Molecular Ultrasound Imaging
Abstract
1. Introduction
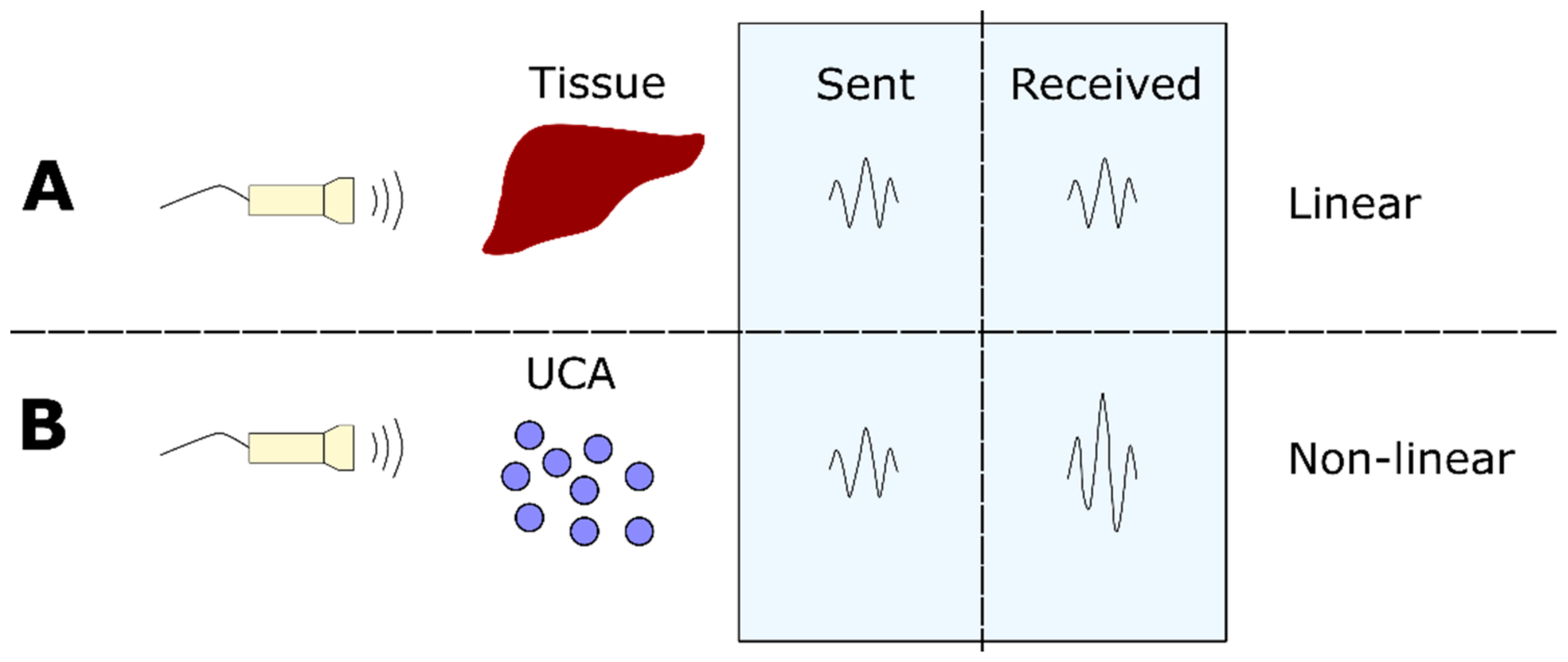
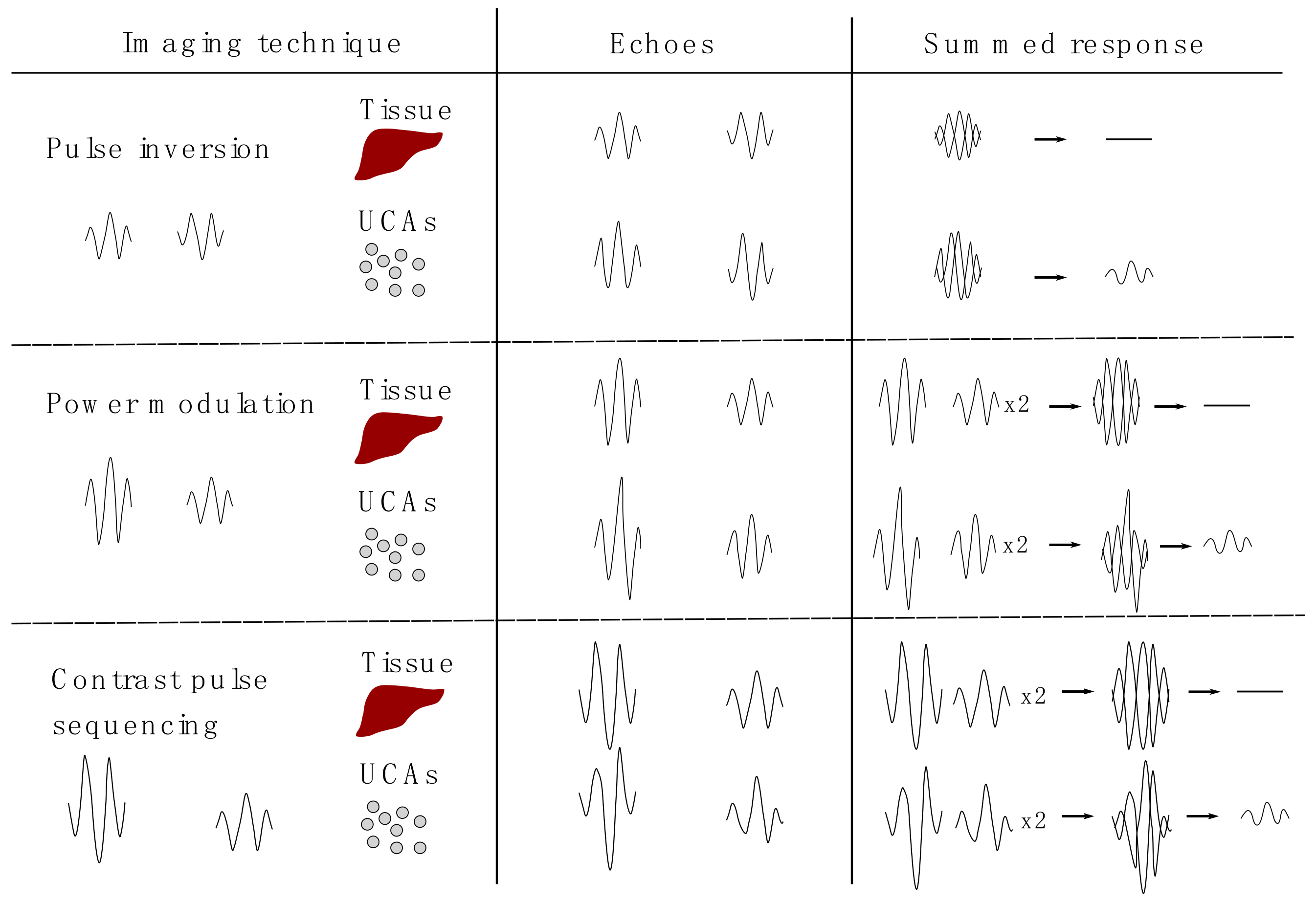
2. Detection Technologies
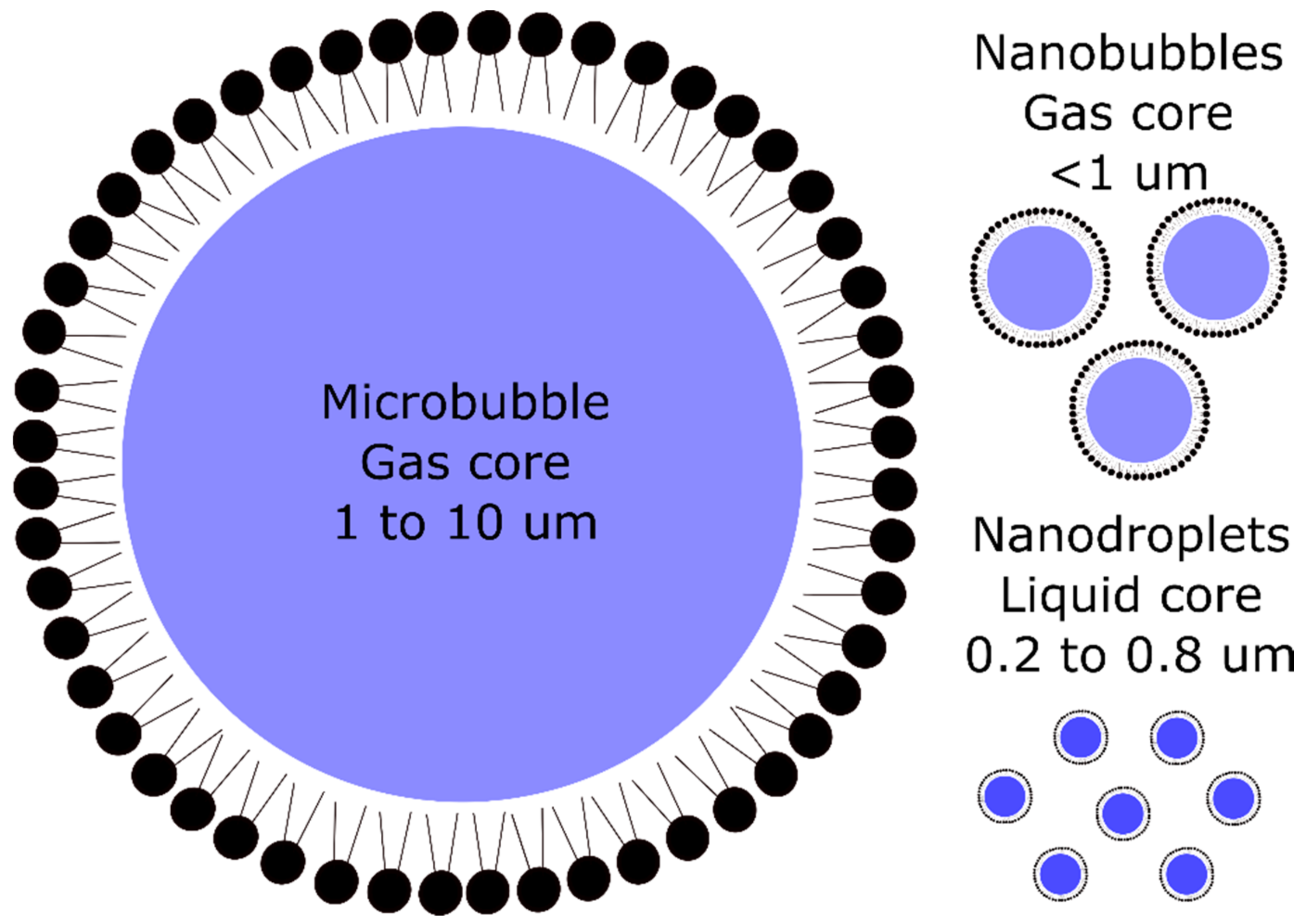
3. Targeted Contrast Agents
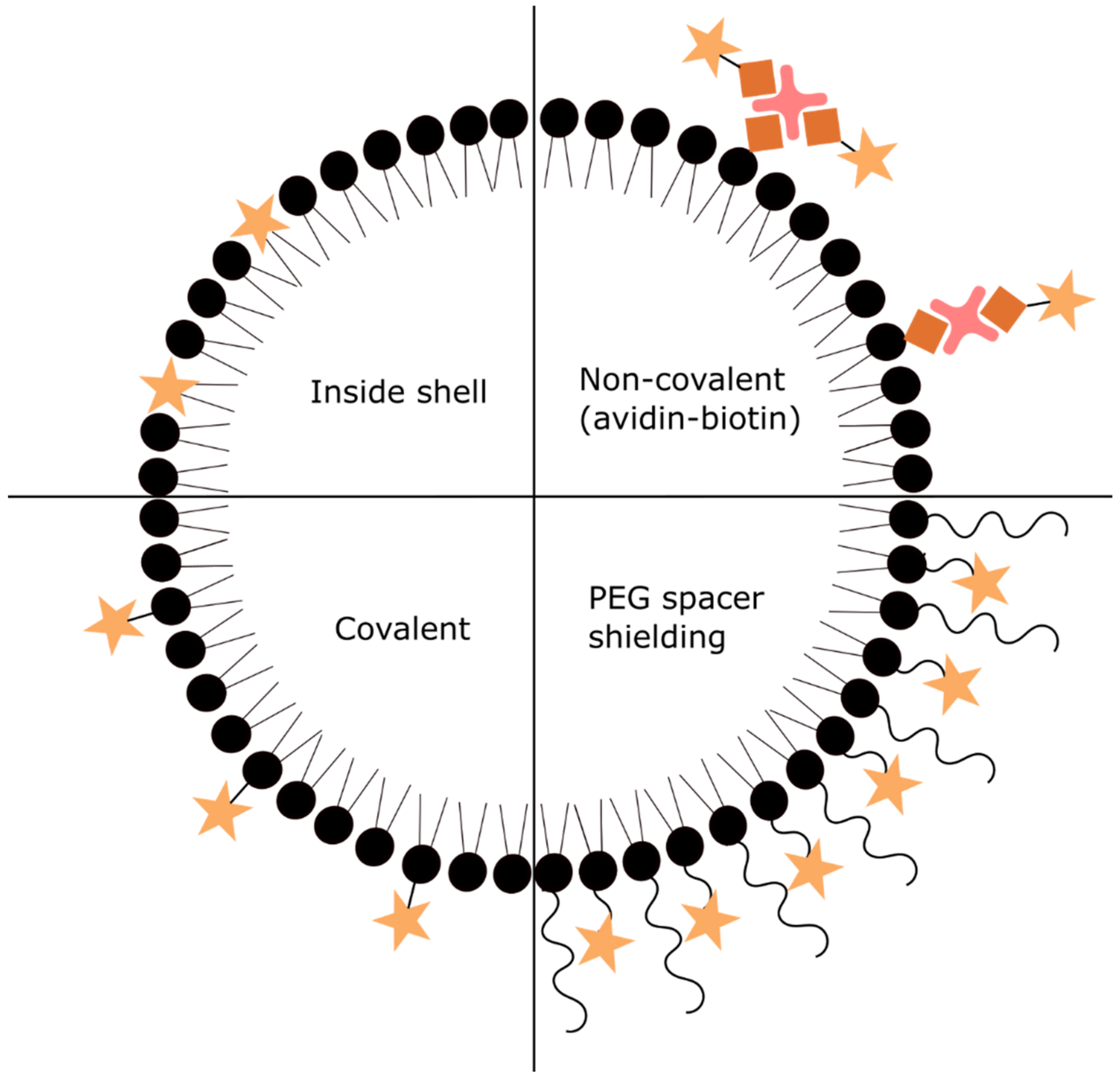
3.1. Functionalization of Contrast Agents
3.2. Intravascular Targeting (MB)
3.2.1. Angiogenesis
3.2.2. Inflammation and Atherosclerosis
3.2.3. Thrombosis
3.2.4. Multiple Targets
3.2.5. Targeted MB in The Clinics
3.3. Extravascular Targeting (NB)
3.4. Phase Shift Nanodroplets
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 18FDG | Fluorodeoxyglucose |
| ACPP | Activatable cell-penetrating peptides |
| ATWLPPR | Alanine-threonine-tryptophan-leucine-proline-proline-arginine |
| CAIX | Carbonic anhydrase IX |
| CEUS | Contrast-enhanced ultrasound |
| CRPPR | Cysteine-arginine-proline-proline-arginine |
| CT | Computed tomography |
| DVT | Deep venous thrombosis |
| EPR | Enhanced permeability and retention |
| GP | Glycoprotein |
| HER2 | Human epidermal growth factor receptor 2 |
| IBD | Inflammatory bowel disease |
| ICAM-1 | Intercellular adhesion molecule - 1 |
| IgG | Immunoglobulin G |
| JAM-A | Junctional adhesion molecule - A |
| KDR | Kinase insert domain receptor |
| KQAGDV | Lysine-glutamine-alanine-glycine-aspartate-valine |
| LOX-1 | Lectin-like oxidized low-density lipoprotein receptor-1 |
| MAdCAM-1 | Mucosal addressin cellular adhesion molecule - 1 |
| MB | Microbubbles |
| MMP | Matrix metalloproteinase |
| MRI | Magnetic resonance imaging |
| NB | Nanobubbles |
| PEG | Polyethylene glycol |
| PET | Positron emission tomography |
| proGRP | Pro-gastrin releasing peptide |
| PSGL-Ig | P-selectin glycoprotein ligand-1 analog |
| PSMA | Prostate specific membrane antigen |
| RGD | Arginine-glycine-aspartate |
| RRL | Arginine-arginine-leucine |
| SFRP2 | Secreted frizzled related protein 2 |
| SPAQ | Sensitive particle acoustic quantification |
| Thy 1 | Thymocyte differentiation antigen 1 |
| TNBS | 2, 4, 6-trinitrobenzene sulfonic acid |
| UCA | Ultrasound contrast agents |
| US | Ultrasound |
| VCAM-1 | Vascular cell adhesion molecule - 1 |
| VEGF | Vascular endothelial growth factor |
| VEGFR2 | Vascular endothelial growth factor receptor 2 |
| VWF | Von Willebrand factor |
References
- Uppal, T. Tissue harmonic imaging. Australas. J. Ultrasound Med. 2010, 13, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Frinking, P.; Segers, T.; Luan, Y.; Tranquart, F. Three Decades of Ultrasound Contrast Agents: A Review of the Past, Present and Future Improvements. Ultrasound Med. Biol. 2020, 46, 892–908. [Google Scholar] [CrossRef] [PubMed]
- Guevener, N.; Appold, L.; de Lorenzi, F.; Golombek, S.K.; Rizzo, L.Y.; Lammers, T.; Kiessling, F. Recent advances in ultrasound-based diagnosis and therapy with micro-and nanometer-sized formulations. Methods 2017, 130, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wang, L.; Liu, Y.; Xu, D.; Fang, K.; Guo, Y. CAIX aptamer-functionalized targeted nanobubbles for ultrasound molecular imaging of various tumors. IJN 2018, 13, 6481–6495. [Google Scholar] [CrossRef]
- Roovers, S.; Segers, T.; Lajoinie, G.; Deprez, J.; Versluis, M.; De Smedt, S.C.; Lentacker, I. The Role of Ultrasound-Driven Microbubble Dynamics in Drug Delivery: From Microbubble Fundamentals to Clinical Translation. Langmuir 2019, 35, 10173–10191. [Google Scholar] [CrossRef]
- Yu, J.; Chen, Z.; Yan, F. Advances in mechanism studies on ultrasonic gene delivery at cellular level. Prog. Biophys. Mol. Biol. 2019, 142, 1–9. [Google Scholar] [CrossRef]
- Omata, D.; Unga, J.; Suzuki, R.; Maruyama, K. Lipid-based microbubbles and ultrasound for therapeutic application. Adv. Drug Deliv. Rev. 2020. [Google Scholar] [CrossRef]
- Mor-Avi, V.; Caiani, E.G.; Collins, K.A.; Korcarz, C.E.; Bednarz, J.E.; Lang, R.M. Combined assessment of myocardial perfusion and regional left ventricular function by analysis of contrast-enhanced power modulation images. Circulation 2001, 104, 352–357. [Google Scholar] [CrossRef]
- Whittingham, T.A. Contrast-Specific Imaging Techniques: Technical Perspective. In Contrast Media in Ultrasonography: Basic Principles and Clinical Applications; Quaia, E., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 43–70. ISBN 978-3-540-27214-4. [Google Scholar]
- Phillips, P.J. Contrast Pulse Sequences (CPS): Imaging Nonlinear Microbubbles. In Proceedings of the 2001 IEEE Ultrasonics Symposium, Atlanta, GA, USA, 7–10 October 2001; pp. 1739–1745. [Google Scholar]
- Caskey, C.F.; Hu, X.; Ferrara, K.W. Leveraging the power of ultrasound for therapeutic design and optimization. J. Control. Release 2011, 156, 297–306. [Google Scholar] [CrossRef][Green Version]
- Reinhardt, M.; Hauff, P.; Briel, A.; Uhlendorf, V.; Linker, R.A.; Mäurer, M.; Schirner, M. Sensitive particle acoustic quantification (SPAQ): A new ultrasound-based approach for the quantification of ultrasound contrast media in high concentrations. Invest. Radiol. 2005, 40, 2–7. [Google Scholar]
- Wei, K.; Jayaweera, A.R.; Firoozan, S.; Linka, A.; Skyba, D.M.; Kaul, S. Quantification of Myocardial Blood Flow With Ultrasound-Induced Destruction of Microbubbles Administered as a Constant Venous Infusion. Circulation 1998, 97, 473–483. [Google Scholar] [CrossRef]
- Pysz, M.A.; Guracar, I.; Tian, L.; Willmann, J.K. Fast microbubble dwell-time based ultrasonic molecular imaging approach for quantification and monitoring of angiogenesis in cancer. Quant. Imaging Med. Surg. 2012, 2, 16. [Google Scholar]
- Zhao, R.; Jiang, J.; Li, H.; Chen, M.; Liu, R.; Sun, S.; Ma, D.; Liang, X.; Wang, S. Phosphatidylserine-microbubble targeting-activated microglia/macrophage in inflammation combined with ultrasound for breaking through the blood–brain barrier. J. Neuroinflamm. 2018, 15, s12974-s018. [Google Scholar] [CrossRef] [PubMed]
- Pochon, S.; Tardy, I.; Bussat, P.; Bettinger, T.; Brochot, J.; von Wronski, M.; Passantino, L.; Schneider, M. BR55: A Lipopeptide-Based VEGFR2-Targeted Ultrasound Contrast Agent for Molecular Imaging of Angiogenesis. Investig. Radiol. 2010, 45, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, F.S.; Jankowski, R.J.; Klibanov, S.; Pina, M.L.; Alber, S.M.; Watkins, S.C.; Brandenburger, G.H.; Wagner, W.R. Microbubbles Targeted to Intercellular Adhesion Molecule-1 Bind to Activated Coronary Artery Endothelial Cells. Circulation 1998, 98, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ham, A.S.; Klibanov, A.L.; Lawrence, M.B. Action at a distance: Lengthening adhesion bonds with poly(ethylene glycol) spacers enhances mechanically stressed affinity for improved vascular targeting of microparticles. Langmuir 2009, 25, 10038–10044. [Google Scholar] [CrossRef]
- Kim, D.H.; Klibanov, A.L.; Needham, D. The Influence of Tiered Layers of Surface-Grafted Poly(ethylene glycol) on Receptor−Ligand-Mediated Adhesion between Phospholipid Monolayer-Stabilized Microbubbles and Coated Glass Beads. Langmuir 2000, 16, 2808–2817. [Google Scholar] [CrossRef]
- Borden, M.A.; Sarantos, M.R.; Stieger, S.M.; Simon, S.I.; Ferrara, K.W.; Dayton, P.A. Ultrasound radiation force modulates ligand availability on targeted contrast agents. Mol. Imaging 2006, 5, 139–147. [Google Scholar] [CrossRef]
- Borden, M.A.; Zhang, H.; Gillies, R.J.; Dayton, P.A.; Ferrara, K.W. A stimulus-responsive contrast agent for ultrasound molecular imaging. Biomaterials 2008, 29, 597–606. [Google Scholar] [CrossRef]
- Ellegala, D.B.; Leong-Poi, H.; Carpenter, J.E.; Klibanov, A.L.; Kaul, S.; Shaffrey, M.E.; Sklenar, J.; Lindner, J.R. Imaging Tumor Angiogenesis With Contrast Ultrasound and Microbubbles Targeted to αvβ3. Circulation 2003, 108, 336–341. [Google Scholar] [CrossRef]
- Leong-Poi, H.; Christiansen, J.; Klibanov, A.L.; Kaul, S.; Lindner, J.R. Noninvasive Assessment of Angiogenesis by Ultrasound and Microbubbles Targeted to αv-Integrins. Circulation 2003, 107, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Leong-Poi, H.; Christiansen, J.; Heppner, P.; Lewis, C.W.; Klibanov, A.L.; Kaul, S.; Lindner, J.R. Assessment of Endogenous and Therapeutic Arteriogenesis by Contrast Ultrasound Molecular Imaging of Integrin Expression. Circulation 2005, 111, 3248–3254. [Google Scholar] [CrossRef] [PubMed]
- Willmann, J.K.; Kimura, R.H.; Deshpande, N.; Lutz, A.M.; Cochran, J.R.; Gambhir, S.S. Targeted Contrast-Enhanced Ultrasound Imaging of Tumor Angiogenesis with Contrast Microbubbles Conjugated to Integrin-Binding Knottin Peptides. J. Nucl. Med. 2010, 51, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.R.; Hu, X.; Zhang, H.; Tlaxca, J.; Declèves, A.-E.; Houghtaling, R.; Sharma, K.; Lawrence, M.; Ferrara, K.W.; Rychak, J.J. Ultrasound Molecular Imaging of Tumor Angiogenesis With an Integrin Targeted Microbubble Contrast Agent. Investig. Radiol. 2011, 46, 215–224. [Google Scholar] [CrossRef]
- Barua, A.; Yellapa, A.; Bahr, J.M.; Machado, S.A.; Bitterman, P.; Basu, S.; Sharma, S.; Abramowicz, J.S. ATL: A Preclinical Model of Spontaneous Ovarian Cancer. Int. J. Gynecol. Cancer 2014, 24, 19–28. [Google Scholar] [CrossRef]
- Palmowski, M.; Peschke, P.; Huppert, J.; Hauff, P.; Reinhardt, M.; Maurer, M.; Karger, C.P.; Scholz, M.; Semmler, W.; Huber, P.E.; et al. Molecular Ultrasound Imaging of Early Vascular Response in Prostate Tumors Irradiated with Carbon Ions. Neoplasia 2009, 11, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Palmowski, M.; Huppert, J.; Ladewig, G.; Hauff, P.; Reinhardt, M.; Mueller, M.M.; Woenne, E.C.; Jenne, J.W.; Maurer, M.; Kauffmann, G.W.; et al. Molecular profiling of angiogenesis with targeted ultrasound imaging: Early assessment of antiangiogenic therapy effects. Mol. Cancer Ther. 2008, 7, 101–109. [Google Scholar] [CrossRef]
- Deshpande, N.; Ren, Y.; Foygel, K.; Rosenberg, J.; Willmann, J.K. Tumor Angiogenic Marker Expression Levels during Tumor Growth: Longitudinal Assessment with Molecularly Targeted Microbubbles and US Imaging. Radiology 2011, 258, 804–811. [Google Scholar] [CrossRef]
- Leguerney, I.; Scoazec, J.-Y.; Gadot, N.; Robin, N.; Pénault-Llorca, F.; Victorin, S.; Lassau, N. Molecular Ultrasound Imaging Using Contrast Agents Targeting Endoglin, Vascular Endothelial Growth Factor Receptor 2 and Integrin. Ultrasound Med. Biol. 2015, 41, 197–207. [Google Scholar] [CrossRef]
- Weller, G.E.R.; Wong, M.K.K.; Modzelewski, R.A.; Lu, E.; Klibanov, A.L.; Wagner, W.R.; Villanueva, F.S. Ultrasonic Imaging of Tumor Angiogenesis Using Contrast Microbubbles Targeted via the Tumor-Binding Peptide Arginine-Arginine-Leucine. Cancer Res. 2005, 65, 533–539. [Google Scholar]
- Rychak, J.J.; Graba, J.; Cheung, A.M.Y.; Mystry, B.S.; Lindner, J.R.; Kerbel, R.S.; Foster, F.S. Microultrasound Molecular Imaging of Vascular Endothelial Growth Factor Receptor 2 in a Mouse Model of Tumor Angiogenesis. Mol. Imaging 2007, 6. [Google Scholar] [CrossRef]
- Wang, S.; Herbst, E.B.; Mauldin, F.W.; Diakova, G.B.; Klibanov, A.L.; Hossack, J.A. Ultra–Low-Dose Ultrasound Molecular Imaging for the Detection of Angiogenesis in a Mouse Murine Tumor Model: How Little Can We See? Investig. Radiol. 2016, 51, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Korpanty, G.; Carbon, J.G.; Grayburn, P.A.; Fleming, J.B.; Brekken, R.A. Monitoring Response to Anticancer Therapy by Targeting Microbubbles to Tumor Vasculature. Clin. Cancer Res. 2007, 13, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Willmann, J.K.; Paulmurugan, R.; Chen, K.; Gheysens, O.; Rodriguez-Porcel, M.; Lutz, A.M.; Chen, I.Y.; Chen, X.; Gambhir, S.S. US Imaging of Tumor Angiogenesis with Microbubbles Targeted to Vascular Endothelial Growth Factor Receptor Type 2 in Mice. Radiology 2008, 246, 508–518. [Google Scholar] [CrossRef]
- Willmann, J.K.; Cheng, Z.; Davis, C.; Lutz, A.M.; Schipper, M.L.; Nielsen, C.H.; Gambhir, S.S. Targeted Microbubbles for Imaging Tumor Angiogenesis: Assessment of Whole-Body Biodistribution with Dynamic Micro-PET in Mice. Radiology 2008, 249, 212–219. [Google Scholar] [CrossRef]
- Lyshchik, A.; Fleischer, A.C.; Huamani, J.; Hallahan, D.E.; Brissova, M.; Gore, J.C. Molecular Imaging of Vascular Endothelial Growth Factor Receptor 2 Expression Using Targeted Contrast-Enhanced High-Frequency Ultrasonography. J. Ultrasound Med. 2007, 26, 1575–1586. [Google Scholar] [CrossRef]
- Bzyl, J.; Lederle, W.; Rix, A.; Grouls, C.; Tardy, I.; Pochon, S.; Siepmann, M.; Penzkofer, T.; Schneider, M.; Kiessling, F.; et al. Molecular and functional ultrasound imaging in differently aggressive breast cancer xenografts using two novel ultrasound contrast agents (BR55 and BR38). Eur. Radiol. 2011, 21, 1988–1995. [Google Scholar] [CrossRef]
- Abou-Elkacem, L.; Wilson, K.E.; Johnson, S.M.; Chowdhury, S.M.; Bachawal, S.; Hackel, B.J.; Tian, L.; Willmann, J.K. Ultrasound Molecular Imaging of the Breast Cancer Neovasculature using Engineered Fibronectin Scaffold Ligands: A Novel Class of Targeted Contrast Ultrasound Agent. Theranostics 2016, 6, 1740–1752. [Google Scholar] [CrossRef]
- Anderson, C.R.; Rychak, J.J.; Backer, M.; Backer, J.; Ley, K.; Klibanov, A.L. scVEGF Microbubble Ultrasound Contrast Agents: A Novel Probe for Ultrasound Molecular Imaging of Tumor Angiogenesis. Investig. Radiol. 2010, 45, 579–585. [Google Scholar] [CrossRef]
- Tardy, I.; Pochon, S.; Theraulaz, M.; Emmel, P.; Passantino, L.; Tranquart, F.; Schneider, M. Ultrasound molecular imaging of VEGFR2 in a rat prostate tumor model using BR55. Investig. Radiol. 2010, 45, 573–578. [Google Scholar] [CrossRef]
- Patel, M.; Vadlapatla, R.K.; Pal, D.; Mitra, A.K. Molecular and functional characterization of riboflavin specific transport system in rat brain capillary endothelial cells. Brain Res. 2012, 1468, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bzyl, J.; Palmowski, M.; Rix, A.; Arns, S.; Hyvelin, J.-M.; Pochon, S.; Ehling, J.; Schrading, S.; Kiessling, F.; Lederle, W. The high angiogenic activity in very early breast cancer enables reliable imaging with VEGFR2-targeted microbubbles (BR55). Eur. Radiol. 2013, 23, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Zafarnia, S.; Bzyl-Ibach, J.; Spivak, I.; Li, Y.; Koletnik, S.; Doleschel, D.; Rix, A.; Pochon, S.; Tardy, I.; Koyadan, S.; et al. Nilotinib Enhances Tumor Angiogenesis and Counteracts VEGFR2 Blockade in an Orthotopic Breast Cancer Xenograft Model with Desmoplastic Response. Neoplasia 2017, 19, 896–907. [Google Scholar] [CrossRef] [PubMed]
- Bachawal, S.V.; Jensen, K.C.; Lutz, A.M.; Gambhir, S.S.; Tranquart, F.; Tian, L.; Willmann, J.K. Earlier detection of breast cancer with ultrasound molecular imaging in a transgenic mouse model. Cancer Res. 2013, 73, 1689–1698. [Google Scholar] [CrossRef] [PubMed]
- Pysz, M.A.; Machtaler, S.B.; Seeley, E.S.; Lee, J.J.; Brentnall, T.A.; Rosenberg, J.; Tranquart, F.; Willmann, J.K. Vascular Endothelial Growth Factor Receptor Type 2–targeted Contrast-enhanced US of Pancreatic Cancer Neovasculature in a Genetically Engineered Mouse Model: Potential for Earlier Detection. Radiology 2015, 274, 790–799. [Google Scholar] [CrossRef]
- Wang, H.; Kaneko, O.F.; Tian, L.; Hristov, D.; Willmann, J.K. Three-Dimensional Ultrasound Molecular Imaging of Angiogenesis in Colon Cancer Using a Clinical Matrix Array Ultrasound Transducer. Investig. Radiol. 2015, 50, 322–329. [Google Scholar] [CrossRef]
- Baetke, S.C.; Rix, A.; Tranquart, F.; Schneider, R.; Lammers, T.; Kiessling, F.; Lederle, W. Squamous Cell Carcinoma Xenografts: Use of VEGFR2-targeted Microbubbles for Combined Functional and Molecular US to Monitor Antiangiogenic Therapy Effects. Radiology 2016, 278, 430–440. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, H.; Zhang, H.; Lutz, A.M.; Tian, L.; Hristov, D.; Willmann, J.K. VEGFR2-Targeted Three-Dimensional Ultrasound Imaging Can Predict Responses to Antiangiogenic Therapy in Preclinical Models of Colon Cancer. Cancer Res. 2016, 76, 4081–4089. [Google Scholar] [CrossRef]
- Eschbach, R.S.; Clevert, D.-A.; Hirner-Eppeneder, H.; Ingrisch, M.; Moser, M.; Schuster, J.; Tadros, D.; Schneider, M.; Kazmierczak, P.M.; Reiser, M.; et al. Contrast-Enhanced Ultrasound with VEGFR2-Targeted Microbubbles for Monitoring Regorafenib Therapy Effects in Experimental Colorectal Adenocarcinomas in Rats with DCE-MRI and Immunohistochemical Validation. PLoS ONE 2017, 12, e0169323. [Google Scholar] [CrossRef]
- Zhang, H.; Tam, S.; Ingham, E.S.; Mahakian, L.M.; Lai, C.-Y.; Tumbale, S.K.; Teesalu, T.; Hubbard, N.E.; Borowsky, A.D.; Ferrara, K.W. Ultrasound molecular imaging of tumor angiogenesis with a neuropilin-1-targeted microbubble. Biomaterials 2015, 56, 104–113. [Google Scholar] [CrossRef]
- Tsuruta, J.K.; Klauber-DeMore, N.; Streeter, J.; Samples, J.; Patterson, C.; Mumper, R.J.; Ketelsen, D.; Dayton, P. Ultrasound Molecular Imaging of Secreted Frizzled Related Protein-2 Expression in Murine Angiosarcoma. PLoS ONE 2014, 9, e86642. [Google Scholar] [CrossRef] [PubMed]
- Bachawal, S.V.; Jensen, K.C.; Wilson, K.E.; Tian, L.; Lutz, A.M.; Willmann, J.K. Breast Cancer Detection by B7-H3-Targeted Ultrasound Molecular Imaging. Cancer Res. 2015, 75, 2501–2509. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ingham, E.S.; Gagnon, M.K.J.; Mahakian, L.M.; Liu, J.; Foiret, J.L.; Willmann, J.K.; Ferrara, K.W. In Vitro Characterization and In Vivo Ultrasound Molecular Imaging of Nucleolin-Targeted Microbubbles. Biomaterials 2017, 118, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Abou-Elkacem, L.; Wang, H.; Chowdhury, S.M.; Kimura, R.H.; Bachawal, S.V.; Gambhir, S.S.; Tian, L.; Willmann, J.K. Thy1-Targeted Microbubbles for Ultrasound Molecular Imaging of Pancreatic Ductal Adenocarcinoma. Clin Cancer Res. 2018, 24, 1574–1585. [Google Scholar] [CrossRef] [PubMed]
- Lindner, J.R.; Song, J.; Xu, F.; Klibanov, A.L.; Singbartl, K.; Ley, K.; Kaul, S. Noninvasive Ultrasound Imaging of Inflammation Using Microbubbles Targeted to Activated Leukocytes. Circulation 2000, 102, 2745–2750. [Google Scholar] [CrossRef]
- Christiansen, J.P.; Leong-Poi, H.; Klibanov, A.L.; Kaul, S.; Lindner, J.R. Noninvasive Imaging of Myocardial Reperfusion Injury Using Leukocyte-Targeted Contrast Echocardiography. Circulation 2002, 105, 1764–1767. [Google Scholar] [CrossRef]
- Weller, G.E.R.; Villanueva, F.S.; Klibanov, A.L.; Wagner, W.R. Modulating Targeted Adhesion of an Ultrasound Contrast Agent to Dysfunctional Endothelium. Ann. Biomed. Eng. 2002, 30, 1012–1019. [Google Scholar] [CrossRef]
- Bachmann, C.; Klibanov, A.L.; Olson, T.S.; Sonnenschein, J.R.; Rivera-Nieves, J.; Cominelli, F.; Ley, K.F.; Lindner, J.R.; Pizarro, T.T. Targeting mucosal addressin cellular adhesion molecule (MAdCAM)-1 to noninvasively image experimental Crohn’s disease. Gastroenterology 2006, 130, 8–16. [Google Scholar] [CrossRef][Green Version]
- Curaj, A.; Wu, Z.; Rix, A.; Gresch, O.; Sternkopf, M.; Alampour-Rajabi, S.; Lammers, T.; van Zandvoort, M.; Weber, C.; Koenen, R.R.; et al. Molecular Ultrasound Imaging of Junctional Adhesion Molecule A Depicts Acute Alterations in Blood Flow and Early Endothelial Dysregulation. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 40–48. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Bai, D.-N.; Du, J.-X.; Jin, L.; Ma, J.; Yang, J.-L.; Cai, W.-B.; Feng, Y.; Xing, C.-Y.; Yuan, L.-J.; et al. Ultrasound-guided imaging of junctional adhesion molecule-A-targeted microbubbles identifies vulnerable plaque in rabbits. Biomaterials 2016, 94, 20–30. [Google Scholar] [CrossRef]
- Liu, Y.; Davidson, B.P.; Yue, Q.; Belcik, T.; Xie, A.; Inaba, Y.; McCarty Owen, J.T.; Tormoen Garth, W.; Zhao, Y.; Ruggeri, Z.M.; et al. Molecular Imaging of Inflammation and Platelet Adhesion in Advanced Atherosclerosis Effects of Antioxidant Therapy With NADPH Oxidase Inhibition. Circ. Cardiovasc. Imaging 2013, 6, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Khanicheh, E.; Qi, Y.; Xie, A.; Mitterhuber, M.; Xu, L.; Mochizuki, M.; Daali, Y.; Jaquet, V.; Krause, K.-H.; Ruggeri, Z.M.; et al. Molecular imaging reveals rapid reduction of endothelial activation in early atherosclerosis with apocynin independent of antioxidative properties. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2187–2192. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, B.A.; Carr, C.L.; Belcik, J.T.; Xie, A.; Yue, Q.; Chadderdon, S.; Caplan, E.S.; Khangura, J.; Bullens, S.; Bunting, S.; et al. Molecular imaging of the initial inflammatory response in atherosclerosis: Implications for early detection of disease. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Khanicheh, E.; Mitterhuber, M.; Xu, L.; Haeuselmann, S.P.; Kuster, G.M.; Kaufmann, B.A. Noninvasive Ultrasound Molecular Imaging of the Effect of Statins on Endothelial Inflammatory Phenotype in Early Atherosclerosis. PLoS ONE 2013, 8, e58761. [Google Scholar] [CrossRef]
- Kaufmann, B.A.; Sanders, J.M.; Davis, C.; Xie, A.; Aldred, P.; Sarembock, I.J.; Lindner, J.R. Molecular imaging of inflammation in atherosclerosis with targeted ultrasound detection of vascular cell adhesion molecule-1. Circulation 2007, 116, 276–284. [Google Scholar] [CrossRef]
- Curaj, A.; Wu, Z.; Fokong, S.; Liehn, E.A.; Weber, C.; Burlacu, A.; Lammers, T.; van Zandvoort, M.; Kiessling, F. Noninvasive Molecular Ultrasound Monitoring of Vessel Healing After Intravascular Surgical Procedures in a Preclinical Setup. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1366–1373. [Google Scholar] [CrossRef]
- Moccetti, F.; Brown, E.; Xie, A.; Packwood, W.; Qi, Y.; Ruggeri, Z.; Shentu, W.; Chen, J.; López, J.A.; Lindner, J.R. Myocardial Infarction Produces Sustained Proinflammatory Endothelial Activation in Remote Arteries. J. Am. Coll. Cardiol. 2018, 72, 1015–1026. [Google Scholar] [CrossRef]
- Wang, S.; Unnikrishnan, S.; Herbst, E.B.; Klibanov, A.L.; Mauldin, F.W.; Hossack, J.A. Ultrasound Molecular Imaging of Inflammation in Mouse Abdominal Aorta. Investig. Radiol 2017, 52, 499–506. [Google Scholar] [CrossRef]
- Masseau, I.; Davis, M.J.; Bowles, D.K. Carotid inflammation is unaltered by exercise in hypercholesterolemic swine. Med. Sci. Sports Exerc. 2012, 44, 2277–2289. [Google Scholar] [CrossRef]
- Koczera, P.; Appold, L.; Shi, Y.; Liu, M.; Dasgupta, A.; Pathak, V.; Ojha, T.; Fokong, S.; Wu, Z.; van Zandvoort, M.; et al. PBCA-based Polymeric Microbubbles for Molecular Imaging and Drug Delivery. J. Control. Release 2017, 259, 128–135. [Google Scholar] [CrossRef]
- Punjabi, M.; Xu, L.; Ochoa-Espinosa, A.; Kosareva, A.; Wolff, T.; Murtaja, A.; Broisat, A.; Devoogdt, N.; Kaufmann, B.A. Ultrasound Molecular Imaging of Atherosclerosis With Nanobodies: Translatable Microbubble Targeting Murine and Human VCAM (Vascular Cell Adhesion Molecule) 1. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 2520–2530. [Google Scholar] [CrossRef] [PubMed]
- Moccetti, F.; Weinkauf, C.C.; Davidson, B.P.; Belcik, J.T.; Marinelli, E.R.; Unger, E.; Lindner, J.R. Ultrasound Molecular Imaging of Atherosclerosis Using Small-Peptide Targeting Ligands Against Endothelial Markers of Inflammation and Oxidative Stress. Ultrasound Med. Biol. 2018, 44, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Weller, G.E.; Wong, M.K.; Modzelewski, R.A.; Lu, E.; Klibanov, A.L.; Wagner, W.R.; Villanueva, F.S. Ultrasound Imaging of Acute Cardiac Transplant Rejection With Microbubbles Targeted to Intercellular Adhesion Molecule-1. Circulation 2003, 108, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Lindner, J.R.; Song, J.; Christiansen, J.; Klibanov, A.L.; Xu, F.; Ley, K. Ultrasound Assessment of Inflammation and Renal Tissue Injury With Microbubbles Targeted to P-Selectin. Circulation 2001, 104, 2107–2112. [Google Scholar] [CrossRef]
- Klibanov, A.L.; Rychak, J.J.; Yang, W.C.; Alikhani, S.; Li, B.; Acton, S.; Lindner, J.R.; Ley, K.; Kaul, S. Targeted ultrasound contrast agent for molecular imaging of inflammation in high-shear flow. Contrast Media Mol. Imaging 2006, 1, 259–266. [Google Scholar] [CrossRef]
- Takalkar, A.M.; Klibanov, A.L.; Rychak, J.J.; Lindner, J.R.; Ley, K. Binding and detachment dynamics of microbubbles targeted to P-selectin under controlled shear flow. J. Control. Release 2004, 96, 473–482. [Google Scholar] [CrossRef]
- Rychak, J.J.; Lindner, J.R.; Ley, K.; Klibanov, A.L. Deformable gas-filled microbubbles targeted to P-selectin. J. Control. Release 2006, 114, 288–299. [Google Scholar] [CrossRef]
- Bettinger, T.; Bussat, P.; Tardy, I.; Pochon, S.; Hyvelin, J.-M.; Emmel, P.; Henrioud, S.; Biolluz, N.; Willmann, J.K.; Schneider, M.; et al. Ultrasound Molecular Imaging Contrast Agent Binding to Both E- and P-Selectin in Different Species. Investig. Radiol. 2012, 47, 516–523. [Google Scholar] [CrossRef]
- Deshpande, N.; Lutz, A.M.; Ren, Y.; Foygel, K.; Tian, L.; Schneider, M.; Pai, R.; Pasricha, P.J.; Willmann, J.K. Quantification and Monitoring of Inflammation in Murine Inflammatory Bowel Disease with Targeted Contrast-enhanced US. Radiology 2012, 262, 172–180. [Google Scholar] [CrossRef]
- El Kaffas, A.; Smith, K.; Pradhan, P.; Machtaler, S.; Wang, H.; von Eyben, R.; Willmann, J.K.; Hristov, D. Molecular Contrast-Enhanced Ultrasound Imaging of Radiation-Induced P-Selectin Expression in Healthy Mice Colon. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 581–585. [Google Scholar] [CrossRef]
- Kaufmann, B.A.; Lewis, C.; Xie, A.; Mirza-Mohd, A.; Lindner, J.R. Detection of recent myocardial ischaemia by molecular imaging of P-selectin with targeted contrast echocardiography. Eur. Heart J. 2007, 28, 2011–2017. [Google Scholar] [CrossRef] [PubMed]
- Davidson, B.P.; Kaufmann, B.A.; Belcik, J.T.; Xie, A.; Qi, Y.; Lindner, J.R. Detection of Antecedent Myocardial Ischemia With Multiselectin Molecular Imaging. J. Am. Coll. Cardiol. 2012, 60, 1690–1697. [Google Scholar] [CrossRef] [PubMed]
- Hyvelin, J.-M.; Tardy, I.; Bettinger, T.; von Wronski, M.; Costa, M.; Emmel, P.; Colevret, D.; Bussat, P.; Lassus, A.; Botteron, C.; et al. Ultrasound Molecular Imaging of Transient Acute Myocardial Ischemia With a Clinically Translatable P- and E-Selectin Targeted Contrast Agent: Correlation With the Expression of Selectins. Investig. Radiol. 2014, 49, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Fokong, S.; Fragoso, A.; Rix, A.; Curaj, A.; Wu, Z.; Lederle, W.; Iranzo, O.; Gätjens, J.; Kiessling, F.; Palmowski, M. Ultrasound Molecular Imaging of E-Selectin in Tumor Vessels Using Poly n-Butyl Cyanoacrylate Microbubbles Covalently Coupled to a Short Targeting Peptide. Investig. Radiol. 2013, 48, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Spivak, I.; Rix, A.; Schmitz, G.; Fokong, S.; Iranzo, O.; Lederle, W.; Kiessling, F. Low-Dose Molecular Ultrasound Imaging with E-Selectin-Targeted PBCA Microbubbles. Mol. Imaging Biol. 2016, 18, 180–190. [Google Scholar] [CrossRef]
- Leng, X.; Wang, J.; Carson, A.; Chen, X.; Fu, H.; Ottoboni, S.; Wagner, W.R.; Villanueva, F.S. Ultrasound Detection of Myocardial Ischemic Memory Using an E-Selectin Targeting Peptide Amenable to Human Application. Mol. Imaging 2014, 13. [Google Scholar] [CrossRef]
- Unger, E.C.; McCreery, T.P.; Sweitzer, R.H.; Shen, D.; Wu, G. In vitro studies of a new thrombus-specific ultrasound contrast agent. Am. J. Cardiol. 1998, 81, 58G–61G. [Google Scholar] [CrossRef]
- Unger, E.; Metzger, P.; Krupinski, E.; Baker, M.; Hulett, R.; Gabaeff, D.; Mills, J.; Ihnat, D.; McCreery, T. The use of a thrombus-specific ultrasound contrast agent to detect thrombus in arteriovenous fistulae. Investig. Radiol. 2000, 35, 86–89. [Google Scholar] [CrossRef]
- Wu, W.; Wang, Y.; Shen, S.; Wu, J.; Guo, S.; Su, L.; Hou, F.; Wang, Z.; Liao, Y.; Bin, J. In Vivo Ultrasound Molecular Imaging of Inflammatory Thrombosis in Arteries With Cyclic Arg-Gly-Asp–Modified Microbubbles Targeted to Glycoprotein IIb/IIIa. Investig. Radiol. 2013, 48, 803–812. [Google Scholar] [CrossRef]
- Hu, G.; Liu, C.; Liao, Y.; Yang, L.; Huang, R.; Wu, J.; Xie, J.; Bundhoo, K.; Liu, Y.; Bin, J. Ultrasound molecular imaging of arterial thrombi with novel microbubbles modified by cyclic RGD in vitro and in vivo. Thromb. Haemost. 2012, 107, 172–183. [Google Scholar] [CrossRef]
- Wang, X.; Hagemeyer, C.E.; Hohmann, J.D.; Leitner, E.; Armstrong, P.C.; Jia, F.; Olschewski, M.; Needles, A.; Peter, K.; Ahrens, I. Novel single-chain antibody-targeted microbubbles for molecular ultrasound imaging of thrombosis: Validation of a unique noninvasive method for rapid and sensitive detection of thrombi and monitoring of success or failure of thrombolysis in mice. Circulation 2012, 125, 3117–3126. [Google Scholar] [CrossRef] [PubMed]
- Metzger, K.; Vogel, S.; Chatterjee, M.; Borst, O.; Seizer, P.; Schönberger, T.; Geisler, T.; Lang, F.; Langer, H.; Rheinlaender, J.; et al. High-frequency ultrasound-guided disruption of glycoprotein VI-targeted microbubbles targets atheroprogressison in mice. Biomaterials 2015, 36, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuka, M.A.; Mattrey, R.F.; Esener, S.C.; Cha, J.N.; Goodwin, A.P. Aptamer-Crosslinked Microbubbles: Smart Contrast Agents for Thrombin-Activated Ultrasound Imaging. Adv. Mater. 2012, 24, 6010–6016. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuka, M.A.; Barback, C.V.; Fitch, K.R.; Farwell, A.R.; Esener, S.C.; Mattrey, R.F.; Cha, J.N.; Goodwin, A.P. In Vivo Ultrasound Visualization of Non-Occlusive Blood Clots with Thrombin-Sensitive Contrast Agents. Biomaterials 2013, 34, 9559–9565. [Google Scholar] [CrossRef] [PubMed]
- Lux, J.; Vezeridis, A.M.; Hoyt, K.; Adams, S.R.; Armstrong, A.M.; Sirsi, S.R.; Mattrey, R.F. Thrombin-Activatable Microbubbles as Potential Ultrasound Contrast Agents for the Detection of Acute Thrombosis. ACS Appl. Mater. Interfaces 2017, 9, 37587–37596. [Google Scholar] [CrossRef] [PubMed]
- Dayton, P.A.; Morgan, K.E.; Klibanov, A.L.; Brandenburger, G.; Nightingale, K.R.; Ferrara, K.W. A preliminary evaluation of the effects of primary and secondary radiation forces on acoustic contrast agents. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1997, 44, 1264–1277. [Google Scholar] [CrossRef]
- Doinikov, A.A. Acoustic radiation forces: Classical theory and recent advances. Recent Res. Dev. Acoust. 2003, 37661, 39–61. [Google Scholar]
- Dayton, P.; Klibanov, A.; Brandenburger, G.; Ferrara, K. Acoustic radiation force in vivo: A mechanism to assist targeting of microbubbles. Ultrasound Med. Biol. 1999, 25, 1195–1201. [Google Scholar] [CrossRef]
- Zhao, S.; Borden, M.; Bloch, S.H.; Kruse, D.; Ferrara, K.W.; Dayton, P.A. Radiation-Force Assisted Targeting Facilitates Ultrasonic Molecular Imaging. Mol. Imaging 2004, 3, 14. [Google Scholar] [CrossRef]
- Gessner, R.C.; Streeter, J.E.; Kothadia, R.; Feingold, S.; Dayton, P.A. An In Vivo Validation of the Application of Acoustic Radiation Force to Enhance the Diagnostic Utility of Molecular Imaging Using 3-D Ultrasound. Ultrasound Med. Biol. 2012, 38, 651–660. [Google Scholar] [CrossRef]
- Wang, S.; Wang, C.Y.; Unnikrishnan, S.; Klibanov, A.L.; Hossack, J.A.; Mauldin, F.W. Optical Verification of Microbubble Response to Acoustic Radiation Force in Large Vessels With In Vivo Results. Investig. Radiol. 2015, 50, 772–784. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, S.; Mauldin, F.W.; Unnikrishnan, S.; Klibanov, A.L.; Hossack, J.A. Ultrasound quantification of molecular marker concentration in large blood vessels. In Proceedings of the 2014 IEEE International Ultrasonics Symposium, Chicago, IL, USA, 3–6 September 2014; pp. 831–834. [Google Scholar]
- Ferrara, N. Vascular Endothelial Growth Factor: Basic Science and Clinical Progress. Endocr. Rev. 2004, 25, 581–611. [Google Scholar] [CrossRef] [PubMed]
- Hicklin, D.J.; Ellis, L.M. Role of the Vascular Endothelial Growth Factor Pathway in Tumor Growth and Angiogenesis. J. Clin. Oncol. 2005, 23, 1011–1027. [Google Scholar] [CrossRef] [PubMed]
- Hood, J.D.; Cheresh, D.A. Role of integrins in cell invasion and migration. Nat. Rev. Cancer 2002, 2, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Brooks, P.C.; Montgomery, A.M.; Rosenfeld, M.; Reisfeld, R.A.; Hu, T.; Klier, G.; Cheresh, D.A. Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell 1994, 79, 1157–1164. [Google Scholar] [CrossRef]
- Brooks, P.C.; Clark, R.A.; Cheresh, D.A. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science 1994, 264, 569–571. [Google Scholar] [CrossRef]
- Van Waes, C. Cell adhesion and regulatory molecules involved in tumor formation, hemostasis, and wound healing. Head Neck 1995, 17, 140–147. [Google Scholar] [CrossRef]
- Fonsatti, E.; Altomonte, M.; Arslan, P.; Maio, M. Endoglin (CD105): A target for anti-angiogenetic cancer therapy. Curr. Drug Targets 2003, 4, 291–296. [Google Scholar] [CrossRef]
- Pircher, A.; Hilbe, W.; Heidegger, I.; Drevs, J.; Tichelli, A.; Medinger, M. Biomarkers in Tumor Angiogenesis and Anti-Angiogenic Therapy. Int. J. Mol. Sci. 2011, 12, 7077–7099. [Google Scholar] [CrossRef]
- Bhati, R.; Patterson, C.; Livasy, C.A.; Fan, C.; Ketelsen, D.; Hu, Z.; Reynolds, E.; Tanner, C.; Moore, D.T.; Gabrielli, F.; et al. Molecular characterization of human breast tumor vascular cells. Am. J. Pathol. 2008, 172, 1381–1390. [Google Scholar] [CrossRef]
- Bergers, G.; Benjamin, L.E. Tumorigenesis and the angiogenic switch. Nat. Rev. Cancer 2003, 3, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ortiz, A.; Jiménez-Borreguero, L.J.; Peñalvo, J.L.; Ordovás, J.M.; Mocoroa, A.; Fernández-Friera, L.; Laclaustra, M.; García, L.; Molina, J.; Mendiguren, J.M.; et al. The Progression and Early detection of Subclinical Atherosclerosis (PESA) study: Rationale and design. Am. Heart J. 2013, 166, 990–998. [Google Scholar] [CrossRef] [PubMed]
- Blankenberg, S.; Zeller, T.; Saarela, O.; Havulinna, A.S.; Kee, F.; Tunstall-Pedoe, H.; Kuulasmaa, K.; Yarnell, J.; Schnabel, R.B.; Wild, P.S.; et al. Contribution of 30 Biomarkers to 10-Year Cardiovascular Risk Estimation in 2 Population Cohorts. Circulation 2010, 121, 2388–2397. [Google Scholar] [CrossRef]
- Nakashima, Y.; Raines, E.W.; Plump, A.S.; Breslow, J.L.; Ross, R. Upregulation of VCAM-1 and ICAM-1 at Atherosclerosis-Prone Sites on the Endothelium in the ApoE-Deficient Mouse. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Iiyama, K.; Hajra, L.; Iiyama, M.; Li, H.; DiChiara, M.; Medoff, B.D.; Cybulsky, M.I. Patterns of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 expression in rabbit and mouse atherosclerotic lesions and at sites predisposed to lesion formation. Circ. Res. 1999, 85, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Babinska, A.; Azari, B.; Salifu, M.; Liu, R.; Jiang, X.-C.; Sobocka, M.; Boo, D.; Khoury, G.; Deitch, J.; Marmur, J.; et al. The F11 receptor (F11R/JAM-A) in atherothrombosis: Overexpression of F11R in atherosclerotic plaques. Thromb. Haemost. 2007, 97, 272–281. [Google Scholar] [CrossRef]
- Schönberger, T.; Siegel-Axel, D.; Bußl, R.; Richter, S.; Judenhofer, M.S.; Haubner, R.; Reischl, G.; Klingel, K.; Münch, G.; Seizer, P.; et al. The immunoadhesin glycoprotein VI-Fc regulates arterial remodelling after mechanical injury in ApoE−/− mice. Cardiovasc. Res. 2008, 80, 131–137. [Google Scholar] [CrossRef]
- Bültmann, A.; Li, Z.; Wagner, S.; Peluso, M.; Schönberger, T.; Weis, C.; Konrad, I.; Stellos, K.; Massberg, S.; Nieswandt, B.; et al. Impact of glycoprotein VI and platelet adhesion on atherosclerosis—A possible role of fibronectin. J. Mol. Cell. Cardiol. 2010, 49, 532–542. [Google Scholar] [CrossRef]
- Ungerer, M.; Li, Z.; Baumgartner, C.; Goebel, S.; Vogelmann, J.; Holthoff, H.-P.; Gawaz, M.; Münch, G. The GPVI – Fc Fusion Protein Revacept Reduces Thrombus Formation and Improves Vascular Dysfunction in Atherosclerosis without Any Impact on Bleeding Times. PLoS ONE 2013, 8, e71193. [Google Scholar] [CrossRef]
- Hamm, C.W.; Goldmann, B.U.; Heeschen, C.; Kreymann, G.; Berger, J.; Meinertz, T. Emergency room triage of patients with acute chest pain by means of rapid testing for cardiac troponin T or troponin I. N. Engl. J. Med. 1997, 337, 1648–1653. [Google Scholar] [CrossRef]
- Pope, J.H.; Aufderheide, T.P.; Ruthazer, R.; Woolard, R.H.; Feldman, J.A.; Beshansky, J.R.; Griffith, J.L.; Selker, H.P. Missed diagnoses of acute cardiac ischemia in the emergency department. N. Engl. J. Med. 2000, 342, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.H.; Eagle, K.A. Missed diagnoses of acute coronary syndromes in the emergency room--continuing challenges. N. Engl. J. Med. 2000, 342, 1207–1210. [Google Scholar] [CrossRef] [PubMed]
- Lev, E.I.; Battler, A.; Behar, S.; Porter, A.; Haim, M.; Boyko, V.; Hasdai, D. Frequency, characteristics, and outcome of patients hospitalized with acute coronary syndromes with undetermined electrocardiographic patterns. Am. J. Cardiol. 2003, 91, 224–227. [Google Scholar] [CrossRef]
- Chukwuemeka, A.O.; Brown, K.A.; Venn, G.E.; Chambers, D.J. Changes in P-selectin expression on cardiac microvessels in blood-perfused rat hearts subjected to ischemia-reperfusion. Ann. Thorac. Surg. 2005, 79, 204–211. [Google Scholar] [CrossRef]
- Villanueva, F.S.; Lu, E.; Bowry, S.; Kilic, S.; Tom, E.; Wang, J.; Gretton, J.; Pacella, J.J.; Wagner, W.R. Myocardial Ischemic Memory Imaging With Molecular Echocardiography. Circulation 2007, 115, 345–352. [Google Scholar] [CrossRef]
- Davidson, B.P.; Chadderdon, S.M.; Belcik, J.T.; Gupta, S.; Lindner, J.R. Ischemic Memory Imaging in Nonhuman Primates with Echocardiographic Molecular Imaging of Selectin Expression. J. Am. Soc. Echocardiogr. 2014, 27, 786–793. [Google Scholar] [CrossRef]
- Wang, H.; Machtaler, S.; Bettinger, T.; Lutz, A.M.; Luong, R.; Bussat, P.; Gambhir, S.S.; Tranquart, F.; Tian, L.; Willmann, J.K. Molecular Imaging of Inflammation in Inflammatory Bowel Disease with a Clinically Translatable Dual-Selectin–targeted US Contrast Agent: Comparison with FDG PET/CT in a Mouse Model. Radiology 2013, 267, 818–829. [Google Scholar] [CrossRef]
- Wang, H.; Felt, S.A.; Machtaler, S.; Guracar, I.; Luong, R.; Bettinger, T.; Tian, L.; Lutz, A.M.; Willmann, J.K. Quantitative Assessment of Inflammation in a Porcine Acute Terminal Ileitis Model: US with a Molecularly Targeted Contrast Agent. Radiology 2015, 276, 809–817. [Google Scholar] [CrossRef]
- Wang, H.; Hyvelin, J.-M.; Felt, S.A.; Guracar, I.; Vilches-Moure, J.G.; Cherkaoui, S.; Bettinger, T.; Tian, L.; Lutz, A.M.; Willmann, J.K. US Molecular Imaging of Acute Ileitis: Anti-Inflammatory Treatment Response Monitored with Targeted Microbubbles in a Preclinical Model. Radiology 2018, 289, 90–100. [Google Scholar] [CrossRef]
- Goodacre, S.; Sampson, F.; Thomas, S.; van Beek, E.; Sutton, A. Systematic review and meta-analysis of the diagnostic accuracy of ultrasonography for deep vein thrombosis. BMC Med. Imaging 2005, 5, 6. [Google Scholar] [CrossRef]
- Nesheim, M. Thrombin and Fibrinolysis. Chest 2003, 124, 33S–39S. [Google Scholar] [CrossRef] [PubMed]
- Kessels, H.; Béguin, S.; Andree, H.; Hemker, H.C. Measurement of thrombin generation in whole blood—The effect of heparin and aspirin. Thromb. Haemost. 1994, 72, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Weller, G.E.R.; Villanueva, F.S.; Tom, E.M.; Wagner, W.R. Targeted ultrasound contrast agents: In vitro assessment of endothelial dysfunction and multi-targeting to ICAM-1 and sialyl Lewisx. Biotechnol. Bioeng. 2005, 92, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, E.A.; Pickard, J.E.; Rychak, J.; Klibanov, A.; Ley, K. Dual targeting improves microbubble contrast agent adhesion to VCAM-1 and P-selectin under flow. J. Control. Release 2009, 140, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Willmann, J.K.; Lutz, A.M.; Paulmurugan, R.; Patel, M.R.; Chu, P.; Rosenberg, J.; Gambhir, S.S. Dual-targeted Contrast Agent for US Assessment of Tumor Angiogenesis in Vivo1. Radiology 2008, 248, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Warram, J.M.; Sorace, A.G.; Saini, R.; Umphrey, H.R.; Zinn, K.R.; Hoyt, K. A Triple-Targeted Ultrasound Contrast Agent Provides Improved Localization to Tumor Vasculature. J. Ultrasound Med. 2011, 30, 921–931. [Google Scholar] [CrossRef]
- Sorace, A.G.; Saini, R.; Mahoney, M.; Hoyt, K. Molecular Ultrasound Imaging Using a Targeted Contrast Agent for Assessing Early Tumor Response to Antiangiogenic Therapy. J. Ultrasound Med. 2012, 31, 1543–1550. [Google Scholar] [CrossRef]
- Ferrara, K.; Pollard, R.; Borden, M. Ultrasound microbubble contrast agents: Fundamentals and application to gene and drug delivery. Annu Rev. Biomed. Eng 2007, 9, 415–447. [Google Scholar] [CrossRef]
- Meyer, D.L.; Schultz, J.; Lin, Y.; Henry, A.; Sanderson, J.; Jackson, J.M.; Goshorn, S.; Rees, A.R.; Graves, S.S. Reduced antibody response to streptavidin through site-directed mutagenesis. Protein Sci. 2001, 10, 491–503. [Google Scholar] [CrossRef]
- Paganelli, G.; Chinol, M.; Maggiolo, M.; Sidoli, A.; Corti, A.; Baroni, S.; Siccardi, A.G. The three-step pretargeting approach reduces the human anti-mouse antibody response in patients submitted to radioimmunoscintigraphy and radioimmunotherapy. Eur. J. Nucl. Med. 1997, 24, 350–351. [Google Scholar] [CrossRef]
- Breitz, H.B.; Weiden, P.L.; Beaumier, P.L.; Axworthy, D.B.; Seiler, C.; Su, F.-M.; Graves, S.; Bryan, K.; Reno, J.M. Clinical Optimization of Pretargeted Radioimmunotherapy with Antibody-Streptavidin Conjugate and 90Y-DOTA-Biotin. J. Nucl. Med. 2000, 11, 131–140. [Google Scholar]
- Stieger, S.M.; Dayton, P.A.; Borden, M.A.; Caskey, C.F.; Griffey, S.M.; Wisner, E.R.; Ferrara, K.W. Imaging of angiogenesis using Cadence contrast pulse sequencing and targeted contrast agents. Contrast Media Mol. Imaging 2008, 3, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Willmann, J.K.; Bonomo, L.; Testa, A.C.; Rinaldi, P.; Rindi, G.; Valluru, K.S.; Petrone, G.; Martini, M.; Lutz, A.M.; Gambhir, S.S. Ultrasound Molecular Imaging With BR55 in Patients With Breast and Ovarian Lesions: First-in-Human Results. J. Clin. Oncol. 2017, 35, 2133–2140. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, S.K.; Monsky, W.L.; Yuan, F.; Roberts, W.G.; Griffith, L.; Torchilin, V.P.; Jain, R.K. Regulation of transport pathways in tumor vessels: Role of tumor type and microenvironment. Proc. Natl. Acad. Sci. USA 1998, 95, 4607. [Google Scholar] [CrossRef]
- Yin, T.; Wang, P.; Zheng, R.; Zheng, B.; Cheng, D.; Zhang, X.; Shuai, X. Nanobubbles for enhanced ultrasound imaging of tumors. Int. J. Nanomed. 2012, 7, 895–904. [Google Scholar] [CrossRef]
- Wang, J.-P.; Zhou, X.-L.; Yan, J.-P.; Zheng, R.-Q.; Wang, W. Nanobubbles as ultrasound contrast agent for facilitating small cell lung cancer imaging. Oncotarget 2017, 8, 78153–78162. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.B.; Yang, H.L.; Zhang, J.; Yin, J.K.; Yang, Y.L.; Yuan, L.J.; Zhang, L.; Duan, Y.Y. The Optimized Fabrication of Nanobubbles as Ultrasound Contrast Agents for Tumor Imaging. Sci. Rep. 2015, 5, 13725. [Google Scholar] [CrossRef]
- Perera, R.; de Leon, A.; Wang, X.; Wang, Y.; Ramamurthy, G.; Peiris, P.; Abenojar, E.; Basilion, J.P.; Exner, A.A. Real Time Ultrasound Molecular Imaging of Prostate Cancer with PSMA-targeted Nanobubbles. Nanomed. Nanotechnol. Biol. Med. 2020, 28, 102213. [Google Scholar] [CrossRef]
- Yang, H.; Cai, W.; Xu, L.; Lv, X.; Qiao, Y.; Li, P.; Wu, H.; Yang, Y.; Zhang, L.; Duan, Y. Nanobubble–Affibody: Novel ultrasound contrast agents for targeted molecular ultrasound imaging of tumor. Biomaterials 2015, 37, 279–288. [Google Scholar] [CrossRef]
- Lv, W.; Shen, Y.; Yang, H.; Yang, R.; Cai, W.; Zhang, J.; Yuan, L.; Duan, Y.; Zhang, L. A Novel Bimodal Imaging Agent Targeting HER2 Molecule of Breast Cancer. J. Immunol. Res. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Jiang, Q.; Hao, S.; Xiao, X.; Yao, J.; Ou, B.; Zhao, Z.; Liu, F.; Pan, X.; Luo, B.; Zhi, H. Production and characterization of a novel long-acting Herceptin-targeted nanobubble contrast agent specific for Her-2-positive breast cancers. Breast Cancer 2016, 23, 445–455. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Y.; Wang, G.; Lv, Q.; Yang, Y.; Wang, J.; Zhang, P.; Liu, J.; Xie, Y.; Zhang, L.; et al. Ultrasound molecular imaging of acute cardiac transplantation rejection using nanobubbles targeted to T lymphocytes. Biomaterials 2018, 162, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Hernandez, C.; Yuan, H.-X.; Lilly, J.; Kota, P.; Zhou, H.; Wu, H.; Exner, A.A. Ultrasound molecular imaging of ovarian cancer with CA-125 targeted nanobubble contrast agents. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2159–2168. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Cai, W.; Yang, H.; Zhang, H.; Hao, M.; Yuan, L.; Liu, J.; Zhang, L.; Yang, Y.; Liu, X.; et al. Annexin V conjugated nanobubbles: A novel ultrasound contrast agent for in vivo assessment of the apoptotic response in cancer therapy. J. Control. Release 2018, 276, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Li, X.-Y.; Hu, H.; Xu, L.; Yang, S.-P.; Li, F.-H. Preparation and Imaging Investigation of Dual-targeted C3F8-filled PLGA Nanobubbles as a Novel Ultrasound Contrast Agent for Breast Cancer. Sci. Rep. 2018, 8, 3887. [Google Scholar] [CrossRef]
- Gorce, J.-M.; Arditi, M.; Schneider, M. Influence of Bubble Size Distribution on the Echogenicity of Ultrasound Contrast Agents: A Study of SonoVue™. Investig. Radiol. 2000, 35, 661–671. [Google Scholar] [CrossRef]
- JafariSojahrood, A.; Nieves, L.; Hernandez, C.; Exner, A.; Kolios, M.C. Theoretical and experimental investigation of the nonlinear dynamics of nanobubbles excited at clinically relevant ultrasound frequencies and pressures: The role oflipid shell buckling. In Proceedings of the 2017 IEEE International Ultrasonics Symposium (IUS), Washington, DC, USA, 6–9 September 2017. [Google Scholar]
- Wu, H.; Rognin, N.G.; Krupka, T.M.; Solorio, L.; Yoshiara, H.; Guenette, G.; Sanders, C.; Kamiyama, N.; Exner, A.A. Acoustic Characterization and Pharmacokinetic Analyses of New Nanobubble Ultrasound Contrast Agents. Ultrasound Med. Biol. 2013, 39, 2137–2146. [Google Scholar] [CrossRef]
- Wang, C.-W.; Yang, S.-P.; Hu, H.; Du, J.; Li, F.-H. Synthesis, characterization and in vitro and in vivo investigation of C3F8-filled poly(lactic-co-glycolic acid) nanoparticles as an ultrasound contrast agent. Mol. Med. Rep. 2015, 11, 1885–1890. [Google Scholar] [CrossRef][Green Version]
- De Leon, A.; Perera, R.; Hernandez, C.; Cooley, M.; Jung, O.; Jeganathan, S.; Abenojar, E.; Fishbein, G.; Sojahrood, A.J.; Emerson, C.C.; et al. Contrast enhanced ultrasound imaging by nature-inspired ultrastable echogenic nanobubbles. Nanoscale 2019, 11, 15647–15658. [Google Scholar] [CrossRef]
- Kiessling, F.; Mertens, M.E.; Grimm, J.; Lammers, T. Nanoparticles for Imaging: Top or Flop? Radiology 2014, 273, 10–28. [Google Scholar] [CrossRef]
- Kee, A.L.Y.; Teo, B.M. Biomedical applications of acoustically responsive phase shift nanodroplets: Current status and future directions. Ultrason. Sonochem. 2019, 56, 37–45. [Google Scholar] [CrossRef]
- Rapoport, N. Phase-shift, stimuli-responsive perfluorocarbon nanodroplets for drug delivery to cancer. Wires Nanomed. Nanobiotechnol. 2012, 4, 492–510. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, B.E.; Rapoport, N. Phase-shift, stimuli-responsive drug carriers for targeted delivery. Ther. Deliv. 2011, 2, 1165–1187. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Pitt, W.G. Acoustic Droplet Vaporization in Biology and Medicine. Biomed Res. Int. 2013, 2013, 404361. [Google Scholar] [CrossRef]
- Rapoport, N.Y.; Kennedy, A.M.; Shea, J.E.; Scaife, C.L.; Nam, K.-H. Controlled and targeted tumor chemotherapy by ultrasound-activated nanoemulsions/microbubbles. J. Control. Release 2009, 138, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, N.; Kennedy, A.M.; Shea, J.E.; Scaife, C.L.; Nam, K.-H. Ultrasonic Nanotherapy of Pancreatic Cancer: Lessons from Ultrasound Imaging. Mol. Pharm. 2010, 7, 22–31. [Google Scholar] [CrossRef]
- Gao, Z.; Kennedy, A.M.; Christensen, D.A.; Rapoport, N.Y. Drug-loaded nano/microbubbles for combining ultrasonography and targeted chemotherapy. Ultrasonics 2008, 48, 260–270. [Google Scholar] [CrossRef]
- Chen, W.-T.; Kang, S.-T.; Lin, J.-L.; Wang, C.-H.; Chen, R.-C.; Yeh, C.-K. Targeted tumor theranostics using folate-conjugated and camptothecin-loaded acoustic nanodroplets in a mouse xenograft model. Biomaterials 2015, 53, 699–708. [Google Scholar] [CrossRef]
- Zhang, G.; Lin, S.; Leow, C.H.; Pang, K.; Hernandez-Gil, J.; Chee, M.; Long, N.J.; Matsunaga, T.O.; Tang, M.-X. Acoustic response of targeted nanodroplets post-activation using high frame rate imaging. In Proceedings of the 2017 IEEE International Ultrasonics Symposium (IUS), Washington, DC, USA, 6–9 September 2017. [Google Scholar]
- Gao, D.; Gao, J.; Xu, M.; Cao, Z.; Zhou, L.; Li, Y.; Xie, X.; Jiang, Q.; Wang, W.; Liu, J. Targeted Ultrasound-Triggered Phase Transition Nanodroplets for Her2-Overexpressing Breast Cancer Diagnosis and Gene Transfection. Mol. Pharm. 2017, 14, 984–998. [Google Scholar] [CrossRef]
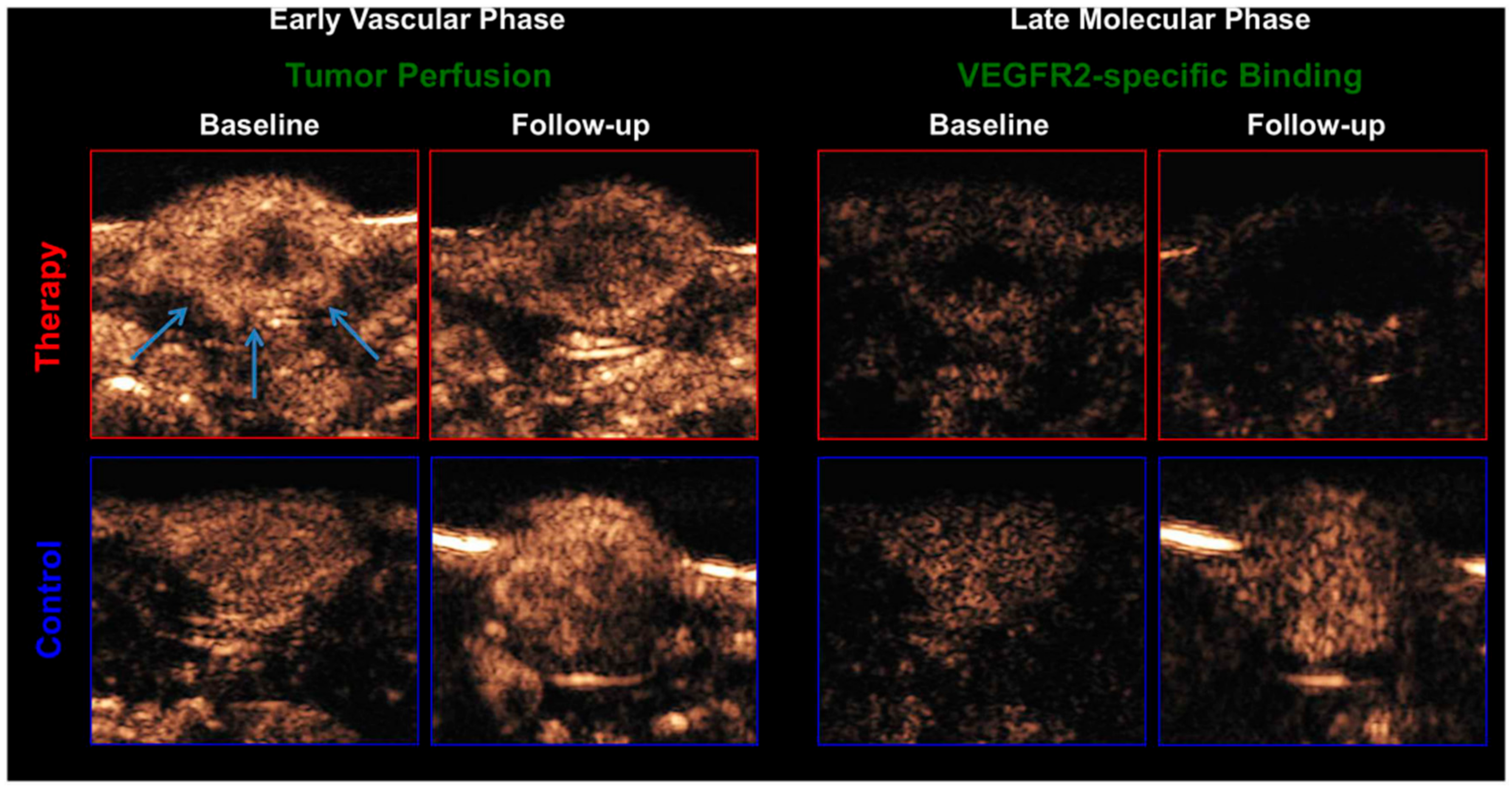

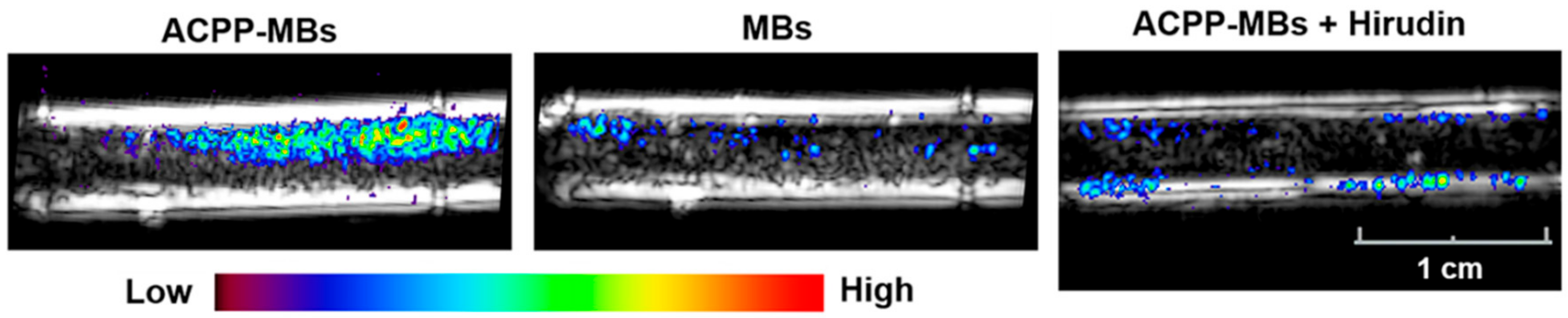
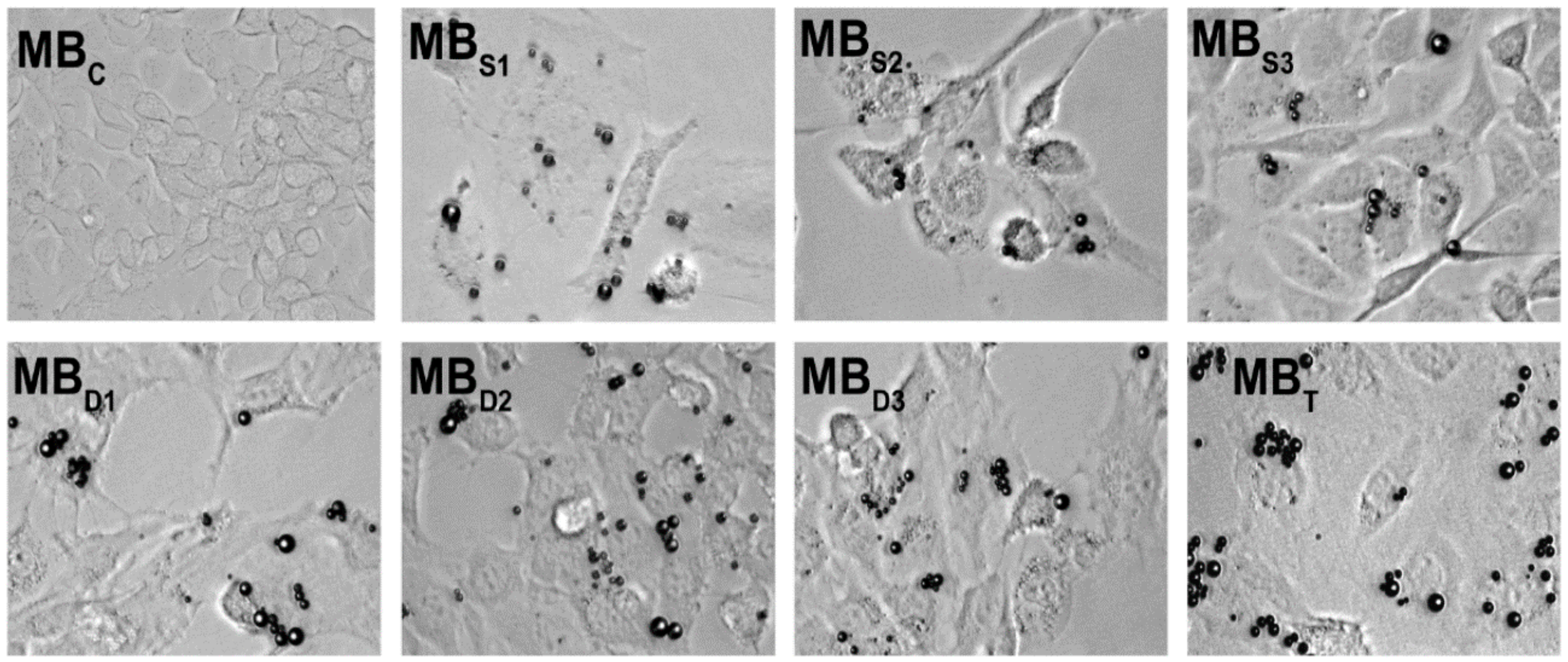
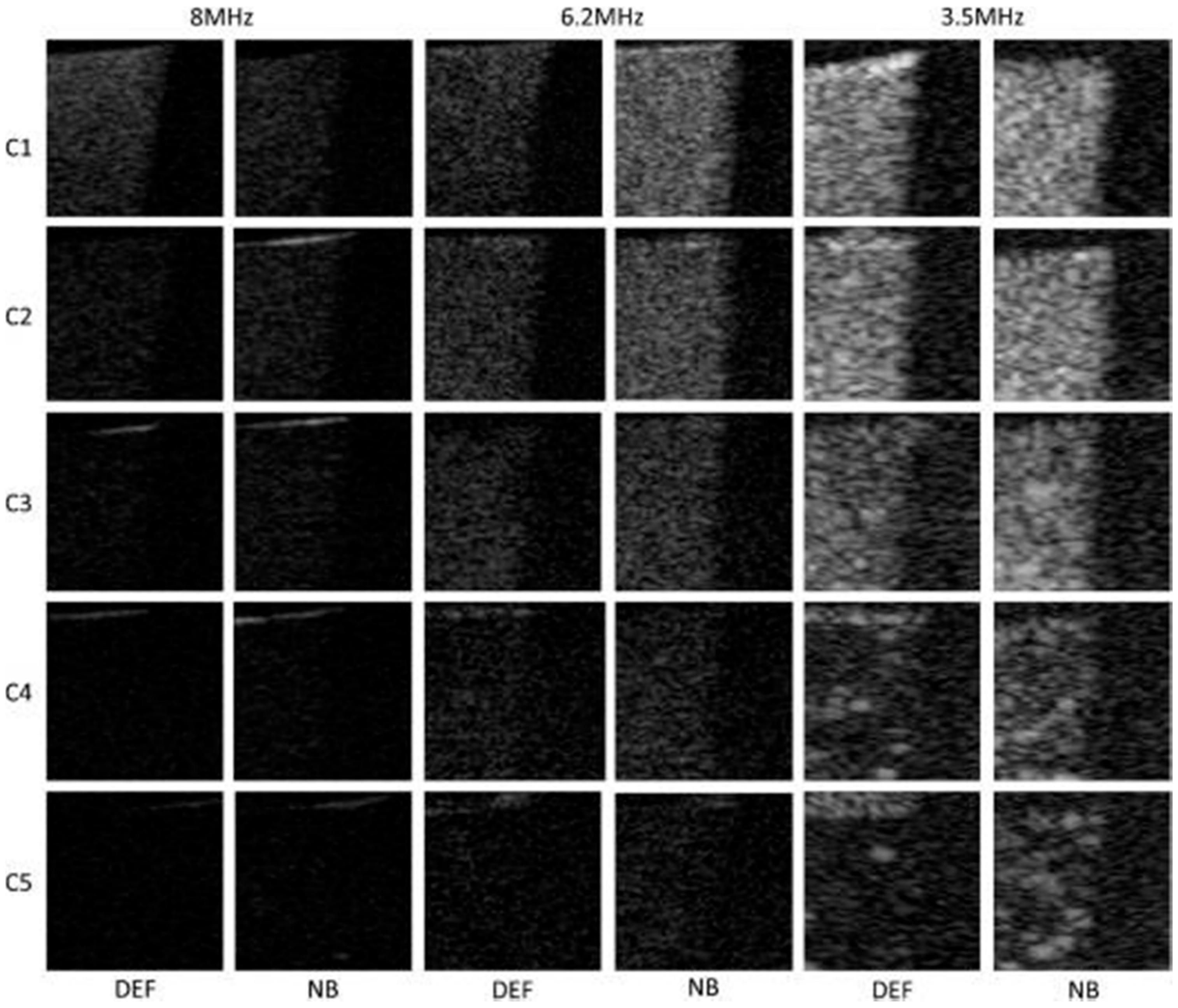
| Target | Binding Ligand | Model |
|---|---|---|
| αvβ3 integrin | Echistatin peptide | Malignant glioma in rats [22] |
| Cremaster muscle in mice [23] | ||
| Ischemic muscle in rats [24] | ||
| Knottin peptide | Ovarian cancer in mice [25] | |
| Cyclic RGD peptide | Breast cancer in mice [26] | |
| Spontaneous model of ovarian cancer in hens [27] | ||
| RGD peptide | Prostate cancer in rats [28] | |
| Squamous cell carcinoma in mice [29] | ||
| Anti-αvβ3 integrin antibody | Breast, ovarian and pancreatic cancers in mice [30] | |
| Skin cancer in mice [31] | ||
| Cremaster muscle in mice [23] | ||
| Cyclic RRL peptide | Prostate cancer in mice [32] | |
| VEGFR2 | Anti-VEGFR2 antibody | Squamous cell carcinoma in mice [29] |
| Ovarian cancers in mice [30] | ||
| Skin cancer in mice [31,33] | ||
| Colon cancer in mice [34] | ||
| Pancreatic cancer in mice [30,35] | ||
| Malignant glioma in mice [36] | ||
| Angiosarcoma in mice [36,37] | ||
| Breast cancers in mice [30,38,39] | ||
| 10th type III domain of human fibronectin | Transgenic breast cancer in mice [40] | |
| Single-chain VEGF construct | Colon cancer in mice [41] | |
| VEGFR2-binding phospholipid-heteropeptides | Prostate cancer in rats [42] | |
| Breast cancer in rats [16] and mice [43,44,45] | ||
| Transgenic breast cancer in mice [46] | ||
| Transgenic pancreatic ductal cancer in mice [47] | ||
| Colon cancer in mice [48] | ||
| Squamous cell carcinoma in mice [49] | ||
| Colon cancer in mice [50,51] | ||
| Neuropilin-1 | CRPPR and ATWLPPR peptides | Pancreatic cancer in mice [35] |
| Endoglin | Anti-endoglin antibody | Breast cancer in mice [30,52] |
| Ovarian cancers in mice [30] | ||
| Pancreatic cancer in mice [30,35] | ||
| Skin cancer in mice [31] | ||
| SFRP2 | Anti-SFRP2 antibody | Angiosarcoma in mice [53] |
| B7-H3 | Anti-B7-H3 antibody | Breast cancer in mice [54] |
| Nucleolin | F3 peptide | Breast cancer in mice [55] |
| Thy1 | Anti-Thy1 antibody | Transgenic and implanted pancreatic cancer in mice [56] |
| Leukocytes | Phosphatidylserine | Inflammation in mice [57] and dogs [58] |
| Anti-ICAM-1 antibody | Activated endothelial cells [59] | |
| MAdCAM-1 | Anti-MAdCAM-1 antibody | Inflammatory bowel disease in mice [60] |
| JAM-A | Anti-JAM-A antibody | Atherosclerosis in mice [61] and rabbits [62] |
| VCAM-1 | Anti-VCAM-1 antibody | Atherosclerosis in mice [63,64,65,66,67,68,69,70] and swine [71] |
| Nanobody targeting VCAM-1 | Epidermoid carcinoma in mice [72] | |
| Atherosclerosis in mice [73] | ||
| HGRANLRILARY peptide | Atherosclerosis in mice [74] | |
| ICAM-1 | Anti-ICAM-1 antibody | Endothelial cells [17] |
| Inflammation in rats [75] | ||
| P-selectin | Anti-P-selectin antibody | Atherosclerosis in mice [63,65,69,70] |
| Inflammation in mice [76,77] and flow chamber [78] | ||
| Muscle inflammation in mice [79,80] | ||
| Inflammatory bowel disease in mice [81,82] | ||
| Myocardial ischemia in mice [83,84] and rats [85] | ||
| LVSVLDLEPLDAAWL peptide | Atherosclerosis in mice [74] | |
| Sialyl Lewis X | Inflammation in mice [77] | |
| E-selectin | IELLQAR peptide | Ovarian carcinoma in mice [86,87] |
| Epidermoid carcinoma in mice [72,87] | ||
| Anti-E-selectin antibody | Muscle inflammation in mice [80] | |
| Myocardial ischemia in rats [85] | ||
| E-selectin affibody | Myocardial ischemia in rats [88] | |
| GP Ibα | Anti-GP Ibα antibody | Atherosclerosis in mice [63,64] |
| Dimeric murine recombinant A1 domain of VWF A1 | Atherosclerosis in mice [69] | |
| GP IIb/IIIa | Linear KQAGDV peptide | Thrombosis in flow chamber [89] and mongrels [90] |
| Cyclic RGD | Thrombosis in mice [91,92] | |
| Anti-GP IIb/IIIa antibody | Thrombosis in mice artery [93] | |
| GP VI | Anti-GP VI antibody | Atherosclerosis in mice [94] |
| VWF | Cell-derived peptide | Atherosclerosis in mice [69] |
| RVVCEYVFGRGAVCS peptide | Atherosclerosis in mice [74] | |
| LOX-1 | LSIPPKA peptide | Atherosclerosis in mice [74] |
| Thrombin | Thrombin aptamer | Thrombosis in rabbit blood [95,96] |
| Thrombin-sensitive ACPP | Thrombosis in rabbit blood [97] |
| Targets | Binding Ligands | Model |
|---|---|---|
| ICAM-1 and selectins | Anti-ICAM-1 antibody and sialyl Lewis X | Flow chamber [136] |
| VCAM-1 and P-selectin | Anti-VCAM-1 and anti-P-selectin antibodies | Flow chamber [137] |
| VEGFR2 and αvβ3 integrin | Anti-VEGFR2 and anti- αvβ3 integrin antibodies | Ovarian cancer in mice [138] |
| Selectins | Sialyl Lewis X | Muscle inflammation in mice [80] |
| Myocardial ischemia in rats [128] | ||
| PSGL-Ig | Muscle inflammation in mice [80] | |
| Inflammatory bowel disease in mice [130] and swine [131,132] | ||
| Myocardial ischemia in mice [84], rats [85] and macaques [129] | ||
| VEGFR2, αvβ3 integrin and P-selectin | Anti-VEGFR2, anti- αvβ3 integrin and anti-P-selectin antibodies | Breast cancer in mice [139,140] |
| Targets | Binding Ligands | Model |
|---|---|---|
| HER2 | HER2-affibody | Breast cancer in mice [152,153] |
| Anti-HER2 antibody | Breast cancer in mice [154] | |
| CAIX | Aptamer | Adenocarcinoma in mice [7] |
| PSMA | PSMA-1 ligand | Prostate cancer in mice [151] |
| CD3 | Anti-CD3 antibody | T-lymphocyte in rats [155] |
| CA-125 | Anti-CA-125 antibody | Epithelial ovarian cancer in mice [156] |
| proGRP | Anti- proGRP antibody | Small cell lung cancer in mice [149] |
| Phosphatidylserine | Annexin V | Apoptosis in mice [157] |
| VEGFR2 and HER2 | Anti-HER2 and anti-VEGFR2 antibodies | Breast cancer in mice [158] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Köse, G.; Darguzyte, M.; Kiessling, F. Molecular Ultrasound Imaging. Nanomaterials 2020, 10, 1935. https://doi.org/10.3390/nano10101935
Köse G, Darguzyte M, Kiessling F. Molecular Ultrasound Imaging. Nanomaterials. 2020; 10(10):1935. https://doi.org/10.3390/nano10101935
Chicago/Turabian StyleKöse, Gurbet, Milita Darguzyte, and Fabian Kiessling. 2020. "Molecular Ultrasound Imaging" Nanomaterials 10, no. 10: 1935. https://doi.org/10.3390/nano10101935
APA StyleKöse, G., Darguzyte, M., & Kiessling, F. (2020). Molecular Ultrasound Imaging. Nanomaterials, 10(10), 1935. https://doi.org/10.3390/nano10101935






