Recent Advances of Graphene-Derived Nanocomposites in Water-Based Drilling Fluids
Abstract
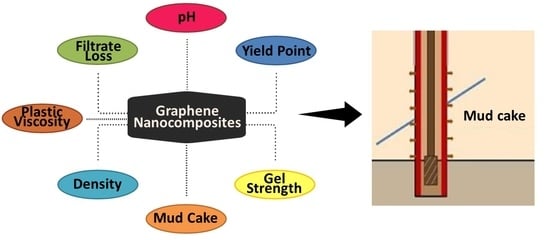
:1. Introduction
2. Role of Significant Nanocomposites in Drilling Fluids
2.1. Effect of Silica Nanocomposites
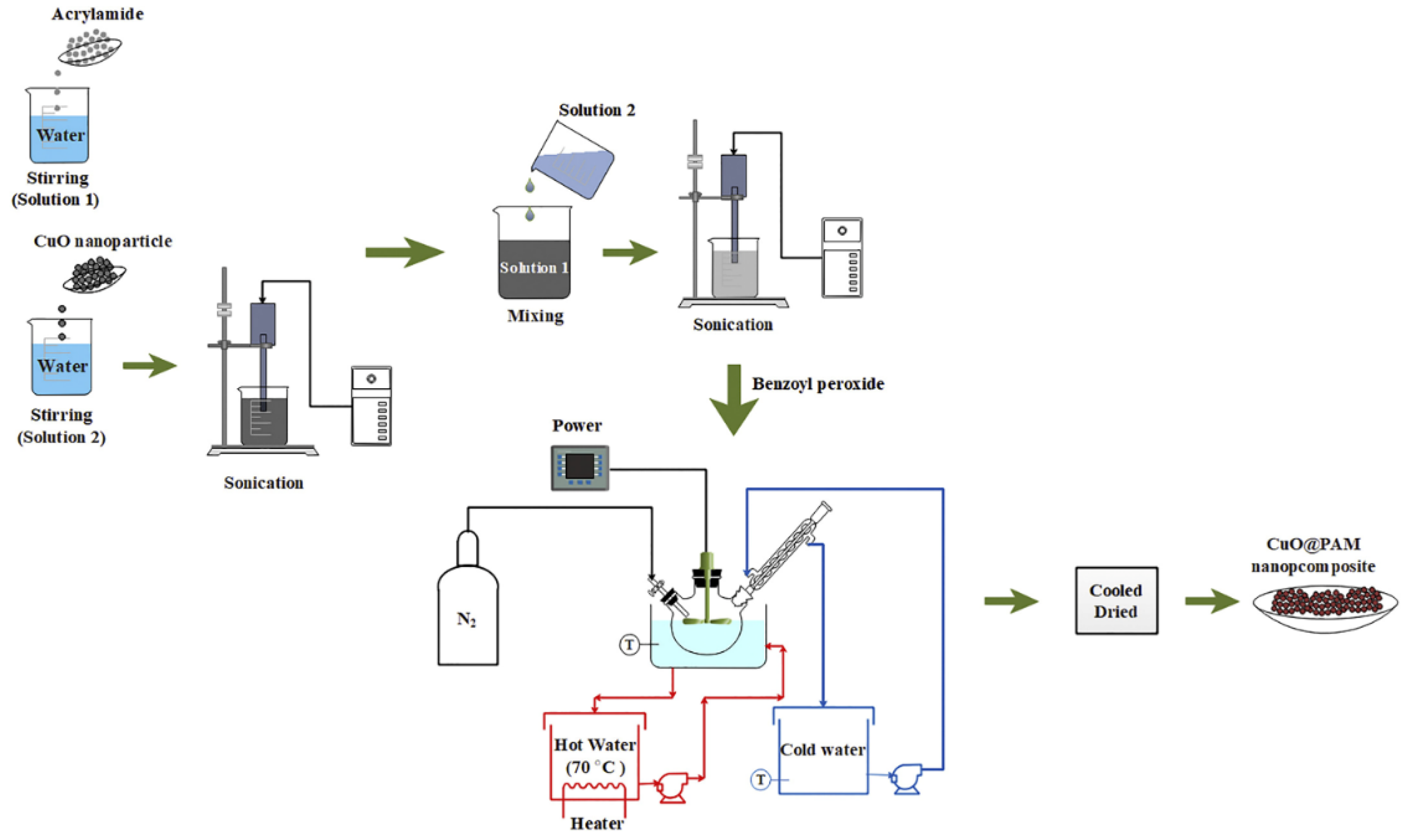
2.2. Effect of Copper Oxide Nanocomposites
2.3. Role of Titanium Dioxide Nanocomposites
2.4. Effect of Grass and Other Additives to Improve Rheological Properties
2.5. Applications of Various Nanocomposites in Drilling Fluids
3. Variation of Rheological Properties by Nanocomposites
3.1. Gel Strength
3.2. Filtrate Loss
3.3. Shear Rate
4. Graphene Impacts in Drilling Operations
4.1. Graphene-Derived Nanocomposites in WBDF
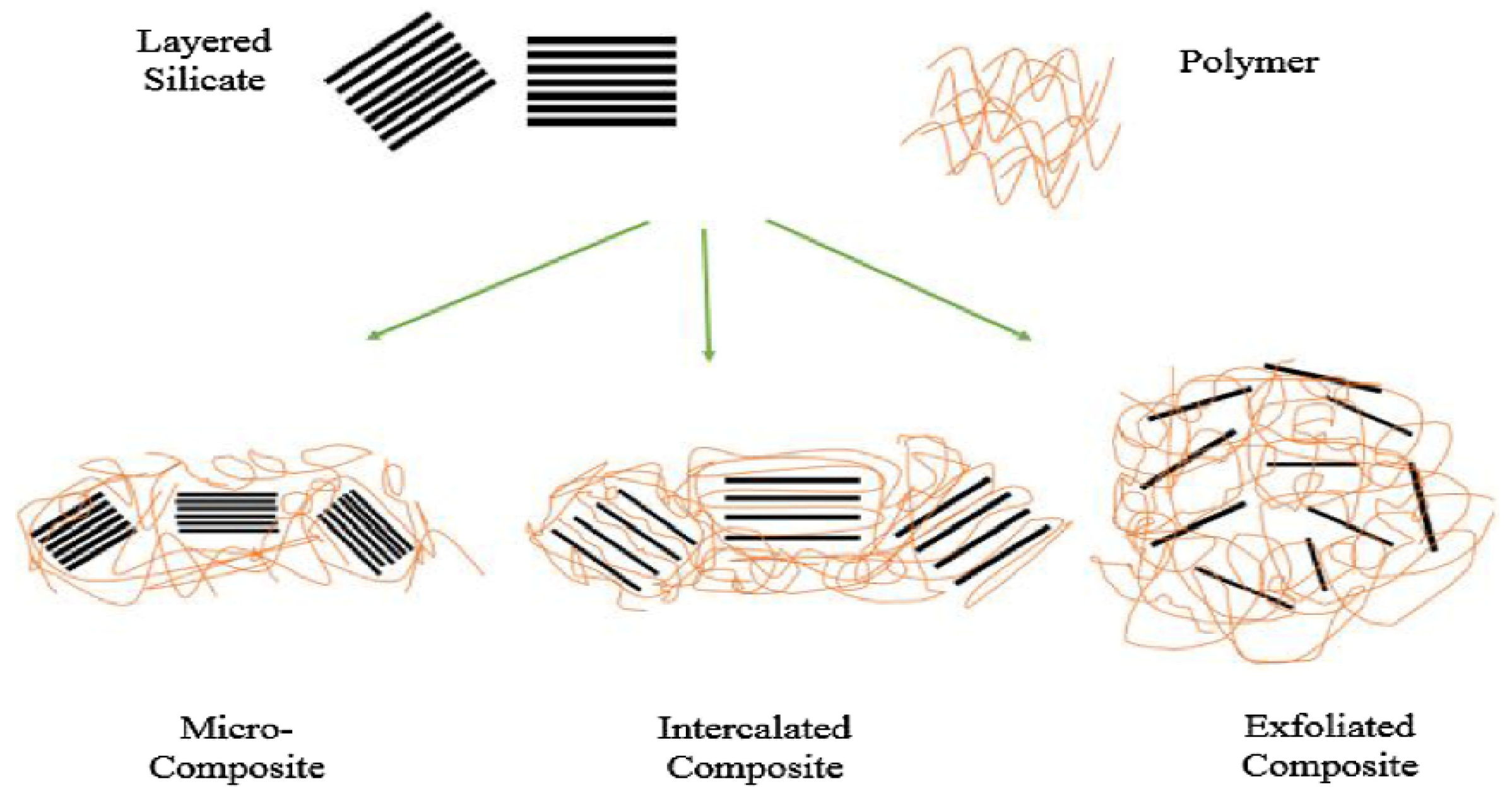
4.1.1. Graphene-Polymer Nanocomposites
4.1.2. Graphene-Activated Carbon Nanocomposites
4.1.3. Graphene-Metal Nanocomposites
4.1.4. Graphene-Metal Oxide Nanocomposites
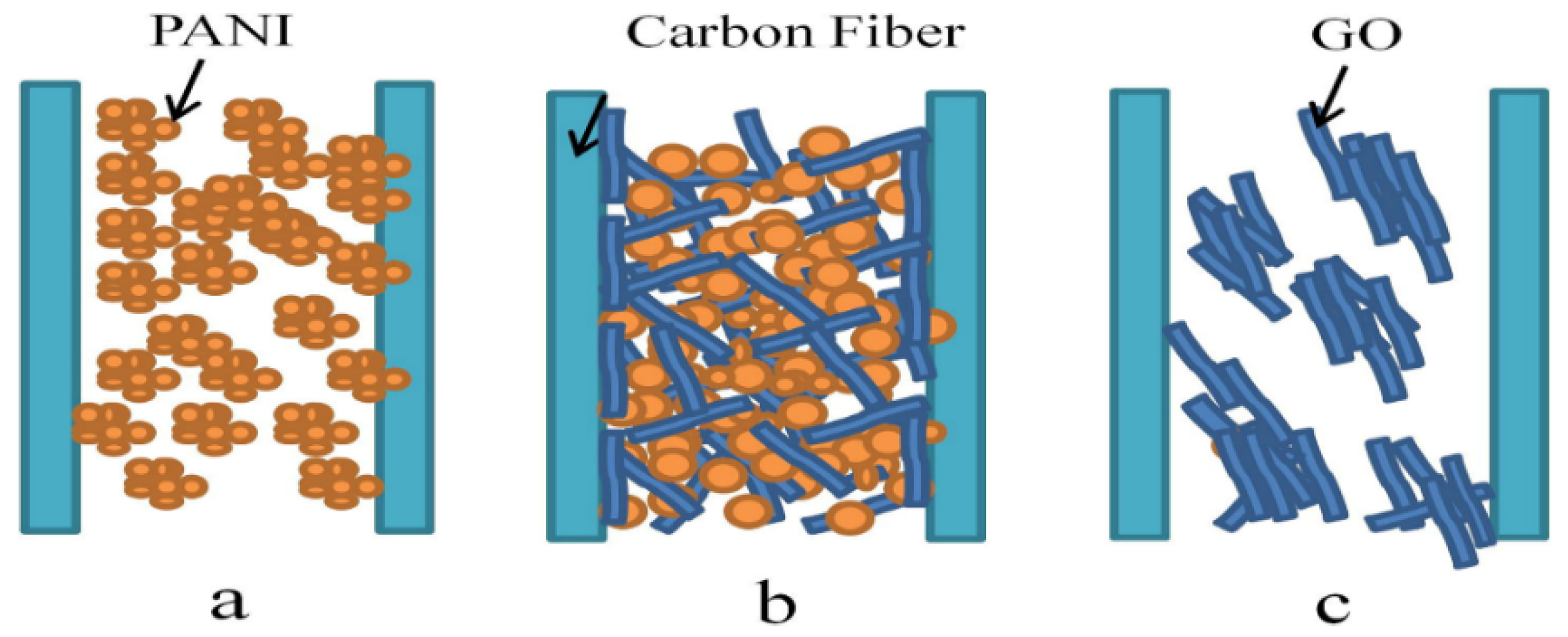
4.1.5. Graphene-Fiber Nanocomposites
| Graphene-Derived Nanocomposites | Synthesis Routes | Conditions & Outcomes | References |
|---|---|---|---|
| Graphene-polypropylene (PP) nanocomposite | Melt mixing | Enhanced PV versus SR, 20~5000 s−1 for nanocomposites at 200 °C. The PV of PP was observed 289 Pa s at 300 s−1 SR, which increased up to 513 Pa s due to the stronger interaction of the PP matrix with GO nanocomposite. | [107] |
| Graphene-acrylonitrile butadiene styrene resin (ABC) nanocomposite | Facile coagulation method | An increase of PV and mechanical modulus was observed due to the graphene nanocomposite. | [108] |
| Graphene-polyester nanocomposite | Partial pyrolysis | An enriched RP was observed due to the nanocomposite as compared to graphite. | [109] |
| Graphene-polyurethane nanocomposite | Solution mixing method | 0.5–3 wt.% qualitative expansion was presented in the frequency of RP. | [110] |
| Graphene-low density polyethylene nanocomposite | Melt extrusion and film casting | Established PV, ST, viscoelasticity at 140 ℃. | [111] |
| GO-Fe2O3/Al2O3 nanocomposite | Vertical bed method | Nanocomposite reduced the FL from 20 mLto 15 mL and MCT from 0.3 mm to 0.1 mm of WBDF with 0.02%. | [112] |
| GO-ZrO nanocomposite | Microwave synthesis | Enriched HPHT applications using a high-temperature range of 330 °F. | [113] |
| rGO-SnO2 nanocomposite | Ultrasonic synthesis | Improved RPs were reported with the effect of vol% of rGO–SnO2 nanocomposite (three different ratios: 1:7, 1:8, 1:10) in base fluid for PV, ST, ranging from 0 to 10,000 s−1 at 25 °C. | [114] |
| GO-ZnO nanocomposite | Chemical synthesis | A desirable increase of PV (5–28%), YP (25–42%), GS (25–33%), and a considerably reduced FL were examined. | [66] |
| rGO-thermally polypyrrole nanocomposite | In situ polymerization | RP of rGO-thermally polypyrrole nanocomposite was determined using a cone-plate method with ratios (100:1, 100:3, and 100:5%) and temperature (25–180 °C), and represented an increase of ST and PV due to the addition of thermally reduced GO sheets into polypyrrole. | [115] |
| GO-polyacrylamide (PAM) nanocomposite | Chemical synthesis (polymerization) | Nanocomposite influenced FL at LPLT and HPHT which was reduced up to 38.96% and 34.36, respectively. A noteworthy decrease in FL, MCT treated with 1.5 wt.% nanocomposite. | [116] |
| GO-hydrolyzed polyacrylamide nanocomposite | Chemical synthesis | Addition of GO increased PV, the effect was notable at elevated temperatures. Addition of 0.1 wt.% of GO enhanced PV by 47% and 36%, respectively, at 85 °C and 25 °C. GO increased the thermal stability due to the electrostatic hydrogen bonding among nanocomposite functional groups. After ageing for 30 days at 80 °C, PV of the composite’s solution decreased very slightly, while a 59% reduction was observed for pure polymer solution. | [117] |
| GO nanocomposite | Chemical synthesis | Reduced FL was observed using low concentration of GO nanocomposite. | [118] |
| Applications of Other Graphenaceous Materials in WBDF | |||
| GO/polyanionic cellulose polymers | Hummers method | FL of 6.1 mL over 30 min, MCT ~20µm/FL of 7.2 mL, MCT ~280 µm, high-temperature stability with better-quality RP. | [16] |
| GO | Hummers method | Concentration of GO increased from 0.2 wt.% to 0.6 wt.%, PV of GO aqueous dispersion noticeably increased, whereas there was no obvious change of YP and GS. | [119] |
| GNP | Hydrothermal technique | Amended RP was presented at HPHT due to the low friction between nanoplates. | [28] |
| Graphene/MgO/TiO2 | Hydrothermal technique | An increase of GS (92%) and PV (253%) by adding MgO (2%) and graphene (75%) was observed. | [120] |
| Graphite–Al2O3 | - | Upgraded drilling mud properties were revealed; thermal conductivity (10%) and zeta potential (13%) in the presence of 0.8 wt.% of graphite-Al2O3. | [121] |
| Graphene | - | Graphene with a concentration of 17.5 mL was reduced polymer usage up to 40% for mud cake formula. Better-quality RP of 13.5lb/gal HPHT was achieved in WBDF without affecting PV and YP. | [122] |
| Graphitized nanotubes | Homogenization | Decrease of PV with an increase in temperature from 25–85 °C. Value-added RP with an increase of temperature were presented. | [123] |
| Nano-graphite nanoparticles | Water-in-oil (w/o) micro emulsions | Decrease in FL and RP were enhanced for WBDF. | [124] |
| Graphene/CNT | Chemical method | Reduced mud filtrate volume up to 18%. Addition of CNT reduced FL, enhanced shale formation. Addition of graphene was decreased friction coefficient from 38–59%. Better lubricity was produced by CNT as compared to graphene at elevated temperature. | [125] |
| Graphene–SiO2 | - | Concentration of nanoparticles (0.75 wt.%) yielded better performance in both LPLT and HPHT filtration tests with a reduction of 20.93% and 27.21%, respectively, as compared to the base fluid. | [126] |
| GO-phosphorylated from welding waste | Chemical synthesis | Addition of GO was tested for improved RP such as PV was reduced from 10 to 7 cp, YP was increased from 11–15 lbs/100 ft2, decreased filtrate volume (6 to 3.6 mL) and reduced MCT (1.06 to 0.33 mm), with enhanced lubricity were presented. | [127] |
4.2. Graphene Oxide on Rheological Properties
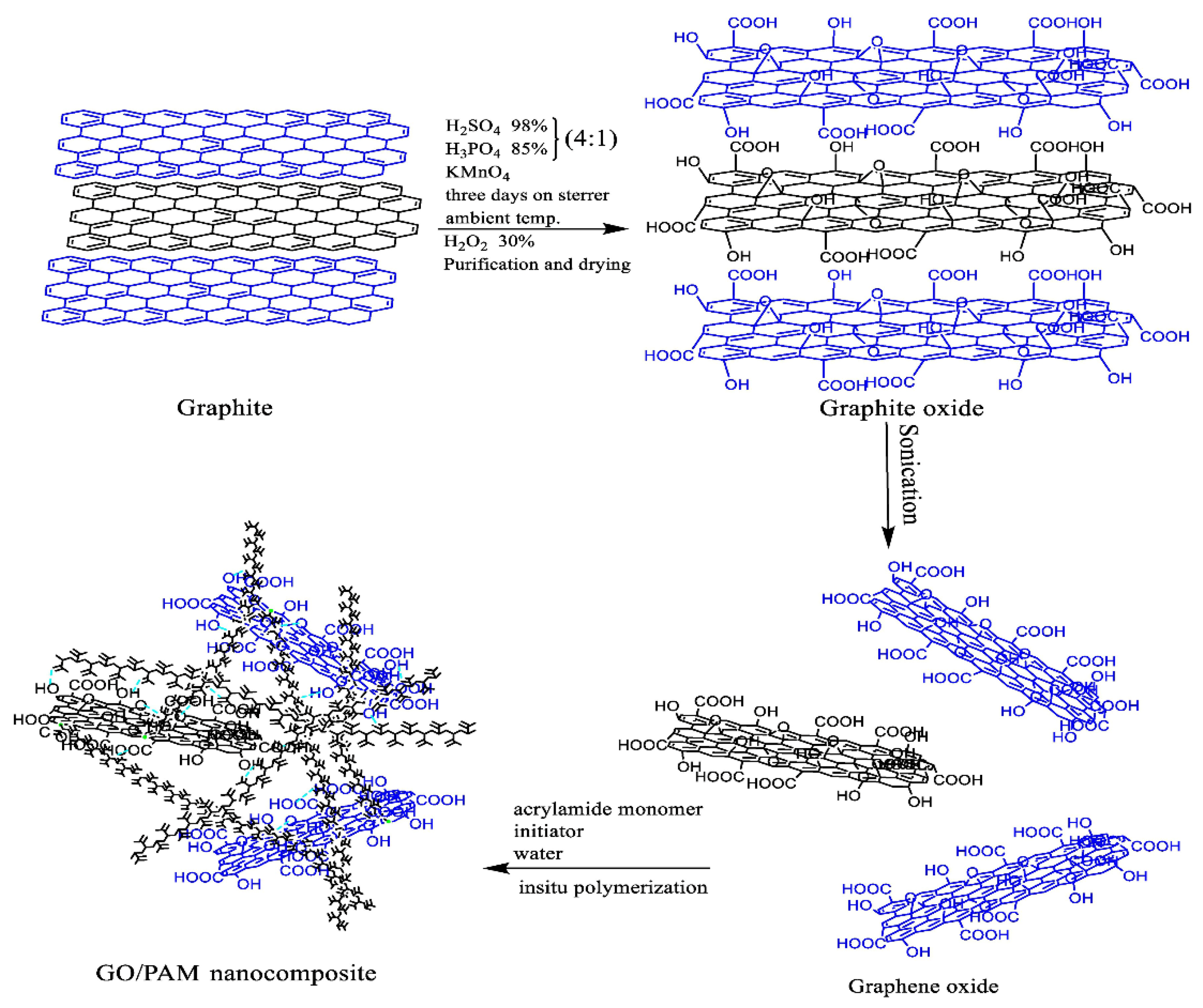
4.3. Graphene Oxide in WBDF
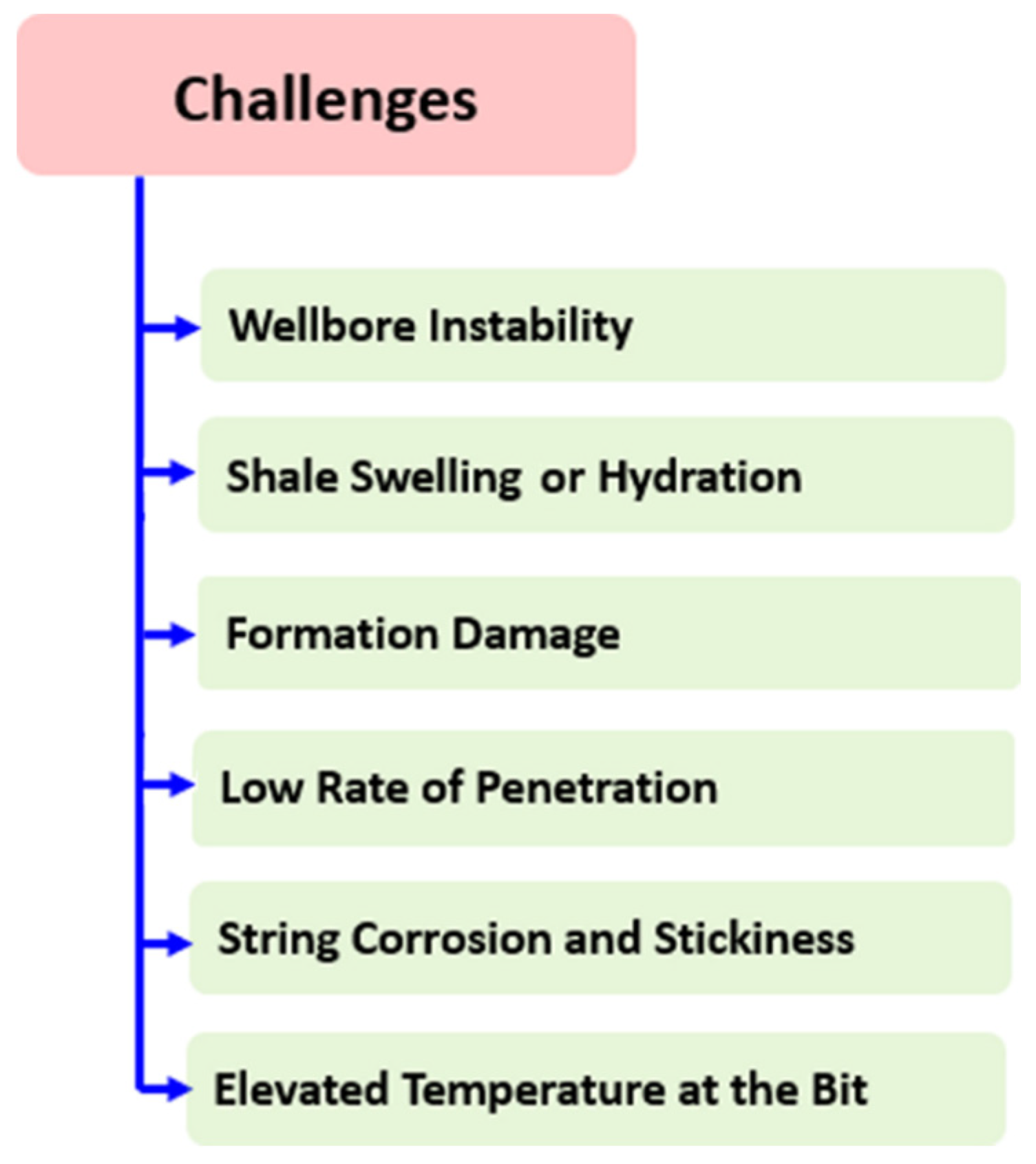
5. Limitations and Challenges
6. Future Prospects
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hajiabadi, S.H.; Aghaei, H.; Kalateh-Aghamohammadi, M.; Shorgasthi, M. An overview on the significance of carbon-based nanomaterials in upstream oil and gas industry. J. Pet. Sci. Eng. 2020, 186, 106783. [Google Scholar] [CrossRef]
- Pastoriza-Santos, I.; Kinnear, C.; Pérez-Juste, J.; Mulvaney, P.; Liz-Marzán, L.M. Plasmonic polymer nanocomposites. Nat. Rev. Mater. 2018, 3, 375–391. [Google Scholar] [CrossRef]
- Cha, G.D.; Lee, W.H.; Lim, C.; Choi, M.K.; Kim, D.H. Materials engineering, processing, and device application of hydrogel nanocomposites. Nanoscale 2020, 12, 10456–10473. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Mechanical properties of graphene and graphene-based nanocomposites. Prog. Mater. Sci. 2017, 90, 75–127. [Google Scholar] [CrossRef]
- Kim, H.; Miura, Y.; Macosko, C.W. Graphene/polyurethane nanocomposites for improved gas barrier and electrical conductivity. Chem. Mater. 2010, 22, 3441–3450. [Google Scholar] [CrossRef]
- Cai, D.; Song, M. Recent advance in functionalized graphene/polymer nanocomposites. J. Mater. Chem. 2010, 20, 7906–7915. [Google Scholar] [CrossRef]
- Abdo, J.; Haneef, M. Clay nanoparticles modified drilling fluids for drilling of deep hydrocarbon wells. Appl. Clay Sci. 2013, 86, 76–82. [Google Scholar] [CrossRef]
- Mao, H.; Yang, Y.; Zhang, H.; Zhang, J.; Huang, Y. A critical review of the possible effects of physical and chemical properties of subcritical water on the performance of water-based drilling fluids designed for ultra-high temperature and ultra-high pressure drilling applications. J. Pet. Sci. Eng. 2020, 187, 106795. [Google Scholar] [CrossRef]
- Bageri, B.S.; Adebayo, A.R.; Al Jaberi, J.; Patil, S. Effect of perlite particles on the filtration properties of high-density barite weighted water-based drilling fluid. Powder Technol. 2020, 360, 1157–1166. [Google Scholar] [CrossRef]
- Elgibaly, A.; Farahat, M.; Abd El Nabbi, M. The Optimum Types and Characteristics of Drilling Fluids Used During Drilling in The Egyption Westren Desert. J. Pet. Min. Eng. 2018, 20, 89–100. [Google Scholar] [CrossRef] [Green Version]
- Neuberger, N.; Adidharma, H.; Fan, M. Graphene: A review of applications in the petroleum industry. J. Pet. Sci. Eng. 2018, 167, 152–159. [Google Scholar] [CrossRef]
- Negin, C.; Ali, S.; Xie, Q. Application of nanotechnology for enhancing oil recovery–A review. Petroleum 2016, 2, 324–333. [Google Scholar] [CrossRef]
- Werner, B.; Myrseth, V.; Saasen, A. Viscoelastic properties of drilling fluids and their influence on cuttings transport. J. Pet. Sci. Eng. 2017, 156, 845–851. [Google Scholar] [CrossRef]
- Ahmad, H.M.; Kamal, M.S.; Al-Harthi, M.A. High molecular weight copolymers as rheology modifier and fluid loss additive for water-based drilling fluids. J. Mol. Liq. 2018, 252, 133–143. [Google Scholar] [CrossRef]
- William, J.K.M.; Ponmani, S.; Samuel, R.; Nagarajan, R.; Sangwai, J.S. Effect of CuO and ZnO nanofluids in xanthan gum on thermal, electrical and high pressure rheology of water-based drilling fluids. J. Pet. Sci. Eng. 2014, 117, 15–27. [Google Scholar] [CrossRef]
- Kosynkin, D.V.; Ceriotti, G.; Wilson, K.C.; Lomeda, J.R.; Scorsone, J.T.; Patel, A.D. Graphene Oxide as a High-Performance Fluid-Loss-Control Additive in Water-Based Drilling Fluids. ACS Appl. Mater. Interfaces 2012, 4, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Qiu, Z.; Shen, Z.; Huang, W. Hydrophobic associated polymer based silica nanoparticles composite with core-shell structure as a filtrate reducer for drilling fluid at utra-high temperature. J. Pet. Sci. Eng. 2015, 129, 1–14. [Google Scholar] [CrossRef]
- Echeverría, C.; Mijangos, C. A Way to Predict Gold Nanoparticles/Polymer Hybrid Microgel Agglomeration Based on Rheological Studies. Nanomaterials 2019, 9, 1499. [Google Scholar] [CrossRef] [Green Version]
- Goyal, R.K. Nanomaterials and Nanocomposites: Synthesis, Properties, Characterization Techniques, and Applications; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Camargo, P.H.C.; Satyanarayana, K.G.; Wypych, F. Nanocomposites: Synthesis, structure, properties and new application opportunities. Mater. Res. 2009, 12, 1–39. [Google Scholar] [CrossRef] [Green Version]
- Oku, T. Carbon/Carbon Composites and Their Properties. In Carbon Alloys; Elsevier: Amsterdam, The Netherlands, 2003; pp. 523–544. [Google Scholar]
- Lepak-Kuc, S.; Milowska, K.Z.; Boncel, S.; Szybowicz, M.; Dychalska, A.; Jozwik, I. Highly Conductive Doped Hybrid Carbon Nanotube–Graphene Wires. ACS Appl. Mater. Interfaces 2019, 11, 33207–33220. [Google Scholar] [CrossRef]
- Jain, R.; Mahto, V. Evaluation of polyacrylamide/clay composite as a potential drilling fluid additive in inhibitive water based drilling fluid system. J. Pet. Sci. Eng. 2015, 133, 612–621. [Google Scholar] [CrossRef]
- Saboori, R.; Sabbaghi, S.; Kalantariasl, A.; Mowla, D. Improvement in filtration properties of water-based drilling fluid by nanocarboxymethyl cellulose/polystyrene core-shell nanocomposite. J. Pet. Explor. Prod. Technol. 2018, 8, 445–454. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, H.M.; Kamal, M.S.; Hussain, S.M.S.; Al-Harthi, M. Synthesis of novel polymer nanocomposite for water-based drilling fluids. In Proceedings of the 35th International Conference of the Polymer Processing Society; AIP Publishing LLC: Melville, NY, USA, 2020. [Google Scholar]
- Sadeghalvaad, M.; Sabbaghi, S. The effect of the TiO2/polyacrylamide nanocomposite on water-based drilling fluid properties. Powder Technol. 2015, 272, 113–119. [Google Scholar] [CrossRef]
- Abdo, J.; Zaier, R.; Hassan, E.; Al-Sharji, H.; Al-Shabibi, A. ZnO-clay nanocomposites for enhance drilling at HTHP conditions. Surf. Interface Anal. 2014, 46, 970–974. [Google Scholar] [CrossRef]
- Aftab, A.; Ismail, A.R.; Khokhar, S.; Ibupoto, Z.H. Novel zinc oxide nanoparticles deposited acrylamide composite used for enhancing the performance of water-based drilling fluids at elevated temperature conditions. J. Pet. Sci. Eng. 2016, 146, 1142–1157. [Google Scholar] [CrossRef]
- Cheraghian, G.; Wu, Q.; Mostofi, M.; Li, M.C.; Afrand, M.; Sangwai, J.S. Effect of a novel clay/silica nanocomposite on water-based drilling fluids: Improvements in rheological and filtration properties. Colloids Surf. A 2018, 555, 339–350. [Google Scholar] [CrossRef]
- Abdo, J.; AL-Sharji, H.; Hassan, E. Effects of nano-sepiolite on rheological properties and filtration loss of water-based drilling fluids. Surf. Interface Anal. 2016, 48, 522–526. [Google Scholar] [CrossRef]
- Saboori, R.; Sabbaghi, S.; Kalantariasl, A. Improvement of rheological, filtration and thermal conductivity of bentonite drilling fluid using copper oxide/polyacrylamide nanocomposite. Powder Technol. 2019, 353, 257–266. [Google Scholar] [CrossRef]
- Liu, M.; Lin, M.C.; Wang, C. Enhancements of thermal conductivities with Cu, CuO, and carbon nanotube nanofluids and application of MWNT/water nanofluid on a water chiller system. Nanoscale Res. Lett. 2011, 6, 297. [Google Scholar] [CrossRef] [Green Version]
- Sahooli, M.; Sabbaghi, S. Investigation of thermal properties of CuO nanoparticles on the ethylene glycol–water mixture. Mater. Lett. 2013, 93, 254–257. [Google Scholar] [CrossRef]
- Das, P.K.; Mallik, A.K.; Ganguly, R.; Santra, A.K. Synthesis and characterization of TiO2–water nanofluids with different surfactants. Int. Commun. Heat Mass Transfer. 2016, 75, 341–348. [Google Scholar] [CrossRef]
- Rafati, R.; Smith, S.R.; Haddad, A.S.; Novara, R.; Hamidi, H. Effect of nanoparticles on the modifications of drilling fluids properties: A review of recent advances. J. Pet. Sci. Eng. 2018, 161, 61–76. [Google Scholar] [CrossRef] [Green Version]
- Krishnakumar, T.; Sheeba, A.; Mahesh, V.; Prakash, M.J. Heat transfer studies on ethylene glycol/water nanofluid containing TiO2 nanoparticles. Int. J. Refrig. 2019, 102, 55–61. [Google Scholar] [CrossRef]
- Ismail, A.R.; Alias, A.; Sulaiman, W.; Jaafar, M.; Ismail, I. Drilling fluid waste management in drilling for oil and gas wells. Chem. Eng. Trans. 2017, 56, 1351–1356. [Google Scholar]
- Onwukwe, S.; Nwakaudu, M. Drilling wastes generation and management approach. Int. J. Environ. Sci. Dev. 2012, 3, 252. [Google Scholar] [CrossRef]
- Al-Hameedi, A.T.T.; Alkinani, H.H.; Dunn-Norman, S.; Al-Alwani, M.A.; Alshammari, A.F.; Albazzaz, H.W. Insights into the application of new eco-friendly drilling fluid additive to improve the fluid properties in water-based drilling fluid systems. J. Pet. Sci. Eng. 2019, 183. [Google Scholar] [CrossRef]
- Song, Y.; Li, Z.; Jiang, L.; Hong, F. The concept and the accumulation characteristics of unconventional hydrocarbon resources. Pet. Sci. 2015, 12, 563–572. [Google Scholar] [CrossRef] [Green Version]
- Oseh, J.O.; Mohd Norddin, M.N.A.; Ismail, I.; Gbadamosi, A.O.; Agi, A.; Mohammed, H.N. A novel approach to enhance rheological and filtration properties of water–based mud using polypropylene–silica nanocomposite. J. Pet. Sci. Eng. 2019, 181. [Google Scholar] [CrossRef]
- Borthakur, A.; Choudhury, S.; Sengupta, P.; Rao, K.; Nihalani, M. Synthesis and evaluation of partially hydrolysed polyacrylamide (PHPA) as viscosifier in water based drilling fluids. Ind. J. Chem. Technol. 1997, 4, 83–88. [Google Scholar]
- Hale, A.; Mody, F. Partially hydrolyzed polyacrylamide (PHPA) mud systems for Gulf of Mexico deepwater prospects. SPE International Symposium on oilfield chemistry. Soc. Pet. Eng. 1993. [Google Scholar]
- Nizamani, A.; Ismail, A.R.; Junin, R.; Dayo, A.; Tunio, A.; Ibupoto, Z. Synthesis of titaniabentonite nano composite and its applications in water-based drilling fluids. Chem. Eng. Trans. 2017, 56, 949–954. [Google Scholar]
- Bhasney, S.M.; Kumar, A.; Katiyar, V. Microcrystalline cellulose, polylactic acid and polypropylene biocomposites and its morphological, mechanical, thermal and rheological properties. Compos. Part B 2020, 184, 107717. [Google Scholar] [CrossRef]
- Jahed, M.; Naderi, G.; Hamid Reza Ghoreishy, M. Microstructure, mechanical, and rheological properties of natural rubber/ethylene propylene diene monomer nanocomposites reinforced by multi-wall carbon nanotubes. Polym. Compos. 2018, 39, E745–E753. [Google Scholar] [CrossRef]
- Zhao, Q.; Finlayson, C.E.; Snoswell, D.R.; Haines, A.; Schäfer, C.; Spahn, P. Large-scale ordering of nanoparticles using viscoelastic shear processing. Nat. Commun. 2016, 7, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivatsa, J.T.; Ziaja, M.B. An experimental investigation on use of nanoparticles as fluid loss additives in a surfactant–polymer based drilling fluid. In Conference Proceedings, IPTC 2012: International Petroleum Technology Conference; European Association of Geoscientists & Engineers: Amsterdam, The Netherlands, 2012; p. 280. [Google Scholar]
- Needaa, A.M.; Pourafshary, P.; Hamoud, A.-H.; Jamil, A. Controlling bentonite-based drilling mud properties using sepiolite nanoparticles. Pet. Explor. Dev. 2016, 43, 717–723. [Google Scholar]
- Jain, R.; Mahto, T.K.; Mahto, V. Rheological investigations of water based drilling fluid system developed using synthesized nanocomposite. Korea-Aust. Rheol. J. 2016, 28, 55–65. [Google Scholar] [CrossRef]
- Xu, J.G.; Qiu, Z.; Zhao, X.; Huang, W. Hydrophobic modified polymer based silica nanocomposite for improving shale stability in water-based drilling fluids. J. Pet. Sci. Eng. 2017, 153, 325–330. [Google Scholar] [CrossRef]
- Xu, J.G.; Qiu, Z.S.; Zhao, X.; Zhong, H.Y.; Li, G.R.; Huang, W.A. Synthesis and characterization of shale stabilizer based on polyethylene glycol grafted nano-silica composite in water-based drilling fluids. J. Pet. Sci. Eng. 2018, 163, 371–377. [Google Scholar] [CrossRef]
- Qiu, Z.; Xu, J.; Yang, P.; Zhao, X.; Mou, T.; Zhong, H. Effect of Amphiphilic Polymer/Nano-Silica Composite on Shale Stability for Water-Based Muds. Appl. Sci. 2018, 8, 1839. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.A.; Al-Saliml, H.; Arsanjani, L.N. Development of high temperature high pressure (HTHP) water based drilling mud using synthetic polymers, and nanoparticles. J. Adv. Res. Fluid Mech. Thermal Sci. 2018, 45, 99–108. [Google Scholar]
- Abdollahi, M.; Pourmahdi, M.; Nasiri, A.R. Synthesis and characterization of lignosulfonate/acrylamide graft copolymers and their application in environmentally friendly water-based drilling fluid. J. Pet. Sci. Eng. 2018, 171, 484–494. [Google Scholar] [CrossRef]
- Mohamadian, N.; Ghorbani, H.; Wood, D.A.; Khoshmardan, M.A. A hybrid nanocomposite of poly (styrene-methyl methacrylate-acrylic acid)/clay as a novel rheology-improvement additive for drilling fluids. J. Polym. Res. 2019, 26, 33. [Google Scholar] [CrossRef]
- Davoodi, S.; SA, A.R.; Soleimanian, A.; Jahromi, A.F. Application of a novel acrylamide copolymer containing highly hydrophobic comonomer as filtration control and rheology modifier additive in water-based drilling mud. J. Pet. Sci. Eng. 2019, 180, 747–755. [Google Scholar] [CrossRef]
- Kök, M.V.; Bal, B. Effects of silica nanoparticles on the performance of water-based drilling fluids. J. Pet. Sci. Eng. 2019, 180, 605–614. [Google Scholar] [CrossRef]
- Kang, Y.; She, J.; Zhang, H.; You, L.; Song, M. Strengthening shale wellbore with silica nanoparticles drilling fluid. Petroleum 2016, 2, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Quintero, L.; Cardenas, A.E.; Clark, D.E. Nanofluids and Methods of Use for Drilling and Completion Fluids. U.S. Patent No. 8,822,386, 2 September 2014. [Google Scholar]
- Putra, P.H.M.; Jan, B.M.; Patah, M.F.A.; Junaidi, M.U.M. Effect of temperature and concentration of industrial waste graphene on rheological properties of water based mud. IOP Conf. Ser. Mater. Sci. Eng. 2020, 778, 012120. [Google Scholar]
- Razi, M.M.; Mazidi, M.; Razi, F.M.; Aligolzadeh, H.; Niazi, S. Artificial neural network modeling of plastic viscosity, yield point, and apparent viscosity for water-based drilling fluids. J. Dispers. Sci. Technol. 2013, 34, 822–827. [Google Scholar] [CrossRef]
- Sajjadian, M.; Sajjadian, V.A.; Rashidi, A. Experimental evaluation of nanomaterials to improve drilling fluid properties of water-based muds HP/HT applications. J. Pet. Sci. Eng. 2020, 190. [Google Scholar] [CrossRef]
- Amani, M.; Al-Jubouri, M. The effect of high pressures and high temperatures on the properties of water based drilling fluids. Energy Sci. Technol. 2012, 4, 27–33. [Google Scholar]
- Smith, S.R.; Rafati, R.; Haddad, A.S.; Cooper, A.; Hamidi, H. Application of aluminium oxide nanoparticles to enhance rheological and filtration properties of water based muds at HPHT conditions. Colloids Surf. A 2018, 537, 361–371. [Google Scholar] [CrossRef] [Green Version]
- Ghayedi, A.; Khosravi, A. Laboratory investigation of the effect of GO-ZnO nanocomposite on drilling fluid properties and its potential on H2S removal in oil reservoirs. J. Pet. Sci. Eng. 2020, 184, 106684. [Google Scholar] [CrossRef]
- Barry, M.M.; Jung, Y.; Lee, J.K.; Phuoc, T.X.; Chyu, M.K. Fluid filtration and rheological properties of nanoparticle additive and intercalated clay hybrid bentonite drilling fluids. J. Pet. Sci. Eng. 2015, 127, 338–346. [Google Scholar] [CrossRef] [Green Version]
- Abduo, M.; Dahab, A.; Abuseda, H.; AbdulAziz, A.M.; Elhossieny, M. Comparative study of using water-based mud containing multiwall carbon nanotubes versus oil-based mud in HPHT fields. Egypt. J. Pet. 2016, 25, 459–464. [Google Scholar] [CrossRef] [Green Version]
- Vallejo, J.P.; Żyła, G.; Fernández-Seara, J.; Lugo, L. Influence of six carbon-based nanomaterials on the rheological properties of nanofluids. Nanomaterials 2019, 9, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monjezi, S.; Jones, J.D.; Nelson, A.K.; Park, J. The effect of weak confinement on the orientation of nanorods under shear flows. Nanomaterials 2018, 8, 130. [Google Scholar] [CrossRef] [Green Version]
- Vallejo, J.P.; Żyła, G.; Fernández-Seara, J.; Lugo, L. Rheological behaviour of functionalized graphene nanoplatelet nanofluids based on water and propylene glycol: Water mixtures. Int. Commun. Heat Mass Transfer. 2018, 99, 43–53. [Google Scholar] [CrossRef]
- Sayindla, S.; Lund, B.; Ytrehus, J.D.; Saasen, A. Hole-cleaning performance comparison of oil-based and water-based drilling fluids. J. Pet. Sci. Eng. 2017, 159, 49–57. [Google Scholar] [CrossRef] [Green Version]
- Mehrali, M.; Sadeghinezhad, E.; Latibari, S.T.; Kazi, S.N.; Mehrali, M.; Zubir, M. Investigation of thermal conductivity and rheological properties of nanofluids containing graphene nanoplatelets. Nanoscale Res. Lett. 2014, 9, 15. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Wu, D.; Feng, X.; Müllen, K. Dispersion of graphene sheets in organic solvent supported by ionic interactions. Adv. Mater. 2009, 21, 1679–1683. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Park, S.; An, J.; Piner, R.D.; Jung, I.; Yang, D.; Velamakanni, A. Aqueous suspension and characterization of chemically modified graphene sheets. Chem. Mater. 2008, 20, 6592–6594. [Google Scholar] [CrossRef]
- Li, D.; Müller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101. [Google Scholar] [CrossRef]
- Lawal, A.T. Graphene-based nano composites and their applications. A review. Biosens. Bioelectron. 2019, 141, 111384. [Google Scholar] [CrossRef] [PubMed]
- Guardia, L.; Fernández-Merino, M.; Paredes, J.; Solis-Fernandez, P.; Villar-Rodil, S.; Martinez-Alonso, A. High-throughput production of pristine graphene in an aqueous dispersion assisted by non-ionic surfactants. Carbon 2011, 49, 1653–1662. [Google Scholar] [CrossRef]
- Su, Y.; Kravets, V.; Wong, S.; Waters, J.; Geim, A.; Nair, R. Impermeable barrier films and protective coatings based on reduced graphene oxide. Nat. Commun. 2014, 5, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.; Seger, B.; Kamat, P.V. TiO2-graphene nanocomposites. UV-assisted photocatalytic reduction of graphene oxide. ACS Nano. 2008, 2, 1487–1491. [Google Scholar] [CrossRef]
- Moon, I.K.; Lee, J.; Ruoff, R.S.; Lee, H. Reduced graphene oxide by chemical graphitization. Nat. Commun. 2010, 1, 1–6. [Google Scholar] [CrossRef] [Green Version]
- George, M.; Chae, M.; Bressler, D.C. Composite materials with bast fibres: Structural, technical, and environmental properties. Prog. Mater. Sci. 2016, 83, 1–23. [Google Scholar] [CrossRef]
- Tesh, S.J.; Scott, T.B. Nano-composites for water remediation: A review. Adv. Mater. 2014, 26, 6056–6068. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Pradeep, K.; Raghul, A.; Senthilkumar, R.; Rangarajan, M.; Kothurkar, N.K. One-step synthesis of Pt-decorated graphene–carbon nanotubes for the electrochemical sensing of dopamine, uric acid and ascorbic acid. Anal. Methods. 2015, 7, 779–786. [Google Scholar] [CrossRef]
- Jiang, L.; Wen, Y.; Zhu, Z.; Su, C.; Ye, S.; Wang, J. Construction of an efficient nonleaching graphene nanocomposites with enhanced contact antibacterial performance. Chem. Eng. J. 2020, 382, 122906. [Google Scholar] [CrossRef]
- Alsaba, M.; Al Marshad, A.; Abbas, A.; Abdulkareem, T.; Al-Shammary, A.; Al-Ajmi, M. Laboratory evaluation to assess the effectiveness of inhibitive nano-water-based drilling fluids for Zubair shale formation. J. Pet. Explor. Prod. Technol. 2020, 10, 419–428. [Google Scholar] [CrossRef] [Green Version]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666. [Google Scholar] [CrossRef] [Green Version]
- Qalandari, R.; Qalandari, E. A review on the potential application of nano graphene as drilling fluid modifier in petroleum industry. Int. Refereed J. Eng. Sci. 2018, 7, 1–7. [Google Scholar]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Ridha, S.; Ibrahim, A.; Shahari, R.; Fonna, S. Graphene nanoplatelets as high-performance filtration control material in water-based drilling fluids. IOP Conf. Ser. Mater. Sci. Eng. 2018, 352, 012025. [Google Scholar] [CrossRef]
- Dideikin, A.T.; Vul, A.Y. Graphene Oxide and Derivatives: The Place in Graphene Family. Front. Phys. 2019, 6, 149. [Google Scholar] [CrossRef]
- Friedheim, J.E.; Young, S.; De Stefano, G.; Lee, J.; Guo, Q. Nanotechnology for oilfield applications-hype or reality? In Proceedings of the SPE International Oilfield Nanotechnology Conference and Exhibition, Noordwijk, The Netherlands, 12–14 June 2012. [Google Scholar]
- Liu, B.; Zhou, K. Recent progress on graphene-analogous 2D nanomaterials: Properties, modeling and applications. Prog. Polym. Sci. 2019, 100, 99–169. [Google Scholar] [CrossRef]
- Hu, K.; Kulkarni, D.D.; Choi, I.; Tsukruk, V.V. Graphene-polymer nanocomposites for structural and functional applications. Prog. Polym. Sci. 2014, 39, 1934–1972. [Google Scholar] [CrossRef]
- Sharma, B.; Malik, P.; Jain, P. Biopolymer reinforced nanocomposites: A comprehensive review. Mater. Today Commun. 2018, 16, 353–363. [Google Scholar] [CrossRef]
- Rana, A.; Arfaj, M.K.; Yami, A.S.; Saleh, T.A. Cetyltrimethylammonium modified graphene as a clean swelling inhibitor in water-based oil-well drilling mud. J. Environ. Chem. Eng. 2020, 8, 103802. [Google Scholar] [CrossRef]
- Li, R.; Liu, Y.; Cheng, L.; Yang, C.; Zhang, J. Photoelectrochemical aptasensing of kanamycin using visible light-activated carbon nitride and graphene oxide nanocomposites. Anal. Chem. 2014, 86, 9372–9375. [Google Scholar] [CrossRef]
- Liu, C.; Wang, K.; Luo, S.; Tang, Y.; Chen, L. Direct electrodeposition of graphene enabling the one-step synthesis of graphene-metal nanocomposite films. Small 2011, 7, 1203–1206. [Google Scholar] [CrossRef] [PubMed]
- Ali, J.A.; Kalhury, A.M.; Sabir, A.N.; Ahmed, R.N.; Ali, N.H.; Abdullah, A.D. A state-of-the-art review of the application of nanotechnology in the oil and gas industry with a focus on drilling engineering. J. Pet. Sci. Eng. 2020, 191, 107118. [Google Scholar] [CrossRef]
- Li, M.C.; Wu, Q.; Song, K.; De Hoop, C.F.; Lee, S.; Qing, Y. Cellulose nanocrystals and polyanionic cellulose as additives in bentonite water-based drilling fluids: Rheological modeling and filtration mechanisms. Ind. Eng. Chem. Res. 2016, 55, 133–143. [Google Scholar] [CrossRef]
- Potts, J.R.; Dreyer, D.R.; Bielawski, C.W.; Ruoff, R.S. Graphene-based polymer nanocomposites. Polymer 2011, 52, 5–25. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Irin, F.; Ma, L.; Bhattacharia, S.K.; Hedden, R.C.; Green, M.J. Rheology and morphology of pristine graphene/polyacrylamide gels. ACS Appl. Mater. Interfaces 2013, 5, 8633–8640. [Google Scholar] [CrossRef]
- Nelson, E.; Guillot, D. Well Cementing 773; Sugar Land: Schlumberger, TX, USA, 2006. [Google Scholar]
- Cheng, X.; Yokozeki, T.; Wu, L.; Wang, H.; Zhang, J.; Koyanagi, J. Electrical conductivity and interlaminar shear strength enhancement of carbon fiber reinforced polymers through synergetic effect between graphene oxide and polyaniline. Compos. Part A Appl. Sci. Manuf. 2016, 90, 243–249. [Google Scholar] [CrossRef]
- Yao, L.; Lu, Y.; Wang, Y.; Hu, L. Effect of graphene oxide on the solution rheology and the film structure and properties of cellulose carbamate. Carbon 2014, 69, 552–562. [Google Scholar] [CrossRef]
- Chen, Y.; Yin, Q.; Zhang, X.; Xue, X.; Jia, H. The crystallization behaviors and rheological properties of polypropylene/graphene nanocomposites: The role of surface structure of reduced graphene oxide. Thermochim. Acta 2018, 661, 124–136. [Google Scholar] [CrossRef]
- Gao, C.; Zhang, S.; Wang, F.; Wen, B.; Han, C.; Ding, Y. Graphene networks with low percolation threshold in ABS nanocomposites: Selective localization and electrical and rheological properties. ACS Appl. Mater. Interfaces 2014, 6, 12252–12260. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Macosko, C.W. Morphology and properties of polyester/exfoliated graphite nanocomposites. Macromolecules 2008, 41, 3317–3327. [Google Scholar] [CrossRef]
- Sadasivuni, K.K.; Ponnamma, D.; Kumar, B.; Strankowski, M.; Cardinaels, R.; Moldenaers, P. Dielectric properties of modified graphene oxide filled polyurethane nanocomposites and its correlation with rheology. Compos. Sci. Technol. 2014, 104, 18–25. [Google Scholar] [CrossRef]
- Gaska, K.; Kádár, R.; Rybak, A.; Siwek, A.; Gubanski, S. Gas barrier, thermal, mechanical and rheological properties of highly aligned graphene-LDPE nanocomposites. Polymers 2017, 9, 294. [Google Scholar] [CrossRef]
- Mohideen, A.A.M.; Saheed, M.S.M.; Mohamed, N.M. Multiwalled carbon nanotubes and graphene oxide as nano-additives in water-based drilling fluid for enhanced fluid-loss-control & gel strength. AIP Conf. Proc. 2019, 2151, 020001. [Google Scholar]
- Almoshin, A.M.; Alsharaeh, E.; Fathima, A.; Bataweel, M. A novel polymer nanocomposite graphene based gel for high temperature water shutoff applications. SPE Kingdom of Saudi Arabia annual technical symposium and exhibition. Soc. Pet. Eng. 2018. [Google Scholar] [CrossRef]
- Chawhan, S.S.; Barai, D.P.; Bhanvase, B.A. Sonochemical preparation of rGO-SnO2 nanocomposite and its nanofluids: Characterization, thermal conductivity, rheological and convective heat transfer investigation. Mater. Today Commun. 2020, 101148. [Google Scholar] [CrossRef]
- Manivel, P.; Kanagaraj, S.; Balamurugan, A.; Ponpandian, N.; Mangalaraj, D.; Viswanathan, C. Rheological behavior and electrical properties of polypyrrole/thermally reduced graphene oxide nanocomposite. Colloids Surf. A 2014, 441, 614–622. [Google Scholar] [CrossRef]
- Gudarzifar, H.; Sabbaghi, S.; Rezvani, A.; Saboori, R. Experimental investigation of rheological & filtration properties and thermal conductivity of water-based drilling fluid enhanced. Powder Technol. 2020. [Google Scholar] [CrossRef]
- Haruna, M.A.; Pervaiz, S.; Hu, Z.; Nourafkan, E.; Wen, D. Improved rheology and high-temperature stability of hydrolyzed polyacrylamide using graphene oxide nanosheet. J. Appl. Polym. Sci. 2019, 136, 47582. [Google Scholar] [CrossRef]
- Jamrozik, A.; Wiśniowski, R.; Czekaj, L.; Pintal, K. Analyses of graphene oxide applicability in drilling mud technology. Int. Multidiscip. Sci. GeoConf. SGEM 2017, 17, 515–522. [Google Scholar]
- Xuan, Y.; Jiang, G.; Li, Y. Nanographite oxide as ultrastrong fluid-loss-control additive in water-based drilling fluids. J. Dispers. Sci. Technol. 2014, 35, 1386–1392. [Google Scholar] [CrossRef]
- Mahmood, D.; Al-Zubaidi, N.; Alwasiti, A. Improving Drilling Fluid Properties by Using Nano-Additives. Eng. Technol. J. 2017, 35, 1034–1041. [Google Scholar]
- Al-Yasiri, M.; Wen, D. Gr-Al2O3 Nanoparticles-Based Multifunctional Drilling Fluid. Ind. Eng. Chem. Res. 2019, 58, 10084–10091. [Google Scholar] [CrossRef]
- Taha, N.M.; Lee, S. Nano graphene application improving drilling fluids performance. Int. Pet. Technol. Conf. 2015. [Google Scholar] [CrossRef]
- Ahmad, H.M.; Kamal, M.S.; Murtaza, M.; Al-Harthi, M.A. Improving the drilling fluid properties using nanoparticles and water-soluble polymers. SPE Kingdom of Saudi Arabia annual technical symposium and exhibition. Soc. Pet. Eng. 2017. [Google Scholar]
- Nasser, J.; Jesil, A.; Mohiuddin, T.; Al Ruqeshi, M.; Devi, G.; Mohataram, S. Experimental investigation of drilling fluid performance as nanoparticles. World J. Nano Sci. 2013, 3, 57. [Google Scholar] [CrossRef] [Green Version]
- Ismail, A.; Rashid, M.; Thameem, B. Application of nanomaterials to enhanced the lubricity and rheological properties of water based drilling fluid. IOP Conf. Ser. Mat. Sci. Eng. 2018, 380, 012021. [Google Scholar] [CrossRef]
- Aramendiz, J.; Imqam, A. Water-based drilling fluid formulation using silica and graphene nanoparticles for unconventional shale applications. J. Pet. Sci. Eng. 2019, 179, 742–749. [Google Scholar] [CrossRef]
- Kusrini, E.; Oktavianto, F.; Usman, A.; Mawarni, D.P.; Alhamid, M.I. Synthesis, characterization, and performance of graphene oxide and phosphorylated graphene oxide as additive in water-based drilling fluids. Appl. Surf. Sci. 2020, 506. [Google Scholar] [CrossRef]
- Aramendiz, J.; Imqam, A.; Fakher, S.M. Design and Evaluation of a Water-Based Drilling Fluid Formulation Using SiO and Graphene Oxide Nanoparticles for Unconventional Shales. Int. Pet. Technol. Conf. 2019. [Google Scholar]
- Smith, A.T.; LaChance, A.M.; Zeng, S.; Liu, B.; Sun, L. Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano Mater. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Singh, P.; Chamoli, P.; Sachdev, S.; Raina, K.; Shukla, R.K. Structural, optical and rheological behavior investigations of graphene oxide/glycerol based lyotropic liquid crystalline phases. Appl. Surf. Sci. 2020, 509, 144710. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773. [Google Scholar] [CrossRef]
- Li, H.; Liu, S.; Lin, L. Rheological study on 3D printability of alginate hydrogel and effect of graphene oxide. Int. J. Bioprint. 2016, 2, 54–66. [Google Scholar] [CrossRef]
- Yi, S.; Li, G.; Ding, S.; Mo, J. Performance and mechanisms of graphene oxide suspended cutting fluid in the drilling of titanium alloy Ti-6Al-4V. J. Manuf. Process. 2017, 29, 182–193. [Google Scholar] [CrossRef]
- Aramendiz, J.; Imqam, A. Silica and Graphene Oxide Nanoparticle Formulation to Improve Thermal Stability and Inhibition Capabilities of Water-Based Drilling Fluid Applied to Woodford Shale. SPE Drill. Complet. 2019. [Google Scholar] [CrossRef]
- Aliabadian, E.; Sadeghi, S.; Moghaddam, A.R.; Maini, B.; Chen, Z.; Sundararaj, U. Application of graphene oxide nanosheets and HPAM aqueous dispersion for improving heavy oil recovery: Effect of localized functionalization. Fuel 2020, 265, 116918. [Google Scholar] [CrossRef]
- Le Ba, T.; Mahian, O.; Wongwises, S.; Szilágyi, I.M. Review on the recent progress in the preparation and stability of graphene-based nanofluids. J. Therm. Anal. Calorim. 2020, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Hoelscher, K.P.; Young, S.; Friedheim, J.; De Stefano, G. Nanotechnology application in drilling fluids. In Proceedings of the Offshore Mediterranean Conference and Exhibition, Ravenna, Italy, 20–22 March 2013. [Google Scholar]
- Alkinani, H.H.; Al-Hameedi, A.T.T.; Dunn-Norman, S.; Lian, D. Application of artificial neural networks in the drilling processes: Can equivalent circulation density be estimated prior to drilling? Egypt. J. Pet. 2019. [Google Scholar] [CrossRef]
- Saleh, T.A.; Ibrahim, M.A. Advances in functionalized Nanoparticles based drilling inhibitors for oil production. Energy Rep. 2019, 5, 1293–1304. [Google Scholar] [CrossRef]
- Rishi, K.; Narayanan, V.; Beaucage, G.; McGlasson, A.; Kuppa, V.; Ilavsky, J. A thermal model to describe kinetic dispersion in rubber nanocomposites: The effect of mixing time on dispersion. Polymer 2019, 175, 272–282. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Q.; Gao, L.; Ma, T.; Qiu, M.; Hu, Y. A molecular dynamics study of lubricating mechanism of graphene nanoflakes embedded in Cu-based nanocomposite. Appl. Surf. Sci. 2020, 511, 145620. [Google Scholar] [CrossRef]
- Viswanathan, V.; Laha, T.; Balani, K.; Agarwal, A.; Seal, S. Challenges and advances in nanocomposite processing techniques. Mater. Sci. Eng. R Rep. 2006, 54, 121–285. [Google Scholar] [CrossRef]
- Harito, C.; Bavykin, D.V.; Yuliarto, B.; Dipojono, H.K.; Walsh, F.C. Polymer nanocomposites having a high filler content: Synthesis, structures, properties, and applications. Nanoscale 2019, 11, 4653–4682. [Google Scholar] [CrossRef]
- Morgani, M.S.; Saboori, R.; Sabbaghi, S. Hydrogen sulfide removal in water-based drilling fluid by metal oxide nanoparticle and ZnO/TiO2 nanocomposite. Mater. Res. Express. 2017, 4, 075501. [Google Scholar] [CrossRef]
- Amr, I.T.; Al-Amer, A.; Al-Harthi, M.; Girei, S.A.; Sougrat, R.; Atieh, M.A. Effect of acid treated carbon nanotubes on mechanical, rheological and thermal properties of polystyrene nanocomposites. Compos. Part B 2011, 42, 1554–1561. [Google Scholar] [CrossRef]
- Tang, W.; Zou, C.; Da, C.; Cao, Y.; Peng, H. A review on the recent development of cyclodextrin-based materials used in oilfield applications. Carbohydr. Polym. 2020, 116321. [Google Scholar] [CrossRef]
- Perween, S.; Beg, M.; Shankar, R.; Sharma, S.; Ranjan, A. Effect of zinc titanate nanoparticles on rheological and filtration properties of water based drilling fluids. J. Pet. Sci. Eng. 2018, 170, 844–857. [Google Scholar] [CrossRef]
- Husin, H.; Elraies, K.A.; Choi, H.J.; Aman, Z. Influence of Graphene Nanoplatelet and Silver Nanoparticle on the Rheological Properties of Water-Based Mud. Appl. Sci. 2018, 8, 1386. [Google Scholar] [CrossRef] [Green Version]
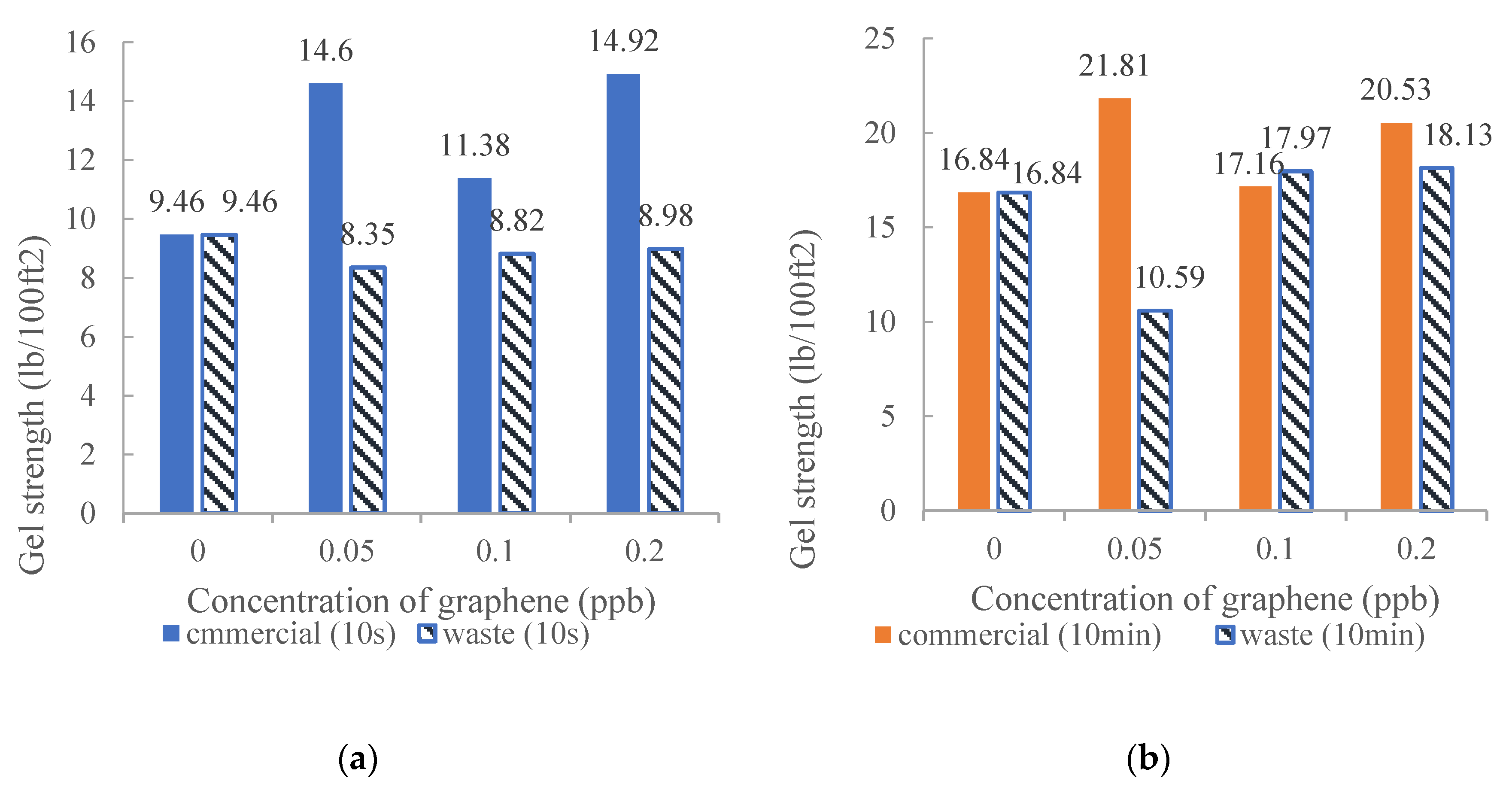
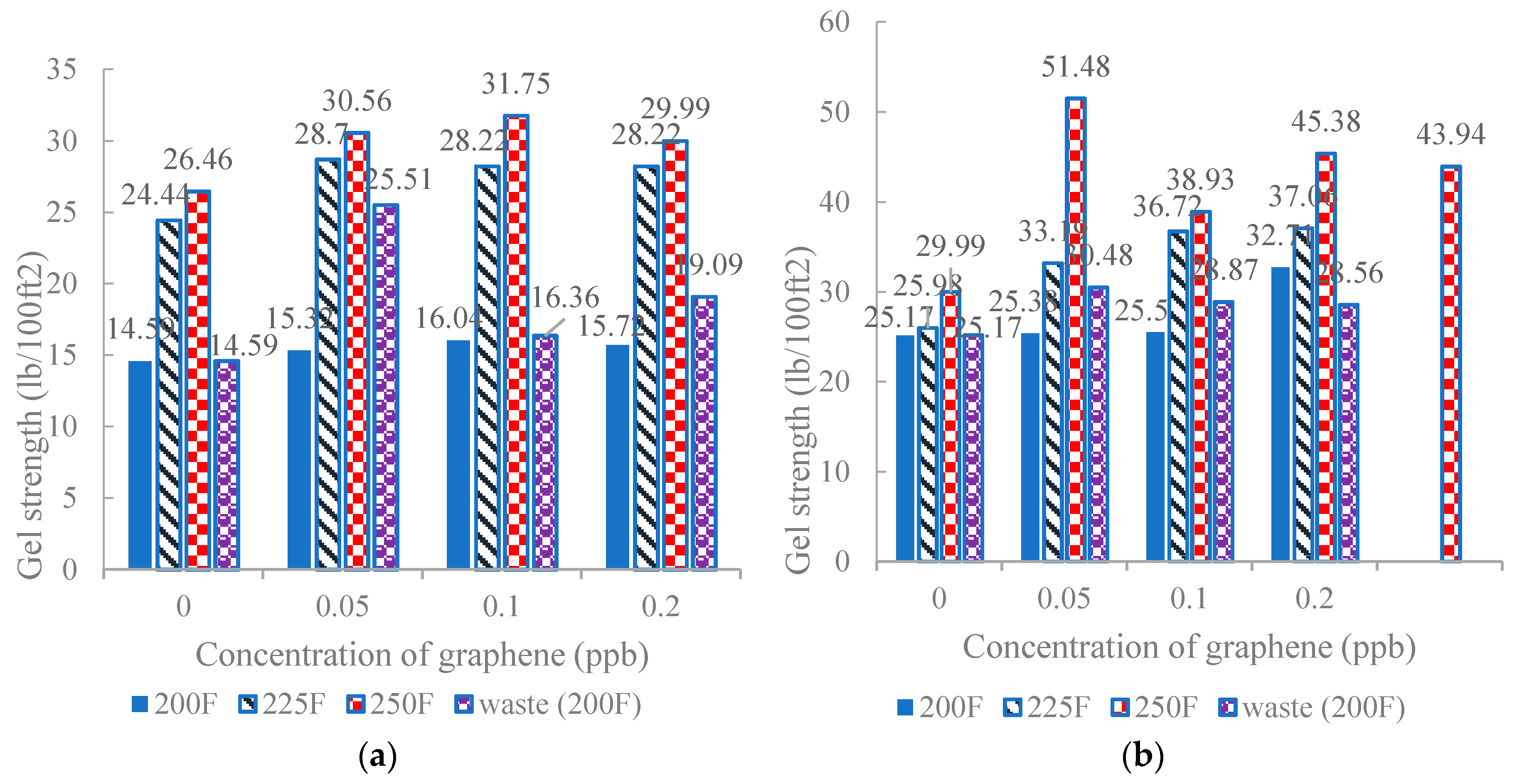
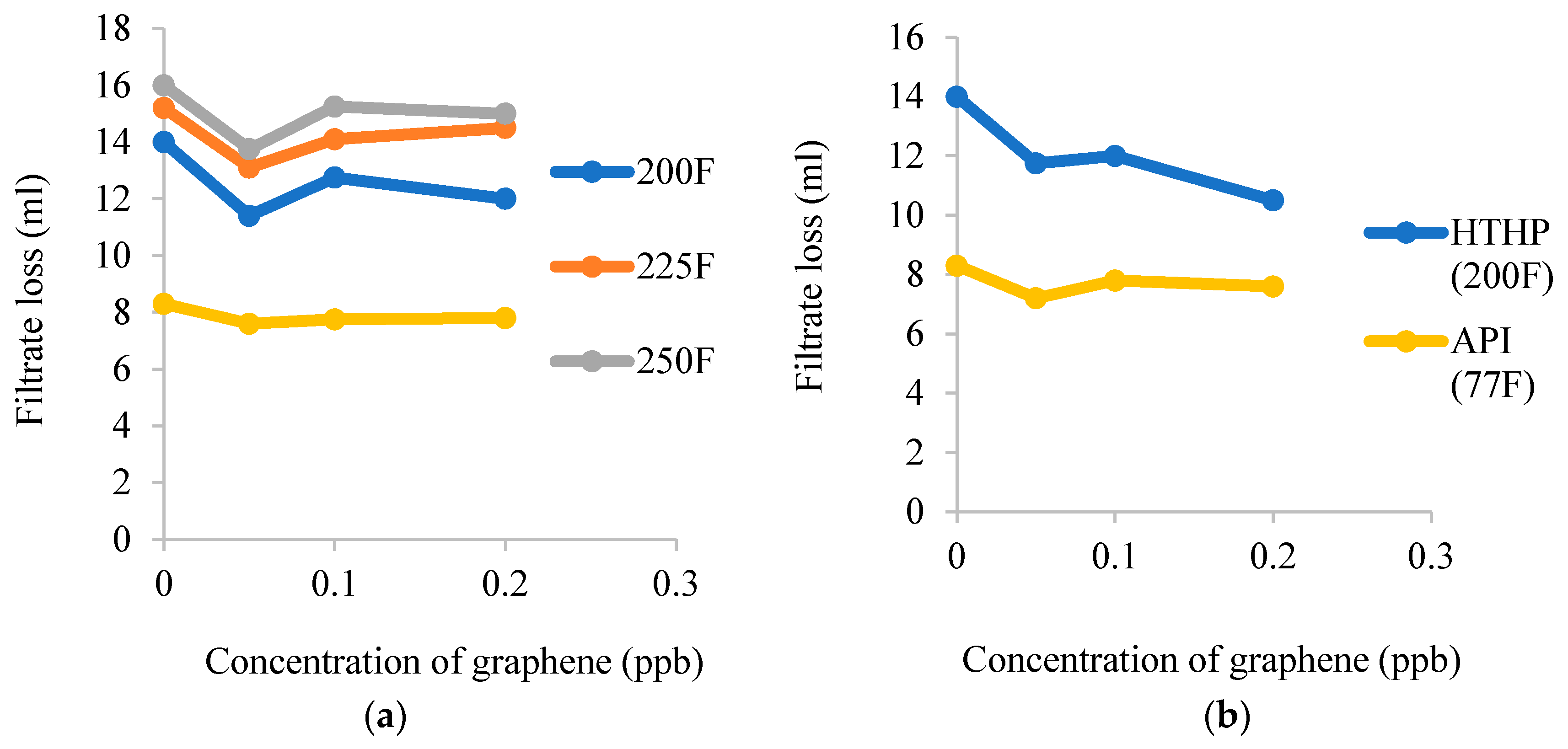
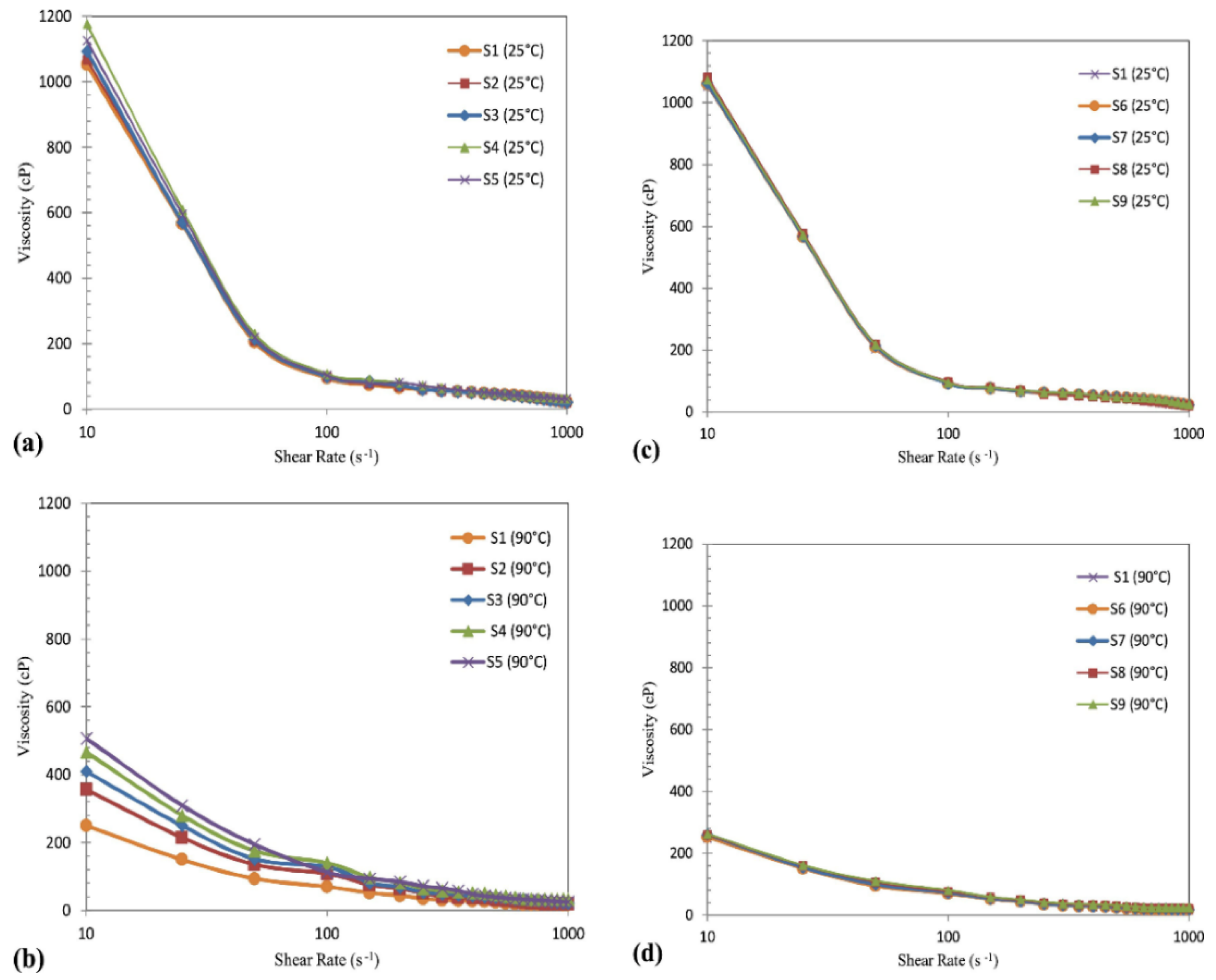
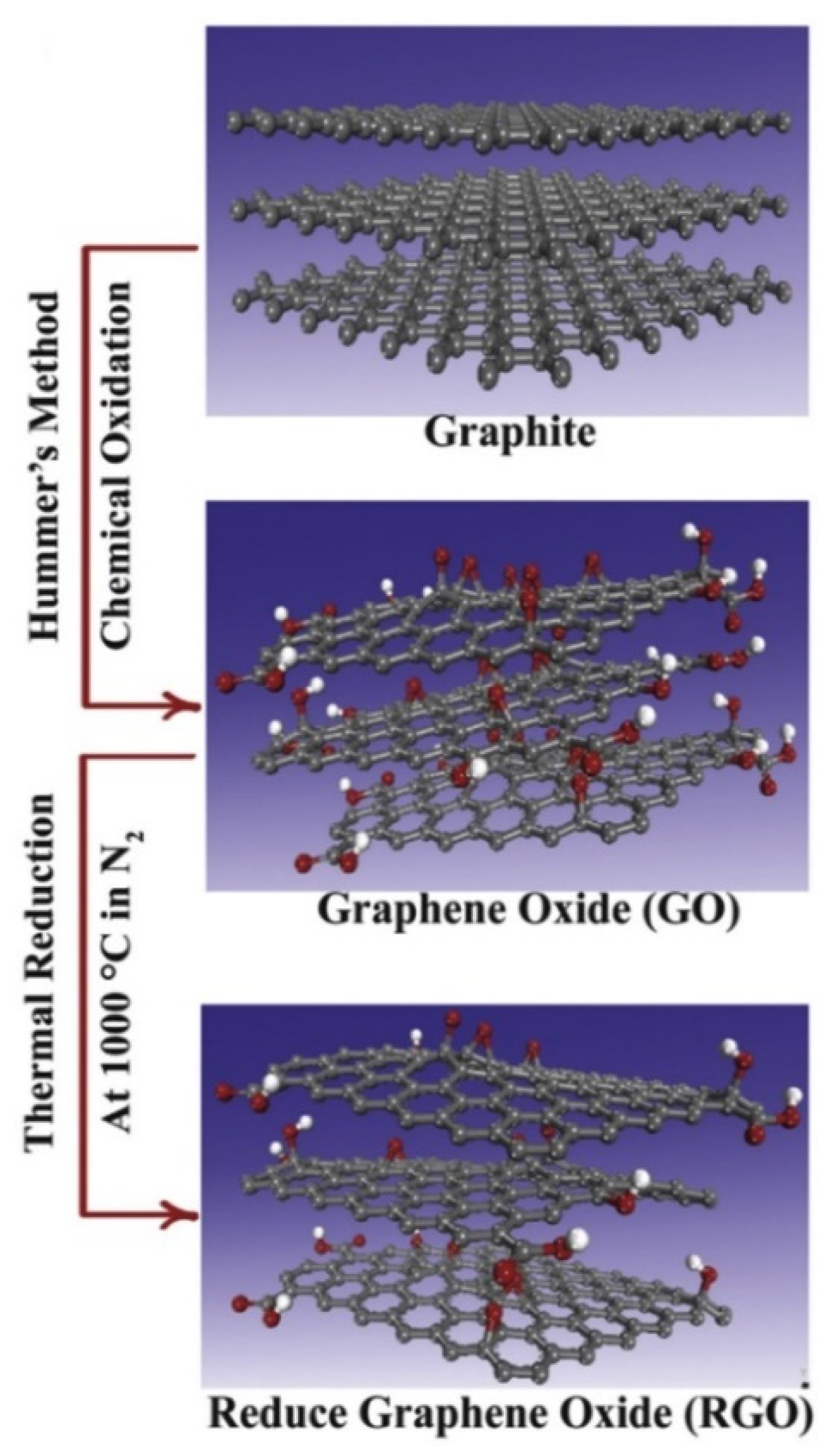
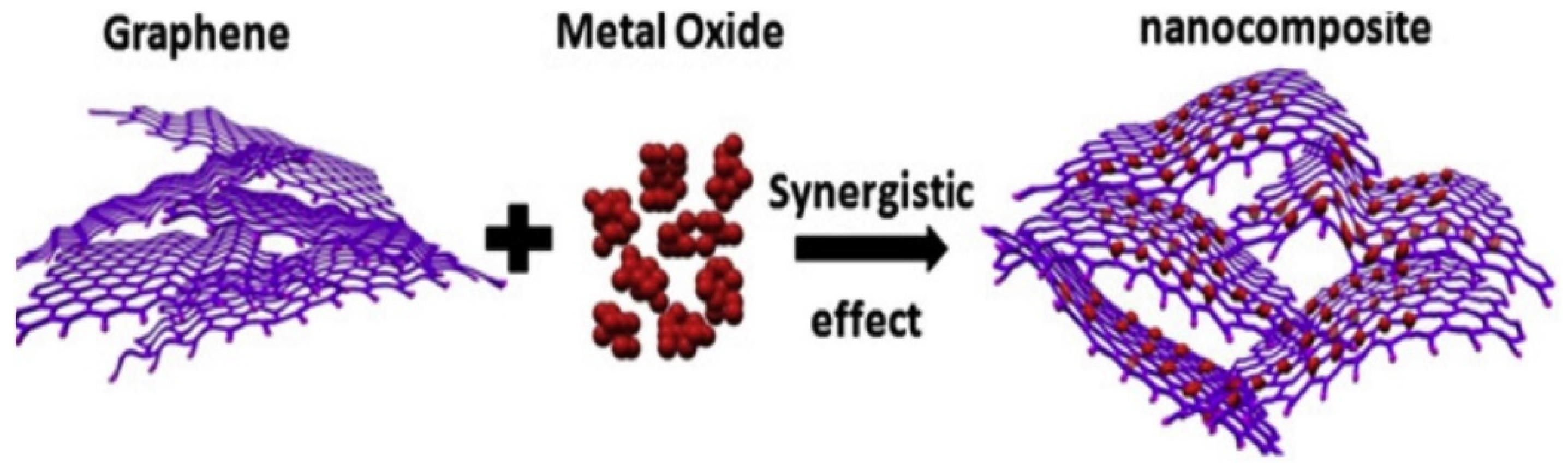
| Nanocomposites | Experimental Conditions | Rheological Properties | Outcomes | References |
|---|---|---|---|---|
| Nanosilica polymer composite | LPLT and high temperature up to 446 °F | PV, YP, GS, FL, MCT | Under LPLT conditions, the usage of nanosilica (1.0 wt.%) has greatly enhanced the rheological and filtration properties. Nanocomposites have shown no decomposition under high temperatures at 392, 410, 428, and 446 °F, proving nanocomposites to be suitable under HPHT conditions. | [17] |
| ZnO nanocomposite | HPHT at 109 to 370 °F and 150 to 18,500 psi | PV, YP | 2.3 wt.% of ZnO nanocomposite (5 to 50 nm) resulted in upgrading the rheological properties under HPHT conditions. | [27] |
| TiO2-polyacrylamide | LPLT | PV, YP, GS, FL | Development in rheological properties and filtration behavior under LPLT condition was observed by using 1–14 g of TiO2-PAM nanocomposite. | [26,48] |
| ZnO-polyacrylamide | At 80 and 150 °F | PV, AV, YP, GS, FL | By adding 0.8 g of ZnO-PAM nanocomposite in the drilling fluid, PV and YP increased by 18.8% and 16.7%, respectively. Fluid loss was reduced by 12.7% and 23% under LPLT and HPHT conditions, respectively, when using 1 g of nanocomposite. | [28] |
| Sepiolite | LPLT and HPHT at 122 to 356 °F and 500 to 6000 psi | - | The experiment showed that WBDF samples with 1.4 wt.% of sepiolite enriched the rheological properties at 6000 psi and temperatures up to 356 °F conditions. | [49] |
| Polyacrylamide-grafted polyethylene glycol nanosilica | High temperature up to 203 °F | PV, YP, GS, FL | Enhancement of rheological and filtration properties was observed with 0.7 wt.% of nanocomposite and the values remained stable under a temperature of 203 °F. | [50] |
| Hydrophobic modified polymer-based silica | LPLT | PV, YP, GS, FL | Rheological and filtration properties were improved by adding 2.0 wt.% before and after hot rolling under 250 °F for 16 h. | [51] |
| Polyethylene glycol grafted nanosilica | LPLT | PV, AV, YP, GS, FL | Results have showed that PV, YP, and AV values were increased while fluid loss volume was decreased to 15.2% by adding 1 g of nanocomposite in drilling fluid. | [52] |
| Amphiphilic polymer/nano-silica | LPLT | PV, AV, YP, GS, FL | PV was enhanced by the addition of 7.1% nanocomposite. Addition of 2 wt.% nanocomposite reduced the fluid loss volume to 6.4 mL. | [53] |
| Nanocarboxylmethyl cellulose/polystyrene core-shell nanocomposite | LPLT | PV, AV, YP, GS, FL | PV and AV increased by up surging the concentration of three additives. YP values were the highest for bulk CMC while core-shell nanocomposites recorded the lowest amount of fluid loss volume. | [24] |
| CuO/ZnO/synthetic polymer nanocomposite | LPLT and high temperature up to 400 °F | PV, YP, GS, FL, MCT | The drilling fluid exhibited stable rheological and filtration properties at 400 °F. Under LPLT conditions, low fluid loss volume was recorded. while the mud cake formed was thin and impermeable. | [54] |
| Lignosulfonate/Acrylamide graft copolymers | 78 °F and 250 °F | PV, YP, GS, FL, MCT | At a temperature of 78 °F and 250 °F, rheological and filtration properties of the drilling fluid were enhanced with the inclusion of nanocomposite (2.4–3.5 g/350 mL water). | [55] |
| Hybrid polymer nanocomposite poly(styrene-methylmethacrylate-acrylic acid)/nanoclay | High temperature up to 250 °F | PV, YP, GS, FL | The nanocomposite presented stable rheology at temperatures up to 250 °F, and the combination of nanocomposite in nanoclay-based drilling fluid was reduced by up to 22% fluid loss under LPLT conditions, and a 65% reduction in the polymer-based drilling fluid. | [56] |
| Novel synthetic based acrylamide-styrene copolymer | High temperature up to 250 °F | PV, YP, GS, FL, MCT | Rheological and filtration properties proved a progressive fluid loss control. An ideal filtration performance at LPLT and HPHT conditions was achieved with the addition of 3 g of nanocomposite into the drilling fluid. | [57,58] |
| Synthesis Routes | Nanofiller Content | Advantages | Limitations |
|---|---|---|---|
| In situ polymerization | 5–70 wt.% | Fabrication and polymerization occur at the same time to produce an efficient interface between filler and polymer | Suitable for limited types of polymers |
| Shear press | 60–70 wt.% | Fine alignment | Restricted to small-scale production |
| Vacuum-assisted polymer infiltration | 5–70 wt.% | Competent at producing large and complex composites | Filler fractions and thickness are challenging to control |
| Spray winding | 50–80 wt.% | Satisfactory alignment and large-scale production | Comparatively complex apparatus |
| Capillary rise infiltration | 40–60 wt.% | User-friendly apparatus | Limited to thermoplastic polymers |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikram, R.; Mohamed Jan, B.; Vejpravova, J.; Choudhary, M.I.; Zaman Chowdhury, Z. Recent Advances of Graphene-Derived Nanocomposites in Water-Based Drilling Fluids. Nanomaterials 2020, 10, 2004. https://doi.org/10.3390/nano10102004
Ikram R, Mohamed Jan B, Vejpravova J, Choudhary MI, Zaman Chowdhury Z. Recent Advances of Graphene-Derived Nanocomposites in Water-Based Drilling Fluids. Nanomaterials. 2020; 10(10):2004. https://doi.org/10.3390/nano10102004
Chicago/Turabian StyleIkram, Rabia, Badrul Mohamed Jan, Jana Vejpravova, M. Iqbal Choudhary, and Zaira Zaman Chowdhury. 2020. "Recent Advances of Graphene-Derived Nanocomposites in Water-Based Drilling Fluids" Nanomaterials 10, no. 10: 2004. https://doi.org/10.3390/nano10102004
APA StyleIkram, R., Mohamed Jan, B., Vejpravova, J., Choudhary, M. I., & Zaman Chowdhury, Z. (2020). Recent Advances of Graphene-Derived Nanocomposites in Water-Based Drilling Fluids. Nanomaterials, 10(10), 2004. https://doi.org/10.3390/nano10102004