pH-Sensitive Dendrimersomes of Hybrid Triazine-Carbosilane Dendritic Amphiphiles-Smart Vehicles for Drug Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. General Information
2.2. Analytical and Spectroscopic Techniques
2.3. Cell Experiments
2.4. Statistical Analysis
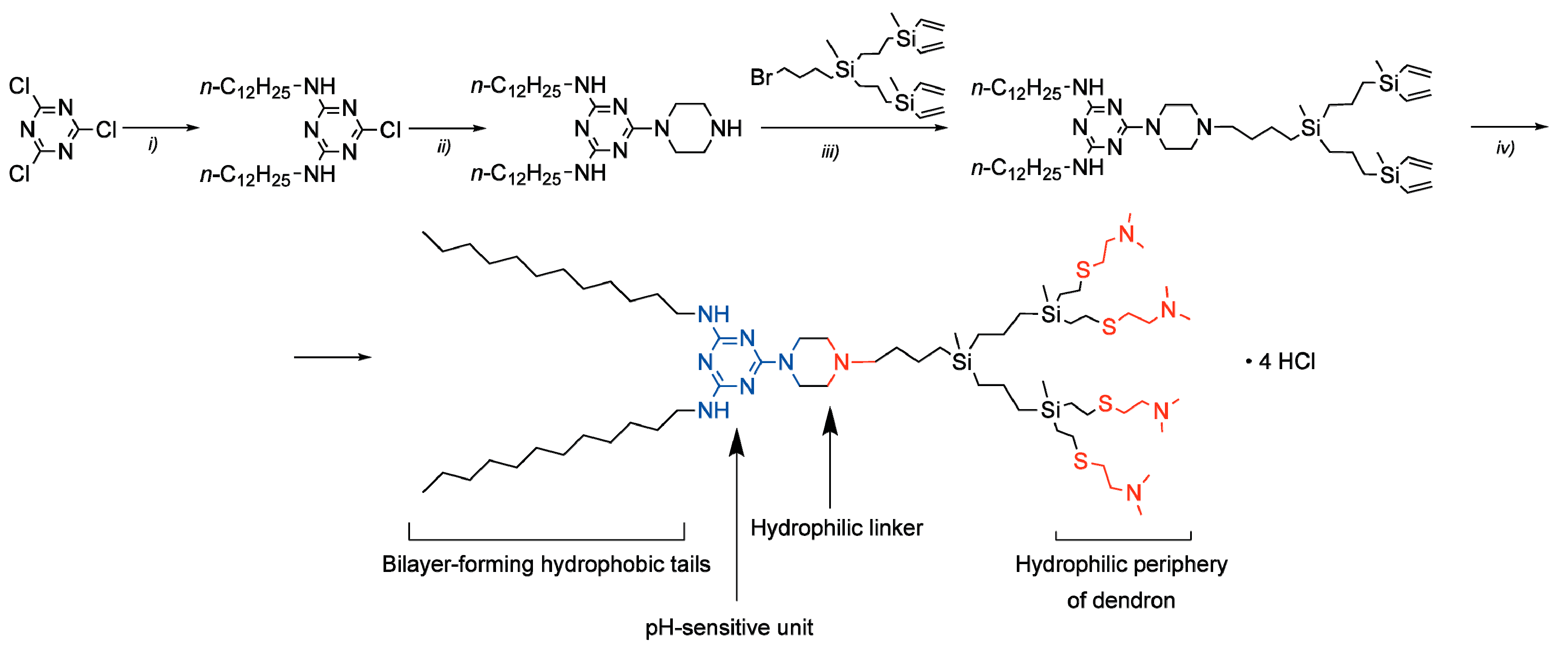
2.5. Synthesis of Dendron Amphiphiles
2.5.1. 2,4-dodecylamino-6-chloro-1,3,5-triazine
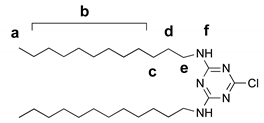
2.5.2. 2,4-dodecylamino-6-piperazino-1,3,5-triazine
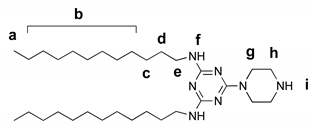
2.5.3. Vinyl-Terminated Dendron G2
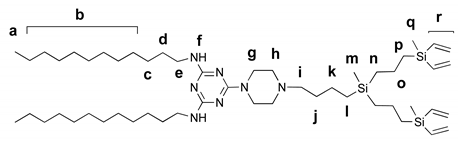
2.5.4. Amphiphilic Dendron G2
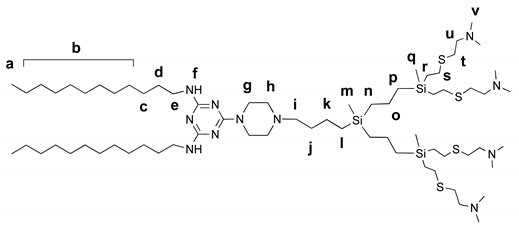
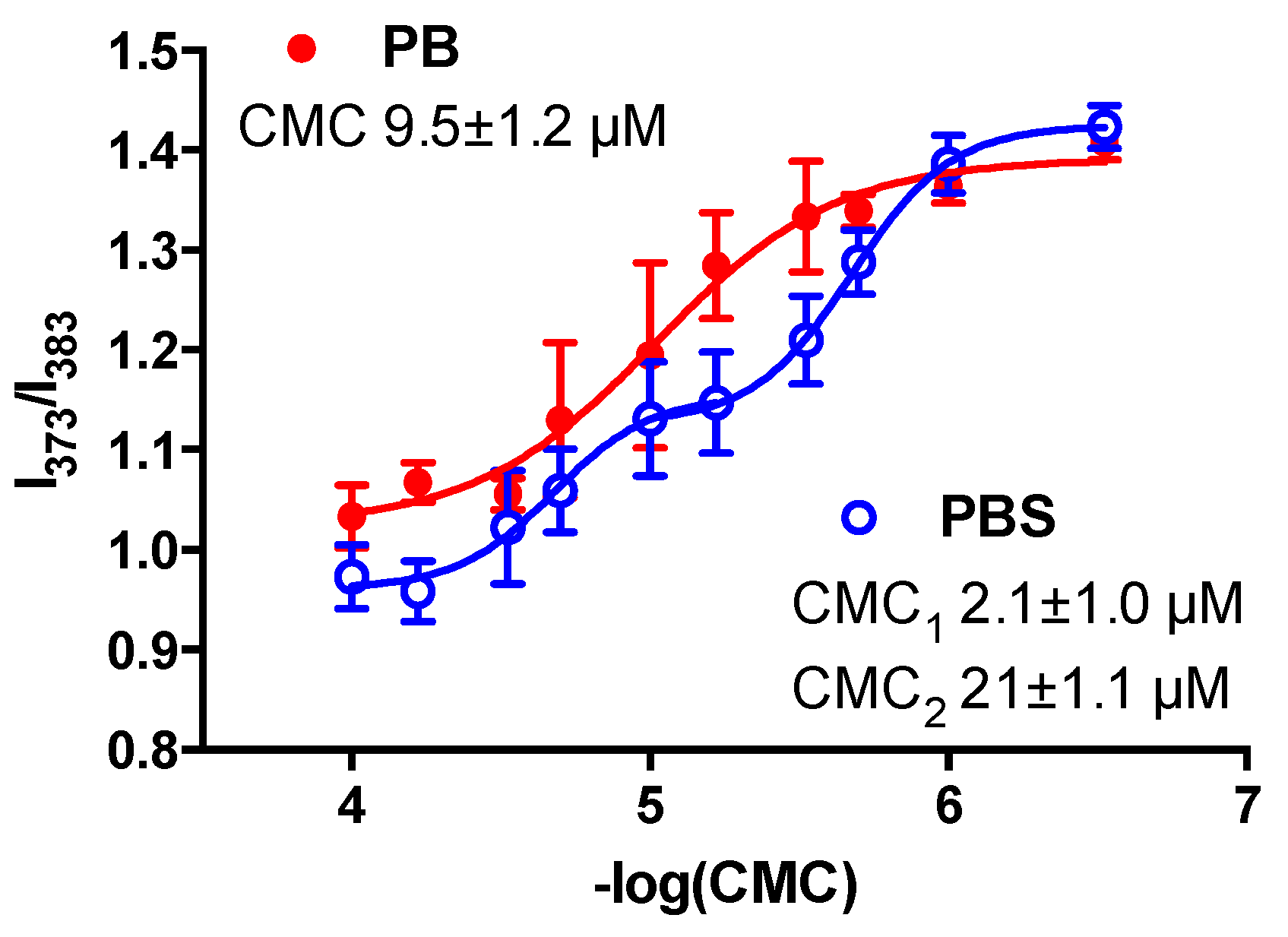
2.6. CMC Measurements
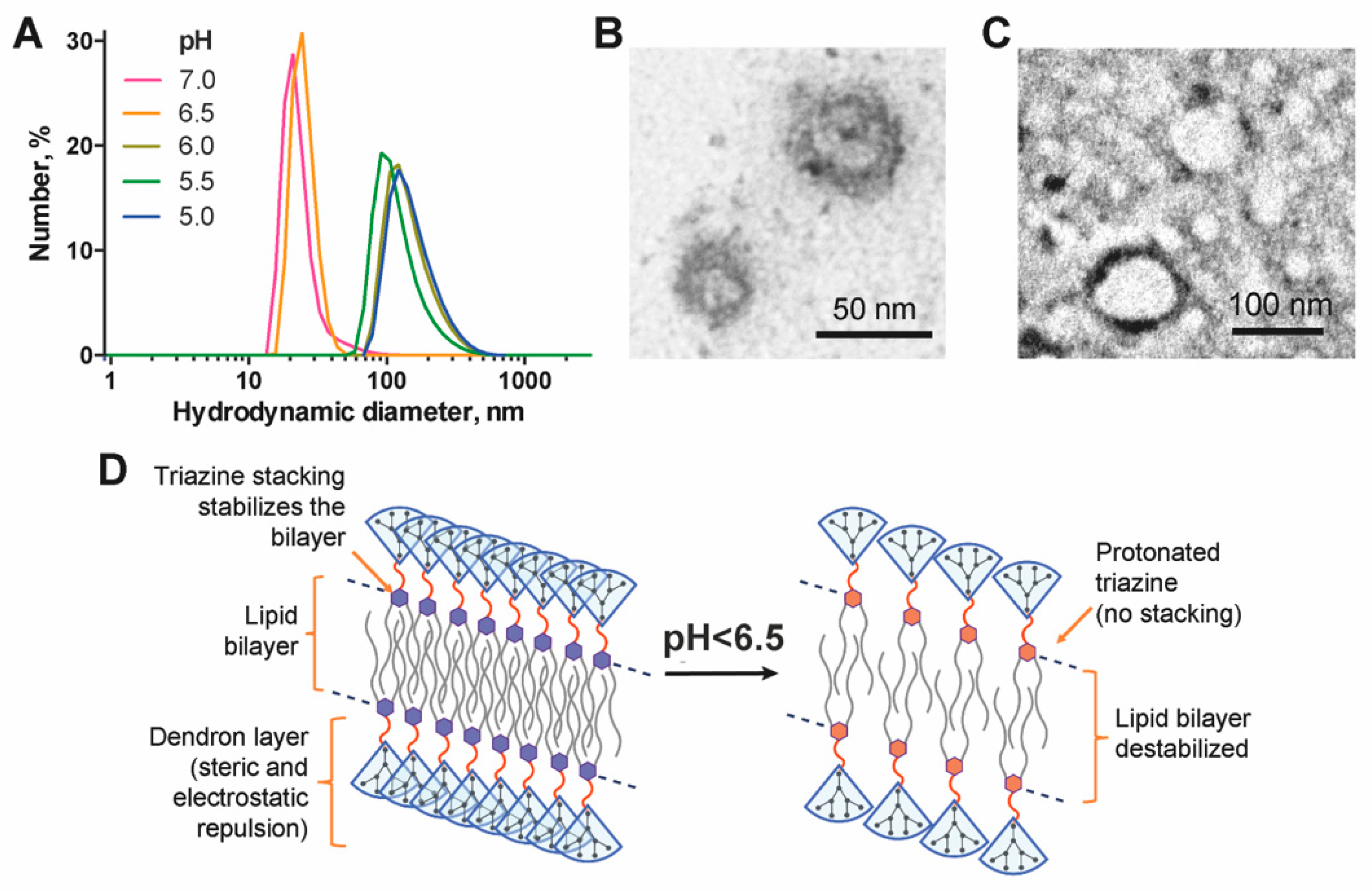
2.7. Study of the pH-Dependence of the Particle Size
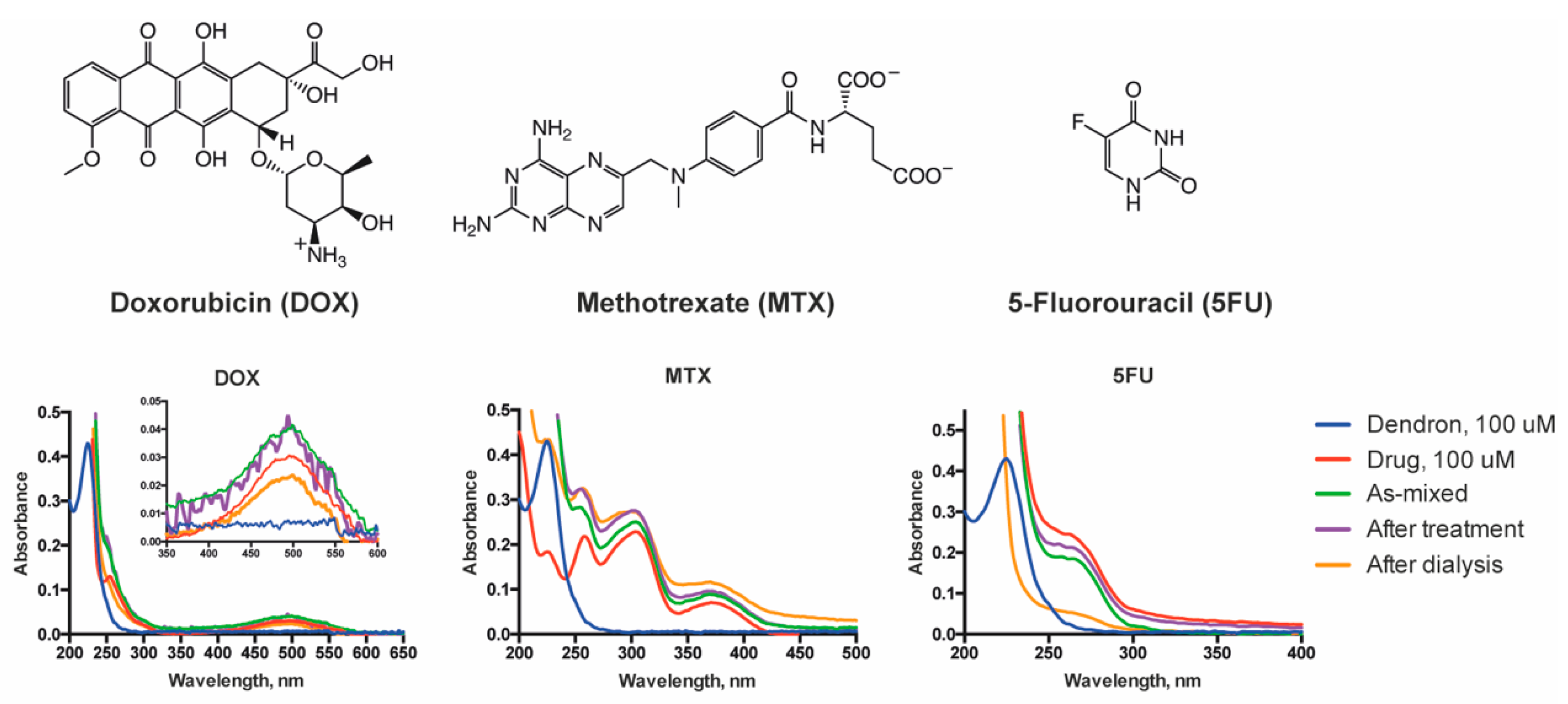
2.8. Drug Encapsulation
2.9. Drug Release from Dendrimersomes
2.10. WST Assay
2.11. Drug Internalization Studies
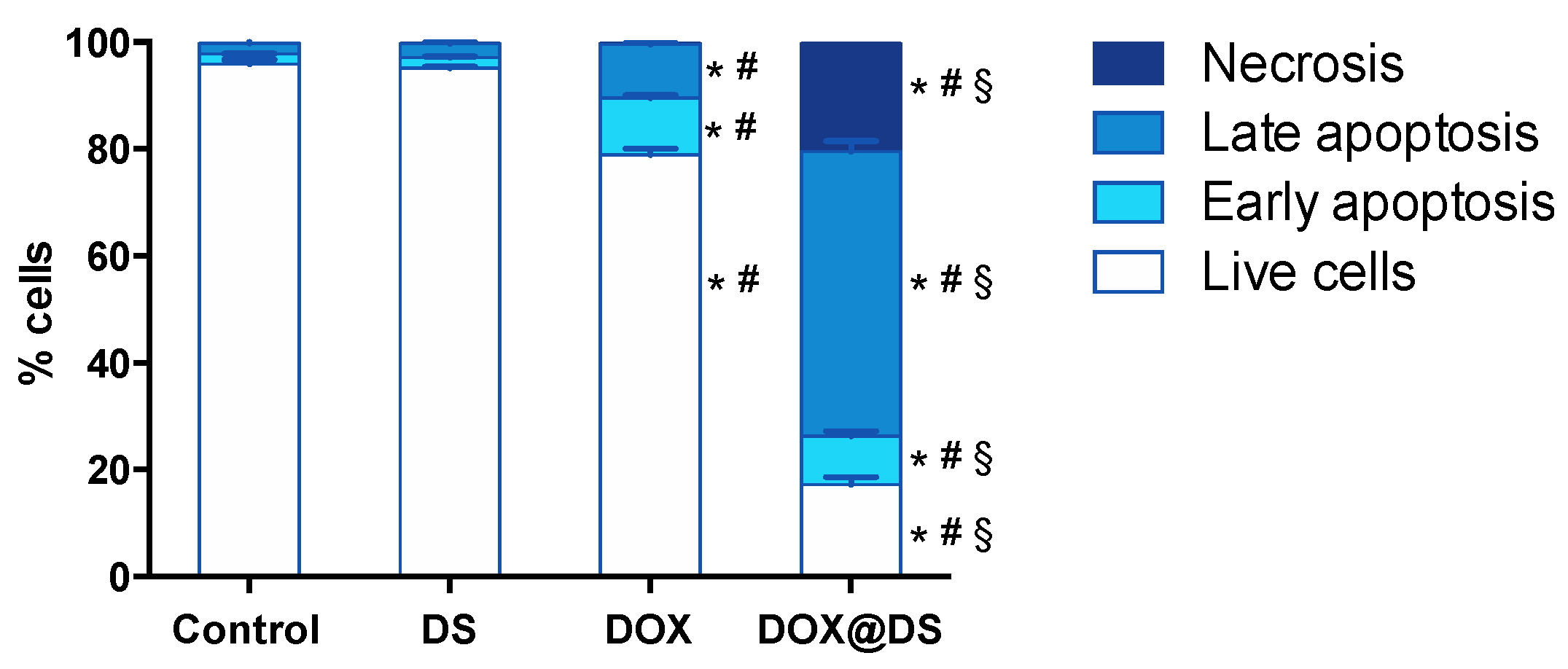
2.12. Apoptosis Induction Studies
3. Results and Discussion
3.1. Synthesis of the Amphiphilic Dendron
3.2. Self-Assembly of Dendrimersomes
3.3. Drug Loading into Dendrimersomes
3.4. Cytotoxic Activity of Drug-Loaded Dendrimersomes Towards Cancer Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jain, K.; Mehra, N.K.; Jain, N.K. Potentials and emerging trends in nanopharmacology. Curr. Opin. Pharmacol. 2014, 15, 97–106. [Google Scholar] [CrossRef]
- Li, Z.; Ye, E.; David; Lakshminarayanan, R.; Loh, X.J. Recent Advances of using hybrid nanocarriers in remotely controlled therapeutic delivery. Small 2016, 12, 4782–4806. [Google Scholar] [CrossRef]
- Movassaghian, S.; Merkel, O.M.; Torchilin, V.P. Applications of polymer micelles for imaging and drug delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 691–707. [Google Scholar] [CrossRef]
- Dzmitruk, V.; Apartsin, E.; Ihnatsyeu-Kachan, A.; Abashkin, V.; Shcharbin, D.; Bryszewska, M. Dendrimers show promise for sirna and microrna therapeutics. Pharmaceutics 2018, 10, 126. [Google Scholar] [CrossRef]
- Knauer, N.; Pashkina, E.; Apartsin, E. Topological aspects of the design of nanocarriers for therapeutic peptides and proteins. Pharmaceutics 2019, 11, 91. [Google Scholar] [CrossRef]
- Palmerston Mendes, L.; Pan, J.; Torchilin, V. Dendrimers as nanocarriers for nucleic acid and drug delivery in cancer therapy. Molecules 2017, 22, 1401. [Google Scholar] [CrossRef]
- Bolu, B.; Sanyal, R.; Sanyal, A. Drug delivery systems from self-assembly of dendron-polymer conjugates. Molecules 2018, 23, 1570. [Google Scholar] [CrossRef]
- Sandoval-Yañez, C.; Castro Rodriguez, C. Dendrimers: Amazing Platforms for Bioactive Molecule Delivery Systems. Materials 2020, 13, 570. [Google Scholar] [CrossRef]
- Fernandez, J.; Acosta, G.; Pulido, D.; Malý, M.; Copa-Patiño, J.L.; Soliveri, J.; Royo, M.; Gómez, R.; Albericio, F.; Ortega, P.; et al. Carbosilane dendron–peptide nanoconjugates as antimicrobial agents. Mol. Pharm. 2019, 16, 2661–2674. [Google Scholar] [CrossRef]
- Sepúlveda-Crespo, D.; de la Mata, F.J.; Gómez, R.; Muñoz-Fernández, M.A. Sulfonate-ended carbosilane dendrimers with a flexible scaffold cause inactivation of HIV-1 virions and gp120 shedding. Nanoscale 2018, 10, 8998–9011. [Google Scholar] [CrossRef]
- Krasheninina, O.; Apartsin, E.; Fuentes, E.; Szulc, A.; Ionov, M.; Venyaminova, A.; Shcharbin, D.; De la Mata, F.; Bryszewska, M.; Gόmez, R. Complexes of pro-apoptotic siRNAs and carbosilane dendrimers: Formation and effect on cancer cells. Pharmaceutics 2019, 11, 25. [Google Scholar] [CrossRef]
- Sánchez-Milla, M.; Muñoz-Moreno, L.; Sánchez-Nieves, J.; Malý, M.; Gómez, R.; Carmena, M.J.; de la Mata, F.J. Anticancer activity of dendriplexes against advanced prostate cancer from protumoral peptides and cationic carbosilane dendrimers. Biomacromolecules 2019, 20, 1224–1234. [Google Scholar] [CrossRef]
- Carloni, R.; Sanz del Olmo, N.; Ortega, P.; Fattori, A.; Gómez, R.; Ottaviani, M.F.; García-Gallego, S.; Cangiotti, M.; de la Mata, F.J. Exploring the interactions of ruthenium (ii) carbosilane metallodendrimers and precursors with model cell membranes through a dual spin-label spin-probe technique using EPR. Biomolecules 2019, 9, 540. [Google Scholar] [CrossRef]
- Gutierrez-Ulloa, C.E.; Buyanova, M.Y.; Apartsin, E.K.; Venyaminova, A.G.; de la Mata, F.J.; Gómez, R. Carbon nanotubes decorated with cationic carbosilane dendrons and their hybrids with nucleic acids. ChemNanoMat 2018, 4, 220–230. [Google Scholar] [CrossRef]
- Pędziwiatr-Werbicka, E.; Gorzkiewicz, M.; Horodecka, K.; Abashkin, V.; Klajnert-Maculewicz, B.; Peña-González, C.E.; Sánchez-Nieves, J.; Gómez, R.; de la Mata, F.J.; Bryszewska, M. Silver nanoparticles surface-modified with carbosilane dendrons as carriers of anticancer siRNA. Int. J. Mol. Sci. 2020, 21, 4647. [Google Scholar] [CrossRef]
- Percec, V.; Wilson, D.A.; Leowanawat, P.; Wilson, C.J.; Hughes, A.D.; Kaucher, M.S.; Hammer, D.A.; Levine, D.H.; Kim, A.J.; Bates, F.S.; et al. Self-Assembly of janus dendrimers into uniform dendrimersomes and other complex architectures. Science 2010, 328, 1009–1014. [Google Scholar] [CrossRef]
- Peterca, M.; Percec, V.; Leowanawat, P.; Bertin, A. Predicting the size and properties of dendrimersomes from the lamellar structure of their amphiphilic janus dendrimers. J. Am. Chem. Soc. 2011, 133, 20507–20520. [Google Scholar] [CrossRef]
- Thota, B.N.S.; Berlepsch, H.v.; Böttcher, C.; Haag, R. Towards engineering of self-assembled nanostructures using non-ionic dendritic amphiphiles. Chem. Commun. 2015, 51, 8648–8651. [Google Scholar] [CrossRef]
- Wei, T.; Chen, C.; Liu, J.; Liu, C.; Posocco, P.; Liu, X.; Cheng, Q.; Huo, S.; Liang, Z.; Fermeglia, M.; et al. Anticancer drug nanomicelles formed by self-assembling amphiphilic dendrimer to combat cancer drug resistance. Proc. Natl. Acad. Sci. USA 2015, 112, 2978–2983. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhou, J.; Yu, T.; Chen, C.; Cheng, Q.; Sengupta, K.; Huang, Y.; Li, H.; Liu, C.; Wang, Y.; et al. Adaptive amphiphilic dendrimer-based nanoassemblies as robust and versatile siRNA delivery systems. Angew. Chem. Int. Ed. 2014, 53, 11822–11827. [Google Scholar] [CrossRef]
- Gutierrez-Ulloa, C.E.; Buyanova, M.Y.; Apartsin, E.K.; Venyaminova, A.G.; de la Mata, F.J.; Valiente, M.; Gómez, R. Amphiphilic carbosilane dendrons as a novel synthetic platform toward micelle formation. Org. Biomol. Chem 2017, 15, 7352–7364. [Google Scholar] [CrossRef] [PubMed]
- Mencia, G.; Lozano-Cruz, T.; Valiente, M.; de la Mata, J.; Cano, J.; Gómez, R. New ionic carbosilane dendrons possessing fluorinated tails at different locations on the skeleton. Molecules 2020, 25, 807. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Ulloa, C.E.; Sepúlveda-Crespo, D.; García-Broncano, P.; Malý, M.; Muñoz-Fernández, M.A.; de la Mata, F.J.; Gómez, R. Synthesis of bow-tie carbosilane dendrimers and their HIV antiviral capacity: A comparison of the dendritic topology on the biological process. Eur. Polym. J. 2019, 119, 200–212. [Google Scholar] [CrossRef]
- Moreno, K.X.; Simanek, E.E. Identification of diamine linkers with differing reactivity and their application in the synthesis of melamine dendrimers. Tetrahedron Lett. 2008, 49, 1152–1154. [Google Scholar] [CrossRef]
- Lim, J.; Simanek, E.E. Triazine dendrimers as drug delivery systems: From synthesis to therapy. Adv. Drug Deliv. Rev. 2012, 64, 826–835. [Google Scholar] [CrossRef]
- Bagul, R.S.; Hosseini, M.M.; Shiao, T.C.; Roy, R. “Onion peel” glycodendrimer syntheses using mixed triazine and cyclotriphosphazene scaffolds. Can. J. Chem. 2017, 95, 975–983. [Google Scholar] [CrossRef]
- Ji, K.; Lee, C.; Janesko, B.G.; Simanek, E.E. Triazine-Substituted and acyl hydrazones: Experiment and computation reveal a stability inversion at low pH. Mol. Pharm. 2015, 12, 2924–2927. [Google Scholar] [CrossRef]
- Poletaeva, J.; Dovydenko, I.; Epanchintseva, A.; Korchagina, K.; Pyshnyi, D.; Apartsin, E.; Ryabchikova, E.; Pyshnaya, I. Non-Covalent associates of siRNAs and AuNPs enveloped with lipid layer and doped with amphiphilic peptide for efficient siRNA delivery. Int. J. Mol. Sci. 2018, 19, 2096. [Google Scholar] [CrossRef] [PubMed]
- Onciu, M. Acute lymphoblastic leukemia. Hematol. Oncol. Clin. N. Am. 2009, 23, 655–674. [Google Scholar] [CrossRef] [PubMed]
- Saußele, S.; Silver, R.T. Management of chronic myeloid leukemia in blast crisis. Ann. Hematol. 2015, 94, 159–165. [Google Scholar] [CrossRef]
- Fuentes-Paniagua, E.; Peña-González, C.E.; Galán, M.; Gómez, R.; De La Mata, F.J.; Sánchez-Nieves, J. Thiol-ene synthesis of cationic carbosilane dendrons: A new family of synthons. Organometallics 2013, 32, 1789–1796. [Google Scholar] [CrossRef]
- Aguiar, J.; Carpena, P.; Molina-Bolívar, J.A.; Carnero Ruiz, C. On the determination of the critical micelle concentration by the pyrene 1:3 ratio method. J. Colloid Interface Sci. 2003, 258, 116–122. [Google Scholar] [CrossRef]
- Sánchez-Nieves, J.; Ortega, P.; Muñoz-Fernández, M.Á.; Gómez, R.; de la Mata, F.J. Synthesis of carbosilane dendrons and dendrimers derived from 1,3,5-trihydroxybenzene. Tetrahedron 2010, 66, 9203–9213. [Google Scholar] [CrossRef]
- Mooibroek, T.J.; Gamez, P. The s-triazine ring, a remarkable unit to generate supramolecular interactions. Inorganica Chim. Acta 2007, 360, 381–404. [Google Scholar] [CrossRef]
- Parkar, N.S.; Akpa, B.S.; Nitsche, L.C.; Wedgewood, L.E.; Place, A.T.; Sverdlov, M.S.; Chaga, O.; Minshall, R.D. Vesicle formation and endocytosis: Function, machinery, mechanisms, and modeling. Antioxid. Redox Signal. 2009, 11, 1301–1312. [Google Scholar] [CrossRef]
- Klein, E.; Vánky, F.; Ben-Bassat, H.; Neumann, H.; Ralph, P.; Zeuthen, J.; Polliack, A. Properties of the K562 cell line, derived from a patient with chronic myeloid leukemia. Int. J. Cancer 1976, 18, 421–431. [Google Scholar] [CrossRef]
- Fahmy, S.A.; Brüßler, J.; Alawak, M.; El-Sayed, M.M.H.; Bakowsky, U.; Shoeib, T. Chemotherapy based on supramolecular chemistry: A promising strategy in cancer therapy. Pharmaceutics 2019, 11, 292. [Google Scholar] [CrossRef]
- Abedi-Gaballu, F.; Dehghan, G.; Ghaffari, M.; Yekta, R.; Abbaspour-Ravasjani, S.; Baradaran, B.; Ezzati Nazhad Dolatabadi, J.; Hamblin, M.R. PAMAM dendrimers as efficient drug and gene delivery nanosystems for cancer therapy. Appl. Mater. Today 2018, 12, 177–190. [Google Scholar] [CrossRef]
- Hossain Sk, U.; Kojima, C. Dendrimers for Drug Delivery of Anticancer Drugs. In Frontiers in Clinical Drug Research-Anti-Cancer Agents; Rahman, A., Ed.; Bentham Science Publishers: Shardjah, UAE, 2015; pp. 3–25. ISBN 978-1-68108-073-4. [Google Scholar]
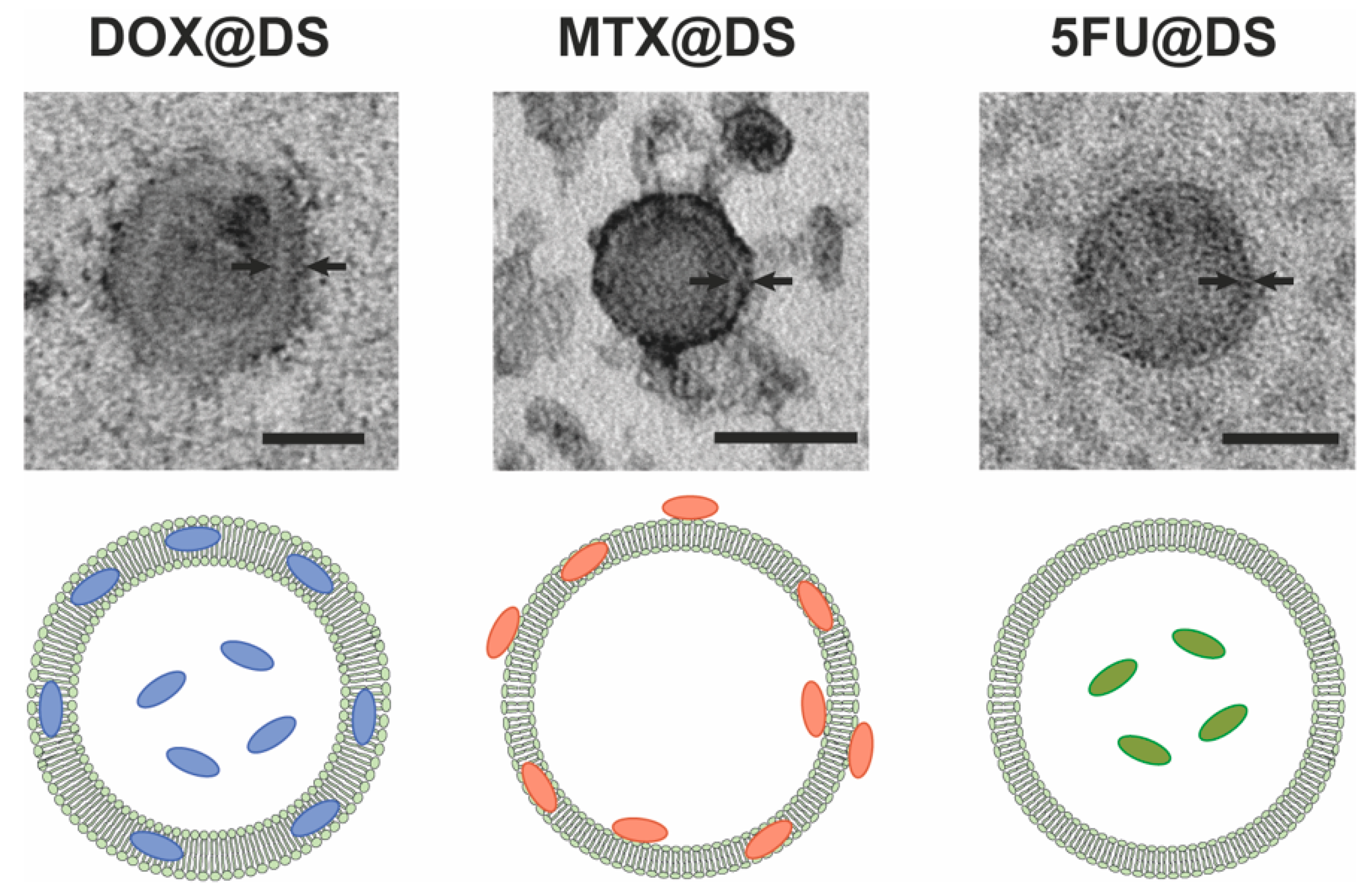

| Sample 1 | Particle Size 2, nm | PDI | Zeta Potential 2, mV | Drug Encapsulation 2, % | Drug Loading Capacity 3, % |
|---|---|---|---|---|---|
| DOX@DS | 92 ± 38 | 0.213 | 41.3 ± 7.3 | 65 ± 10 | >20.0 |
| MTX@DS | 46 ± 17 | 0.292 | 1.2 ± 4.5 | 75 ± 10 | >20.0 |
| 5FU@DS | 75 ± 30 | 0.216 | 11.0 ± 5.7 | 25 ± 5 | >2.1 |
| Sample 1 | 1301 | K562 |
|---|---|---|
| DS | 5.4 ± 1.4 µM | 4.6 ± 2.6 µM |
| DOX | 0.059 ± 0.021 µM 2 | 0.20 ± 0.08 µM |
| DOX@DS | 0.71 ± 0.13 µM | 1.2 ± 0.6 µM |
| MTX | 0.99 ± 0.34 µM | 0.046 ± 0.03 µM 2 |
| MTX@DS | 0.99 ± 0.40 µM | 0.15 ± 0.08 µM |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apartsin, E.; Knauer, N.; Arkhipova, V.; Pashkina, E.; Aktanova, A.; Poletaeva, J.; Sánchez-Nieves, J.; de la Mata, F.J.; Gómez, R. pH-Sensitive Dendrimersomes of Hybrid Triazine-Carbosilane Dendritic Amphiphiles-Smart Vehicles for Drug Delivery. Nanomaterials 2020, 10, 1899. https://doi.org/10.3390/nano10101899
Apartsin E, Knauer N, Arkhipova V, Pashkina E, Aktanova A, Poletaeva J, Sánchez-Nieves J, de la Mata FJ, Gómez R. pH-Sensitive Dendrimersomes of Hybrid Triazine-Carbosilane Dendritic Amphiphiles-Smart Vehicles for Drug Delivery. Nanomaterials. 2020; 10(10):1899. https://doi.org/10.3390/nano10101899
Chicago/Turabian StyleApartsin, Evgeny, Nadezhda Knauer, Valeria Arkhipova, Ekaterina Pashkina, Alina Aktanova, Julia Poletaeva, Javier Sánchez-Nieves, Francisco Javier de la Mata, and Rafael Gómez. 2020. "pH-Sensitive Dendrimersomes of Hybrid Triazine-Carbosilane Dendritic Amphiphiles-Smart Vehicles for Drug Delivery" Nanomaterials 10, no. 10: 1899. https://doi.org/10.3390/nano10101899
APA StyleApartsin, E., Knauer, N., Arkhipova, V., Pashkina, E., Aktanova, A., Poletaeva, J., Sánchez-Nieves, J., de la Mata, F. J., & Gómez, R. (2020). pH-Sensitive Dendrimersomes of Hybrid Triazine-Carbosilane Dendritic Amphiphiles-Smart Vehicles for Drug Delivery. Nanomaterials, 10(10), 1899. https://doi.org/10.3390/nano10101899







