Rational Regulation of Surface Free Radicals on TiO2 Nanotube Arrays via Ag2O–AgBiO3 towards Enhanced Selective Photoelectrochemical Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Fabrication
2.2. Characterizations
2.3. Photoelectrochemical Measurements
3. Results
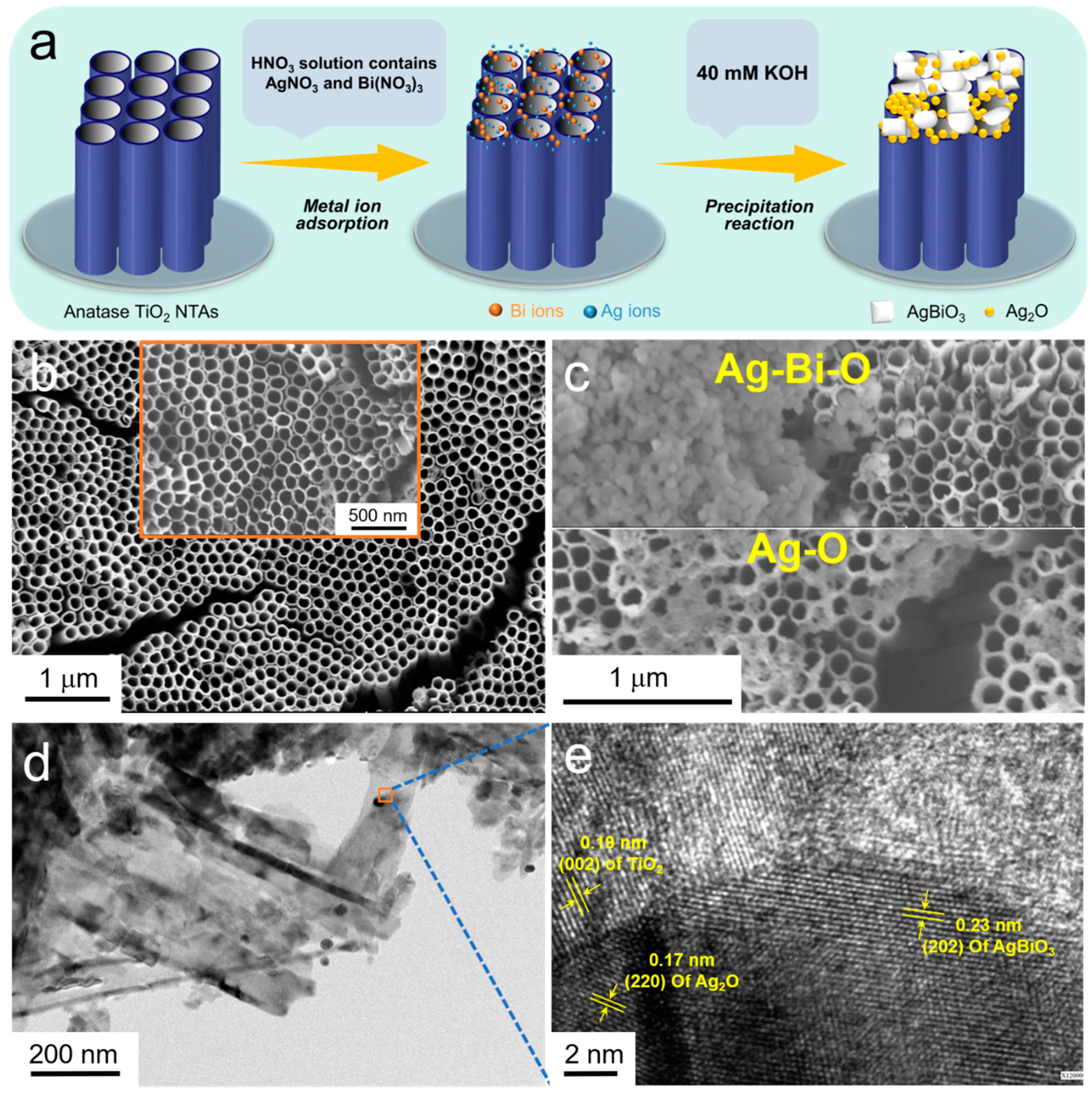
3.1. Microscopic Morphologies
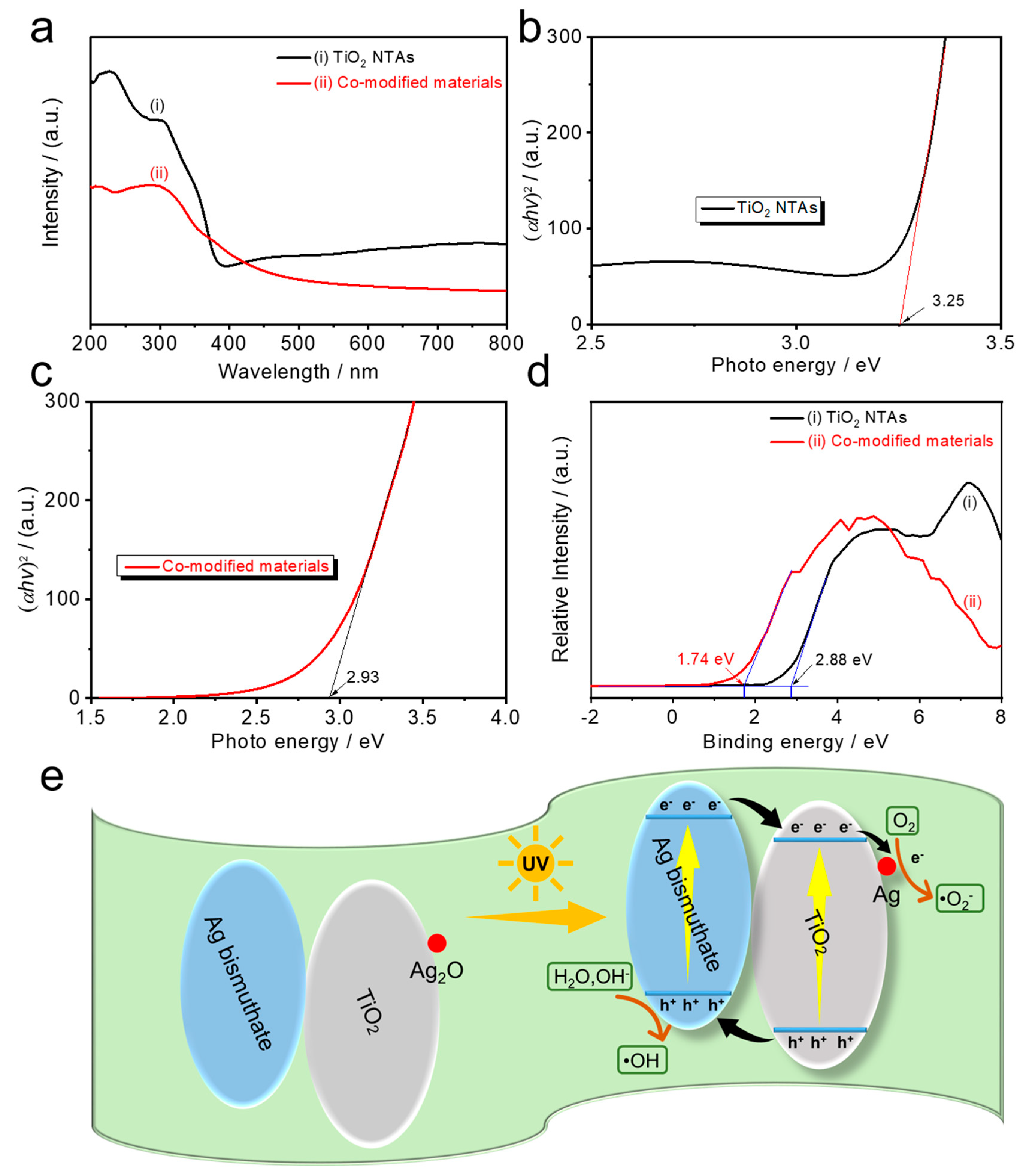
3.2. Crystalline Properties and Component Analysis
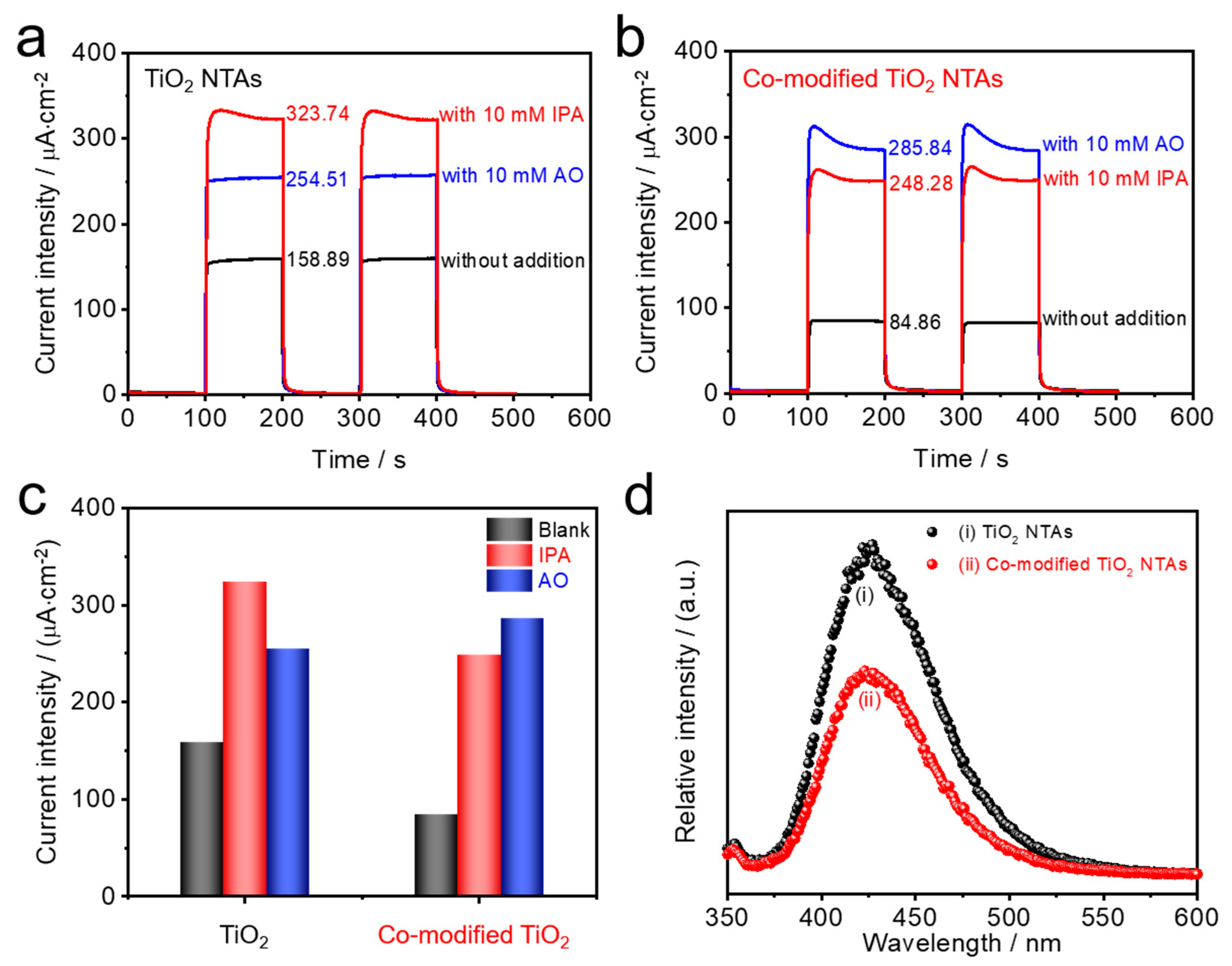
3.3. Surface Electrochemical Reactions and Related Free Radicals
3.4. Photoelectrochemical Detection Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ma, Y.; Wang, X.; Jia, Y.; Chen, X.; Han, H.; Li, C. Titanium Dioxide-Based Nanomaterials for Photocatalytic Fuel Generations. Chem. Rev. 2014, 114, 9987–10043. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Guven, H.; Dereli, R.K.; Ozgun, H.; Ersahin, M.E.; Öztürk, I. Towards sustainable and energy efficient municipal wastewater treatment by up-concentration of organics. Prog. Energy Combust. Sci. 2019, 70, 145–168. [Google Scholar] [CrossRef]
- Tian, J.; Li, Y.; Dong, J.; Huang, M.; Lu, J. Photoelectrochemical TiO2 nanotube arrays biosensor for asulam determination based on in-situ generation of quantum dots. Biosens. Bioelectron. 2018, 110, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Pan, X.; Zhang, G.; Liao, X.; Zhou, X.; Yan, M.; Xu, L.; Mai, L. Nanowires in Energy Storage Devices: Structures, Synthesis, and Applications. Adv. Energy Mater. 2018, 8, 1802369. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Zhu, X.; Duan, X.; Pan, A. Composition modulation in one-dimensional and two-dimensional chalcogenide semiconductor nanostructures. Chem. Soc. Rev. 2018, 47, 7504–7521. [Google Scholar] [CrossRef]
- Qiu, Y.; Pan, Z.; Chen, H.; Ye, D.; Guo, L.; Fan, Z.; Yang, S.; Lin, G. Current progress in developing metal oxide nanoarrays-based photoanodes for photoelectrochemical water splitting. Sci. Bull. 2019, 64, 1348–1380. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Liu, G.; Wang, L. Crystal Facet Engineering of Photoelectrodes for Photoelectrochemical Water Splitting. Chem. Rev. 2019, 119, 5192–5247. [Google Scholar] [CrossRef]
- Pang, Y.; Zang, W.; Kou, Z.; Zhang, L.; Xu, G.; Lv, J.; Gao, X.; Pan, Z.; Wang, J.; Wu, Y.-C. Assembling of Bi atoms on TiO2 nanorods boosts photoelectrochemical water splitting of semiconductors. Nanoscale 2020, 12, 4302–4308. [Google Scholar] [CrossRef]
- Yu, J.; Wang, B. Effect of calcination temperature on morphology and photoelectrochemical properties of anodized titanium dioxide nanotube arrays. Appl. Catal. B Environ. 2010, 94, 295–302. [Google Scholar] [CrossRef]
- Li, Z.; Bian, H.; Xiao, X.; Shen, J.; Zhao, C.; Lu, J.; Li, Y.Y. Defective Black TiO2 Nanotube Arrays for Enhanced Photocatalytic and Photoelectrochemical Applications. ACS Appl. Nano Mater. 2019, 2, 7372–7378. [Google Scholar] [CrossRef]
- Xu, X.; Cai, J.; Zhou, M.; Du, X.; Zhang, Y.; Xuedong, D. Photoelectrochemical degradation of 2,4-dichlorophenoxyacetic acid using electrochemically self-doped Blue TiO2 nanotube arrays with formic acid as electrolyte. J. Hazard. Mater. 2019, 382, 121096. [Google Scholar] [CrossRef] [PubMed]
- Pancielejko, A.; Mazierski, P.; Lisowski, W.; Zaleska-Medynska, A.; Łuczak, J. Ordered TiO2 Nanotubes with Improved Photoactivity through Self-organizing Anodization with the Addition of an Ionic Liquid: Effects of the Preparation Conditions. ACS Sustain. Chem. Eng. 2019, 7, 15585–15596. [Google Scholar] [CrossRef]
- Zhou, J.; Guo, M.; Wang, L.; Ding, Y.; Zhang, Z.; Tang, Y.; Liu, C.; Luo, S. 1T-MoS2 nanosheets confined among TiO2 nanotube arrays for high performance supercapacitor. Chem. Eng. J. 2019, 366, 163–171. [Google Scholar] [CrossRef]
- Ye, Y.; Bruning, H.; Li, X.; Yntema, D.; Rijnaarts, H.H. Significant enhancement of micropollutant photocatalytic degradation using a TiO2 nanotube array photoanode based photocatalytic fuel cell. Chem. Eng. J. 2018, 354, 553–562. [Google Scholar] [CrossRef]
- Ji, L.; Zhou, X.; Schmuki, P. Electrochemically Faceted Bamboo-type TiO2 Nanotubes Provide Enhanced Open-Circuit Hydrogen Evolution. ChemElectroChem 2018, 6, 114–120. [Google Scholar] [CrossRef]
- Byrne, C.; Moran, L.; Hermosilla, D.; Merayo, N.; Blanco, Á.; Rhatigan, S.; Hinder, S.; Ganguly, P.; Nolan, M.; Pillai, S.C. Effect of Cu doping on the anatase-to-rutile phase transition in TiO2 photocatalysts: Theory and experiments. Appl. Catal. B Environ. 2019, 246, 266–276. [Google Scholar] [CrossRef]
- Wang, K.; Chen, Q.; Hu, Y.; Wei, W.; Wang, S.; Shen, Q.; Qu, P. Crystalline Ru0.33 Se Nanoparticles-Decorated TiO2 Nanotube Arrays for Enhanced Hydrogen Evolution Reaction. Small 2018, 14, 1802132. [Google Scholar] [CrossRef]
- Motola, M.; Čaplovičová, M.; Krbal, M.; Sopha, H.; Thirunavukkarasu, G.K.; Gregor, M.; Plesch, G.; Macak, J.M. Ti3+ doped anodic single-wall TiO2 nanotubes as highly efficient photocatalyst. Electrochim. Acta 2020, 331, 135374. [Google Scholar] [CrossRef]
- Shin, Y.; Lee, S. Self-Organized Regular Arrays of Anodic TiO2 Nanotubes. Nano Lett. 2008, 8, 3171–3173. [Google Scholar] [CrossRef]
- Ai, C.; Xie, P.; Zhang, X.; Zheng, X.; Li, J.; Kafizas, A.; Lin, S. Explaining the Enhanced Photoelectrochemical Behavior of Highly Ordered TiO2 Nanotube Arrays: Anatase/Rutile Phase Junction. ACS Sustain. Chem. Eng. 2019, 7, 5274–5282. [Google Scholar] [CrossRef]
- Pang, Y.; Li, Y.; Xu, G.; Hu, Y.; Kou, Z.; Feng, Q.; Lv, J.; Zhang, Y.; Wang, J.; Wu, Y. Z-scheme carbon-bridged Bi2O3/TiO2 nanotube arrays to boost photoelectrochemical detection performance. Appl. Catal. B 2019, 248, 255–263. [Google Scholar] [CrossRef]
- Wang, R.; Zu, M.; Yang, S.; Zhang, S.; Zhou, W.; Mai, Z.; Ge, C.; Xu, Y.-H.; Fang, Y.; Zhang, S. Visible-light-driven photoelectrochemical determination of Cu2+ based on CdS sensitized hydrogenated TiO2 nanorod arrays. Sens. Actuators B Chem. 2018, 270, 270–276. [Google Scholar] [CrossRef]
- Si, H.; Pan, N.; Zhang, X.; Liao, J.; Rumyantseva, M.; Gaskov, A.; Lin, S. A real-time on-line photoelectrochemical sensor toward chemical oxygen demand determination based on field-effect transistor using an extended gate with 3D TiO2 nanotube arrays. Sens. Actuators B Chem. 2019, 289, 106–113. [Google Scholar] [CrossRef]
- Pang, Y.; Feng, Q.; Kou, Z.; Xu, G.; Gao, F.; Wang, B.; Pan, Z.; Lv, J.; Zhang, Y.; Wu, Y. A surface precleaning strategy intensifies the interface coupling of the Bi2O3/TiO2 heterostructure for enhanced photoelectrochemical detection properties. Mater. Chem. Front. 2020, 4, 638–644. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, T.; Zhu, Y.; Liang, B.; Jiang, W. KBiO3 as an Effective Visible-Light-Driven Photocatalyst: Degradation Mechanism for Different Organic Pollutants. Chem. Photo. Chem. 2018, 2, 442–449. [Google Scholar] [CrossRef]
- He, R.; Xu, D.; Cheng, B.; Yu, J.; Ho, W.-K. Review on nanoscale Bi-based photocatalysts. Nanoscale Horiz. 2018, 3, 464–504. [Google Scholar] [CrossRef]
- Dhandole, L.K.; Chung, H.-S.; Ryu, J.; Jang, J.S. Vertically Aligned Titanate Nanotubes Hydrothermally Synthesized from Anodized TiO2 Nanotube Arrays: An Efficient Adsorbent for the Repeatable Recovery of Sr Ions. ACS Sustain. Chem. Eng. 2018, 6, 16139–16150. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, T.; Zhang, M.; Li, Q.; Yang, J. Interfacial Construction of Zero-Dimensional/One-Dimensional g-C3N4 Nanoparticles/TiO2 Nanotube Arrays with Z-Scheme Heterostructure for Improved Photoelectrochemical Water Splitting. ACS Sustain. Chem. Eng. 2018, 7, 2483–2491. [Google Scholar] [CrossRef]
- Ran, R.; Meng, X.; Zhang, Z. Facile Preparation of Novel Graphene Oxide-Modified Ag2O/Ag3VO4/AgVO3 Composites with High Photocatalytic Activities under Visible Light Irradiation. Appl. Catal. B 2016, 196, 1–15. [Google Scholar] [CrossRef]
- Yue, X.; Miao, X.; Shen, X.; Ji, Z.; Zhou, H.; Sun, Y.; Xu, K.; Zhu, G.-X.; Kong, L.; Chen, Q.; et al. Flower-like silver bismuthate supported on nitrogen-doped carbon dots modified graphene oxide sheets with excellent degradation activity for organic pollutants. J. Colloid Interface Sci. 2019, 540, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Mahadevegowda, A.; Young, N.P.; Grant, P. Engineering the nanostructure of a polymer-nanocomposite film containing Ti-based core-shell particles to enhance dielectric response. Nanoscale 2015, 7, 15727–15733. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wang, P.; Sun, Y.; Zhu, B.; Pan, J. Preparation of Nano-Ag4Bi2O5/graphene oxide composite and study of its catalytic performance for oxygen reduction reaction. Int. J. Electrochem. Sci. 2017, 12, 1263–1271. [Google Scholar] [CrossRef]
- Gong, J.; Lee, C.-S.; Kim, E.-J.; Kim, J.-H.; Lee, W.; Chang, Y.-S. Self-Generation of Reactive Oxygen Species on Crystalline AgBiO3 for the Oxidative Remediation of Organic Pollutants. ACS Appl. Mater. Interfaces 2017, 9, 28426–28432. [Google Scholar] [CrossRef]
- Yu, H.; Liu, R.; Wang, X.; Wang, P.; Yu, J. Enhanced visible-light photocatalytic activity of Bi2WO6 nanoparticles by Ag2O cocatalyst. Appl. Catal. B Environ. 2012, 326–333. [Google Scholar] [CrossRef]
- Shayeh, J.S.; Salari, H.; Daliri, A.; Omidi, M. Decorative reduced graphene oxide/C3N4/Ag2O/conductive polymer as a high performance material for electrochemical capacitors. Appl. Surf. Sci. 2018, 447, 374–380. [Google Scholar] [CrossRef]
- Hu, X.; Zhu, Q.; Wang, X.; Kawazoe, N.; Yang, Y. Nonmetal-metal-semiconductor-promoted P/Ag/Ag2O/Ag3PO4/TiO2 photocatalyst with superior photocatalytic activity and stability. J. Mater. Chem. A 2015, 3, 17858–17865. [Google Scholar] [CrossRef]
- Zhu, Q.; Hu, X.; Stanislaus, M.S.; Zhang, N.; Xiao, R.; Liu, N.; Yang, Y. A novel P/Ag/Ag2O/Ag3PO4/TiO2 composite film for water purification and antibacterial application under solar light irradiation. Sci. Total Environ. 2017, 577, 236–244. [Google Scholar] [CrossRef]
- Wang, P.; Zhou, Q.; Xia, Y.; Zhan, S.; Li, Y. Understanding the charge separation and transfer in mesoporous carbonate-doped phase-junction TiO2 nanotubes for photocatalytic hydrogen production. Appl. Catal. B Environ. 2018, 225, 433–444. [Google Scholar] [CrossRef]
- Yang, G.; Ding, H.; Chen, D.; Feng, J.; Hao, Q.; Zhu, Y. Construction of urchin-like ZnIn2S4-Au-TiO2 heterostructure with enhanced activity for photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2018, 234, 260–267. [Google Scholar] [CrossRef]
- Hu, Y.; Qian, H.; Liu, Y.; Du, G.; Zhang, F.; Wang, L.; Hu, X. A microwave-assisted rapid route to synthesize ZnO/ZnS core–shell nanostructures via controllable surface sulfidation of ZnO nanorods. CrystEngComm 2011, 13, 3438–3443. [Google Scholar] [CrossRef]
- Liu, W.; Cao, L.; Cheng, W.; Cao, Y.; Liu, X.; Zhang, W.; Mou, X.; Jin, L.; Zheng, X.; Che, W.; et al. Single-Site Active Cobalt-Based Photocatalyst with a Long Carrier Lifetime for Spontaneous Overall Water Splitting. Angew. Chem. 2017, 56, 9312–9317. [Google Scholar] [CrossRef]
- Hu, R.; Xiao, X.; Tu, S.; Zuo, X.; Nan, J. Synthesis of flower-like heterostructured β-Bi2O3/Bi2O2CO3 microspheres using Bi2O2CO3 self-sacrifice precursor and its visible-light-induced photocatalytic degradation of o-phenylphenol. Appl. Catal. B 2015, 163, 510–519. [Google Scholar] [CrossRef]
- Yang, L.; Liu, H.; Wang, J.; Liu, D.; Du, G.; Cui, J. Ag2O/TiO2 Nanobelts Heterostructure with Enhanced Ultraviolet and Visible Photocatalytic Activity. ACS Appl. Mater. Interfaces 2010, 2, 2385–2392. [Google Scholar] [CrossRef]
- Behara, D.K.; Ummireddi, A.K.; Aragonda, V.; Gupta, P.K.; Pala, R.G.S.; Sivakumar, S. Coupled optical absorption, charge carrier separation, and surface electrochemistry in surface disordered/hydrogenated TiO2 for enhanced PEC water splitting reaction. Phys. Chem. Chem. Phys. 2016, 18, 8364–8377. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Chen, X.; Ong, W.-J.; Zhao, X.; Li, N. Surface and Heterointerface Engineering of 2D MXenes and Their Nanocomposites: Insights into Electro- and Photocatalysis. Chem 2019, 5, 18–50. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Guo, P.; Dou, M.; Wang, J.; Cheng, Y.; Jönssona, P.G.; Zhao, Z. Visible light-driven g-C3N4/m-Ag2Mo2O7 composite photocatalysts: Synthesis, enhanced activity and photocatalytic mechanism. Rsc Adv. 2014, 4, 51008–51015. [Google Scholar] [CrossRef]
- Aškrabić, S.; Araújo, V.D.; Passacantando, M.; Bernardi, M.I.B.; Tomić, N.; Dojčinović, B.; Manojlović, D.; Čalija, B.; Miletić, M.; Dohčević-Mitrović, Z.D. Nitrate-assisted photocatalytic efficiency of defective Eu-doped Pr(OH)3 nanostructures. Phys. Chem. Chem. Phys. 2017, 19, 31756–31765. [Google Scholar] [CrossRef] [Green Version]
- Pang, Y.; Xu, G.; Feng, Q.; Liu, J.; Lv, J.; Zhang, Y.; Wu, Y. Synthesis of α-Bi2Mo3O12/TiO2 Nanotube Arrays for Photoelectrochemical COD Detection Application. Langmuir 2017, 33, 8933–8942. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, Y.; Chen, H.; Yang, J.; Wang, B.; Yang, Z.; Lv, J.; Pan, Z.; Xu, G.; Shen, Z.; Wu, Y. Rational Regulation of Surface Free Radicals on TiO2 Nanotube Arrays via Ag2O–AgBiO3 towards Enhanced Selective Photoelectrochemical Detection. Nanomaterials 2020, 10, 2002. https://doi.org/10.3390/nano10102002
Pang Y, Chen H, Yang J, Wang B, Yang Z, Lv J, Pan Z, Xu G, Shen Z, Wu Y. Rational Regulation of Surface Free Radicals on TiO2 Nanotube Arrays via Ag2O–AgBiO3 towards Enhanced Selective Photoelectrochemical Detection. Nanomaterials. 2020; 10(10):2002. https://doi.org/10.3390/nano10102002
Chicago/Turabian StylePang, Yajun, Hao Chen, Jin Yang, Bo Wang, Zhenyu Yang, Jun Lv, Zhenghui Pan, Guangqing Xu, Zhehong Shen, and Yucheng Wu. 2020. "Rational Regulation of Surface Free Radicals on TiO2 Nanotube Arrays via Ag2O–AgBiO3 towards Enhanced Selective Photoelectrochemical Detection" Nanomaterials 10, no. 10: 2002. https://doi.org/10.3390/nano10102002




