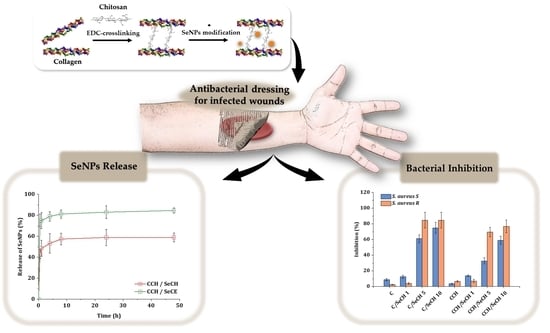
Synergistic Effect of Chitosan and Selenium Nanoparticles on Biodegradation and Antibacterial Properties of Collagenous Scaffolds Designed for Infected Burn Wounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Both Collagen and Collagen–Chitosan Scaffolds
2.2.2. Preparation of Collagen and Collagen–Chitosan Scaffolds with SeNPs
2.2.3. Microstructure Imaging and Evaluation
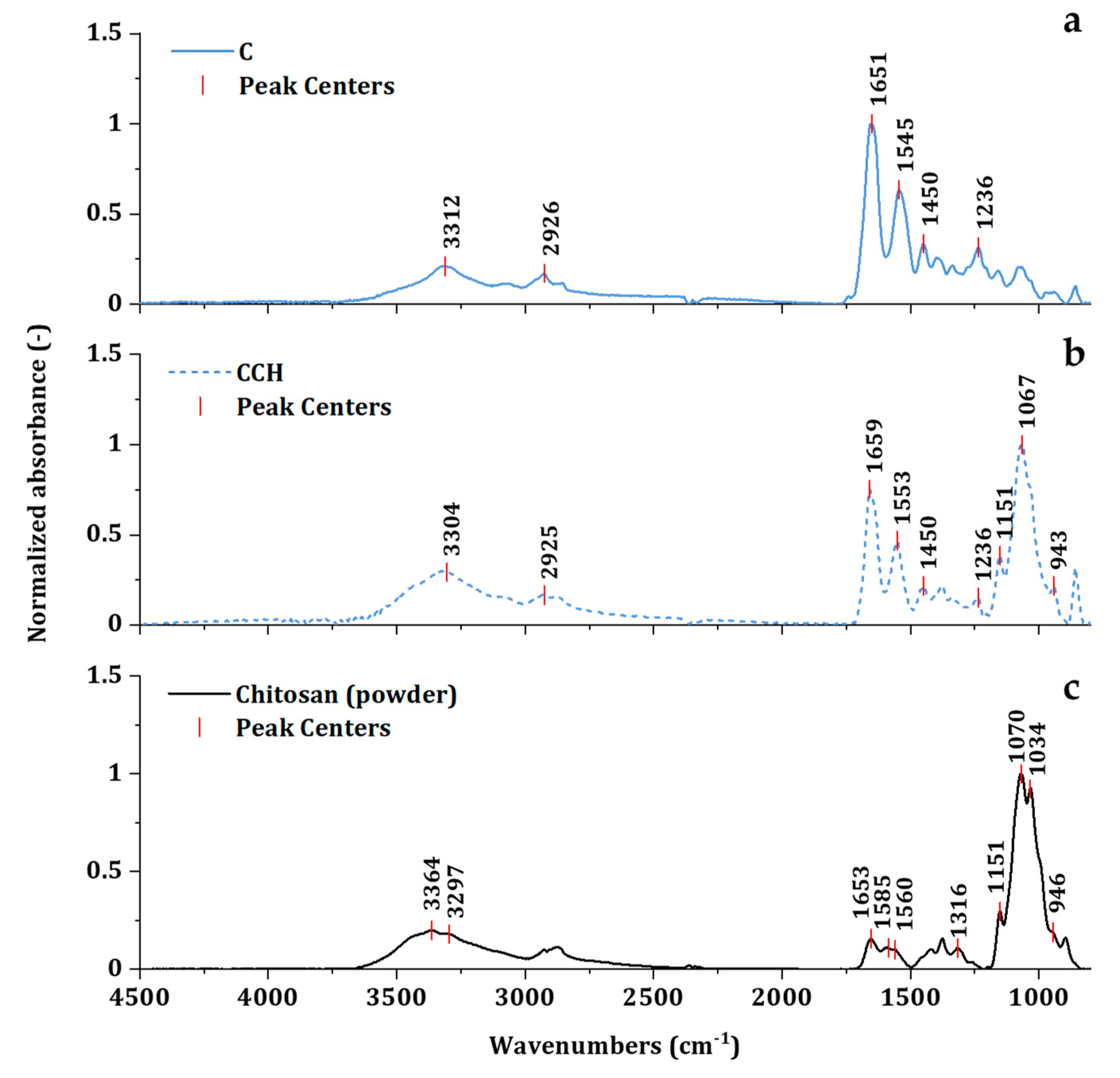
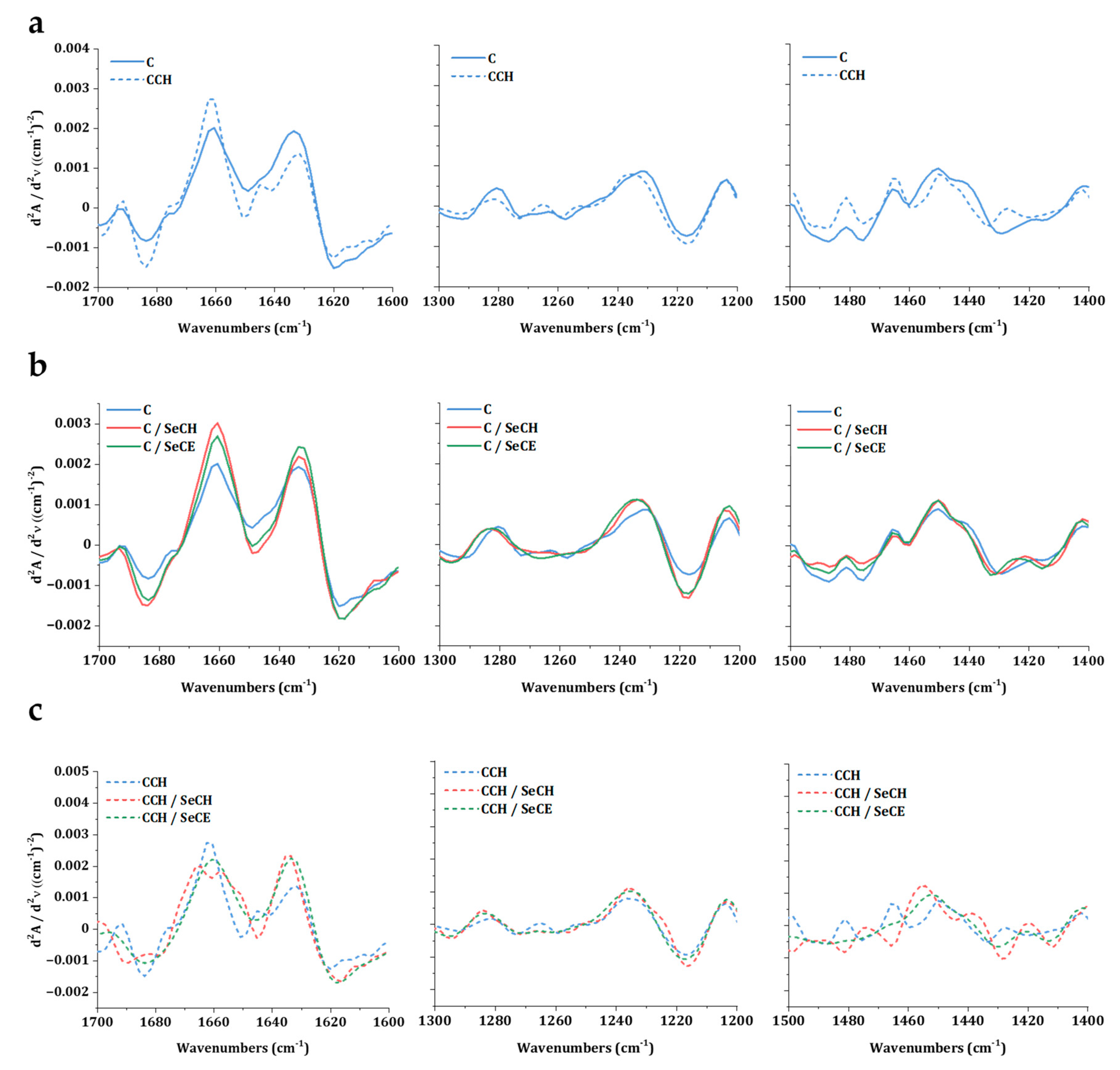
2.2.4. Investigation of Collagen Secondary Structure
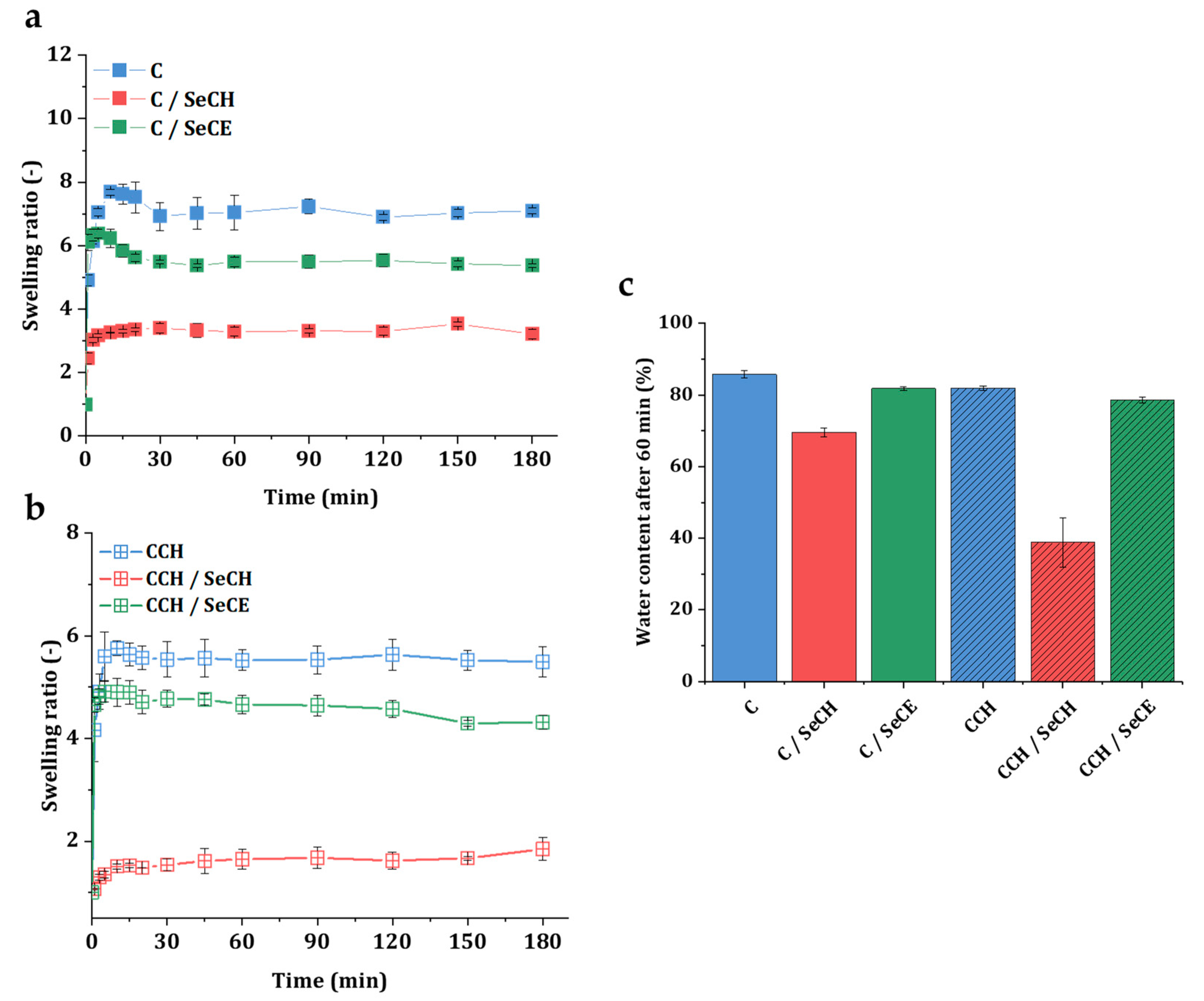
2.2.5. Swelling and Water Absorption Studies
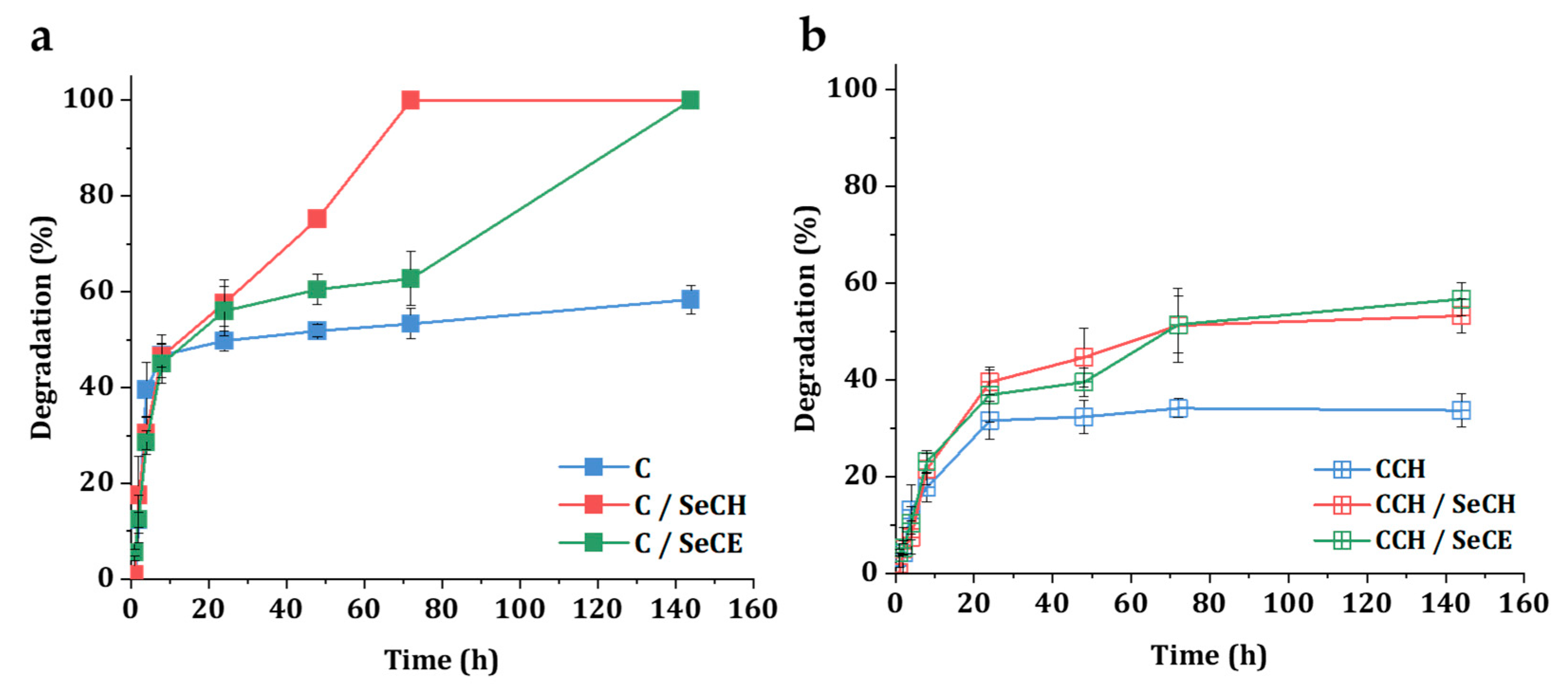
2.2.6. Degradation in a Simulated Physiological Environment
2.2.7. Release Kinetics of SeNPs
2.2.8. Antibacterial Activity Evaluation
3. Results and Discussion
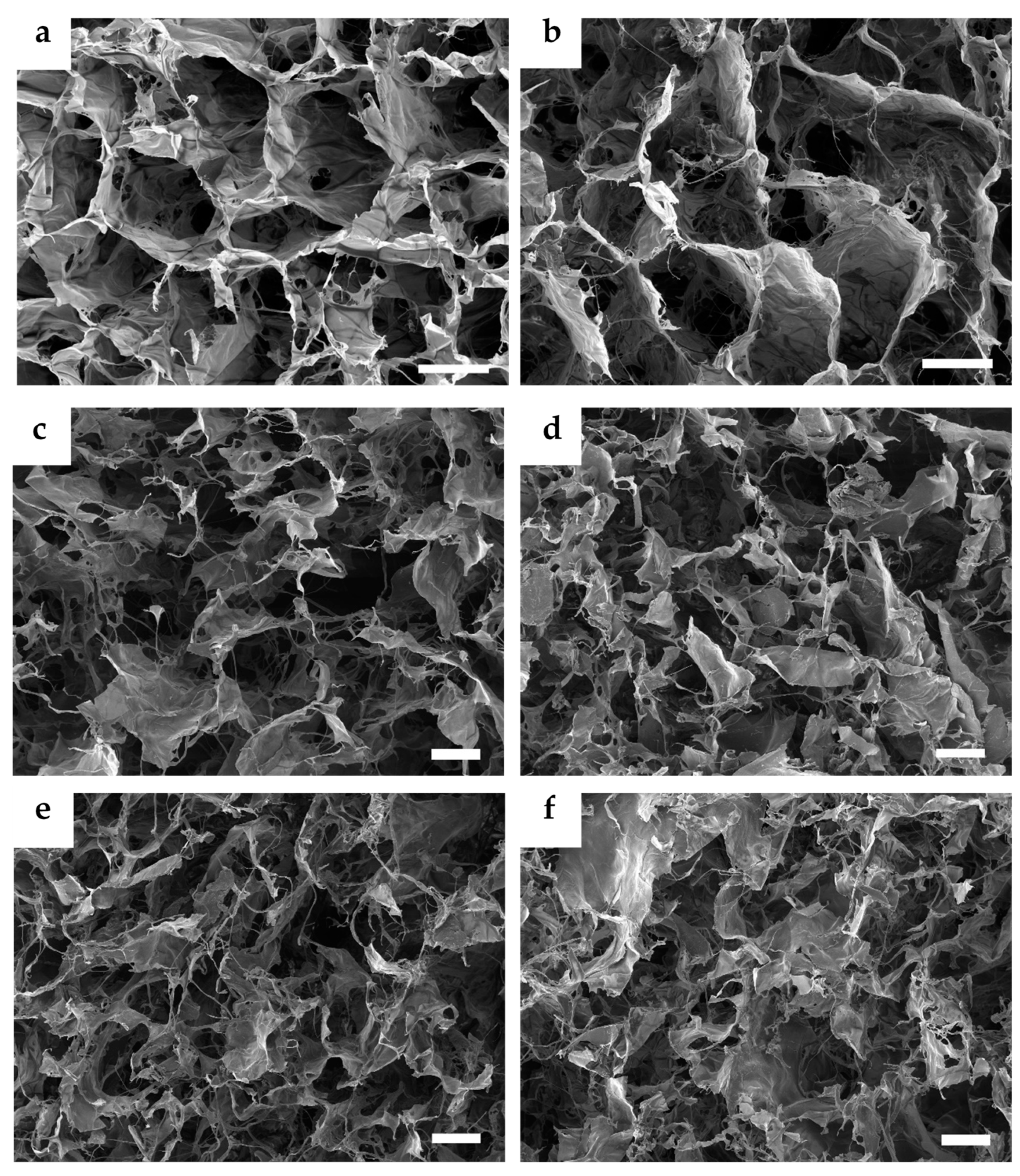
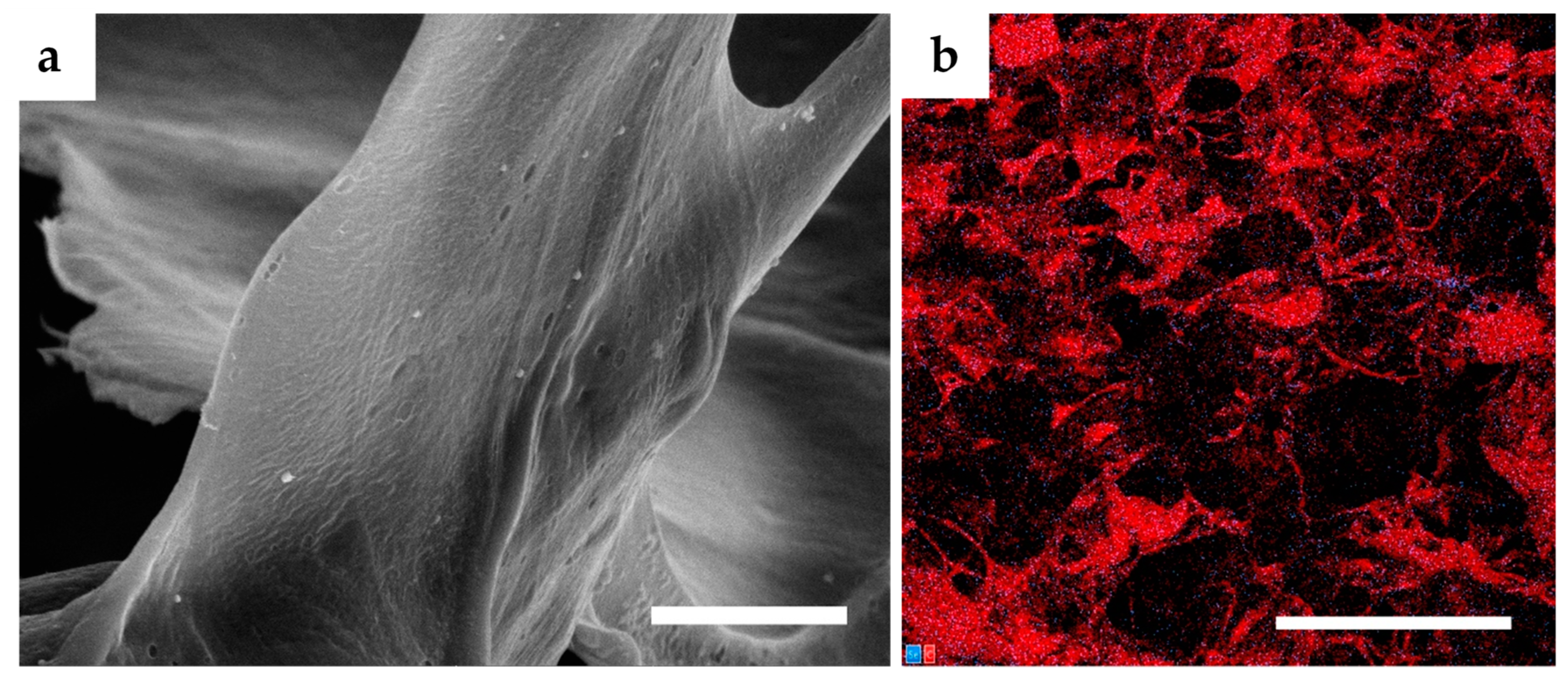
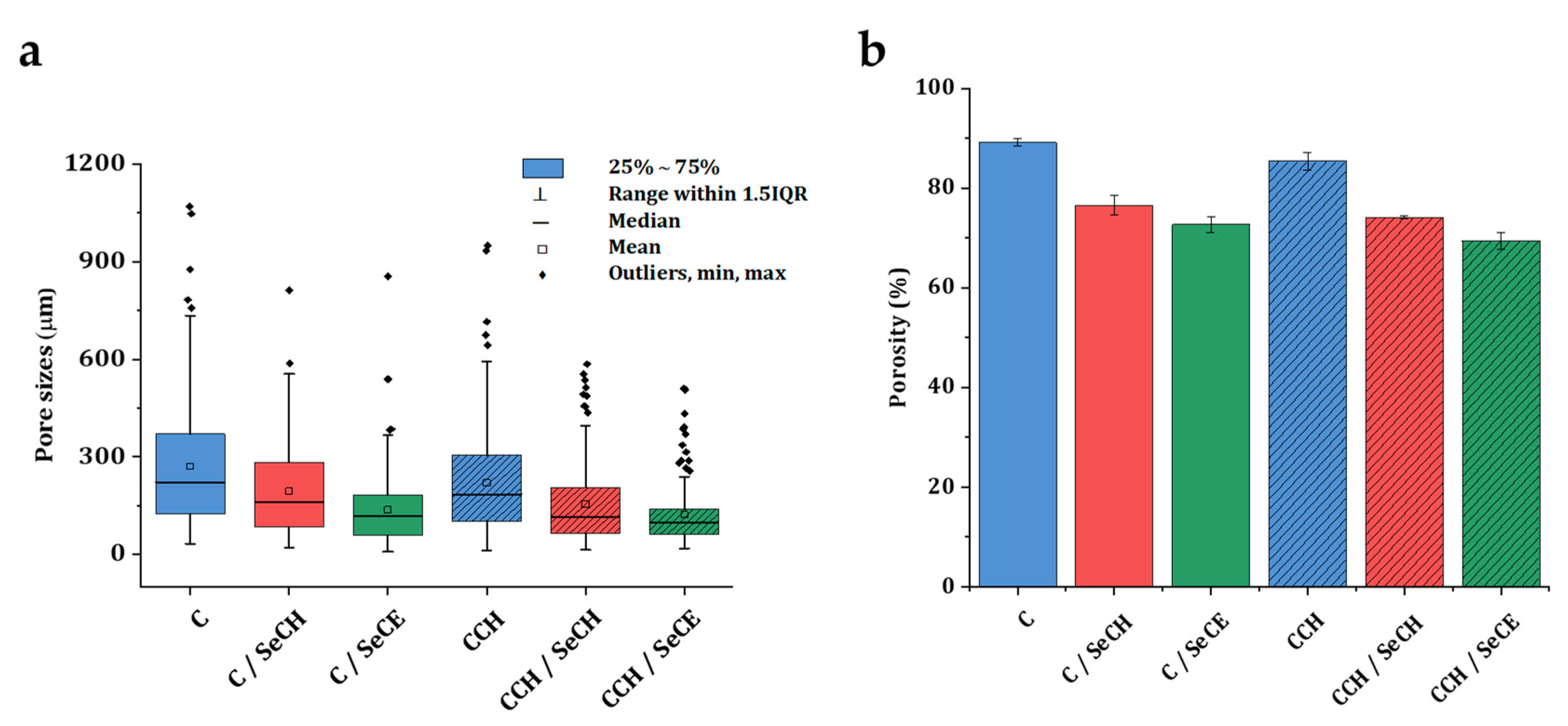
3.1. Microstructure Imaging and Evaluation
3.2. Investigation of Intact Collagen Secondary Structure
3.3. Swelling Kinetics
3.4. Degradation in a Simulated Physiological Environment
3.5. Release Kinetics of SeNPs
3.6. Antibacterial Testing
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mayet, N.; Choonara, Y.E.; Chellappan, D.K.; Tomar, L.K.; Tyagi, C.; Du Toit, L.C.; Pillay, V. A Comprehensive Review of Advanced Biopolymeric Wound Healing Systems. J. Pharm. Sci. 2014, 103, 2211–2230. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.C.D.O.; Freire, T.F.C.; Andrade, Z.D.A.; Medrado, A.P. Wound healing—A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malone, C.H.; McLaughlin, J.M.; Ross, L.S.; Phillips, L.G.; Wagner, J.R.F. Progressive Tightening of Pulley Sutures for Primary Repair of Large Scalp Wounds. Plast. Reconstr. Surg. Glob. Open 2017, 5, e1592. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.D.; Choi, D.S.; Cho, Y.K.; Kim, T.K.; Lee, J.W.; Choi, K.Y.; Chung, H.Y.; Cho, B.C.; Byun, J.S. Effect of Amniotic Fluid Stem Cells and Amniotic Fluid Cells on the Wound Healing Process in a White Rat Model. Arch. Plast. Surg. 2013, 40, 496–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, P.; Guo, B.; Hui, Q.; Liu, X.; Tao, K. A bioartificial dermal regeneration template promotes skin cell proliferation in vitro and enhances large skin wound healing in vivo. Oncotarget 2017, 8, 25226–25241. [Google Scholar] [CrossRef] [Green Version]
- Bessa, L.J.; Fazii, P.; Di Giulio, M.; Cellini, L. Bacterial isolates from infected wounds and their antibiotic susceptibility pattern: Some remarks about wound infection. Int. Wound J. 2013, 12, 47–52. [Google Scholar] [CrossRef]
- Méric, G.; Mageiros, L.; Pensar, J.; Laabei, M.; Yahara, K.; Pascoe, B.; Kittiwan, N.; Tadee, P.; Post, V.; Lamble, S.; et al. Disease-associated genotypes of the commensal skin bacterium Staphylococcus epidermidis. Nat. Commun. 2018, 9, 5034. [Google Scholar] [CrossRef] [Green Version]
- Tsige, Y.; Tadesse, S.; G/Eyesus, T.; Tefera, M.M.; Amsalu, A.; Menberu, M.A.; Gelaw, B. Prevalence of Methicillin-Resistant Staphylococcus aureus and Associated Risk Factors among Patients with Wound Infection at Referral Hospital, Northeast Ethiopia. J. Pathog. 2020, 2020, 3168325. [Google Scholar] [CrossRef]
- Murray, R.Z.; West, Z.; Cowin, A.J.; Farrugia, B.L. Development and use of biomaterials as wound healing therapies. Burn. Trauma 2019, 7, 2. [Google Scholar] [CrossRef] [Green Version]
- Park, S.-B.; Lih, E.; Park, K.-S.; Joung, Y.K.; Han, D.K. Biopolymer-based functional composites for medical applications. Prog. Polym. Sci. 2017, 68, 77–105. [Google Scholar] [CrossRef]
- Rajabi, M.; Ali, A.; McConnell, M.; Cabral, J. Keratinous materials: Structures and functions in biomedical applications. Mater. Sci. Eng. C 2020, 110, 110612. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, D.; Mandal, B.B. Silk biomaterials in wound healing and skin regeneration therapeutics: From bench to bedside. Acta Biomater. 2020, 103, 24–51. [Google Scholar] [CrossRef] [PubMed]
- Goh, K.L.; Holmes, D.F. Collagenous Extracellular Matrix Biomaterials for Tissue Engineering: Lessons from the Common Sea Urchin Tissue. Int. J. Mol. Sci. 2017, 18, 901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussey, G.S.; Dziki, J.L.; Badylak, S.F. Extracellular matrix-based materials for regenerative medicine. Nat. Rev. Mater. 2018, 3, 159–173. [Google Scholar] [CrossRef]
- Carson, A.E.; Barker, T.H. Emerging concepts in engineering extracellular matrix variants for directing cell phenotype. Regen. Med. 2009, 4, 593–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogel, S.; Ullm, F.; Müller, C.D.; Pompe, T.; Hempel, U. Remodeling of 3D collagen I matrices by human bone marrow stromal cells during osteogenic differentiation in vitro. ACS Appl. Bio Mater. 2020. [Google Scholar] [CrossRef]
- Nečas, A.; Plánka, L.; Srnec, R.; Crha, M.; Hlučilová, J.; Klíma, J.; Starý, D.; Křen, L.; Amler, E.; Vojtová, L.; et al. Quality of newly formed cartilaginous tissue in defects of articular surface after transplantation of mesenchymal stem cells in a composite scaffold based on collagen I with chitosan micro- and nanofibres. Physiol. Res. 2009, 59, 605–614. [Google Scholar]
- Jančář, J.; Vojtová, L.; Nečas, A.; Srnec, R.; Urbanová, L.; Crha, M. Stability of Collagen Scaffold Implants for Animals with Iatrogenic Articular Cartilage Defects. Acta Veter-Brno 2009, 78, 643–648. [Google Scholar] [CrossRef]
- Prosecká, E.; Rampichová, M.; Litvinec, A.; Tonar, Z.; Králíčková, M.; Vojtová, L.; Kochová, P.; Plencner, M.; Buzgo, M.; Míčková, A.; et al. Collagen/hydroxyapatite scaffold enriched with polycaprolactone nanofibers, thrombocyte-rich solution and mesenchymal stem cells promotes regeneration in large bone defect in vivo. J. Biomed. Mater. Res. Part. A 2014, 103, 671–682. [Google Scholar] [CrossRef]
- Prosecká, E.; Rampichová, M.; Vojtová, L.; Tvrdík, D.; Melčáková, Š.; Juhasová, J.; Plencner, M.; Jakubová, R.; Jančář, J.; Nečas, A.; et al. Optimized conditions for mesenchymal stem cells to differentiate into osteoblasts on a collagen/hydroxyapatite matrix. J. Biomed. Mater. Res. Part. A 2011, 99, 307–315. [Google Scholar] [CrossRef]
- Selvakumar, G.; Suguna, L. Fabrication and characterization of collagen-oxidized pullulan scaffold for biomedical applications. Int. J. Biol. Macromol. 2020, 164, 1592–1599. [Google Scholar] [CrossRef]
- El-Fiqi, A.; Kim, J.-H.; Kim, H.-W. Novel bone-mimetic nanohydroxyapatite/collagen porous scaffolds biomimetically mineralized from surface silanized mesoporous nanobioglass/collagen hybrid scaffold: Physicochemical, mechanical and in vivo evaluations. Mater. Sci. Eng. C 2020, 110, 110660. [Google Scholar] [CrossRef]
- Si, J.; Yang, Y.; Xing, X.; Yang, F.; Shan, P. Controlled degradable chitosan/collagen composite scaffolds for application in nerve tissue regeneration. Polym. Degrad. Stab. 2019, 166, 73–85. [Google Scholar] [CrossRef]
- Berdichevski, A.; Birch, M.; Markaki, A.E. Collagen scaffolds with tailored pore geometry for building three-dimensional vascular networks. Mater. Lett. 2019, 248, 93–96. [Google Scholar] [CrossRef]
- Ge, L.; Xu, Y.; Li, X.; Yuan, L.; Tan, H.; Li, D.; Mu, C. Fabrication of Antibacterial Collagen-Based Composite Wound Dressing. ACS Sustain. Chem. Eng. 2018, 6, 9153–9166. [Google Scholar] [CrossRef]
- Oryan, A.; Sahvieh, S. Effectiveness of chitosan scaffold in skin, bone and cartilage healing. Int. J. Biol. Macromol. 2017, 104, 1003–1011. [Google Scholar] [CrossRef]
- Freier, T.; Koh, H.S.; Kazazian, K.; Shoichet, M.S. Controlling cell adhesion and degradation of chitosan films by N-acetylation. Biomaterials 2005, 26, 5872–5878. [Google Scholar] [CrossRef]
- Chatelet, C. Influence of the degree of acetylation on some biological properties of chitosan films. Biomaterials 2001, 22, 261–268. [Google Scholar] [CrossRef]
- Li, J.; Wu, Y.; Zhao, L. Antibacterial activity and mechanism of chitosan with ultra high molecular weight. Carbohydr. Polym. 2016, 148, 200–205. [Google Scholar] [CrossRef]
- Li, B.; Wang, J.; Gui, Q.; Yang, H. Continuous production of uniform chitosan beads as hemostatic dressings by a facile flow injection method. J. Mater. Chem. B 2020, 8, 7941–7946. [Google Scholar] [CrossRef]
- Li, B.; Wang, J.; Gui, Q.; Yang, H. Drug-loaded chitosan film prepared via facile solution casting and air-drying of plain water-based chitosan solution for ocular drug delivery. Bioact. Mater. 2020, 5, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Fairweather-Tait, S.; Bao, Y.; Broadley, M.R.; Collings, R.; Ford, D.; Hesketh, J.E.; Hurst, R. Selenium in Human Health and Disease. Antioxidants Redox Signal. 2011, 14, 1337–1383. [Google Scholar] [CrossRef] [PubMed]
- Wadhwani, S.A.; Shedbalkar, U.U.; Singh, R.; Chopade, B.A. Biogenic selenium nanoparticles: Current status and future prospects. Appl. Microbiol. Biotechnol. 2016, 100, 2555–2566. [Google Scholar] [CrossRef] [PubMed]
- Chudobova, D.; Cihalova, K.; Dostalova, S.; Ruttkay-Nedecky, B.; Rodrigo, M.A.M.; Tmejova, K.; Kopel, P.; Nejdl, L.; Kudr, J.; Gumulec, J.; et al. Comparison of the effects of silver phosphate and selenium nanoparticles onStaphylococcus aureusgrowth reveals potential for selenium particles to prevent infection. FEMS Microbiol. Lett. 2013, 351, 195–201. [Google Scholar] [CrossRef] [Green Version]
- Mulla, N.A.; Otari, S.V.; Bohara, R.A.; Yadav, H.M.; Pawar, S. Rapid and size-controlled biosynthesis of cytocompatible selenium nanoparticles by Azadirachta indica leaves extract for antibacterial activity. Mater. Lett. 2020, 264, 127353. [Google Scholar] [CrossRef]
- Shakibaie, M.; Forootanfar, H.; Golkari, Y.; Mohammadi-Khorsand, T.; Shakibaie, M.R. Anti-biofilm activity of biogenic selenium nanoparticles and selenium dioxide against clinical isolates of Staphylococcus aureus, Pseudomonas aeruginosa, and Proteus mirabilis. J. Trace Elements Med. Biol. 2015, 29, 235–241. [Google Scholar] [CrossRef]
- Huang, X.; Chen, X.; Chen, Q.; Yu, Q.; Sun, N.; Liu, J. Investigation of functional selenium nanoparticles as potent antimicrobial agents against superbugs. Acta Biomater. 2016, 30, 397–407. [Google Scholar] [CrossRef]
- Guisbiers, G.; Lara, H.H.; Mendoza-Cruz, R.; Naranjo, G.; Vincent, B.A.; Peralta, X.G.; Nash, K.L. Inhibition of Candida albicans biofilm by pure selenium nanoparticles synthesized by pulsed laser ablation in liquids. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1095–1103. [Google Scholar] [CrossRef] [Green Version]
- Yip, J.; Liu, L.; Wong, K.-H.; Leung, P.H.M.; Yuen, C.-W.M.; Cheung, M.-C. Investigation of antifungal and antibacterial effects of fabric padded with highly stable selenium nanoparticles. J. Appl. Polym. Sci. 2014, 131, 131. [Google Scholar] [CrossRef]
- Sloviková, A.; Vojtova, L.; Jančař, J. Preparation and modification of collagen-based porous scaffold for tissue engineering. Chem. Pap. 2008, 62, 417–422. [Google Scholar] [CrossRef]
- Cihalova, K.; Chudobova, D.; Michálek, P.; Moulick, A.; Guran, R.; Kopel, P.; Adam, V.; Kizek, R. Staphylococcus aureus and MRSA Growth and Biofilm Formation after Treatment with Antibiotics and SeNPs. Int. J. Mol. Sci. 2015, 16, 24656–24672. [Google Scholar] [CrossRef] [PubMed]
- Heger, Z.; Vesely, R.; Cihalova, K.; Kopel, P.; Milosavljevic, V.; Heger, Z.; Hynek, D.; Guran, R.; Vaculovicova, M.; Sedlacek, P.; et al. Antimicrobial Agent Based on Selenium Nanoparticles and Carboxymethyl Cellulose for the Treatment of Bacterial Infections. J. Biomed. Nanotechnol. 2017, 13, 767–777. [Google Scholar] [CrossRef]
- Sendrea, C.; Carsote, C.; Badea, E.; Buda, A.A.; Niculescu, M.; Iovu, H. Non-Invasive Characterisation of Collagen-Based Materials by NMR-Mouse and ATR-FTIR. Sci. Bull. B Chem. Mater. Sci. UPB 2016, 78, 27–38. [Google Scholar]
- Bauer, E.A.; Cooper, T.W.; Huang, J.S.; Altman, J.; Deuel, T.F. Stimulation of in vitro human skin collagenase expression by platelet-derived growth factor. Proc. Natl. Acad. Sci. USA 1985, 82, 4132–4136. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Xu, S.; Shen, L.; Li, G. Factors affecting thermal stability of collagen from the aspects of extraction, processing and modification. J. Leather Sci. Eng. 2020, 2, 1–29. [Google Scholar] [CrossRef]
- Davidenko, N.; Schuster, C.; Bax, D.; Raynal, N.; Farndale, R.; Best, S.; Cameron, R.E. Control of cross-linking for tailoring collagen-based scaffolds stability and mechanics. Acta Biomater. 2015, 25, 131–142. [Google Scholar] [CrossRef] [Green Version]
- Grabarek, Z.; Gergely, J. Zero-length cross-linking procedure with the use of active esters. Anal. Biochem. 1990, 185, 131–135. [Google Scholar] [CrossRef]
- Yang, C. Enhanced physicochemical properties of collagen by using EDC/NHS-cross-linking. Bull. Mater. Sci. 2012, 35, 913–918. [Google Scholar] [CrossRef]
- Babrnáková, J.; Pavliňáková, V.; Brtníková, J.; Sedlacek, P.; Prosecká, E.; Rampichová, M.; Filová, E.; Hearnden, V.; Vojtová, L. Synergistic effect of bovine platelet lysate and various polysaccharides on the biological properties of collagen-based scaffolds for tissue engineering: Scaffold preparation, chemo-physical characterization, in vitro and ex ovo evaluation. Mater. Sci. Eng. C 2019, 100, 236–246. [Google Scholar] [CrossRef]
- McHale, M.K.; Bergmann, N.M.; West, J.L. Histogenesis in Three-Dimensional Scaffolds. In Handbook of Stem Cells; Elsevier BV: London, UK, 2013; pp. 951–963. [Google Scholar]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen–glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-Dimensional Scaffolds for Tissue Engineering Applications: Role of Porosity and Pore Size. Tissue Eng. Part. B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [Green Version]
- George, J.; Onodera, J.; Miyata, T. Biodegradable honeycomb collagen scaffold for dermal tissue engineering. J. Biomed. Mater. Res. Part A 2008, 87, 1103–1111. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen Structure and Stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [Green Version]
- Nagai, T.; Suzuki, N.; Tanoue, Y.; Kai, N.; Takeshi, N.; Nobutaka, S.; Yasuhiro, T.; Norihisa, K. Collagen from Tendon of Yezo Sika Deer (Cervus nippon yesoensis) as By-Product. Food Nutr. Sci. 2012, 3, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Stani, C.; Vaccari, L.; Mitri, E.; Giovanni, B. FTIR investigation of the secondary structure of type I collagen: New insight into the amide III band. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 229, 118006. [Google Scholar] [CrossRef]
- Nikonenko, N.A.; Buslov, D.K.; Sushko, N.I.; Zhbankov, R.G. Investigation of stretching vibrations of glycosidic linkages in disaccharides and polysaccarides with use of IR spectra deconvolution. Biopolymers 2000, 57, 257–262. [Google Scholar] [CrossRef]
- Queiroz, M.F.; Melo, K.R.T.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O. Does the Use of Chitosan Contribute to Oxalate Kidney Stone Formation? Mar. Drugs 2014, 13, 141–158. [Google Scholar] [CrossRef]
- Soubhagya, A.; Moorthi, A.; Prabaharan, M. Preparation and characterization of chitosan/pectin/ZnO porous films for wound healing. Int. J. Biol. Macromol. 2020, 157, 135–145. [Google Scholar] [CrossRef]
- Muta, H.; Miwa, M.; Satoh, M. Ion-specific swelling of hydrophilic polymer gels. Polymer 2001, 42, 6313–6316. [Google Scholar] [CrossRef]
- Egawa, M.; Arimoto, H.; Hirao, T.; Takahashi, M.; Ozaki, Y. Regional Difference of Water Content in Human Skin Studied by Diffuse-Reflectance Near-Infrared Spectroscopy: Consideration of Measurement Depth. Appl. Spectrosc. 2006, 60, 24–28. [Google Scholar] [CrossRef]
- Postlethwaite, A.E.; Seyer, J.M.; Kang, A.H. Chemotactic attraction of human fibroblasts to type I, II, and III collagens and collagen-derived peptides. Proc. Natl. Acad. Sci. USA 1978, 75, 871–875. [Google Scholar] [CrossRef] [Green Version]
- Lauer-Fields, J.L.; Juska, D.; Fields, G.B. Matrix metalloproteinases and collagen catabolism. Biopolymers 2002, 66, 19–32. [Google Scholar] [CrossRef]
- Karakiulakis, G.; Papadimitriu, E.; Missirlis, E.; Maragoudakis, M.E. Effect of divalent metal ions on collagenase from Clostridium histolyticum. Biochem. Int. 1991, 24, 397–404. [Google Scholar]
- Gimeno, M.; Pinczowski, P.; Pérez, M.; Giorello, A.; Martínez, M.Á.; Santamaria, J.; Arruebo, M.; Luján, L. A controlled antibiotic release system to prevent orthopedic-implant associated infections: An in vitro study. Eur. J. Pharm. Biopharm. 2015, 96, 264–271. [Google Scholar] [CrossRef] [Green Version]
- Kourmouli, A.; Valenti, M.; Van Rijn, E.; Beaumont, H.J.E.; Kalantzi, O.-I.; Schmidt-Ott, A.; Biskos, G. Can disc diffusion susceptibility tests assess the antimicrobial activity of engineered nanoparticles? J. Nanoparticle Res. 2018, 20, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Stocks, S.M. Mechanism and use of the commercially available viability stain, BacLight. Cytom. Part A 2003, 61, 189–195. [Google Scholar] [CrossRef]
- Zgurskaya, H.I.; Lopez, C.A.; Gnanakaran, S. Permeability Barrier of Gram-Negative Cell Envelopes and Approaches To Bypass It. ACS Infect. Dis. 2015, 1, 512–522. [Google Scholar] [CrossRef] [Green Version]
- Geoffrion, L.D.; Hesabizadeh, T.; Medina-Cruz, D.; Kusper, M.; Taylor, P.; Vernet-Crua, A.; Chen, J.; Ajo, A.; Webster, T.J.; Guisbiers, G. Naked Selenium Nanoparticles for Antibacterial and Anticancer Treatments. ACS Omega 2020, 5, 2660–2669. [Google Scholar] [CrossRef]
- Huang, T.; Holden, J.A.; Heath, D.E.; O’Brien-Simpson, N.M.; O’Connor, A.J. Engineering highly effective antimicrobial selenium nanoparticles through control of particle size. Nanoscale 2019, 11, 14937–14951. [Google Scholar] [CrossRef]
- Giacometti, A.; Cirioni, O.; Schimizzi, A.M.; Del Prete, M.S.; Barchiesi, F.; D’Errico, M.M.; Petrelli, E.; Scalise, G. Epidemiology and Microbiology of Surgical Wound Infections. J. Clin. Microbiol. 2000, 38, 918–922. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.E.; Fischbach, M.A.; Belkaid, Y. Skin microbiota–host interactions. Nat. Cell Biol. 2018, 553, 427–436. [Google Scholar] [CrossRef] [PubMed]
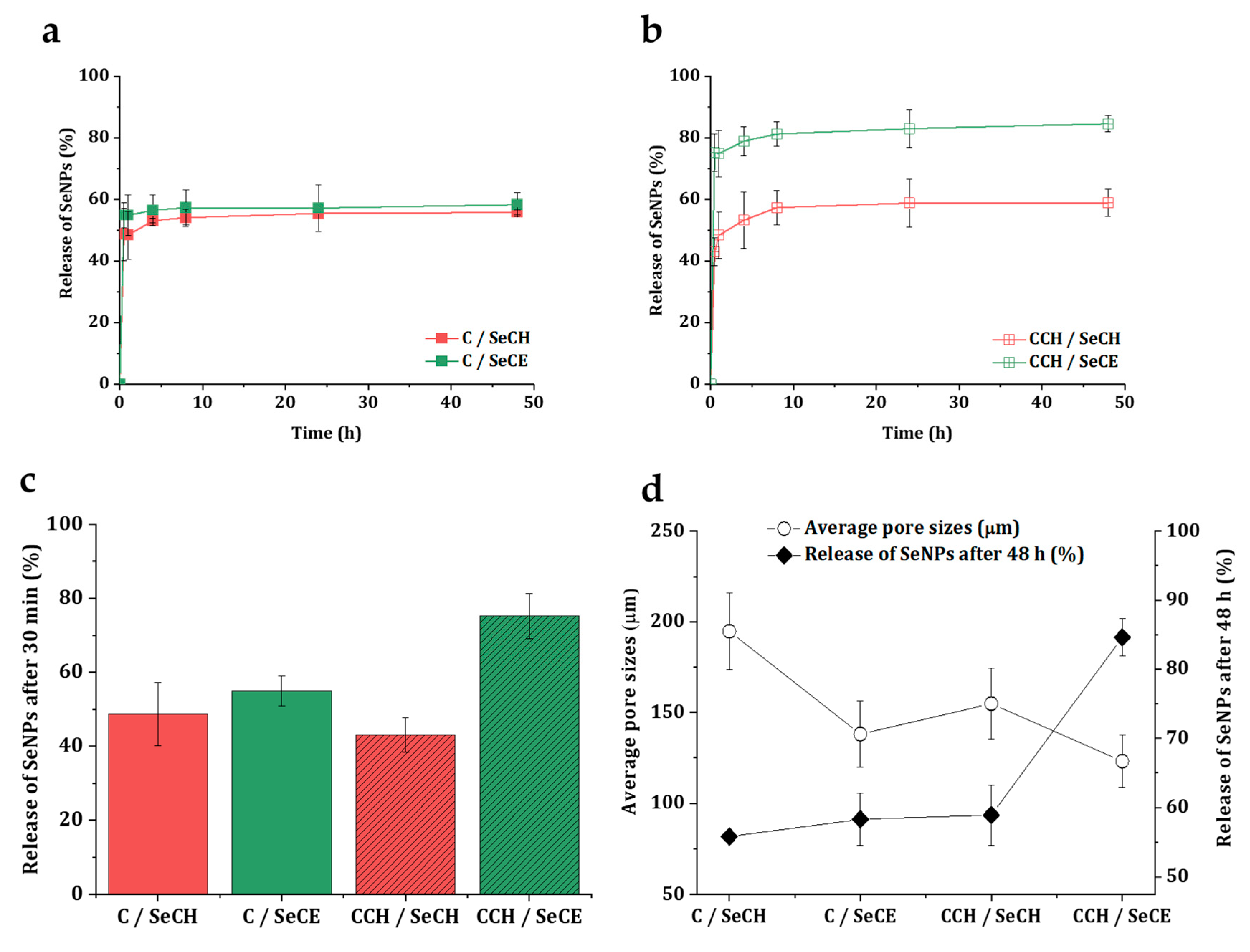
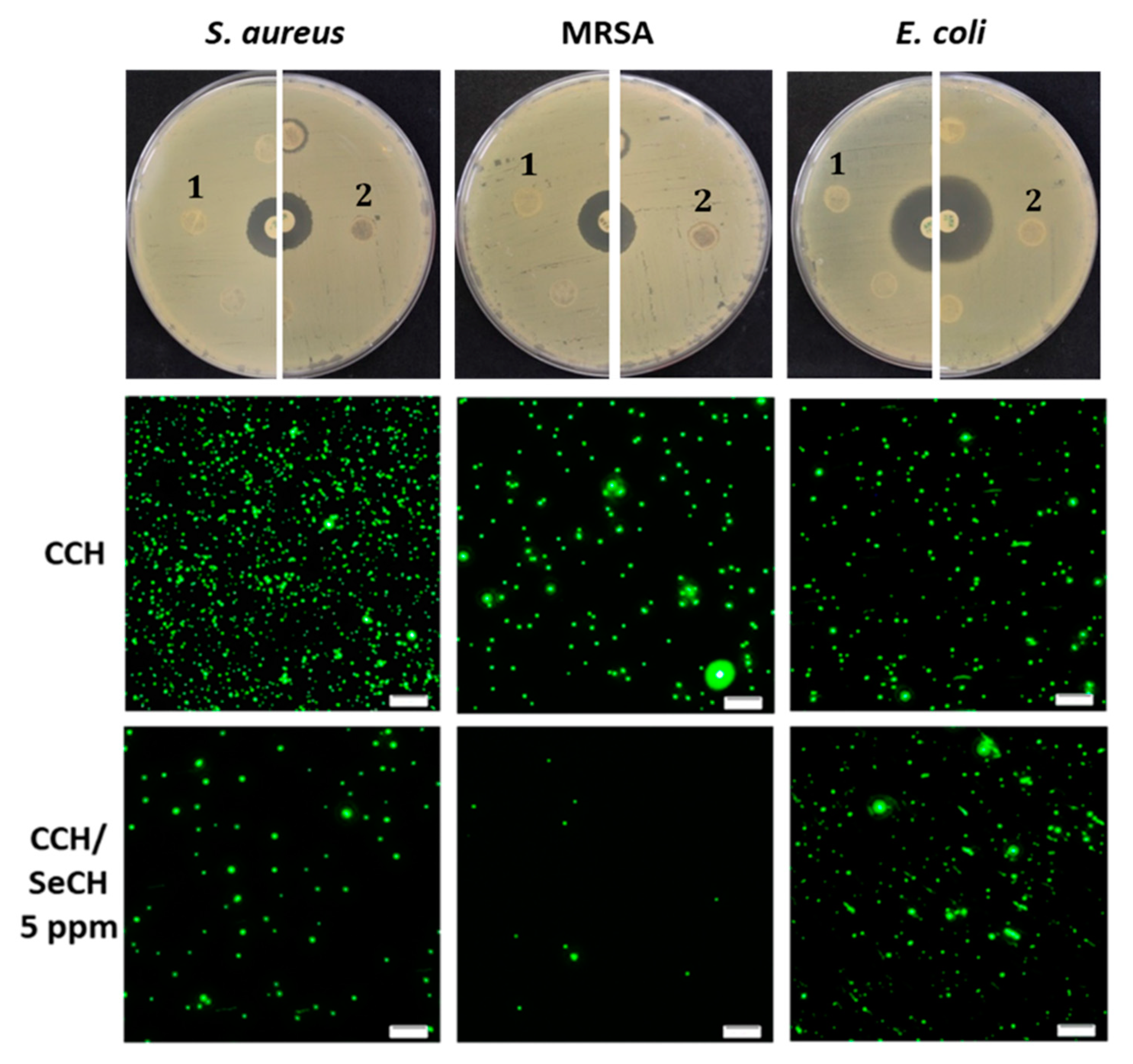

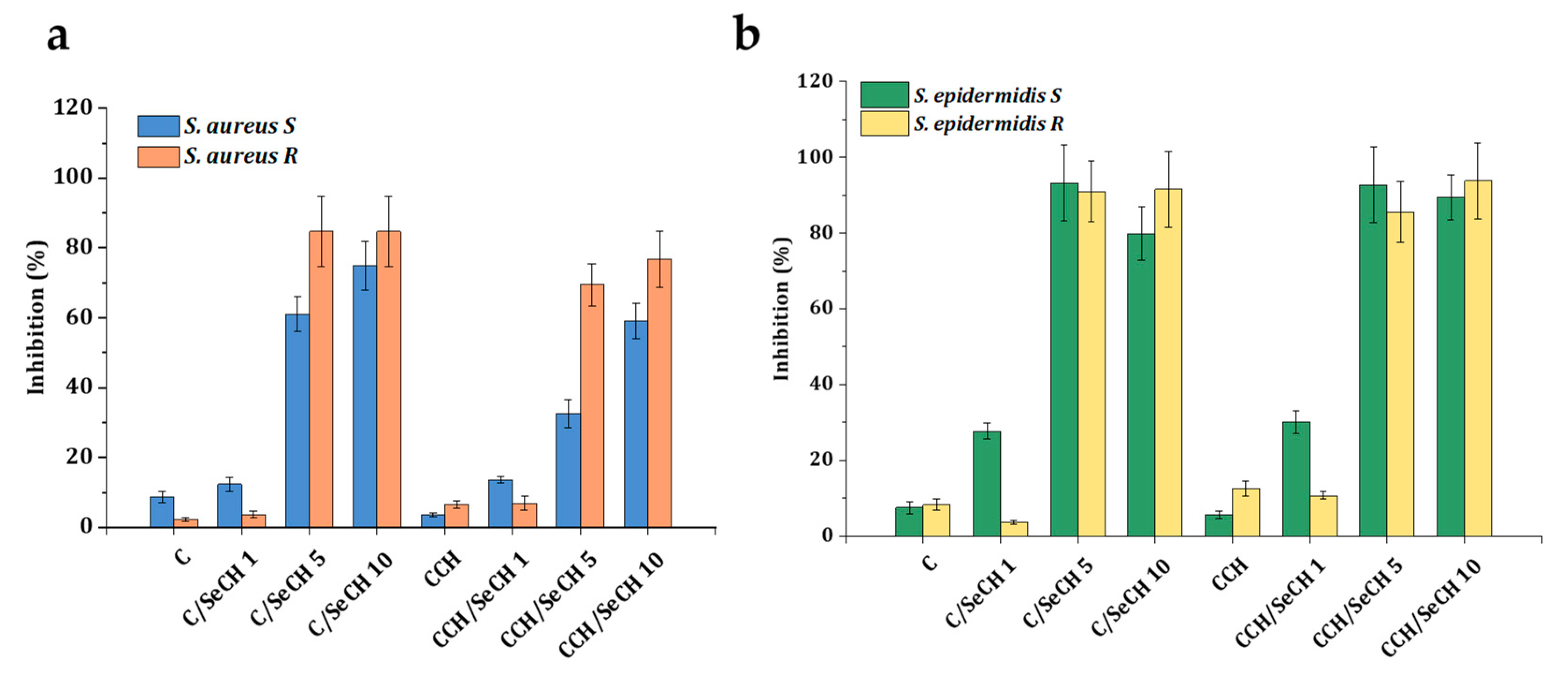
| Sample Name | Matrix composition | |||
|---|---|---|---|---|
| Collagen (%) | Chitosan (%) | SeCH (ppm) | SeCE (ppm) | |
| C | 100 | - | - | - |
| C/SeCH | 100 | - | 1, 5, 10 | - |
| C/SeCE | 100 | - | - | 1, 5, 10 |
| CCH | 50 | 50 | - | - |
| CCH/SeCH | 50 | 50 | 1, 5, 10 | - |
| CCH/SeCE | 50 | 50 | - | 1, 5, 10 |
| Scaffold | Wavenumbers [cm−1] | Triple-Helix Ratio | |||||
|---|---|---|---|---|---|---|---|
| Amide A | Amide B | Amide I | Amide II | Amide III | Pyrrolidine | ||
| C | 3312 | 2926 | 1651 | 1545 | 1236 | 1450 | 0.94 |
| C/SeCH | 3325 | 2932 | 1657 | 1551 | 1236 | 1452 | 0.95 |
| C/SeCE | 3319 | 2930 | 1657 | 1551 | 1238 | 1452 | 0.96 |
| CCH | 3304 | 2925 | 1659 | 1553 | 1236 | 1451 | 0.70 |
| CCH/SeCH | 3325 | 2928 | 1655 | 1551 | 1236 | 1454 | 0.87 |
| CCH/SeCE | 3321 | 2935 | 1655 | 1553 | 1239 | 1452 | 0.80 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dorazilová, J.; Muchová, J.; Šmerková, K.; Kočiová, S.; Diviš, P.; Kopel, P.; Veselý, R.; Pavliňáková, V.; Adam, V.; Vojtová, L. Synergistic Effect of Chitosan and Selenium Nanoparticles on Biodegradation and Antibacterial Properties of Collagenous Scaffolds Designed for Infected Burn Wounds. Nanomaterials 2020, 10, 1971. https://doi.org/10.3390/nano10101971
Dorazilová J, Muchová J, Šmerková K, Kočiová S, Diviš P, Kopel P, Veselý R, Pavliňáková V, Adam V, Vojtová L. Synergistic Effect of Chitosan and Selenium Nanoparticles on Biodegradation and Antibacterial Properties of Collagenous Scaffolds Designed for Infected Burn Wounds. Nanomaterials. 2020; 10(10):1971. https://doi.org/10.3390/nano10101971
Chicago/Turabian StyleDorazilová, Jana, Johana Muchová, Kristýna Šmerková, Silvia Kočiová, Pavel Diviš, Pavel Kopel, Radek Veselý, Veronika Pavliňáková, Vojtěch Adam, and Lucy Vojtová. 2020. "Synergistic Effect of Chitosan and Selenium Nanoparticles on Biodegradation and Antibacterial Properties of Collagenous Scaffolds Designed for Infected Burn Wounds" Nanomaterials 10, no. 10: 1971. https://doi.org/10.3390/nano10101971
APA StyleDorazilová, J., Muchová, J., Šmerková, K., Kočiová, S., Diviš, P., Kopel, P., Veselý, R., Pavliňáková, V., Adam, V., & Vojtová, L. (2020). Synergistic Effect of Chitosan and Selenium Nanoparticles on Biodegradation and Antibacterial Properties of Collagenous Scaffolds Designed for Infected Burn Wounds. Nanomaterials, 10(10), 1971. https://doi.org/10.3390/nano10101971