapped liquid depending on the period of the structure. For experimentally obtained deposits, that would be an added loading that depends on the morphology of aggregated cells. Summarizing both experimental and theoretical results, it can be stated that an accurate evaluation of the amount of deposited cells using frequency shift measurement requires an evaluation of the topology of the formed cellular deposits. The reason is the multiple contributions from different loading mechanisms that are challenging to decouple from the measured frequency shift of the shear bulk acoustic resonator.
The results presented in this contribution are discussed only in terms of topological effects of cellular deposits and do not consider the complex nature of the cell and its changes of material properties. All these effects are likely to contribute to the frequency shift and should also be carefully studied.
5. Conclusions
This work was focused on experimental and theoretical studies of living cells assessment with a shear bulk acoustic resonator biosensor. The study showed that the resonator frequency shift depends not only on the amount of blood platelets deposits but also on their topology. First, the frequency shift was different for different topologies formed by equal average thickness of platelet deposits. This is the result of differences in the slope of frequency shift and in liquid trapping contribution depending on the cell deposits topology at the sensor surface. Second, the deposit coverage controls the range of thicknesses where the sensor remains sensitive to viscoelastic loads. The lower the coverage, the lower the deposit thickness at which the biosensor response reaches saturation. This is directly linked to the constant penetration depth of the acoustic waves in the viscoelastic deposit.
Our studies showed that the frequency shift measurement obtained for different viscoelastic deposits at the sensor surface have to be carefully calibrated with respect to the deposit topologies. It makes the assessment of living cells using an acoustic biosensor alone a very challenging task. In fact, the simple interpretation of the frequency shift as a measure of the amount of cells is prone to errors as the shift depends on cell deposits topology as well.
The herein-presented study was performed with human blood platelets but the results can be extended to other types of cells as soon as they are assessed with a shear bulk acoustic resonator based biosensor.