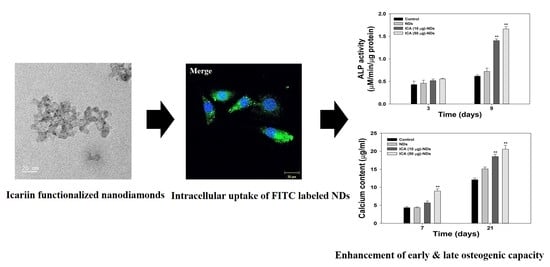
Icariin-Functionalized Nanodiamonds to Enhance Osteogenic Capacity In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Icariin-Functionalized Nanodiamonds
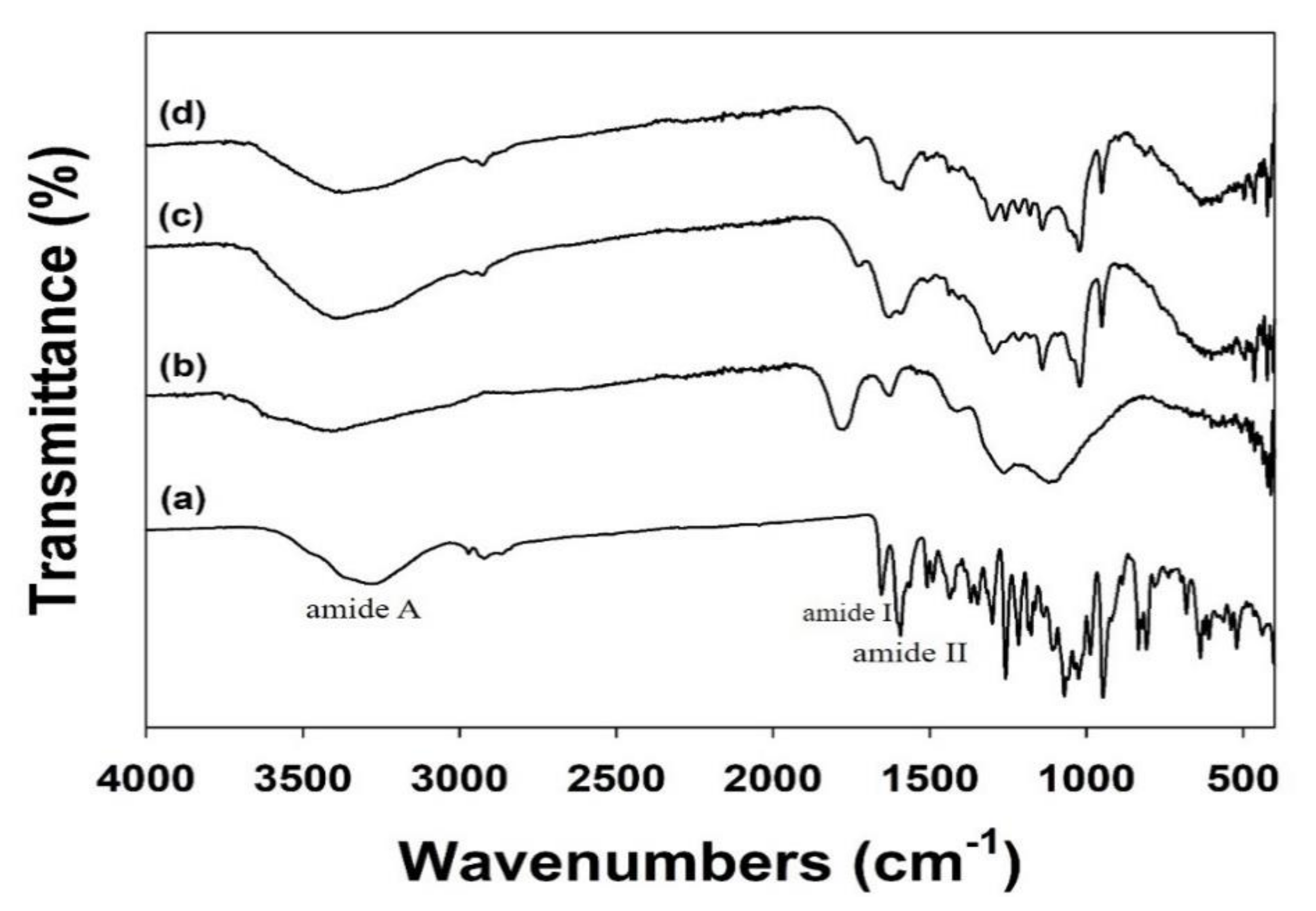
2.3. Characterization of Plain NDs and Modified NDs
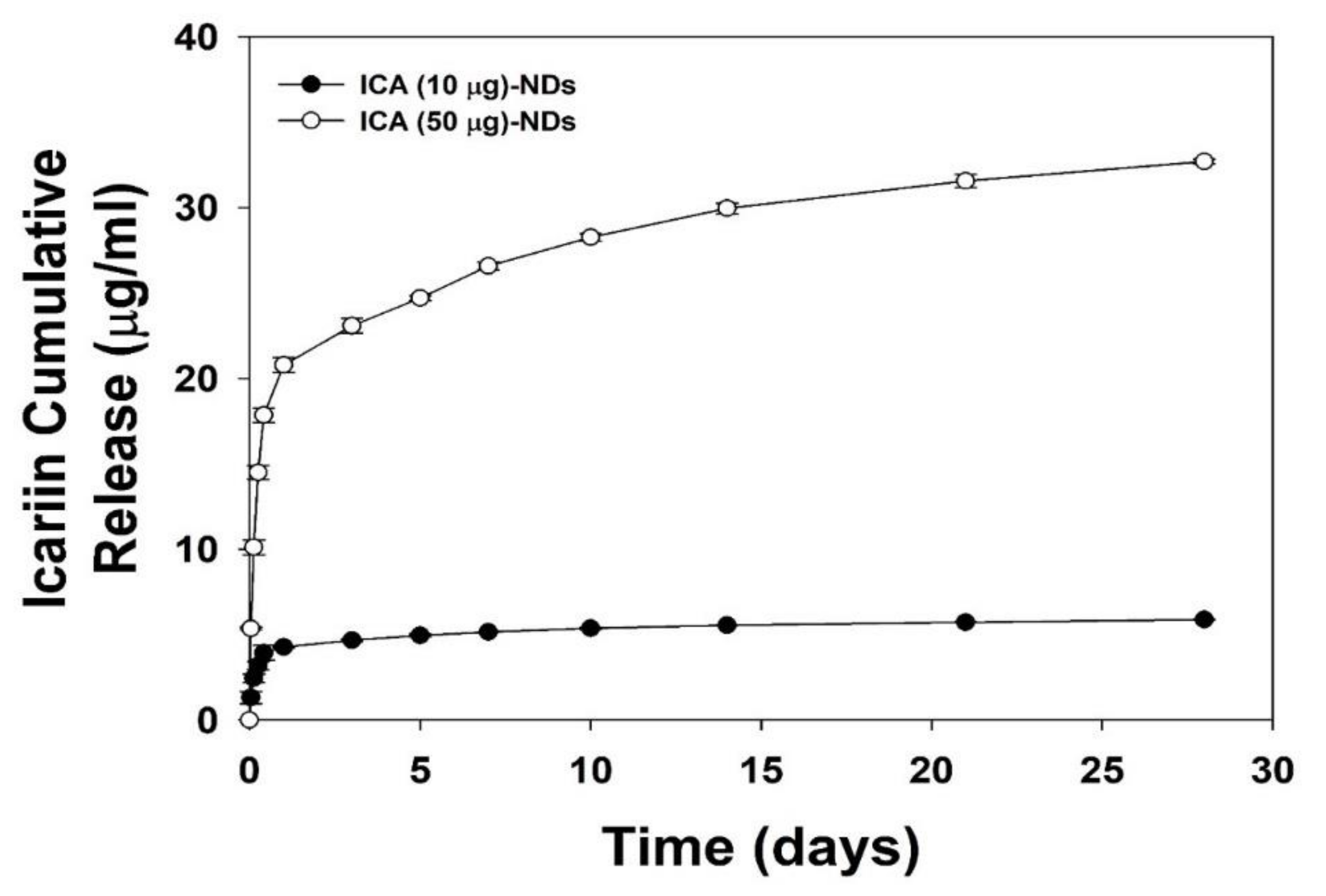
2.4. ICA Release
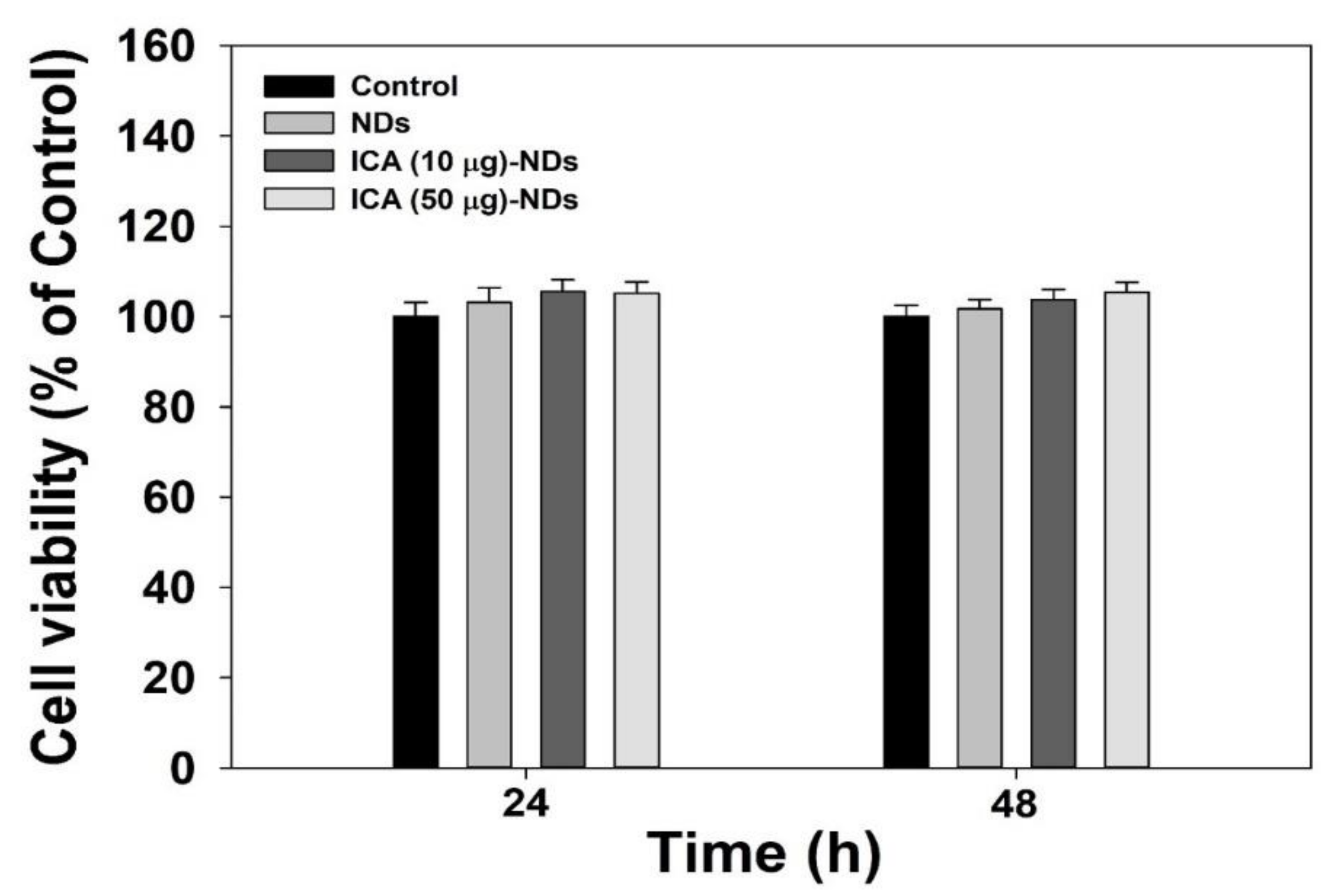
2.5. Cell Viability Analysis
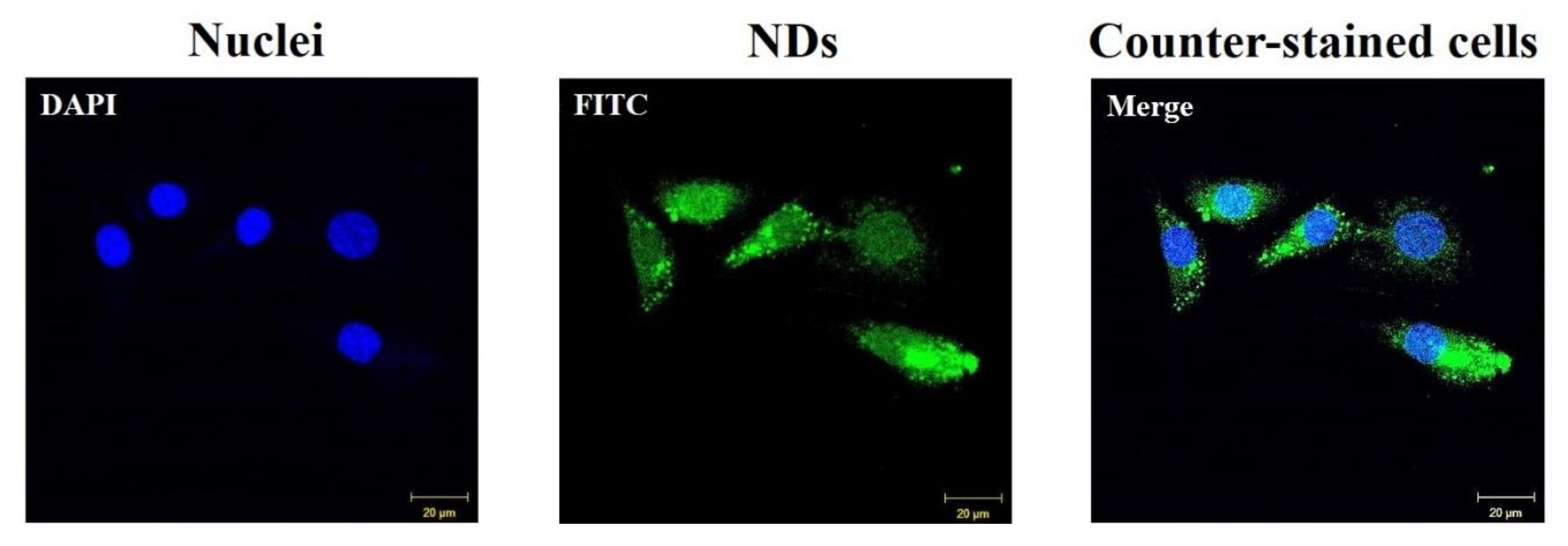
2.6. Infiltration of Nanodiamonds into Cells
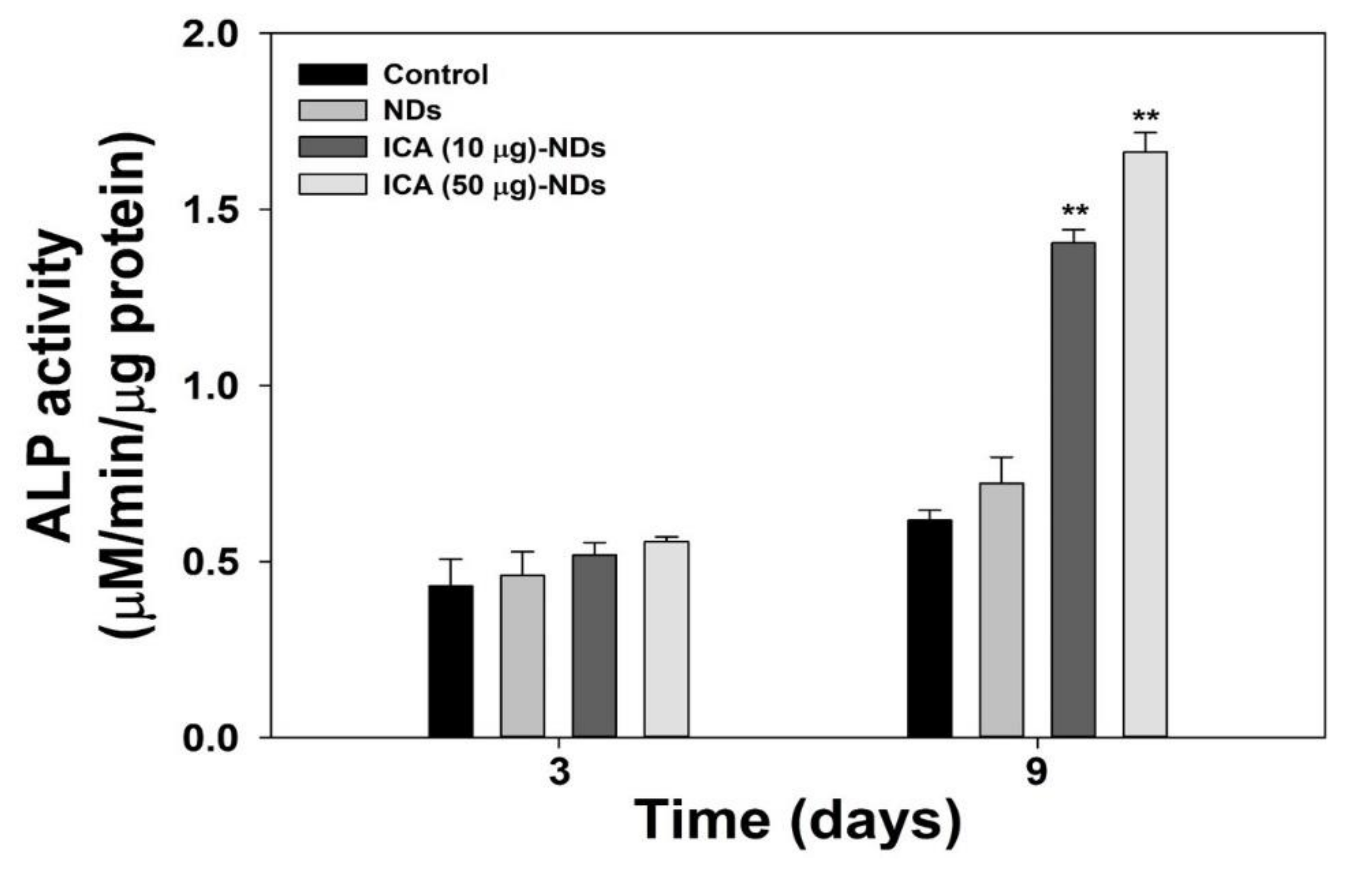
2.7. Early Osteogenic Differentiation Marker Analysis
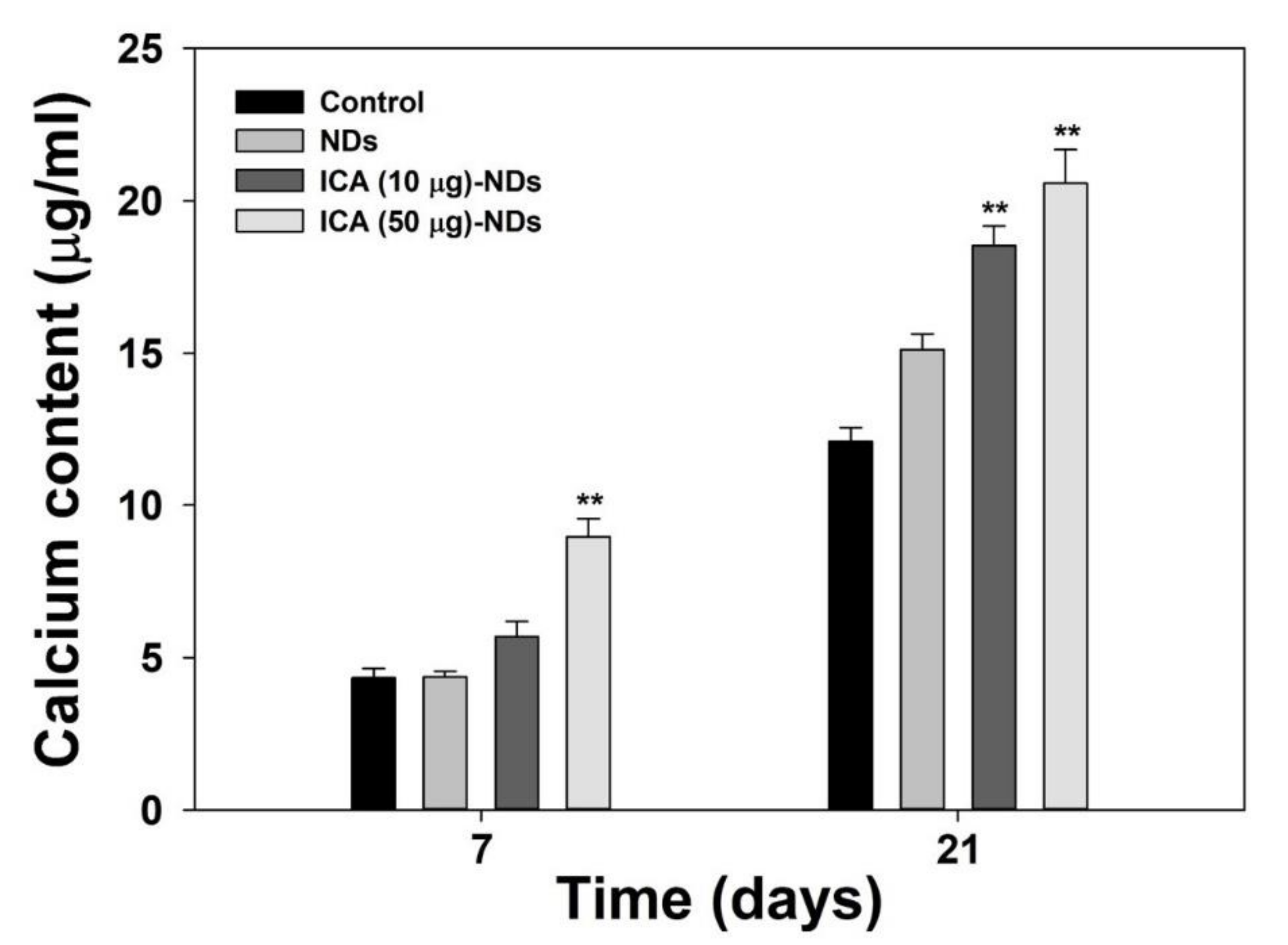
2.8. Late Osteogenic Differentiation Marker Analysis
2.9. Evaluation of Osteogenic-Related Genes
2.10. Statistical Analysis
3. Results
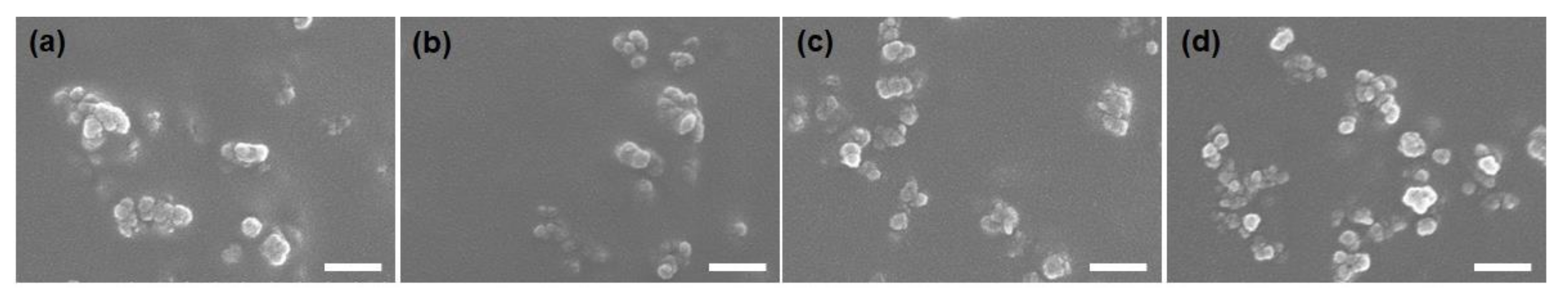
3.1. Characterizations
3.2. In Vitro ICA Release
3.3. Cytotoxic Test and Cellular Internalization
3.4. Early Osteogenic Differentiation Marker Analysis
3.5. Late Osteogenic Differentiation Marker Analysis
3.6. Osteogenic-Related Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Webster, T.J.; Ahn, E.S. Nanostructured biomaterials for tissue engineering bone. Adv. Biochem. Eng. Biotechnol. 2007, 103, 275–308. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C. Nanomaterial-based bone regeneration. Nanoscale 2017, 9, 4862–4874. [Google Scholar] [CrossRef] [PubMed]
- Bauer, T.W.; Muschler, G.F. Bone graft materials. An overview of the basic science. Clin. Orthop. Relat. Res. 2000, 371, 10–27. [Google Scholar] [CrossRef]
- Sohn, H.S.; Oh, J.K. Review of bone graft and bone substitutes with an emphasis on fracture surgeries. Biomater. Res. 2019, 23, 9. [Google Scholar] [CrossRef] [PubMed]
- Perrott, D.H.; Smith, R.A.; Kaban, L.B. The Use of Fresh-Frozen Allogeneic Bone for Maxillary and Mandibular Reconstruction. Int. J. Oral Max. Surg. 1992, 21, 260–265. [Google Scholar] [CrossRef]
- Gocke, D.J. Tissue donor selection and safety. Clin. Orthop. Relat. Res. 2005, 435, 17–21. [Google Scholar] [CrossRef]
- Holtkamp, K.C.; Vos, E.M.; Rigter, T.; Lakeman, P.; Henneman, L.; Cornel, M.C. Stakeholder perspectives on the implementation of genetic carrier screening in a changing landscape. BMC Health Serv. Res. 2017, 17, 146. [Google Scholar] [CrossRef]
- Nandi, S.K.; Roy, S.; Mukherjee, P.; Kundu, B.; De, D.K.; Basu, D. Orthopaedic applications of bone graft & graft substitutes: A review. Indian J. Med. Res. 2010, 132, 15–30. [Google Scholar]
- Athanasiou, V.T.; Papachristou, D.J.; Panagopoulos, A.; Saridis, A.; Scopa, C.D.; Megas, P. Histological comparison of autograft, allograft-DBM, xenograft, and synthetic grafts in a trabecular bone defect: An experimental study in rabbits. Med. Sci. Monit. 2010, 16, BR24–BR31. [Google Scholar]
- Talebian, S.; Mehrali, M.; Mohan, S.; Raghavendran, H.R.B.; Mehrali, M.; Khanlou, H.M.; Kamarul, T.; Afifi, A.M.; Abbas, A.A. Chitosan (PEO)/bioactive glass hybrid nanofibers for bone tissue engineering (vol 4, pg 49144, 2014). Rsc. Adv. 2015, 5, 5054. [Google Scholar] [CrossRef]
- Shirazi, F.S.; Moghaddam, E.; Mehrali, M.; Oshkour, A.A.; Metselaar, H.S.; Kadri, N.A.; Zandi, K.; Abu, N.A. In Vitro characterization and mechanical properties of beta-calcium silicate/POC composite as a bone fixation device. J. Biomed. Mater. Res. A 2014, 102, 3973–3985. [Google Scholar] [CrossRef] [PubMed]
- Puvaneswary, S.; Talebian, S.; Raghavendran, H.B.; Murali, M.R.; Mehrali, M.; Afifi, A.M.; Kasim, N.H.; Kamarul, T. Fabrication and In Vitro biological activity of betaTCP-Chitosan-Fucoidan composite for bone tissue engineering. Carbohydr. Polym. 2015, 134, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.; Lee, J.; Oh, S.; Ham, B.; Chung, S.; Lee, J.K.; Ku, J. Bone graft materials for current implant dentistry. J. Dent. Implant Res. 2020, 39, 1–10. [Google Scholar]
- Barbeck, M.; Udeabor, S.; Lorenz, J.; Schlee, M.; Holthaus, M.G.; Raetscho, N.; Choukroun, J.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. High-Temperature Sintering of Xenogeneic Bone Substitutes Leads to Increased Multinucleated Giant Cell Formation: In Vivo and Preliminary Clinical Results. J. Oral Implantol. 2015, 41, e212–e222. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.S.; Aaboe, M.; Pinholt, E.M.; Hjorting-Hansen, E.; Melsen, F.; Ruyter, I.E. Tissue reaction and material characteristics of four bone substitutes. Int. J. Oral Maxillofac. Implants 1996, 11, 55–66. [Google Scholar] [PubMed]
- Ye, Y.; Jing, X.; Li, N.; Wu, Y.; Li, B.; Xu, T. Icariin promotes proliferation and osteogenic differentiation of rat adipose-derived stem cells by activating the RhoA-TAZ signaling pathway. Biomed. Pharmacother. 2017, 88, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, W.; Luo, B.; Chen, X.; Wen, W.; Zhou, C. Icariin immobilized electrospinning poly(l-lactide) fibrous membranes via polydopamine adhesive coating with enhanced cytocompatibility and osteogenic activity. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 79, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.Q.; Li, D.D.; Huang, C.; Lu, D.S.; Zhang, C.; Zhou, S.Y.; Liu, J.; Zhang, F. Icariin Reduces Dopaminergic Neuronal Loss and Microglia-Mediated Inflammation in Vivo and in Vitro. Front. Mol. Neurosci. 2017, 10, 441. [Google Scholar] [CrossRef]
- Ke, Z.; Liu, J.; Xu, P.; Gao, A.; Wang, L.; Ji, L. The Cardioprotective Effect of Icariin on Ischemia-Reperfusion Injury in Isolated Rat Heart: Potential Involvement of the PI3K-Akt Signaling Pathway. Cardiovasc. Ther. 2015, 33, 134–140. [Google Scholar] [CrossRef]
- Fan, C.; Yang, Y.; Liu, Y.; Jiang, S.; Di, S.; Hu, W.; Ma, Z.; Li, T.; Zhu, Y.; Xin, Z.; et al. Icariin displays anticancer activity against human esophageal cancer cells via regulating endoplasmic reticulum stress-mediated apoptotic signaling. Sci. Rep. 2016, 6, 21145. [Google Scholar] [CrossRef]
- Zhao, J.; Ohba, S.; Shinkai, M.; Chung, U.I.; Nagamune, T. Icariin induces osteogenic differentiation in vitro in a BMP- and Runx2-dependent manner. Biochem. Biophys. Res. Commun. 2008, 369, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Zhou, W.; Liu, S.; Chen, P.; Wu, H. Icariin stimulates the proliferation of rat bone mesenchymal stem cells via ERK and p38 MAPK signaling. Int. J. Clin. Exp. Med. 2015, 8, 7125–7133. [Google Scholar]
- Song, L.G.; Zhao, J.S.; Zhang, X.Z.; Li, H.; Zhou, Y. Icariin induces osteoblast proliferation, differentiation and mineralization through estrogen receptor-mediated ERK and JNK signal activation. Eur. J. Pharmacol. 2013, 714, 15–22. [Google Scholar] [CrossRef]
- Feng, R.; Feng, L.; Yuan, Z.; Wang, D.; Wang, F.; Tan, B.; Han, S.; Li, T.; Li, D.; Han, Y. Icariin protects against glucocorticoid-induced osteoporosis in vitro and prevents glucocorticoid-induced osteocyte apoptosis in vivo. Cell Biochem. Biophys. 2013, 67, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.N.; Zhou, J.; Ge, B.F.; Zhen, P.; Ma, H.P.; Shi, W.G.; Cheng, K.; Xian, C.J.; Chen, K.M. Icariin Induces Osteoblast Differentiation and Mineralization without Dexamethasone In Vitro. Planta Med. 2013, 79, 1501–1508. [Google Scholar] [CrossRef]
- Cui, J.F.; Zhu, M.; Zhu, S.J.; Wang, G.X.; Xu, Y.Z.; Geng, D.C. Inhibitory effect of icariin on Ti-induced inflammatory osteoclastogenesis. J. Surg. Res. 2014, 192, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tao, Y.; Ping, Z.; Zhang, W.; Hu, X.; Wang, Y.; Wang, L.; Shi, J.; Wu, X.; Yang, H.; et al. Icariin attenuates titanium-particle inhibition of bone formation by activating the Wnt/beta-catenin signaling pathway In Vivo and In Vitro. Sci. Rep. 2016, 6, 23827. [Google Scholar] [CrossRef]
- Choi, S.; Lee, Y.S.; Jo, H.S.; Jeong, W.K.; Kim, H.J.; Song, M.H.; Park, K.; Kim, S.E. Investigating the In Vitro Osteogenic Properties of the Inclusion Nanocarrier of Icariin with Beta-Cyclodextrin-Alginate. Appl. Sci.-Basel 2020, 10, 4137. [Google Scholar] [CrossRef]
- Turcheniuk, K.; Mochalin, V.N. Biomedical applications of nanodiamond (Review). Nanotechnology 2017, 28, 252001. [Google Scholar] [CrossRef]
- Gao, G.; Guo, Q.; Zhi, J. Nanodiamond-Based Theranostic Platform for Drug Delivery and Bioimaging. Small 2019, 15, e1902238. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.K.; Zheng, W.W.; Wang, C.C.; Chiu, Y.C.; Cheng, C.L.; Lo, Y.S.; Chen, C.; Chao, J.I. Covalent linkage of nanodiamond-paclitaxel for drug delivery and cancer therapy. Nanotechnology 2010, 21, 315106. [Google Scholar] [CrossRef]
- Li, X.X.; Shao, J.Q.; Qin, Y.; Shao, C.; Zheng, T.T.; Ye, L. TAT-conjugated nanodiamond for the enhanced delivery of doxorubicin. J. Mater. Chem. 2011, 21, 7966–7973. [Google Scholar] [CrossRef]
- Zhang, Q.; Mochalin, V.N.; Neitzel, I.; Knoke, I.Y.; Han, J.; Klug, C.A.; Zhou, J.G.; Lelkes, P.I.; Gogotsi, Y. Fluorescent PLLA-nanodiamond composites for bone tissue engineering. Biomaterials 2011, 32, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.; Gatica, M.; Kim, H.; Osawa, E.; Ho, D. Multi-protein delivery by nanodiamonds promotes bone formation. J. Dent. Res. 2013, 92, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Ryu, T.K.; Kang, R.H.; Jeong, K.Y.; Jun, D.R.; Koh, J.M.; Kim, D.; Bae, S.K.; Choi, S.W. Bone-targeted delivery of nanodiamond-based drug carriers conjugated with alendronate for potential osteoporosis treatment. J. Control. Release 2016, 232, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Choi, S.; Hong, J.Y.; Shim, K.S.; Kim, T.H.; Park, K.; Lee, S.H. Accelerated Osteogenic Differentiation of MC3T3-E1 Cells by Lactoferrin-Conjugated Nanodiamonds through Enhanced Anti-Oxidant and Anti-Inflammatory Effects. Nanomaterials-Basel 2020, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, Y.; Li, D.X.; Wang, R.; Fan, H.S.; Xiao, Y.M.; Zhang, L.; Zhang, X.D. The effect of loading icariin on biocompatibility and bioactivity of porous beta-TCP ceramic. J. Mater. Sci. Mater. Med. 2011, 22, 371–379. [Google Scholar] [CrossRef]
- Xia, L.; Li, Y.; Zhou, Z.; Dai, Y.; Liu, H.; Liu, H. Icariin delivery porous PHBV scaffolds for promoting osteoblast expansion In Vitro. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 3545–3552. [Google Scholar] [CrossRef]
- Lai, Y.; Cao, H.; Wang, X.; Chen, S.; Zhang, M.; Wang, N.; Yao, Z.; Dai, Y.; Xie, X.; Zhang, P.; et al. Porous composite scaffold incorporating osteogenic phytomolecule icariin for promoting skeletal regeneration in challenging osteonecrotic bone in rabbits. Biomaterials 2018, 153, 1–13. [Google Scholar] [CrossRef]
- Wu, Y.; Cao, L.; Xia, L.; Wu, Q.; Wang, J.; Wang, X.; Xu, L.; Zhou, Y.; Xu, Y.; Jiang, X. Evaluation of Osteogenesis and Angiogenesis of Icariin in Local Controlled Release and Systemic Delivery for Calvarial Defect in Ovariectomized Rats. Sci. Rep. 2017, 7, 5077. [Google Scholar] [CrossRef]
- Huang, Z.; Nelson, E.R.; Smith, R.L.; Goodman, S.B. The sequential expression profiles of growth factors from osteoprogenitors [correction of osteroprogenitors] to osteoblasts In Vitro. Tissue Eng. 2007, 13, 2311–2320. [Google Scholar] [CrossRef] [PubMed]
- Tenenbaum, H.C. Levamisole and inorganic pyrophosphate inhibit beta-glycerophosphate induced mineralization of bone formed In Vitro. Bone Miner. 1987, 3, 13–26. [Google Scholar] [PubMed]
- Aubin, J.E. Regulation of osteoblast formation and function. Rev. Endocr. Metab. Disord. 2001, 2, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Yun, Y.P.; Han, Y.K.; Lee, D.W.; Ohe, J.Y.; Lee, B.S.; Song, H.R.; Park, K.; Choi, B.J. Osteogenesis induction of periodontal ligament cells onto bone morphogenic protein-2 immobilized PCL fibers. Carbohydr. Polym. 2014, 99, 700–709. [Google Scholar] [CrossRef]
- Gong, M.; Chi, C.; Ye, J.J.; Liao, M.H.; Xie, W.Q.; Wu, C.G.; Shi, R.; Zhang, L.Q. Icariin-loaded electrospun PCL/gelatin nanofiber membrane as potential artificial periosteum. Colloids Surf. B 2018, 170, 201–209. [Google Scholar] [CrossRef]
- Graneli, C.; Thorfve, A.; Ruetschi, U.; Brisby, H.; Thomsen, P.; Lindahl, A.; Karlsson, C. Novel markers of osteogenic and adipogenic differentiation of human bone marrow stromal cells identified using a quantitative proteomics approach. Stem Cell Res. 2014, 12, 153–165. [Google Scholar] [CrossRef]
- Thiagarajan, L.; Abu-Awwad, H.A.D.M.; Dixon, J.E. Osteogenic Programming of Human Mesenchymal Stem Cells with Highly Efficient Intracellular Delivery of RUNX2. Stem Cell Transl. Med. 2017, 6, 2146–2159. [Google Scholar] [CrossRef]
- Blair, H.C.; Larrouture, Q.C.; Li, Y.N.; Lin, H.; Beer-Stoltz, D.; Liu, L.; Tuan, R.S.; Robinson, L.J.; Schlesinger, P.H.; Nelson, D.J. Osteoblast Differentiation and Bone Matrix Formation In Vivo and In Vitro. Tissue Eng. Part B-Rev. 2017, 23, 268–280. [Google Scholar] [CrossRef]
- Huang, Z.F.; Cheng, C.; Wang, J.; Liu, X.Z.; Wei, H.; Han, Y.; Yang, S.H.; Wang, X. Icariin regulates the osteoblast differentiation and cell proliferation of MC3T3-E1 cells through microRNA-153 by targeting Runt-related transcription factor 2. Exp. Ther. Med. 2018, 15, 5159–5166. [Google Scholar] [CrossRef]
- Li, M.; Zhang, C.; Zhong, Y.; Zhao, J.Y. A Novel Approach to Utilize Icariin as Icariin-Derived ECM on Small Intestinal Submucosa Scaffold for Bone Repair. Ann. Biomed. Eng. 2017, 45, 2673–2682. [Google Scholar] [CrossRef]

| Elements | C1s (%) | N1s (%) | O1s (%) | Total (%) | |
|---|---|---|---|---|---|
| Groups | |||||
| NDs | 91.60 | - | 8.40 | 100 | |
| NH2-NDs | 89.00 | 2.15 | 8.85 | 100 | |
| ICA (10 μg)-NDs | 89.20 | 1.90 | 8.90 | 100 | |
| ICA (50 μg)-NDs | 89.55 | 1.73 | 8.72 | 100 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.; Noh, S.H.; Lim, C.O.; Kim, H.-J.; Jo, H.-S.; Min, J.S.; Park, K.; Kim, S.E. Icariin-Functionalized Nanodiamonds to Enhance Osteogenic Capacity In Vitro. Nanomaterials 2020, 10, 2071. https://doi.org/10.3390/nano10102071
Choi S, Noh SH, Lim CO, Kim H-J, Jo H-S, Min JS, Park K, Kim SE. Icariin-Functionalized Nanodiamonds to Enhance Osteogenic Capacity In Vitro. Nanomaterials. 2020; 10(10):2071. https://doi.org/10.3390/nano10102071
Chicago/Turabian StyleChoi, Somang, Sung Hyun Noh, Chae Ouk Lim, Hak-Jun Kim, Han-Saem Jo, Ji Seon Min, Kyeongsoon Park, and Sung Eun Kim. 2020. "Icariin-Functionalized Nanodiamonds to Enhance Osteogenic Capacity In Vitro" Nanomaterials 10, no. 10: 2071. https://doi.org/10.3390/nano10102071
APA StyleChoi, S., Noh, S. H., Lim, C. O., Kim, H.-J., Jo, H.-S., Min, J. S., Park, K., & Kim, S. E. (2020). Icariin-Functionalized Nanodiamonds to Enhance Osteogenic Capacity In Vitro. Nanomaterials, 10(10), 2071. https://doi.org/10.3390/nano10102071