Au–Nitrogen-Doped Graphene Quantum Dot Composites as “On–Off” Nanosensors for Sensitive Photo-Electrochemical Detection of Caffeic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Apparatus
2.2. Preparation of Au, NGQDs, and Au/NGQD Composites
2.3. Fabrication of Au, NGQD, and Au/NGQD Composite Modified Electrodes
3. Results and Discussion
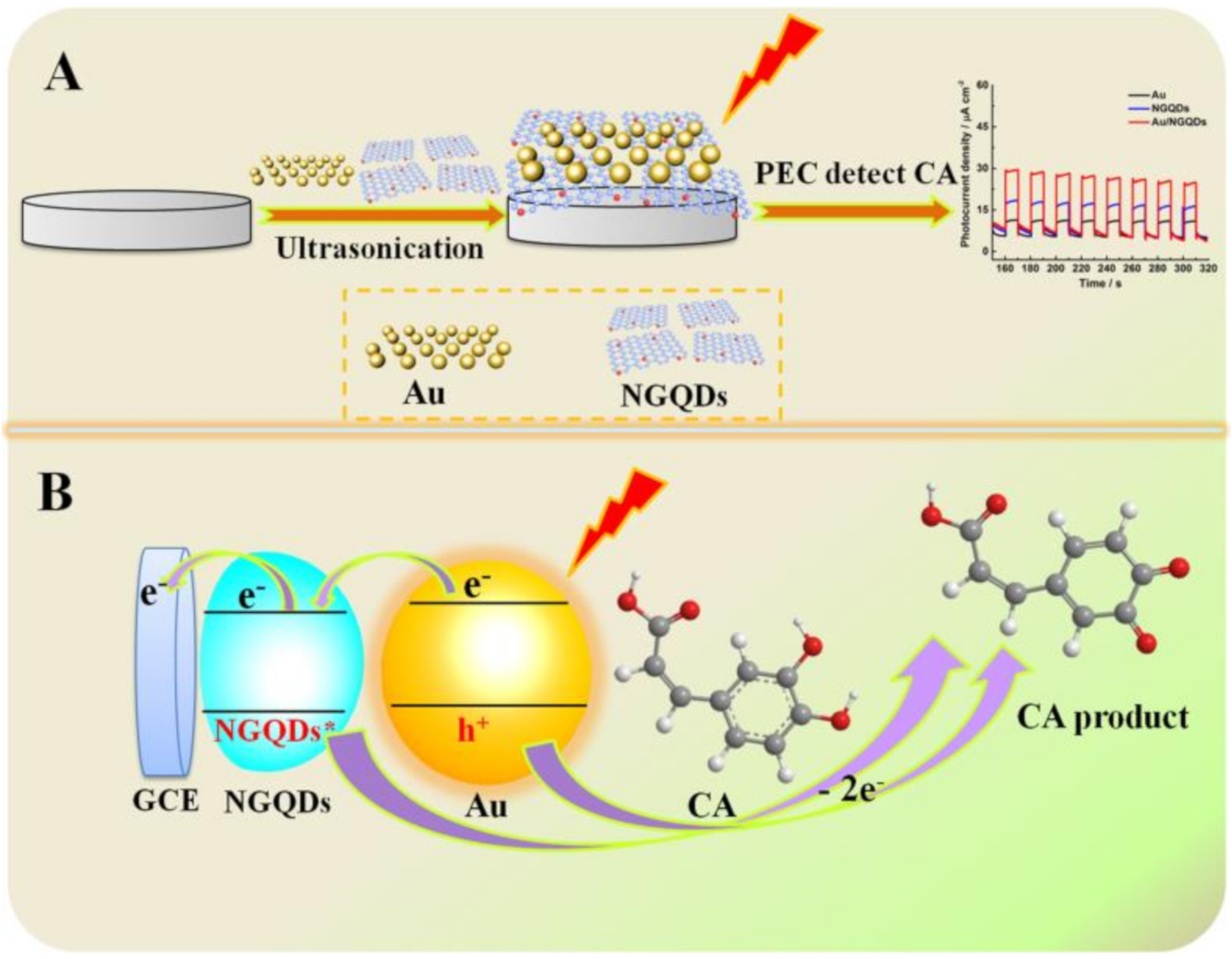
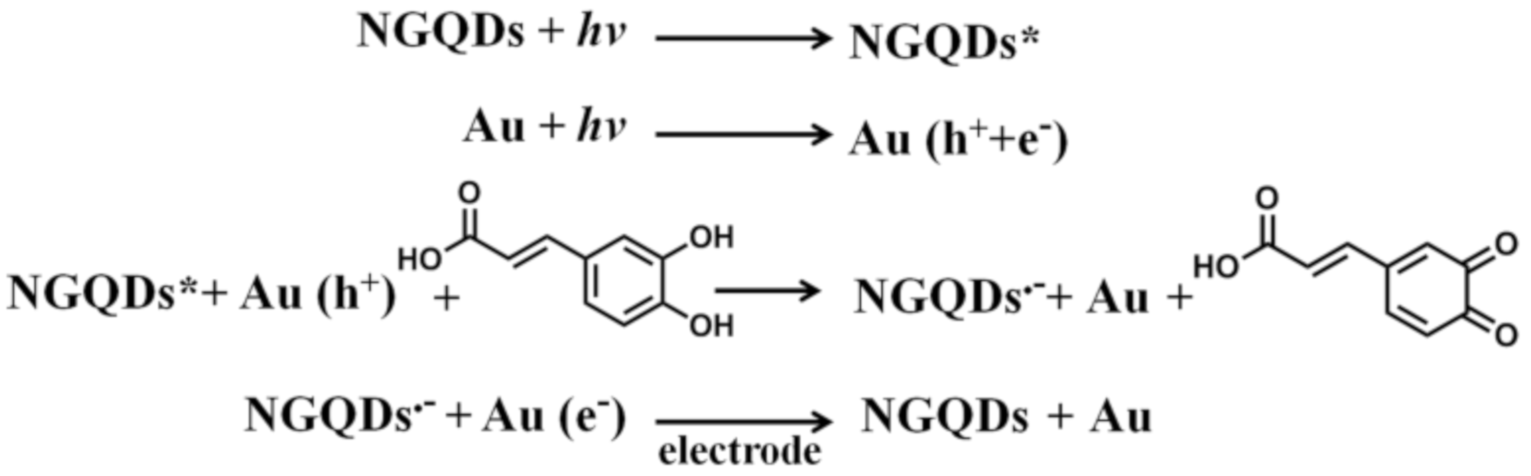
3.1. Detection Strategy of Au/NGQD Composites for CA
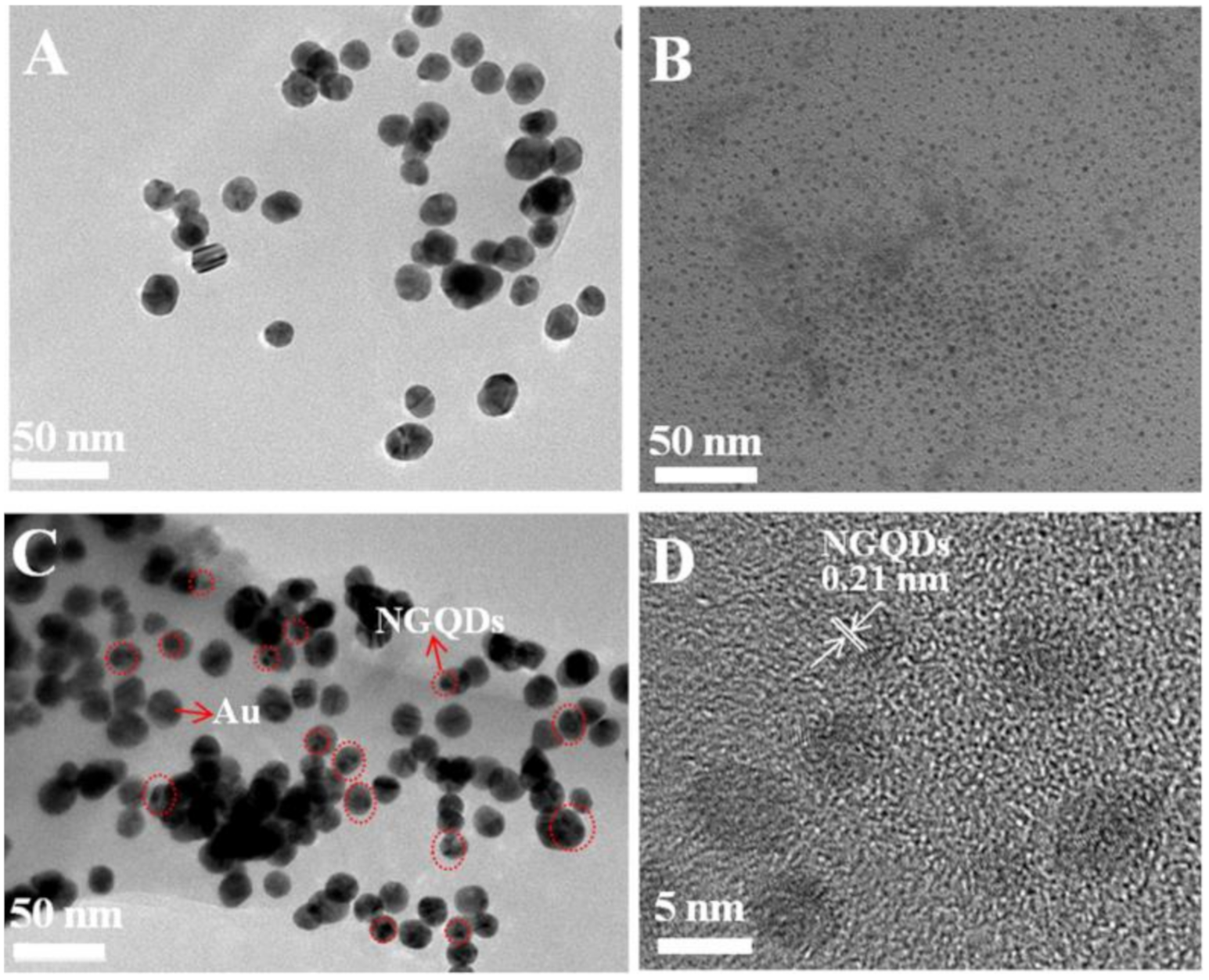
3.2. Characterization of Au, NGQDs, and Au/NGQDs Composite Modified Electrodes
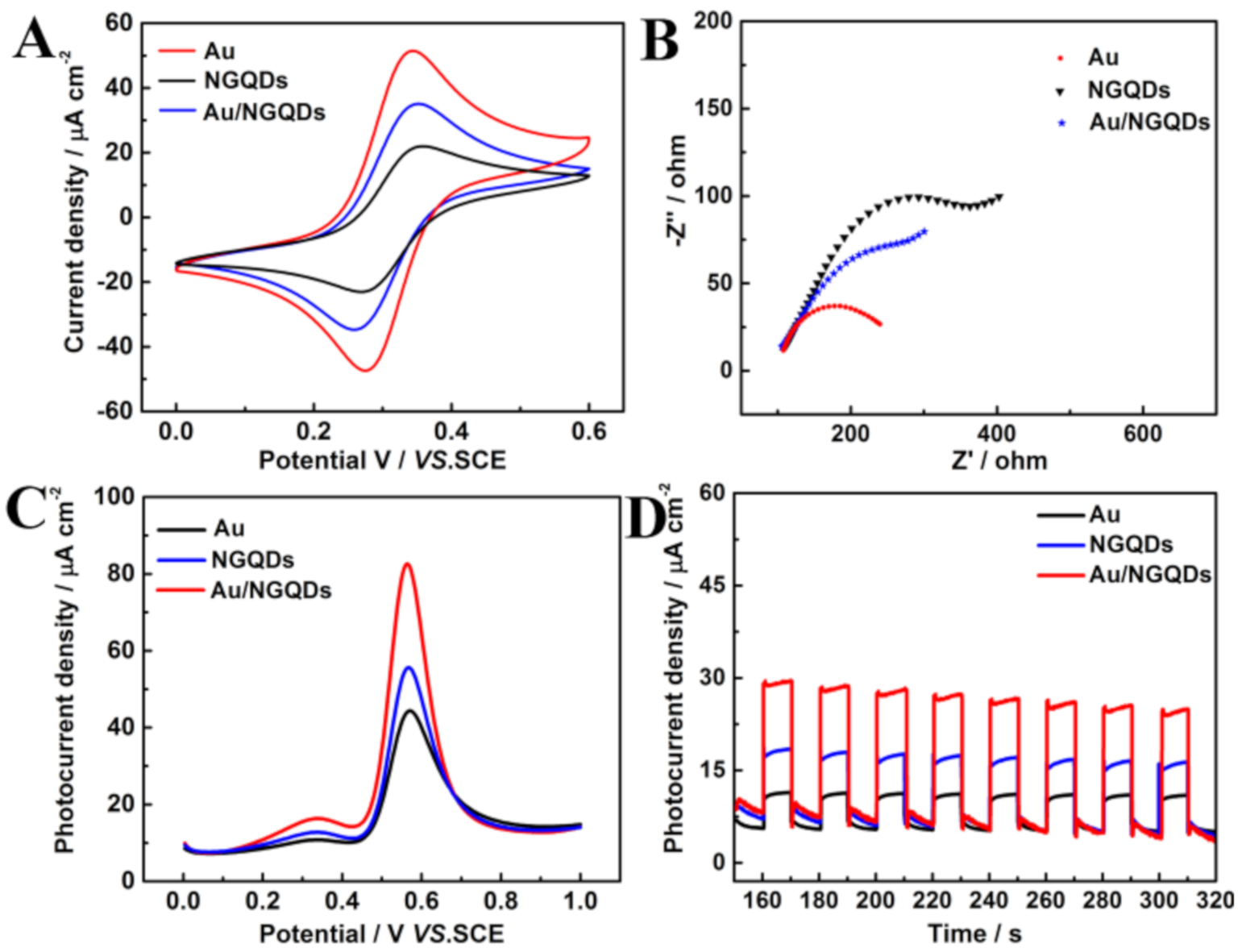
3.3. Electrochemical andPhoto-Electrochemical Characterization of the Sensing Platform
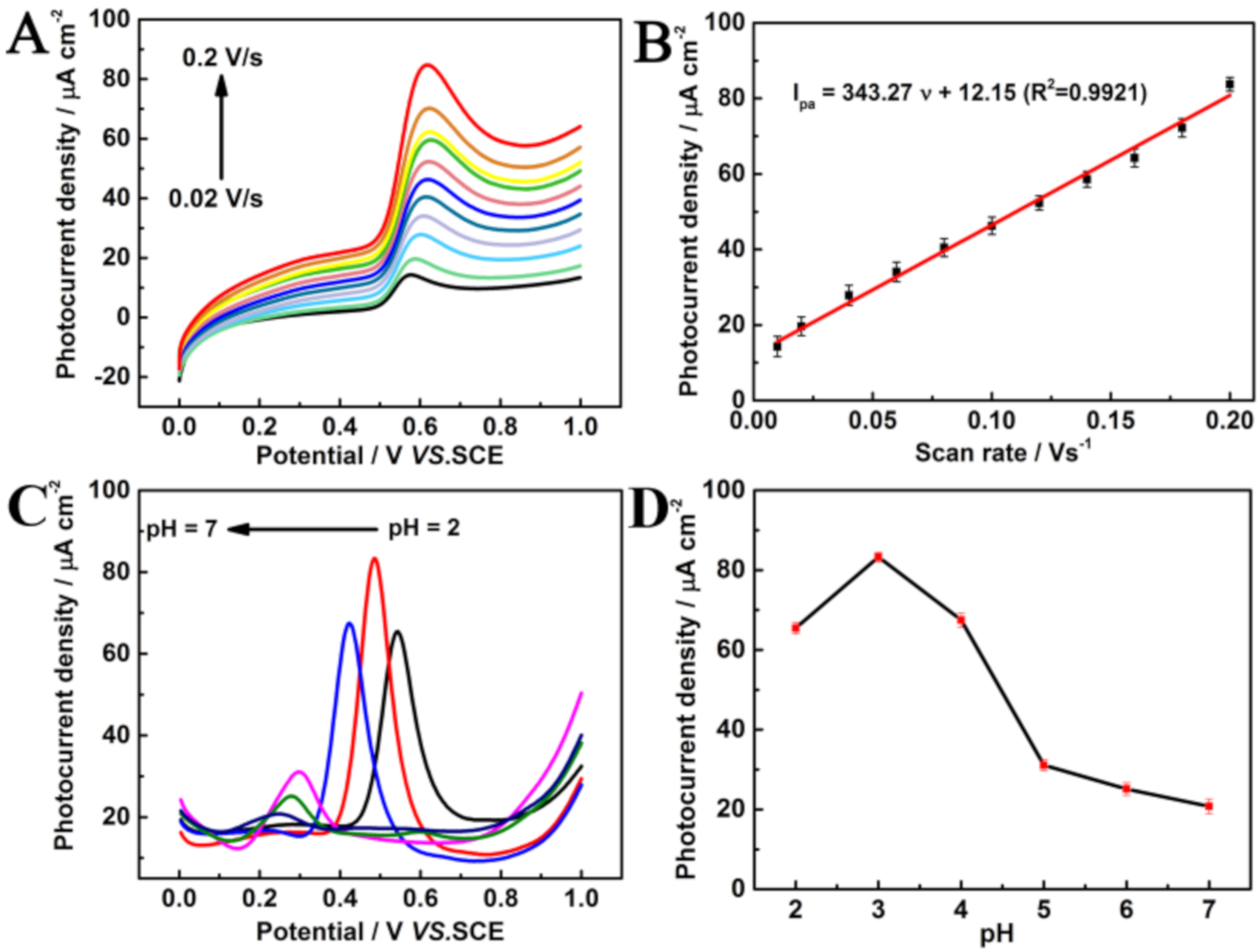
3.4. Optimization of Experimental Conditions
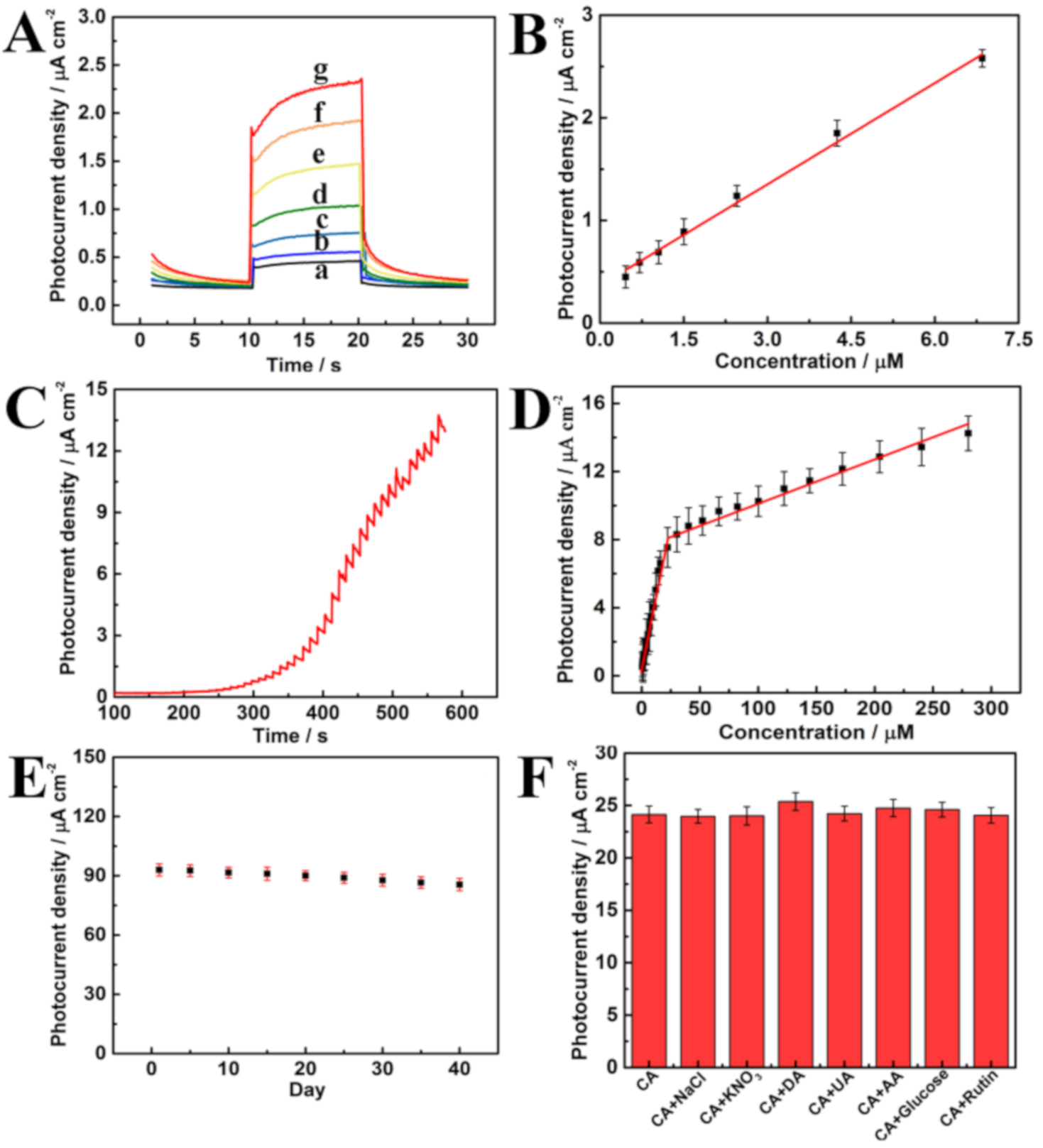
3.5. Analytical Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Leite, F.R.F.; Santos, W.D.J.R.; Kubota, L.T. Selective determination of caffeic acid in wines with electrochemical sensor based on molecularly imprinted siloxanes. Sensors Actuators B: Chem. 2014, 193, 238–246. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, J.; Yue, R.; Yang, T.; Gao, L. Facile one-pot synthesis of Au–PEDOT/rGO nanocomposite for highly sensitive detection of caffeic acid in red wine sample. Electrochim. Acta 2016, 196, 1–12. [Google Scholar] [CrossRef]
- Filik, H.; Çetintaş, G.; Avan, A.A.; Aydar, S.; Koç, S.N.; Boz, I. Square-wave stripping voltammetric determination of caffeic acid on electrochemically reduced graphene oxide–Nafion composite film. Talanta 2013, 116, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Cai, N.; Li, Y.; Chen, S.; Su, X. A fluorometric assay platform for caffeic acid detection based on the G-quadruplex/hemin DNAzyme. Analyst 2016, 141, 4456–4462. [Google Scholar] [CrossRef]
- Khezeli, T.; Daneshfar, A.; Sahraei, R. A green ultrasonic-assisted liquid–liquid microextraction based on deep eutectic solvent for the HPLC-UV determination of ferulic, caffeic and cinnamic acid from olive, almond, sesame and cinnamon oil. Talanta 2016, 150, 577–585. [Google Scholar] [CrossRef]
- Konar, N.; Dalabasmaz, S.; Poyrazoglu, E.S.; Artik, N.; Colak, A. The determination of the caffeic acid derivatives of Echinacea purpurea aerial parts under various extraction conditions by supercritical fluid extraction (SFE). J. Supercrit. Fluids 2014, 89, 128–136. [Google Scholar] [CrossRef]
- Wang, J.; Lu, C.; Chen, T.; Hu, L.; Du, Y.; Yao, Y.; Goh, M.C. Simply synthesized nitrogen-doped graphene quantum dot (NGQD)-modified electrode for the ultrasensitive photoelectrochemical detection of dopamine. Nanophotonics 2019. [Google Scholar] [CrossRef]
- Lin, Y.; Adilbekova, B.; Firdaus, Y.; Yengel, E.; Faber, H.; Sajjad, M.; Zheng, X.; Yarali, E.; Seitkhan, A.; Bakr, O.M.; et al. 17% Efficient Organic Solar Cells Based on Liquid Exfoliated WS 2 as a Replacement for PEDOT:PSS. Adv. Mater. 2019, 31, e1902965. [Google Scholar] [CrossRef]
- Zhang, K.; Shi, M.; Wu, Y.; Wang, C. Constructing FeCoSe2/Co0.85Se heterostructure catalysts for efficient oxygen evolution. J. Alloy. Compd. 2020, 825, 154073. [Google Scholar] [CrossRef]
- Aldalbahi, A.; Velázquez, R.; Zhou, A.F.; Rahaman, M.; Feng, P.X. Bandgap-Tuned 2D Boron Nitride/Tungsten Nitride Nanocomposites for Development of High-Performance Deep Ultraviolet Selective Photodetectors. Nanomaterials 2020, 10, 1433. [Google Scholar] [CrossRef]
- Cheng, W.; Zengab, X.; Chen, H.; Li, Z.; Zeng, W.; Meiab, L.; Zhao, Y. Versatile Polydopamine Platforms: Synthesis and Promising Applications for Surface Modification and Advanced Nanomedicine. ACS Nano 2019, 13, 8537–8565. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Kim, S.J.; Sohn, J.S.; Nam, M.S.; Kang, S.; Jun, S.C. Surface plasmon enhancement of photoluminescence in photo-chemically synthesized graphene quantum dot and Au nanosphere. Nano Res. 2016, 9, 1866–1875. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, X.; Zhang, W.; Lv, F.; Guo, S. Recent progress in two-dimensional inorganic quantum dots. Chem. Soc. Rev. 2018, 47, 586–625. [Google Scholar] [CrossRef]
- Yan, M.; Hua, Y.; Zhu, F.; Gu, W.; Jiang, J.; Shen, H.; Shi, W. Fabrication of nitrogen doped graphene quantum dots-BiOI/MnNb2O6 p-n junction photocatalysts with enhanced visible light efficiency in photocatalytic degradation of antibiotics. Appl. Catal. B Environ. 2017, 202, 518–527. [Google Scholar] [CrossRef]
- Wu, J.; Ma, S.; Sun, J.; Gold, J.I.; Tiwary, C.; Kim, B.; Zhu, L.; Chopra, N.; Odeh, I.N.; Vajtai, R.; et al. A metal-free electrocatalyst for carbon dioxide reduction to multi-carbon hydrocarbons and oxygenates. Nat. Commun. 2016, 7, 13869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, X.; Wen, S.; Su, Y.; Fu, J.; Luo, X.; Wu, P.; Cai, C.; Jelinek, R.; Jiang, L.; Zhu, J.-J. Graphene Quantum Dots Wrapped Gold Nanoparticles with Integrated Enhancement Mechanisms as Sensitive and Homogeneous Substrates for Surface-Enhanced Raman Spectroscopy. Anal. Chem. 2019, 91, 7295–7303. [Google Scholar] [CrossRef]
- Yan, M.; Hua, Y.; Zhu, F.; Sun, L.; Gu, W.; Shi, W. Constructing nitrogen doped graphene quantum dots-ZnNb2O6/g-C3N4 catalysts for hydrogen production under visible light. Appl. Catal. B Environ. 2017, 206, 531–537. [Google Scholar] [CrossRef]
- Luo, L.; Ma, S.; Li, L.; Liu, X.; Zhang, J.; Li, X.; Liu, N.; You, T. Monitoring zearalenone in corn flour utilizing novel self-enhanced electrochemiluminescence aptasensor based on NGQDs-NH2-Ru@SiO2 luminophore. Food Chem. 2019, 292, 98–105. [Google Scholar] [CrossRef]
- Lee, H.-E.; Ahn, H.-Y.; Mun, J.; Lee, Y.Y.; Kim, M.; Cho, N.H.; Chang, K.; Kim, W.S.; Rho, J.; Nam, K.T. Amino-acid- and peptide-directed synthesis of chiral plasmonic gold nanoparticles. Nature 2018, 556, 360–365. [Google Scholar] [CrossRef]
- Duchene, J.S.; Tagliabue, G.; Welch, A.J.; Cheng, W.-H.; Atwater, H.A. Hot Hole Collection and Photoelectrochemical CO2 Reduction with Plasmonic Au/p-GaN Photocathodes. Nano Lett. 2018, 18, 2545–2550. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Li, N.; Goebl, J.; Lu, Z.; Yin, Y. A Systematic Study of the Synthesis of Silver Nanoplates: Is Citrate a “Magic” Reagent? J. Am. Chem. Soc. 2011, 133, 18931–18939. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Wang, Y.; Song, C.; Zheng, L.; Ma, N.; Liu, X.; Li, S.; Lin, L.; Li, M.; Xu, Y.; et al. Hybrid Au–Ag Nanostructures for Enhanced Plasmon-Driven Catalytic Selective Hydrogenation through Visible-light irradiation and Surface-Enhanced Raman Scattering. J. Am. Chem. Soc. 2018, 140, 864–867. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Han, S.W.; Min, D.-H. Graphene Oxide Sheath on Ag Nanoparticle/Graphene Hybrid Films as an Antioxidative Coating and Enhancer of Surface-Enhanced Raman Scattering. ACS Appl. Mater. Interfaces 2012, 4, 6545–6551. [Google Scholar] [CrossRef] [PubMed]
- Fei, X.; Liu, Z.; Li, Y.; Yang, G.; Su, C.; Zhong, H.; Zhuang, Z.; Guo, Z. One-pot green synthesis of flower-liked Au NP@GQDs nanocomposites for surface-enhanced Raman scattering. J. Alloy. Compd. 2017, 725, 1084–1090. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, K.; Xu, H.; Yan, B.; Gao, F.; Shi, Y.; Du, Y. Engineered photoelectrochemical platform for the ultrasensitive detection of caffeic acid based on flower-like MoS2 and PANI nanotubes nanohybrid. Sensors Actuators B Chem. 2018, 276, 322–330. [Google Scholar] [CrossRef]
- Li, M.; Chen, T.; Gooding, J.J.; Liu, J. Review of Carbon and Graphene Quantum Dots for Sensing. ACS Sensors 2019, 4, 1732–1748. [Google Scholar] [CrossRef] [PubMed]
- Thanh, T.D.; Balamurugan, J.; Lee, S.H.; Kim, N.H.; Lee, J.H. Effective seed-assisted synthesis of gold nanoparticles anchored nitrogen-doped graphene for electrochemical detection of glucose and dopamine. Biosens. Bioelectron. 2016, 81, 259–267. [Google Scholar] [CrossRef]
- Wei, D.; Liu, Y.; Wang, Y.; Zhang, H.; Huang, L.; Yu, G. Synthesis of N-Doped Graphene by Chemical Vapor Deposition and Its Electrical Properties. Nano Lett. 2009, 9, 1752–1758. [Google Scholar] [CrossRef]
- Chaicham, C.; Tuntulani, T.; Promarak, V.; Tomapatanaget, B. Effective GQD/AuNPs nanosensors for selectively bifunctional detection of lysine and cysteine under different photophysical properties. Sensors Actuators B Chem. 2019, 282, 936–944. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Q.; Zhou, L.; Li, X.; He, J.; Huang, W.; Cai, Y.; Wang, J.; Chen, T.; Du, Y.; Yao, Y. Au–Nitrogen-Doped Graphene Quantum Dot Composites as “On–Off” Nanosensors for Sensitive Photo-Electrochemical Detection of Caffeic Acid. Nanomaterials 2020, 10, 1972. https://doi.org/10.3390/nano10101972
Zhao Q, Zhou L, Li X, He J, Huang W, Cai Y, Wang J, Chen T, Du Y, Yao Y. Au–Nitrogen-Doped Graphene Quantum Dot Composites as “On–Off” Nanosensors for Sensitive Photo-Electrochemical Detection of Caffeic Acid. Nanomaterials. 2020; 10(10):1972. https://doi.org/10.3390/nano10101972
Chicago/Turabian StyleZhao, Qiyuan, Lin Zhou, Xue Li, Jiaqi He, Weichun Huang, Yan Cai, Jin Wang, Tingting Chen, Yukou Du, and Yong Yao. 2020. "Au–Nitrogen-Doped Graphene Quantum Dot Composites as “On–Off” Nanosensors for Sensitive Photo-Electrochemical Detection of Caffeic Acid" Nanomaterials 10, no. 10: 1972. https://doi.org/10.3390/nano10101972
APA StyleZhao, Q., Zhou, L., Li, X., He, J., Huang, W., Cai, Y., Wang, J., Chen, T., Du, Y., & Yao, Y. (2020). Au–Nitrogen-Doped Graphene Quantum Dot Composites as “On–Off” Nanosensors for Sensitive Photo-Electrochemical Detection of Caffeic Acid. Nanomaterials, 10(10), 1972. https://doi.org/10.3390/nano10101972







