Abstract
The association between chemotherapeutic drugs and metal oxide nanoparticles has sparked a rapidly growing interest in cancer nanomedicine. The elaboration of new engineered docetaxel (DTX)-nanocarriers based on titanate nanotubes (TiONts) was reported. The idea was to maintain the drug inside cancer cells and avoid multidrug resistance mechanisms, which often limit drug efficacy by decreasing their intracellular concentrations in tumor cells. HS-PEGn-COOH (PEG: polyethylene glycol, n = 3000, 5000, 10,000) was conjugated, in an organic medium by covalent linkages, on TiONts surface. This study aimed to investigate the influence of different PEG derivatives chain lengths on the TiONts colloidal stability, on the PEGn density and conformation, as well as on the DTX biological activity in a prostate cancer model (human PC-3 prostate adenocarcinoma cells). In vitro tests highlighted significant cytotoxicities of the drug after loading DTX on PEGn-modified TiONts (TiONts-PEGn-DTX). Higher grafting densities for shorter PEGylated chains were most favorable on DTX cytotoxicity by promoting both colloidal stability in biological media and cells internalization. This promising strategy involves a better understanding of nanohybrid engineering, particularly on the PEGylated chain length influence, and can thus become a potent tool in nanomedicine to fight against cancer.
1. Introduction
In the last two decades, titanate nanotubes (TiONts) have generated increased attention for their great potential since their discovery by Kasuga et al. [1,2]. Recently, they have been considered with strengthened interest by our group and others for biomedical applications [3]. It includes the dopamine detection [4], DNA transfection [5] and adsorption [6], orthopedics and dental implants [7], bioimaging, when conjugated with superparamagnetic iron oxide nanoparticles (SPIONs) [8], with dithiolated diethylenetriaminepentaacetic acid-modified gold nanoparticles (Au@DTDTPA NPs) [9] or with fluorescent probes (phthalocyanine) [10], safe nanocarrier [5,11,12], drug delivery (genistein and docetaxel) [9,13,14,15] and cancer cell radiosensitization [9,15,16]. TiONts present a needle-shaped morphology with an internal cavity [9,14] and they can be internalized with no cytotoxicity induction and maintained inside cells for at least 10 days in vitro [5,12,16]. Indeed, the tubular nanoparticles (NPs) are more readily internalized than their spherical counterparts of a similar specific surface area [5,17,18]. It has also been shown that the shape and functionalization of NPs, used as carriers, affect the colloidal stability in suspension [14,19,20,21], attachment of selective groups [22,23], biodistribution [24,25,26,27] and interaction mode between nano-objects and cells [19,20,26,28,29]. Hence, the surface chemistry of TiONt-nanocarriers allows via hydroxyl groups further functionalization and complementary functionalities such as stability and biocompatibility in physiological conditions, which are a mandatory requirement for biomedical applications [9,11,14,21,27].
To date, very few studies have investigated the functionalization of TiONts for drug delivery purposes. Multidrug resistance mechanisms often limit drug efficacy by decreasing intracellular concentrations of drugs in tumor cells [30]. Hence, the development of engineered nanocarriers to maintain the drug inside cancer cells and thus improve treatment efficacy seems highly relevant [27,31]. The most used type of stabilization is the steric hindrance which improves the long-term colloidal stability under biological conditions and thus increases the blood circulation time of NPs [19,20,32]. Indeed, this strategy with bulky molecules is much more efficient at higher salt concentrations than the one using electrostatic interactions [33]. Polyethylene glycol (PEG) grafting has become the most widely used approach to provide stealth properties to drug nanocarrier against the reticuloendothelial system. It avoids the adsorption of opsonin proteins due to the neutrality, lipophilicity at the same time as hydrophilicity, and capacity for hydration of the PEG moiety [34,35,36,37]. PEG are also biocompatible and biodegradable polymers used in a wide range of molecular weights (usually from 160 to 20,000 g∙mol−1 for nanomedicine) and approved by the Food and Drug Administration (FDA) [32,36]. Besides, it has already been proven to promote the passive targeting of NPs by the enhanced permeability and retention (EPR) effect [38,39] and to improve the therapeutic efficacy [20,40]. PEGylated chain length of the polymer allows a screening effect to be observed [9], which enables the renal clearance of NPs as well as a reduced liver uptake [38]. Several studies reported the impact of grafting density and PEGylated chain length on the biological behavior of different NPs [19,20,35,36], such as liposomes [29,41,42], polymeric [43,44,45] and inorganic NPs (quantum dots, gold NPs, iron oxide NPs, silica NPs, etc.) [20,46,47,48,49,50] as well as carbon nanotubes [51,52]. Nevertheless, no study, to the best of our knowledge, has been conducted on PEG-modified TiONts. It has been established that both higher PEG density and PEGylated chain length onto the nano-object surface allows improving the colloidal stability while reducing surface interaction with its environment (in particular nonspecific adsorption of proteins), hence minimizing its detection by the immune system, as well as their uptake by cells [47,53]. Adsorbed proteins may either facilitate cancer cell entry or mark inorganic NPs for macrophage detection followed by their clearance from the body [47]. For instance, higher grafting densities lead to less protein adsorption and lower NPs uptake by cell lines while shorter PEG chain lengths result in higher cellular uptake in cell lines at the cost of greater nonspecific protein adsorption. Thus, it is necessary to find a compromise between the PEG density and chain length used.
In the present study, the core strategy is based on the use of different PEG spacer (HS-PEGn-COOH; n = 3000, 5000, 10,000) between TiONts and one chemotherapeutic agent, herein docetaxel (DTX), to observe the influence of PEGylated length on nanotube properties and promote the interactions of DTX-functionalized TiONts with the tubulins present into microtubules [54,55]. DTX is a clinically well-established cytotoxic drug with inhibitory properties on mitosis [31]. It has been approved by FDA especially for the treatment of hormone-refractory prostate cancers [56,57]. In previous studies, TiONts have been synthesized by a hydrothermal process and functionalized with (3-aminopropyl)triethoxysilane (TiONts-APTES) to provide additional amine functions for PEG3000 and DTX conjugation, making them promising drug carriers [9,14,15]. This report describes the functionalization of TiONts-APTES with different PEGn molecular weights in an organic medium to analyze the influence of PEGylated chain lengths on the colloidal stability and PEGn density as well as the cell survival after loading DTX molecules on PEGn-modified TiONts (TiONts-PEGn-DTX). In vitro efficacy of these latter is evaluated on a human PC-3 prostate adenocarcinoma cells using 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-ulfophenyl)-2H-tetrazolium (MTS) assay. The analysis of each elaboration step of TiONts-PEGn-DTX is performed using several characterization techniques such as thermogravimetric analysis (TGA), transmission electron microscopy (TEM), ζ-potential measurement, X-ray photoelectron spectroscopy (XPS), Fourier-transformed infrared (FTIR), and UV-visible spectroscopies.
2. Materials and Methods
2.1. Materials
Titanium dioxide (TiO2) rutile was obtained from Tioxide (Calais, France). 1-ethyl-3-(dimethylaminopropyl) carbodiimide hydrochloride (EDC), N-hydroxysuccinimide (NHS), tris (2-carboxyethyl)-phosphine hydrochloride (TCEP), and p-maleimidophenyl isocyanate (PMPI) were acquired from Thermo Scientific (Illkirch, France). (3-aminopropyl) triethoxysilane (APTES), sodium hydroxide (NaOH), benzotriazole 1-yl oxytripyrrolidinophosphonium hexafluorophosphate (PyBOP), ethanol, and N,N diisopropylethylamine (DIEA) were purchased from Sigma Aldrich (Saint Quentin Fallavier, France). Alpha-Thio-Omega-carboxy polyethylene glycol (HS-PEG3000-COOH, MW = 3073 g∙mol−1; HS-PEG5000-COOH, MW = 4847 g∙mol−1; HS-PEG10,000-COOH, MW = 9515 g∙mol−1) were obtained from Iris Biotech GmbH (Marktredwitz, Germany). Docetaxel (DTX) was purchased by BIOTREND Chemikalien GmbH (Cologne, Germany). Phosphate buffered saline (PBS) 1× solution (Acros Organics BVBA, Geel, Belgium), and dimethyl sulfoxide (DMSO extra dry, anhydrous 99.99%) (Acroseal) were acquired from Fisher Chemicals (Illkirch, France). Borate buffered saline (0.1 M; pH 8.5) was prepared from boric acid (99.8%). The ultrafiltration stirred cell (Model 8400, 400 mL) and membranes (regenerated cellulose 100 kDa) were purchased from Merck Millipore (Molsheim, France). Only ultrapure water was used for the preparation of aqueous solutions (ρ = 18 MΩ.cm).
2.2. Titanate Nanotube (TiONts) Synthesis
Bare TiONts were synthesized by a hydrothermal process in a basic medium, according to ref [9,14]. Briefly, TiO2 rutile as a precursor (1 g) was ultrasonicated in a NaOH aqueous solution (10 M, 250 mL) for 30 min at 375 W (Sonics Vibra-Cells, Newton, CT, USA). Then, the mixture was added into a Teflon reactor with mechanical stirring at 120 rpm and heating at 155 °C for 36 h. After cooling to room temperature, TiONts were washed and purified by centrifugation (24,000× g, 10 min), dialysis (Cellu·Sep tubular membranes of 12–14 kDa), and ultrafiltration (regenerated cellulose membranes with a molecular weight cut-off (MWCO) of 100 kDa) with ultrapure water.
2.3. Amine-Functionalized TiONts (TiONts-APTES) Preparation
Amine-functionalized TiONts (TiONts-APTES) were prepared from a silane-coupling agent, presenting high reactivity with hydroxyl groups on the surface of the material. Subsequently, TiONts were functionalized by APTES via hydrolysis and condensation process in an ethanol/water mixture (50:50 v:v) under reflux and magnetic agitation (60 °C, 5 h) [9,14,58]. The molar ratio between hydroxyl functions of TiONts and APTES was 1:3. After the synthesis, the suspension was ultrafiltered (100 kDa) and freeze-dried.
2.4. Functionalization of TiONts-APTES by Polyethylene Glycol with Different Ethylene Oxide Chain Lengths (PEG3000/5000/10,000)
The same method was used for the grafting of different heterobifunctional polymers (HS-PEG3000-COOH: PEG3000; HS-PEG5000-COOH: PEG5000; HS-PEG10,000-COOH: PEG10,000) onto the TiONts-APTES surface. First, carboxyl groups of heterobifunctional polyethylene glycols have been activated with PyBOP (molar ratio was 1:1) in DMSO in the presence of DIEA (in 6 × excess) under nitrogen flow and magnetic stirring for 30 min. Then, TiONts-APTES were dispersed in DMSO before adding in the activation solution, for 24 h under magnetic agitation and nitrogen flow. Polymers were attached to the amine groups of APTES with a molar ratio of 1:1. Finally, the products (TiONts-PEGn: TiONts-PEG3000, TiONts-PEG5000, and TiONts-PEG10,000) were washed by centrifugation (20,000× g, 20 min, mainly to remove DMSO less compatible with the ultrafiltration membrane), then purified by ultrafiltration (500 kDa) with ultrapure water.
2.5. Modification and Grafting of Docetaxel on TiONts-PEGn
The modification and grafting of the therapeutic agent (docetaxel, DTX) were described by Loiseau et al. [9,14] and occurred in the same conditions for each TiONts-PEGn. Briefly, DTX and the PMPI crosslinker were dissolved in DMSO (the molar ratio of DTX to PMPI was 1:4) and then added in borate buffered saline (0.1 M; pH 8.5) under magnetic agitation at 25 °C for 24 h. The resulting PMPI-activated DTX (DTX-PMPI) solution was then dialyzed (0.5–1 kDa) to remove unreacted PMPI and lyophilized to obtain a yellowish powder. TiONts-PEGn-DTX were synthesized from TiONts-PEGn and DTX-PMPI (large excess) using TCEP for cleavage of disulfides in PBS (0.1 M; pH 7.4). The mixture was homogenized beforehand in an ultrasonic bath and placed under magnetic stirring at 25 °C for 24 h. TiONts-PEGn-DTX were washed and purified by dialysis and ultrafiltration (500 kDa), and then, freeze-dried.
2.6. Surface Area Measurements
Specific surface area measurements were performed using a Micromeritics Tristar II apparatus (Micromeritics Instrument Corp., Norcross, GA, USA). Samples were outgassed in situ under 20 mTorr pressure for 16 h at 100 °C. The specific surface area value (SBET) from N2 gas adsorption was calculated from Brunauer–Emmett–Teller (BET) method.
2.7. Thermogravimetric Analysis (TGA)
TGA (TA instrument, Discovery TGA, Newcastle, UK) was used to determine the amount of the molecules on the surface of the TiONts after each grafting step. All powders were analyzed with a temperature ramp of 10 °C∙min−1 from 50 to 800 °C under an airflow rate of 25 mL∙min−1. The experiments were reproduced from 2 to 10 times for each sample.
2.8. ζ-Potential Measurements
Zeta potentials of nanoparticle suspensions were measured with a Malvern Nano ZS instrument (Worcestershire, UK) supplied by DTS Nano V7.11 software (Worcestershire, UK). pH titrations from 3 to 11 were carried out using aqueous solutions of HCl (0.1 M), NaOH (0.1 M), or NaOH (0.01 M). Before each measurement, the powder was dispersed in an aqueous NaCl solution (10−2 M) and sonicated for 10 min.
2.9. UV-Visible Absorbance Measurements
UV-visible absorbances at 600 nm were measured using Shimadzu UV-2550 UV-visible spectrophotometer (Tokyo, Japan). Turbidimetric studies of nanoparticle suspensions were made in PBS (0.1 M; pH 7.4) at 25 °C (one measurement/5 min).
2.10. X-ray Photoelectron Spectroscopy (XPS)
A PHI 5000 Versaprobe apparatus (ULVAC-PHI, Osaka, Japan) from a monochromatic Al Kα1 X-ray source (EKα1 (Al) = 1486.7 eV with a 200 μm diameter spot size, an accelerating voltage of 12 kV, and a power of 200 W) was used to record XPS measurements. Powders were pressed on an indium sheet before analysis. Data analysis and curve fittings were realized with CasaXPS processing, and MultiPak software (ver. 9.0.1, Osaka, Japan) was employed for quantitative analysis. A Shirley background was subtracted and Gauss (70%)–Lorentz (30%) profiles were applied. The charge effects were minimized by a neutralization process and the Ti2p peak at 458.7 eV was used as a reference to correct the charge effects. The resolution was 2.0 eV for global spectra and 1.3 eV for windows corresponding to selected lines.
2.11. Transmission Electron Microscopy (TEM)
Nanotube morphology and agglomeration state characterization were performed using a JEOL JEM-2100F (Tokyo, Japan), with an accelerating voltage of 200 kV and fitted with an ultra-high pole-piece achieving a point-to-point resolution of 0.19 nm. Samples were prepared by evaporating a diluted suspension of nanoparticles onto the carbon-coated copper grids.
2.12. Fourier Transformed Infrared (FTIR) Spectroscopy
A Bruker Vertex 70v (Billerica, MA, USA) supplied by OPUS version 3.1 software (Billerica, MA, USA) was used to record FTIR spectra using the KBr method. The pellets were made by mixing 2 mg of the sample within 198 mg of dried KBr.
2.13. Inductively Coupled Plasma (ICP) Spectroscopy
Determination of titanium content in nanohybrids in contact with cells was performed by ICP coupled to mass spectrometry (ICP-MS) analysis (ThermoScientific iCAP 6000 series ICP Spectrometer (Waltham, MA, USA)). A dried sample of centrifuged cells in contact with nanohybrids (TiONts-PEG3000-DTX or TiONts-PEG10,000-DTX) were dissolved in 1 mL aqua regia during 72 h in a PTFE reactor submitted to microwaves. The resulting solutions were diluted to a total volume of 5 mL with 2% HNO3 before analysis at the Ti wavelength (336.121 nm). The measured concentration (in ppt) allowed the calculation of the Ti concentration (in µg∙L−1) and deducing the mass of nanohybrids per cell (knowing the number of cells per sample).
2.14. Cell Culture of Human PC-3 Prostate Adenocarcinoma
Human PC-3 prostate adenocarcinoma cells (ATCC, Manassas, VA, USA) were cultured in Dulbecco’s modified Eagle medium (DMEM) with 10% fetal calf serum (Deutscher, France) at 37 °C, 5% CO2, and 95% humidity.
2.15. In Vitro Evaluation of Nanohybrid Cytotoxicity
Androgen-independent PC-3 prostate cancer cells were seeded in 96-well plates at a concentration of 3000 cells/well to determine the DTX cytotoxicity on the nanohybrid surface. Cells were incubated at 37 °C in 190 μL of drug-free culture medium (DMEM), with 10% fetal calf serum for 24 h before treatment (when the cells were at around 20% confluence). Cytotoxicity tests were performed with five samples at each concentration of free DTX (positive control), DTX-PMPI, TiONts-PEG3000-DTX, TiONts-PEG5000-DTX, and TiONts-PEG10,000-DTX. PC-3 cells were then incubated (+ 10 μL of drug in 190 μL of culture medium) with a range of equivalent DTX concentrations from 0.5 to 500 nM (100 nM of DTX corresponds to 0.18 μg, 0.23 μg, and 2 μg of TiONts-PEG3000-DTX, TiONts-PEG5000-DTX, and TiONts-PEG10,000-DTX per well from TGA, i.e., nanohybrid concentrations of 0.9 μg∙mL−1, 1.15 μg∙mL−1 and 10 μg∙mL−1, respectively). After 96 h of incubation corresponding approximately to 4 cycles, cell viability was evaluated using MTS assay (Promega Corporation, Madison, WI, USA) according to Mirjolet et al. [9,14,15,16]. Results were expressed as relative absorption at 490 nm relative to the untreated control.
3. Results and Discussion
The titanate-based nanohybrid has been elaborated step-by-step following the strategy described in Figure 1 to create a very attractive nanocarrier for chemotherapy.
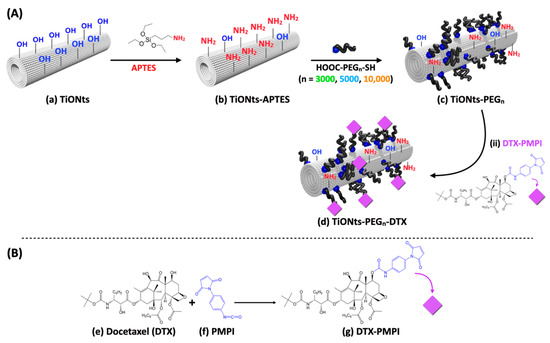
Figure 1.
Illustration of (A) TiONts (a) and step-by-step pre-functionalization with APTES (b) and α-acid-ω-thiol-polyethylene glycol (c) using one of the three different carbon chain lengths (PEGn; n = 3000, 5000, or 10,000); (B) in the second step, DTX (e) and PMPI (f) were combined to form PMPI-modified DTX (g) to create the final nanohybrid TiONts-PEGn-DTX (d).
Transmission electron microscopy (TEM) is used to highlight the formation of TiONts with a coiled spiral-shaped structure and an internal cavity, as presented in Figure 2 and described in previous work [12,14,59]. They are 10 ± 1 nm in outer diameter, 4 ± 1 nm in inner diameter, as well as 170 ± 50 nm in length and they, present a high specific surface area due to their specific morphology (SBET = 174 ± 1 m2∙g−1).
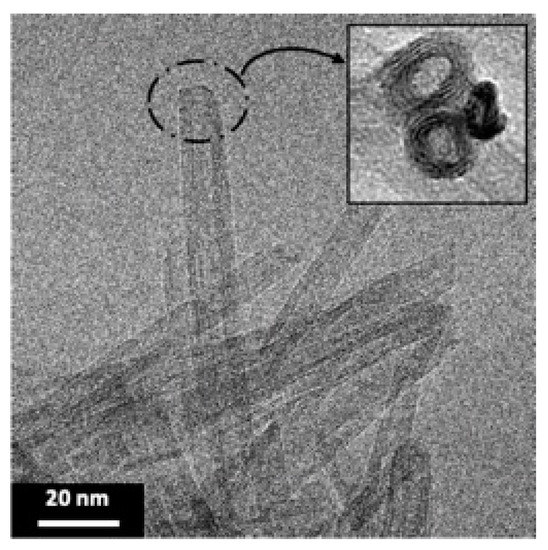
Figure 2.
TEM micrograph shows the elongated morphology of TiONts with a coiled spiral-shaped structure and an internal cavity.
TGA results have first demonstrated the effective synthesis of the TiONts-PEGn nanohybrids (Figure 3; Table 1). TGA analysis shows a more consequent mass loss after PEGn grafting due to the additional organic matter on TiONts as is the case at each successive step of grafting (details on the calculation are given in Figure S1). Moreover, as expected, the greater the molecular weight (MW) of polymer, the more important the mass losses in TGA but, on the contrary, the lower the grafting yields (0.09 PEG3000∙nm−2; 0.05 PEG5000∙nm−2; 0.03 PEG10,000∙nm−2). The PEG density of each polymer grafted onto the TiONts surface seems to be related to the polymer chain length (the longer the chains, the lower the PEG density), in particular for reasons of steric hindrance: the anchoring group might be embedded into the organic layer limiting thus the ease of access for grafting. PEGylation density is commonly related to the Flory radius (RF) of the grafted PEG, the distance between grafted PEG (D =), or the length/thickness of the grafted PEG layer. There are two main conformations that PEG chains can acquire based on these parameters: “mushroom” (RF < D) or “brush” (RF > D) conformation [36,60,61,62,63]. The determined coverage rates () obtained by the DMSO/PyBOP pathway correspond to areas of 11, 20, and 33 nm2 relative to PEG3000, PEG5000, and PEG10,000, respectively. These values are approximately 5 to 8-fold lower than the theoretical projected surface areas (πRF2) for polymer chains of 3, 5, and 10 kg∙mol−1, for which the values reported in the literature of RF are about 4.4, 6.0, and 9.1 nm [63,64], respectively, which corresponds to a covering surface of 61, 113 and 260 nm2. Moreover, the calculated distances (D) between grafted PEGn are 3.7, 5.0, and 6.5 nm relative to PEG3000, PEG5000, and PEG10,000. These results then indicate a PEGn brush conformation (RF > D), relatively sparse, less for the PEG10,000 chains grafted which seem more stretched than those of PEG3000 and PEG5000 (RF/D = 1.2, 1.2 and 1.4 for PEG3000, PEG5000, and PEG10,000, respectively). Considering these ratios close to 1, the conformation of the PEG coatings would be more likely between little stretched chains and loose mushrooms.
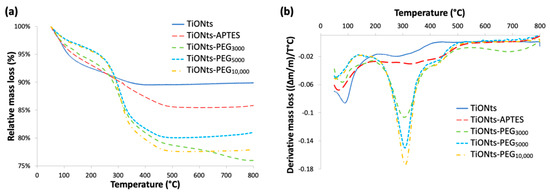
Figure 3.
(a) TGA and (b) derivative curves of bare TiONts and functionalized-TiONts under air atmosphere.

Table 1.
Detailed analysis of relative mass loss and graft ratio of TiONts and modified-TiONts.
As shown in Figure 4a, ζ-potential measurements highlight the presence of PEGn on the surface of APTES-modified TiONts with the observation of a significant charge shielding for the different PEGylated chain lengths. This effect is clearly pronounced when the PEGylated chain length increases and even more in this synthesis pathway leading to higher PEG grafting yields when compared to the water pathway published earlier [14]. These measurements confirm TGA results by proving a PEG brush conformation as the carbon chain length tends to hide the charges present on the TiONts surface. ζ-potential is then close to 0 mV over the entire studied pH range. Thus, ζ-potential for TiONts-PEGn indicates an isoelectric point (IEP) at pH 3.5 and varies between 0 mV and −5 mV at physiological pH (pH 7.4) by suggesting that the steric effect mainly governed the colloidal stability at this pH value.
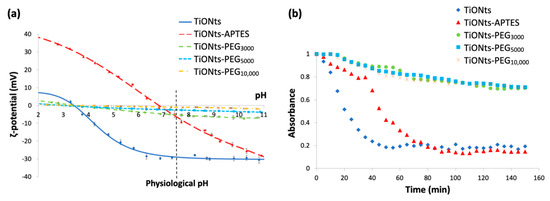
Figure 4.
(a) ζ-potential curves as a function of pH in NaCl (10−2 M) of bare TiONts and different functionalized-TiONts (the vertical dashed line corresponds to the physiological pH). (b) Turbidimetric studies: colloidal stability of functionalized-TiONts suspensions (PBS 0.1 M; pH 7.4) over 150 min following their absorbance at 600 nm as a function of time.
Turbidimetric analyses in Figure 4b highlight the colloidal stability of the different PEGn-functionalized TiONts’ suspensions under physiological conditions (PBS 0.1 M; pH 7.4), which is correlated with TEM images Figure 5. The absorbance measurements as a function of time (150 min; λ = 600 nm) demonstrate better colloidal stability for TiONts-PEGn suspensions in PBS (0.1 M; pH 7.4) than bare TiONts or TiONts-APTES. Nevertheless, the colloidal stability remains substantially the same whatever the molecular weight of PEG.
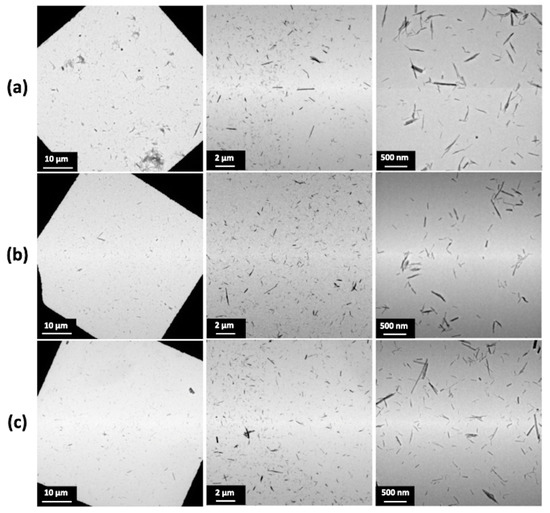
Figure 5.
TEM images of TiONts-PEGn dispersion state: (a) TiONts-PEG3000, (b) TiONts-PEG5000, and (c) TiONts-PEG10,000.
Figure 5 shows the dispersion of TiONts-PEGn in the same suspension concentration. The PEGn grafting on the nanotube surface greatly enhances nanohybrid individualization, regardless of the molecular weight used, as compared with TiONts and TiONts-APTES (Figure S2). Despite the different grafting densities of PEGn on TiONts, the PEGylated chain length offsets the number of grafted molecules and leads to a similar dispersion state of the nanohybrids. Namely, a decrease in the PEG density does not lead to a greater agglomeration of nanotubes. Thus, this excellent dispersion state highlights the effectiveness of the peptidic coupling with PyBOP on the grafting yields and the colloidal stability of nanotubes functionalized by PEG chains in a brush conformation.
XPS analyses have been carried out to evaluate the chemical composition on the surface of the different nanohybrids (Table 2). The XPS quantitative analysis reveals the presence of PEGn on nanotubes, with a significant increase in carbon and oxygen rates compared to TiONts-APTES and as the PEG MW is greater. In addition, XPS shows that sulfur (originally at the end of PEG chains and involved in the C–S coupling between PEG and DTX-PMPI) is only observed for both TiONts-PEG3000 (0.3%) and TiONts-PEG5000 (0.2%) samples. This could be explained by a thinner layer of lower MW PEG (3000 and 5000) on the TiONts’ surface even if their grafting density is higher compared to greater PEG MW in TiONts-PEG10,000 samples grafted in smaller amounts (Table 1). This is correlated by a significant decrease in the NaKLL component as the corresponding chemical element, belonging to the chemical composition of TiONts, becomes more hidden when using a polymer with increasing molecular weight, until complete disappearance (Table 2). This is probably related to either the depth of XPS analysis (a few nanometers) as the grafting of PEGn partially hides the titanate core or the repeated washings (ultrafiltration after each grafting step) which decrease the Na content below the detection limit. By comparison with the literature, it is interesting to note that the grafting of PEG3000 on TiONts using a PyBOP crosslinker rather than EDC/NHS carbodiimide coupling [14] leads to higher concentrations of carbon and oxygen in the resulting TiONts-PEG3000 nanohybrid: C/Ti = 1.5 and O/Ti = 3.5 for PyBOP versus C/Ti = 1.0 and O/Ti = 2.5 for EDC/NHS. Besides, from the TGA study, the grafting ratio of PEG3000 on TiONts-APTES is almost doubled with the use of PyBOP versus carbodiimide coupling (0.09 PEG3000∙nm−2, as shown in Table 1, versus 0.05 PEG3000∙nm−2, respectively) and the improved grafting yield confirms that the PEGn grafting is more efficient in the organic medium than in water.

Table 2.
XPS ratio of chemical elements of bare TiONts, TiONts-APTES, TiONts-PEG3000, TiONts-PEG5000, and TiONts-PEG10,000.
The decomposition of the C1s and O1s threshold of the TiONts-PEGn samples also highlights the formation of the characteristic bonds of the PEGn grafting on TiONts (Figure 6). The C1s peak of the different TiONts-PEGn shows one component located at 286.4 eV (C–OPEG, C–OH and (C=O)–NH–C) and the appearance of a new component at 288.2 eV ((C=O)–OH and (C=O)–NH–C) attributed to the formation of secondary amide bonds characterizing the PEGn grafting on TiONts-APTES and the polymers proper bonds. The appearance of a new component in the O1s peak attributed to the carboxyl function of polymers ((C=O)–OH; 533.2 eV) and the slight shift of the component located at 532.2 eV, in comparison with TiONts-APTES, show the presence of new characteristic grafting bonds. This contribution of the latter corresponds to (C=O)–OH, (C=O)–NH–C, and C–OPEG, proving once again the presence of polymers as well as the formation of the covalent amide bonds. The proportions of components for the C1s and O1s thresholds vary according to the carbon chain length of PEGn (from n = 3000 to 10,000). Indeed, a decrease is observed for the components located at 530.3 eV (O2−, from 67.6% to 64.7%) and 533.2 eV ((C=O)–OH, from 10% to 7%) on the O1s threshold and at 288.2 eV ((C=O)–OH; (C=O)–NH–C) for the C1s peak (4.5%, 3.1%, and 1.9%, respectively, for n = 3000 to 10,000). This observation demonstrates not only a shielding effect when the size of the PEG carbon chain increase by hiding in-depth bonds but also a decrease of the grafted PEG amount due to a higher steric hindrance ((C=O)–OH; (C=O)–NH–C).
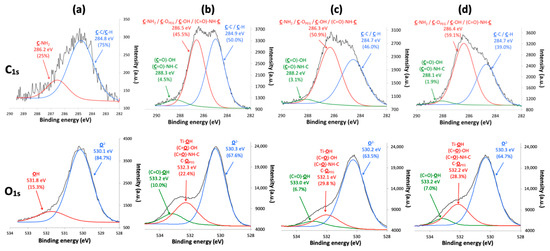
Figure 6.
XPS spectra with the fitted peaks of C1s and O1s for (a) TiONts-APTES, (b) TiONts-PEG3000, (c) TiONts-PEG5000, and (d) TiONts-PEG10,000.
The FTIR spectra correlate with the TGA and XPS results by confirming the PEGn presence on TiONts surface, notably with a new C-OPEG specific band to the ethylene glycol repeat units (Figure 7). Indeed, the most intense vibration bands, attributed to C-OPEG at 1100 cm−1 (red highlight on FTIR spectra) and aliphatic carbons (CH/CH2/CH3) between 3000–2800 cm−1 and 1550–1250 cm−1 (dark gray highlight on FTIR spectra), can be assigned to the characteristic bonds of PEG. Once more the PEGn grafting thanks to DMSO/PyBOP showed more intense vibration bands by comparison with the EDC/NHS coupling in our previous study [14].
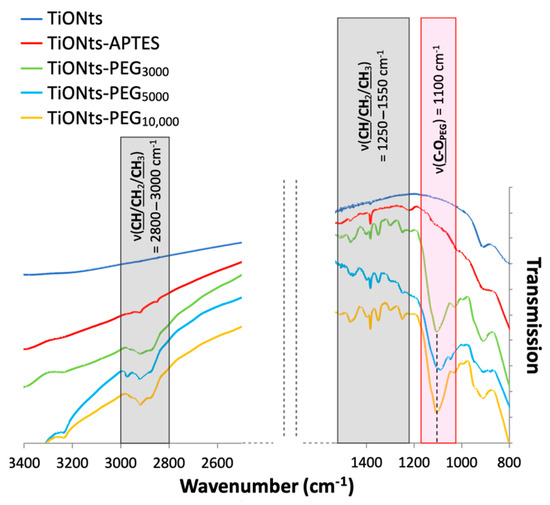
Figure 7.
FTIR spectra of TiONts, TiONts-APTES, TiONts-PEG3000, TiONts-PEG5000 and TiONts-PEG10,000 between 3400–2500 cm−1 and 1600–800 cm−1.
DTX pristine molecule is not readily reactive to be attached as is to TiONt nanohybrids. To activate the coupling of DTX, p-maleimidophenyl isocyanate (PMPI) crosslinkers have been reacted thanks to their isocyanate groups on one of the hydroxyl groups of DTX. In a second step, the maleimide groups of PMPI crosslinkers have been used to form new carbon-sulfur bonds with the end thiols of PEGs to eventually form TiONts-PEGn preferentially at pH 7.4 in PBS. Whereas the reaction is possible with both thiol and amine functions above pH 7.5, the specificity of maleimide for thiols is preserved when the reaction is carried out between pH 6.5 and 7.5 [65] (Figure S3).
The grafting yields of DTX-PMPI on the surface of TiONts-PEGn were determined by TGA and are presented in Table 1 and Figure 8a. The amount of grafted therapeutic molecules, in the case of TiONts-PEG3000 prepared thanks to DMSO/PyBOP, is higher than the one obtained, in a previous study, by EDC/NHS coupling in water [14]: 0.32 DTX-PMPI∙nm−2 versus 0.24 DTX-PMPI∙nm-2, respectively. Indeed, the number of grafted polymers on the TiONts surface is also higher, promoted by our new strategy of TiONts-DTX nanohybrid elaboration. Thus, the smaller the PEGylated chain size, the greater the DTX-PMPI amount. Furthermore, the number of DTX on TiONts-PEG3000 and TiONts-PEG5000 have been found greater than that of sulfur groups initially present at the nanotube surface: 0.32 DTX-PMPI∙nm−2 versus 0.09 PEG3000∙nm−2 for TiONts-PEG3000-DTX and 0.24 DTX-PMPI∙nm−2 versus 0.05 PEG5000∙nm−2 for TiONts-PEG5000-DTX. To some extent, DTX-PMPI seems to have another strong interaction with the surface (in addition to the one with PEGn thiols). Additional experiments are then carried out and confirm the presence of modified-DTX on simple contact with the use of an inactive polymer grafted onto TiONts-APTES, preventing any chemical reaction with PMPI-DTX (Figure S4). As its grafting ratio does not vary despite repeated purifications, these DTX-PMPI molecules being thus less likely embedded within PEG chains. DTX-PMPI molecules could presumably interact with the remaining amine groups of APTES (not already functionalized by PEGn), or could also be trapped inside the cavity of nanotubes. Indeed for DTX-PMPI grafting, the reaction carried out at pH 7.4 used to promote the formation of covalent bonds with the thiol groups of PEGn, might have undergone a pH drift to higher values resulting in unexpected coupling with the amine function of APTES [65], as described above. It should be noted that the grafting ratio of DTX-PMPI on TiONts-PEG10,000 corresponds to the same as that of PEG10,000 (0.03 DTX-PMPI∙nm−2 and 0.03 PEG10,000∙nm−2 for TiONts-PEG10,000-DTX). This observation seems to be a direct consequence of the total shielding of APTES by the PEG10,000 layer. Thus, DTX-PMPI tends to attach mainly to the thiol groups provided by the PEG10,000, contrary to PEG3000 and PEG5000, which would also have another way of adhesion, improving the DTX grafting yields and probably, the therapeutic effect of nanohybrids on tumor cells.
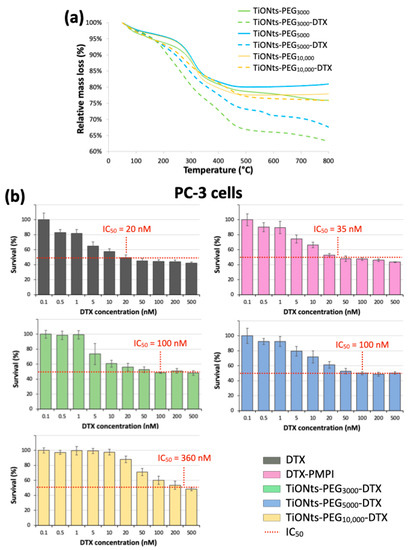
Figure 8.
(a) TGA curves of TiONts-PEGn and TiONts-PEGn-DTX under air atmosphere. (b) Survival curves (MTS cytotoxicity assays) on PC-3 cell lines after incubation of DTX, DTX-PMPI, and TiONts-PEGn-DTX (mean ± SD) (the horizontal dotted lines allow for an estimate of the different nanohybrids’ IC50). The studied range was from 0.5 to 500 nM in DTX concentration, which corresponded to a concentration range of 4.5 × 10−3 to 4.5 μg∙mL−1, 5.75 × 10−3 to 5.75 μg∙mL−1, and 5 × 10−2 to 50 μg∙mL−1 for TiONts-PEG3000-DTX, TiONts-PEG5000-DTX and TiONts-PEG10,000-DTX, respectively.
Additionally, ICP-MS measurements have been realized on cells incubated with TiONts-PEGn-DTX, the results of which are presented in Table 3. First, a dose-effect can be noticed with an increase in the mass of nanohybrids per cell when the DTX concentration is doubled (10 to 20 nM). However, this effect is more pronounced when the PEG chain is shorter: when DTX concentration varies from 10 to 20 nM, the TiONts-PEG3000-DTX mass is increased 5-fold compared to the 2.5-fold increase in the case of TiONts-PEG10,000-DTX nanohybrids. This may be due to the steric hindrance of the polymer shell, bigger in the case of MW = 10,000 g∙mol−1. The thinner shell could thus improve interaction with cells.

Table 3.
Masses of PEGylated TiONt-based DTX nanohybrids were calculated from ICP-MS analyses and according to the PEG length (MW = 3000 or 10,000) and the DTX concentration (10 nM or 20 nM).
MTS assays on a PC-3 human prostate cancer cell lines evaluate the cytotoxicity of DTX (modified or not) and associated nanohybrids (Figure 8b). The first results reveal that TiONts-PEGn-DTX nanohybrids have a cytotoxic activity according to their half-maximum inhibitory concentration (IC50). DTX-nanohybrids cytotoxicity are lower than that of DTX (IC50: 20 nM; black curve) and DTX-PMPI (IC50: 35 nM; pink curve), as described in a previous report [9,14,15]. Note that PMPI-activated DTX molecules have a slightly lower cytotoxic activity compared with free DTX, most probably because of the covalent bond formed between PMPI and DTX. Nevertheless, the survival curves show also a “plateau effect” at high doses around the IC50 for each TiONts-PEGn-DTX nanohybrid and suggest a phenomenon called mitotic catastrophe, which is a type of cell death, as already observed in the literature [66,67,68]. Indeed, DTX binding can inhibit mitotic cell division by blocking the microtubule repolymerization and prevent further cell proliferation, but some cells could continue to replicate their DNA without dividing [69]. This leads to a higher survival rate for high doses in DTX due to hypermetabolic cells before inducing the apoptosis or necrosis shortly after mitosis dysfunction. Even with this phenomenon, the cytotoxic activity of TiONts-PEG3000-DTX (green curve) is more than three times greater than that observed for the same nanohybrids elaborated in water (IC50: 100 nM versus 360 nM in ref [14], respectively) and is close to that of a previous study by using the same organic way (IC50: 82 nM) [9]. PEG brush conformation may affect the biological performance of these nanohybrids, as already demonstrated with PEG-modified carbon nanotubes [70], being most favorable on DTX cytotoxicity while promoting both colloidal stability in biological media and the interaction of the therapeutic agent with microtubules. In addition, the IC50 of TiONts-PEG10,000-DTX (IC50: 360 nM; yellow curve) is greater than the IC50 of TiONts-PEG3000-DTX and TiONts-PEG5000-DTX (IC50: 100 nM; green and blue curves, respectively). Therefore, the PEGylated chain length plays an important role in the DTX interactions with the PC-3 prostate cancer cells and, as corroborated by the ICP-MS results, these results show that DTX has a better efficiency when attached on shorter-chains (PEG3000/5000 versus PEG10,000) which tends indeed to promote the interaction between DTX and microtubules. Despite a similar surface charge and dispersion state for all TiONts-PEGn-DTX nanohybrids, a lower internalization rate for TiONts-PEG10,000-DTX can occur in comparison with TiONts-PEG3000/5000-DTX. Thus, about the PEGylated chain, the higher the grafting densities, the shorter the lengths, the greater the cytotoxicity. These achievements are then very promising to understand the influence of the PEGylated chain length in nanomedicine to improve tumor cell internalization and might ensure better access of DTX to microtubules to fight prostate cancer.
4. Conclusions
TiONts were successfully functionalized to develop docetaxel-grafted nanohybrids using a step-by-step process. Each grafting step was characterized by different techniques to prove their success. The grafting of PEGn has been achieved via a peptide coupling with PyBOP in DMSO on the surface of TiONts-APTES and the resulting TiONts-PEGn nanohybrids have significantly improved the colloidal stability of suspensions under physiological conditions consistent with the targeted biomedical applications. The high number of polymers on the nanotube surface (0.09 PEG3000∙nm−2; 0.05 PEG5000∙nm−2; 0.03 PEG10,000∙nm−2) allowed to significantly improve the amount of therapeutic agent (DTX) modified by PMPI on nanohybrids, especially with the use of smaller PEGylated chain lengths (PEG3000: 0.32 DTX∙nm−2; PEG5000: 0.24 DTX∙nm−2; PEG10,000: 0.03 DTX∙nm−2). Indeed, the steric hindrance generated by PEG on nanotubes was progressively improved with increasing molecular weight. In vitro (MTS) tests highlighted satisfactory cytotoxicities of DTX for each TiONts-PEGn-DTX nanohybrids on human PC-3 prostate adenocarcinoma cells. Finally, this study demonstrated that the PEG grafting with high molecular weights on TiONts (in our case: PEG10,000) decreased the cytotoxic activity of DTX and reduce the internalization in cells. Thus, higher grafting densities of shorter PEG chain lengths have been found more favorable to greater cytotoxicities. Understanding the influence of PEGylated chain lengths allows one to move towards an approach to fight tumor cells and in this context, titanate nanotubes appear as new theranostic tools to improve the efficacy of cancer treatment.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/nano11102733/s1, Figure S1: (a) TGA curves of bare TiONts under air atmosphere and theoretical calculation of the hydroxyl rates. (b) Theoretical calculation for the graft ratios of functionalized-TiONts; Figure S2: TEM images show the evolution of the dispersion (a) before and (b) after APTES grafting on TiONts surface; Figure S3: (a) Maleimide reacts specifically with a thiol function at pH < 7.5 and (b) lose its specificity to react either with a thiol function or with amine function at pH > 7.5; Figure S4: (a) Polymer (Boc-NH-PEG3000-COOH; M = 3173 g∙mol−1) having an inactive function (Boc) and carboxyl function to react with an amine group via peptide coupling and (b) TGA curves showing the adsorption of DTX-PMPI upon contact between TiONts-PEG3000-Boc and DTX-PMPI (TiONts-DTX were washed by dialysis and ultrafiltration (100 kDa)). (c) Results of relative mass loss and graft ratio of bare TiONts, TiONts-APTES, TiONts-PEG3000-Boc and after mixing DTX-PMPI with TiONts-PEG3000-Boc.
Author Contributions
Conceptualization, A.L., J.B. and N.M.; J.B. and N.M. contributed equally to this work and have supervised this project; A.L. and J.B. designed the chemical experiments; A.L. performed the synthesis and characterization of nanohybrids; V.M. and C.M. realized the biological experiments; A.L. wrote the manuscript (original draft preparation); all authors are reviewed and edited the manuscript; All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Cancéropôle Est through the call “Emergence 2015” (Région Bourgogne Franche Comté) and by the European Union through the PO FEDER-FSE Bourgogne 2014/2020 programs. This work has been also supported by the EIPHI Graduate School (contract “ANR-17-EURE-0002”).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the authors.
Acknowledgments
The authors would like to thank Olivier Heintz (ICB) for XPS measurements, Rémi Chassagnon for TEM images, and Myriam Heydel (Sayens) for ICP measurements.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kasuga, T.; Hiramatsu, M.; Hoson, A.; Sekino, T.; Niihara, K. Formation of titanium oxide nanotube. Langmuir 1998, 14, 3160–3163. [Google Scholar] [CrossRef]
- Kasuga, T.; Hiramatsu, M.; Hoson, A.; Sekino, T.; Niihara, K. Titania Nanotubes Prepared by Chemical Processing. Adv. Mater. 1999, 11, 1307–1311. [Google Scholar] [CrossRef]
- Bavykin, D.V.; Walsh, F.C. Titanate and Titania Nanotubes: Synthesis, Properties and Applications; Royal Society of Chemistry: Cambridge, UK, 2010; p. 154. [Google Scholar]
- Niu, L.; Shao, M.; Wang, S.; Lu, L.; Gao, H.; Wang, J. Titanate nanotubes: Preparation, characterization, and application in the detection of dopamine. J. Mater. Sci. 2008, 43, 1510–1514. [Google Scholar] [CrossRef]
- Papa, A.L.; Dumont, L.; Vandroux, D.; Millot, N. Titanate nanotubes: Towards a novel and safer nanovector for cardiomyocytes. Nanotoxicology 2013, 7, 1131–1142. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Cai, Y. Preparation of amino-modified titanate nanotubes and its striking adsorption ability to duplex DNA. J. Nanopart. Res. 2011, 13, 39–43. [Google Scholar] [CrossRef]
- Oh, S.-H.; Finõnes, R.R.; Daraio, C.; Chen, L.-H.; Jin, S. Growth of nano-scale hydroxyapatite using chemically treated titanium oxide nanotubes. Biomaterials 2005, 26, 4938–4943. [Google Scholar] [CrossRef] [PubMed]
- Papa, A.-L.; Maurizi, L.; Vandroux, D.; Walker, P.; Millot, N. Synthesis of Titanate Nanotubes Directly Coated with USPIO in Hydrothermal Conditions: A New Detectable Nanocarrier. J. Phys. Chem. C 2011, 115, 19012–19017. [Google Scholar] [CrossRef]
- Loiseau, A.; Boudon, J.; Oudot, A.; Moreau, M.; Boidot, R.; Chassagnon, R.; Saïd, N.M.; Roux, S.; Mirjolet, C.; Millot, N. Titanate Nanotubes Engineered with Gold Nanoparticles and Docetaxel to Enhance Radiotherapy on Xenografted Prostate Tumors. Cancers 2019, 11, 1962. [Google Scholar] [CrossRef] [Green Version]
- Paris, J.; Bernhard, Y.; Boudon, J.; Heintz, O.; Millot, N.; Decreau, R.A. Phthalocyanine-titanate nanotubes: A promising nanocarrier detectable by optical imaging in the so-called imaging window. RSC Adv. 2015, 5, 6315–6322. [Google Scholar] [CrossRef]
- Sallem, F.; Boudon, J.; HEINTZ, O.; Séverin, I.; Megriche, A.; Millot, N. Synthesis and characterization of chitosan-coated titanate nanotubes: Towards a new safe nanocarrier. Dalton Trans. 2017, 46, 15386–15398. [Google Scholar] [CrossRef]
- Sruthi, S.; Loiseau, A.; Boudon, J.; Sallem, F.; Maurizi, L.; Mohanan, P.V.; Lizard, G.; Millot, N. In vitro interaction and biocompatibility of titanate nanotubes with microglial cells. Toxicol. Appl. Pharmacol. 2018, 353, 74–86. [Google Scholar] [CrossRef]
- Baati, T.; Kefi, B.B.; Aouane, A.; Njim, L.; Chaspoul, F.; Heresanu, V.; Kerkeni, A.; Neffati, F.; Hammami, M. Biocompatible titanate nanotubes with high loading capacity of genistein: Cytotoxicity study and anti-migratory effect on U87-MG cancer cell lines. RSC Adv. 2016, 6, 101688–101696. [Google Scholar] [CrossRef]
- Loiseau, A.; Boudon, J.; Mirjolet, C.; Crehange, G.; Millot, N. Taxane-Grafted Metal-Oxide Nanoparticles as a New Theranostic Tool against Cancer: The Promising Example of Docetaxel-Functionalized Titanate Nanotubes on Prostate Tumors. Adv. Healthc. Mater. 2017, 6, 1700245. [Google Scholar] [CrossRef]
- Mirjolet, C.; Boudon, J.; Loiseau, A.; Chevrier, S.; Boidot, R.; Oudot, A.; Collin, B.; Martin, E.; Joy, P.A.; Millot, N. Docetaxel-titanate nanotubes enhance radiosensitivity in an androgen-independent prostate cancer model. Int. J. Nanomed. 2017, 12, 6357. [Google Scholar] [CrossRef] [Green Version]
- Mirjolet, C.; Papa, A.-L.; Créhange, G.; Raguin, O.; Seignez, C.; Paul, C.; Truc, G.; Maingon, P.; Millot, N. The radiosensitization effect of titanate nanotubes as a new tool in radiation therapy for glioblastoma: A proof-of-concept. Radiother. Oncol. 2013, 108, 136–142. [Google Scholar] [CrossRef]
- Gratton, S.E.A.; Ropp, P.A.; Pohlhaus, P.D.; Luft, J.C.; Madden, V.J.; Napier, M.E.; DeSimone, J.M. The effect of particle design on cellular internalization pathways. Proc. Natl. Acad. Sci. USA 2008, 105, 11613–11618. [Google Scholar] [CrossRef] [Green Version]
- Barua, S.; Yoo, J.-W.; Kolhar, P.; Wakankar, A.; Gokarn, Y.R.; Mitragotri, S. Particle shape enhances specificity of antibody-displaying nanoparticles. Proc. Natl. Acad. Sci. USA 2013, 110, 3270–3275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howard, M.D.; Jay, M.; Dziubla, T.D.; Lu, X. PEGylation of nanocarrier drug delivery systems: State of the art. J. Biomed. Nanotechnol. 2008, 4, 133–148. [Google Scholar] [CrossRef]
- Karakoti, A.S.; Das, S.; Thevuthasan, S.; Seal, S. PEGylated Inorganic Nanoparticles. Angew. Chem. Int. Ed. 2011, 50, 1980–1994. [Google Scholar] [CrossRef] [PubMed]
- Papa, A.-L.; Boudon, J.; Bellat, V.; Loiseau, A.; Bisht, H.; Sallem, F.; Chassagnon, R.; Berard, V.; Millot, N. Dispersion of titanate nanotubes for nanomedicine: Comparison of PEI and PEG nanohybrids. Dalton Trans. 2015, 44, 739–746. [Google Scholar] [CrossRef]
- Sperling, R.A.; Parak, W.J. Surface modification, functionalization and bioconjugation of colloidal inorganic nanoparticles. Philos. Trans. R. Soc. A: Math. Phys. Eng. Sci. 2010, 368, 1333–1383. [Google Scholar] [CrossRef]
- Pujari, S.P.; Scheres, L.; Marcelis, A.T.M.; Zuilhof, H. Covalent Surface Modification of Oxide Surfaces. Angew. Chem. Int. Ed. 2014, 53, 6322–6356. [Google Scholar] [CrossRef] [PubMed]
- Decuzzi, P.; Godin, B.; Tanaka, T.; Lee, S.Y.; Chiappini, C.; Liu, X.; Ferrari, M. Size and shape effects in the biodistribution of intravascularly injected particles. J. Control. Release 2010, 141, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Maldiney, T.; Richard, C.; Seguin, J.; Wattier, N.; Bessodes, M.; Scherman, D. Effect of Core Diameter, Surface Coating, and PEG Chain Length on the Biodistribution of Persistent Luminescence Nanoparticles in Mice. ACS Nano 2011, 5, 854–862. [Google Scholar] [CrossRef]
- Ernsting, M.J.; Murakami, M.; Roy, A.; Li, S.-D. Factors controlling the pharmacokinetics, biodistribution and intratumoral penetration of nanoparticles. J. Control. Release 2013, 172, 782–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, T.; Zhang, Y.S.; Pang, B.; Hyun, D.C.; Yang, M.; Xia, Y. Engineered Nanoparticles for Drug Delivery in Cancer Therapy. Angew. Chem. Int. Ed. 2014, 53, 12320–12364. [Google Scholar] [CrossRef] [PubMed]
- Chithrani, B.D.; Ghazani, A.A.; Chan, W.C. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006, 6, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, D.; Colapicchioni, V.; Caracciolo, G.; Piovesana, S.; Capriotti, A.L.; Palchetti, S.; De Grossi, S.; Riccioli, A.; Amenitsch, H.; Laganà, A. Effect of polyethyleneglycol (PEG) chain length on the bio–nano-interactions between PEGylated lipid nanoparticles and biological fluids: From nanostructure to uptake in cancer cells. Nanoscale 2014, 6, 2782–2792. [Google Scholar] [CrossRef] [PubMed]
- Brigger, I.; Dubernet, C.; Couvreur, P. Nanoparticles in cancer therapy and diagnosis. Adv. Drug Deliv. Rev. 2002, 54, 631–651. [Google Scholar] [CrossRef]
- Zhao, P.; Astruc, D. Docetaxel nanotechnology in anticancer therapy. ChemMedChem 2012, 7, 952–972. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Stellacci, F. Effect of Surface Properties on Nanoparticle–Cell Interactions. Small 2010, 6, 12–21. [Google Scholar] [CrossRef]
- Sakura, T.; Takahashi, T.; Kataoka, K.; Nagasaki, Y. One-pot preparation of mono-dispersed and physiologically stabilized gold colloid. Colloid Polym. Sci. 2005, 284, 97–101. [Google Scholar] [CrossRef]
- Otsuka, H.; Nagasaki, Y.; Kataoka, K. PEGylated nanoparticles for biological and pharmaceutical applications. Adv. Drug Deliv. Rev. 2003, 55, 403–419. [Google Scholar] [CrossRef]
- Li, S.-D.; Huang, L. Stealth Nanoparticles: High Density but Sheddable PEG is a Key for Tumor Targeting. J. Control Release 2010, 145, 178–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jokerst, J.V.; Lobovkina, T.; Zare, R.N.; Gambhir, S.S. Nanoparticle PEGylation for imaging and therapy. Nanomedicine 2011, 6, 715–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amoozgar, Z.; Yeo, Y. Recent advances in stealth coating of nanoparticle drug delivery systems. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2012, 4, 219–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nano 2007, 2, 751–760. [Google Scholar] [CrossRef]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef] [Green Version]
- Hwu, J.R.; Lin, Y.S.; Josephrajan, T.; Hsu, M.-H.; Cheng, F.-Y.; Yeh, C.-S.; Su, W.-C.; Shieh, D.-B. Targeted Paclitaxel by Conjugation to Iron Oxide and Gold Nanoparticles. J. Am. Chem. Soc. 2008, 131, 66–68. [Google Scholar] [CrossRef]
- Dos Santos, N.; Allen, C.; Doppen, A.-M.; Anantha, M.; Cox, K.A.K.; Gallagher, R.C.; Karlsson, G.; Edwards, K.; Kenner, G.; Samuels, L.; et al. Influence of poly(ethylene glycol) grafting density and polymer length on liposomes: Relating plasma circulation lifetimes to protein binding. Biochim. Biophys. Acta-Biomembr. 2007, 1768, 1367–1377. [Google Scholar] [CrossRef] [Green Version]
- Ren, H.; He, Y.; Liang, J.; Cheng, Z.; Zhang, M.; Zhu, Y.; Hong, C.; Qin, J.; Xu, X.; Wang, J. Role of Liposome Size, Surface Charge, and PEGylation on Rheumatoid Arthritis Targeting Therapy. ACS Appl. Mater. Interfaces 2019, 11, 20304–20315. [Google Scholar] [CrossRef]
- Hu, Y.; Xie, J.; Tong, Y.W.; Wang, C.-H. Effect of PEG conformation and particle size on the cellular uptake efficiency of nanoparticles with the HepG2 cells. J. Control. Release 2007, 118, 7–17. [Google Scholar] [CrossRef]
- Bertrand, N.; Grenier, P.; Mahmoudi, M.; Lima, E.M.; Appel, E.A.; Dormont, F.; Lim, J.-M.; Karnik, R.; Langer, R.; Farokhzad, O.C. Mechanistic understanding of in vivo protein corona formation on polymeric nanoparticles and impact on pharmacokinetics. Nat. Commun. 2017, 8, 777. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-L.; Du, X.-J.; Yang, J.-X.; Shen, S.; Li, H.-J.; Luo, Y.-L.; Iqbal, S.; Xu, C.-F.; Ye, X.-D.; Cao, J.; et al. The effect of surface poly(ethylene glycol) length on in vivo drug delivery behaviors of polymeric nanoparticles. Biomaterials 2018, 182, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Faure, A.C.; Dufort, S.; Josserand, V.; Perriat, P.; Coll, J.L.; Roux, S.; Tillement, O. Control of the in vivo biodistribution of hybrid nanoparticles with different poly(ethylene glycol) coatings. Small 2009, 5, 2565–2575. [Google Scholar] [CrossRef] [PubMed]
- Cruje, C.; Chithrani, B. Polyethylene glycol density and length affects nanoparticle uptake by cancer cells. J. Nanomed. Res. 2014, 1, 00006. [Google Scholar]
- Arami, H.; Khandhar, A.; Liggitt, D.; Krishnan, K.M. In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles. Chem. Soc. Rev. 2015, 44, 8576–8607. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; De Rose, R.; Alt, K.; Alcantara, S.; Paterson, B.M.; Liang, K.; Hu, M.; Richardson, J.J.; Yan, Y.; Jeffery, C.M. Engineering poly (ethylene glycol) particles for improved biodistribution. ACS Nano 2015, 9, 1571–1580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, Y.; Zhong, Y.; Dong, J.; Qian, C.; Sun, S.; Gao, L.; Yang, D. The effect of PEG functionalization on the in vivo behavior and toxicity of CdTe quantum dots. RSC Adv. 2019, 9, 12218–12225. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Davis, C.; Cai, W.; He, L.; Chen, X.; Dai, H. Circulation and long-term fate of functionalized, biocompatible single-walled carbon nanotubes in mice probed by Raman spectroscopy. Proc. Natl. Acad. Sci. USA 2008, 105, 1410–1415. [Google Scholar] [CrossRef] [Green Version]
- Bottini, M.; Rosato, N.; Bottini, N. PEG-modified carbon nanotubes in biomedicine: Current status and challenges ahead. Biomacromolecules 2011, 12, 3381–3393. [Google Scholar] [CrossRef] [PubMed]
- Mosqueira, V.C.F.; Legrand, P.; Morgat, J.-L.; Vert, M.; Mysiakine, E.; Gref, R.; Devissaguet, J.-P.; Barratt, G. Biodistribution of Long-Circulating PEG-Grafted Nanocapsules in Mice: Effects of PEG Chain Length and Density. Pharm. Res. 2001, 18, 1411–1419. [Google Scholar] [CrossRef] [PubMed]
- Rowinsky, M.; Eric, K. The development and clinical utility of the taxane class of antimicrotubule chemotherapy agents. Annu. Rev. Med. 1997, 48, 353–374. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, K.; Liu, H.; Kim, S.; Guo, M.; Navarro, V.; Bander, N.H. Docetaxel down-regulates the expression of androgen receptor and prostate-specific antigen but not prostate-specific membrane antigen in prostate cancer cell lines: Implications for PSA surrogacy. Prostate 2009, 69, 1579–1585. [Google Scholar] [CrossRef]
- Galsky, M.D.; Vogelzang, N.J. Docetaxel-based combination therapy for castration-resistant prostate cancer. Ann. Oncol. 2010, 21, 2135–2144. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.V.; Liu, S.; Pinski, J. Luteinizing hormone-releasing hormone receptor targeted agents for prostate cancer. Expert Opin. Investig. Drugs 2011, 20, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Pontón, P.I.; d’Almeida, J.R.M.; Marinkovic, B.A.; Savić, S.M.; Mancic, L.; Rey, N.A.; Morgado, E.; Rizzo, F.C. The effects of the chemical composition of titanate nanotubes and solvent type on 3-aminopropyltriethoxysilane grafting efficiency. Appl. Surf. Sci. 2014, 301, 315–322. [Google Scholar] [CrossRef]
- Papa, A.-L.; Millot, N.; Saviot, L.; Chassagnon, R.; Heintz, O. Effect of Reaction Parameters on Composition and Morphology of Titanate Nanomaterials. J. Phys. Chem. C 2009, 113, 12682–12689. [Google Scholar] [CrossRef]
- Peracchia, M.T.; Vauthier, C.; Passirani, C.; Couvreur, P.; Labarre, D. Complement consumption by poly(ethylene glycol) in different conformations chemically coupled to poly(isobutyl 2-cyanoacrylate) nanoparticles. Life Sci. 1997, 61, 749–761. [Google Scholar] [CrossRef]
- Fang, C.; Shi, B.; Pei, Y.-Y.; Hong, M.-H.; Wu, J.; Chen, H.-Z. In vivo tumor targeting of tumor necrosis factor-α-loaded stealth nanoparticles: Effect of MePEG molecular weight and particle size. Eur. J. Pharm. Sci. 2006, 27, 27–36. [Google Scholar] [CrossRef]
- Damodaran, V.B.; Fee, C.J.; Ruckh, T.; Popat, K.C. Conformational Studies of Covalently Grafted Poly(ethylene glycol) on Modified Solid Matrices Using X-ray Photoelectron Spectroscopy. Langmuir 2010, 26, 7299–7306. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.L.; Reuter, K.G.; Kai, M.P.; Herlihy, K.P.; Jones, S.W.; Luft, J.C.; Napier, M.; Bear, J.E.; DeSimone, J.M. PEGylated PRINT nanoparticles: The impact of PEG density on protein binding, macrophage association, biodistribution, and pharmacokinetics. Nano Lett. 2012, 12, 5304–5310. [Google Scholar] [CrossRef] [PubMed]
- Movileanu, L.; Cheley, S.; Bayley, H. Partitioning of Individual Flexible Polymers into a Nanoscopic Protein Pore. Biophys. J. 2003, 85, 897–910. [Google Scholar] [CrossRef] [Green Version]
- Shen, G.; Horgan, A.; Levicky, R. Reaction of N-phenyl maleimide with aminosilane monolayers. Colloids Surf. B Biointerfaces 2004, 35, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Galletti, G.; Matov, A.; Beltran, H.; Fontugne, J.; Mosquera, J.M.; Cheung, C.; MacDonald, T.Y.; Sung, M.; O’Toole, S.; Kench, J.G. ERG induces taxane resistance in castration-resistant prostate cancer. Nat. Commun. 2014, 5, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, H.; Cao, S.; Yang, Z.; Zhang, S.; Zhang, Q.; Jiang, X. Preparation, characterization and anti-glioma effects of docetaxel-incorporated albumin-lipid nanoparticles. J. Biomed. Nanotechnol. 2015, 11, 2137–2147. [Google Scholar] [CrossRef]
- Kim, C.H.; Kang, T.H.; Kim, B.D.; Lee, T.H.; Yoon, H.Y.; Goo, Y.T.; Choi, Y.S.; Kang, M.J.; Choi, Y.W. Enhanced Docetaxel Delivery Using Sterically Stabilized RIPL Peptide-Conjugated Nanostructured Lipid Carriers: In Vitro and In Vivo Antitumor Efficacy Against SKOV3 Ovarian Cancer Cells. Int. J. Pharm. 2020, 119393. [Google Scholar] [CrossRef]
- Morse, D.L.; Gray, H.; Payne, C.M.; Gillies, R.J. Docetaxel induces cell death through mitotic catastrophe in human breast cancer cells. Mol. Cancer Ther. 2005, 4, 1495–1504. [Google Scholar] [CrossRef] [Green Version]
- Sacchetti, C.; Motamedchaboki, K.; Magrini, A.; Palmieri, G.; Mattei, M.; Bernardini, S.; Rosato, N.; Bottini, N.; Bottini, M. Surface polyethylene glycol conformation influences the protein corona of polyethylene glycol-modified single-walled carbon nanotubes: Potential implications on biological performance. ACS Nano 2013, 7, 1974–1989. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).