Recent Advances in Nanomedicine for the Diagnosis and Therapy of Liver Fibrosis
Abstract
:1. Introduction
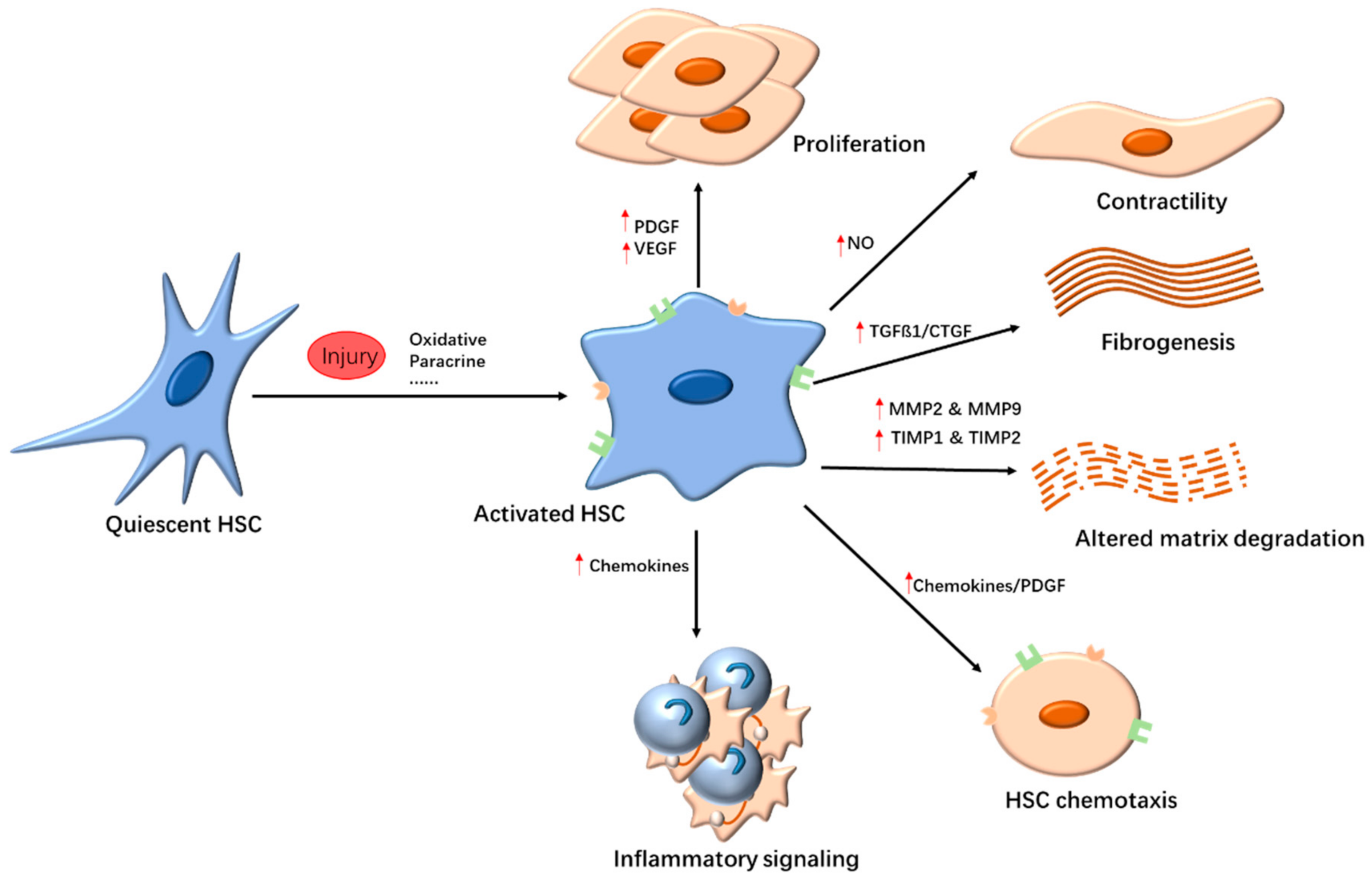
2. Potential Targets of Liver Fibrosis
2.1. Targeting HSCs
2.2. Anti-Inflammatory Response
2.3. Inhibition of Collagen Deposition
3. Nanomedicine in Liver Fibrosis Diagnosis
4. Nanomedicine for Liver Fibrosis Therapy
4.1. NPs as Therapeutic Agents
4.2. NPs as Drug Carriers without Targeting Ligand for the Treatment of Liver Fibrosis
4.3. NPs as Drug Carriers with Targeting Ligands for the Treatment of Liver Fibrosis
5. Nanomedicine in Liver Fibrosis Theranostics
6. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, Y.A.; Wallace, M.C.; Friedman, S.L. Pathobiology of liver fibrosis: A translational success story. Gut 2015, 64, 830–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baffy, G.; Brunt, E.M.; Caldwell, S.H. Hepatocellular carcinoma in non-alcoholic fatty liver disease: An emerging menace. J. Hepatol. 2012, 56, 1384–1391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mieli-Vergani, G.; Vergani, D.; Czaja, A.J.; Manns, M.P.; Krawitt, E.L.; Vierling, J.M.; Lohse, A.W.; Montano-Loza, A.J. Autoimmune hepatitis. Nat. Rev. Dis. Primers 2018, 4, 18017. [Google Scholar] [CrossRef] [PubMed]
- Sarin, S.K.; Kumar, M.; Eslam, M.; George, J.; Al Mahtab, M.; Akbar, S.M.F.; Jia, J.; Tian, Q.; Aggarwal, R.; Muljono, D.H.; et al. Liver diseases in the Asia-Pacific region: A Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol. Hepatol. 2020, 5, 167–228. [Google Scholar]
- Trautwein, C.; Friedman, S.L.; Schuppan, D.; Pinzani, M. Hepatic fibrosis: Concept to treatment. J. Hepatol. 2015, 62, S15–S24. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.Y.; Friedman, S.L. Fibrosis-dependent mechanisms of hepatocarcinogenesis. Hepatology 2012, 56, 769–775. [Google Scholar] [CrossRef]
- Manning, D.S.; Afdhal, N.H. Diagnosis and Quantitation of Fibrosis. Gastroenterology 2008, 134, 1670–1681. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, C.P.M.S.; Stefano, J.T.; De Siqueira, E.R.F.; Silva, L.S.; De Campos Mazo, D.F.; Lima, V.M.R.; Furuya, C.K.; Mello, E.S.; Souza, F.G.; Rabello, F.; et al. Combination of N-acetylcysteine and metformin improves histological steatosis and fibrosis in patients with non-alcoholic steatohepatitis. Hepatol. Res. 2008, 38, 159–165. [Google Scholar] [CrossRef]
- Peres, W.; Tuñón, M.J.; Collado, P.S.; Herrmann, S.; Marroni, N.; González-Gallego, J. The flavonoid quercetin ameliorates liver damage in rats with biliary obstruction. J. Hepatol. 2000, 33, 742–750. [Google Scholar] [CrossRef]
- Ogawa, S.; Ochi, T.; Shimada, H.; Inagaki, K.; Fujita, I.; Nii, A.; Moffat, M.A.; Katragadda, M.; Violand, B.N.; Arch, R.H.; et al. Anti-PDGF-B monoclonal antibody reduces liver fibrosis development. Hepatol. Res. 2010, 40, 1128–1141. [Google Scholar] [CrossRef]
- Schuppan, D.; Kim, Y.O. Evolving therapies for liver fibrosis. J. Clin. Investig. 2013, 123, 1887–1901. [Google Scholar] [CrossRef] [Green Version]
- Silva, C.; Pinho, J.; Lopes, J.M.; Almeida, A.J.; Reis, C.P. Current Trends in Cancer Nanotheranostics: Metallic, Polymeric, and Lipid-Based Systems. Pharmaceutics 2019, 11, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pucek, A.; Tokarek, B.; Waglewska, E.; Bazylińska, U. Recent Advances in the Structural Design of Photosensitive Agent Formulations Using “Soft” Colloidal Nanocarriers. Pharmaceutics 2020, 12, 587. [Google Scholar] [CrossRef] [PubMed]
- Wawrzyńczyk, D.; Cichy, B.; Zaręba, J.K.; Bazylińska, U. On the interaction between up-converting NaYF4:Er3+,Yb3+ nanoparticles and Rose Bengal molecules constrained within the double core of multifunctional nanocarriers. J. Mater. Chem. C 2019, 7, 15021–15034. [Google Scholar] [CrossRef]
- Doane, T.L.; Burda, C. The unique role of nanoparticles in nanomedicine: Imaging, drug delivery and therapy. Chem. Soc. Rev. 2012, 41, 2885–2911. [Google Scholar] [CrossRef] [PubMed]
- Surendran, S.P.; Thomas, R.G.; Moon, M.J.; Jeong, Y.Y. Nanoparticles for the treatment of liver fibrosis. Int. J. Nanomed. 2017, 12, 6997–7006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, L.H.; Couvreur, P. Nanotechnology for therapy and imaging of liver diseases. J. Hepatol. 2011, 55, 1461–1466. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Shang, W.; Liang, X.; Zeng, C.; Liu, M.; Wang, S.; Li, H.; Tian, J. The diagnosis of hepatic fibrosis by magnetic resonance and near-infrared imaging using dual-modality nanoparticles. RSC Adv. 2018, 8, 6699–6708. [Google Scholar] [CrossRef] [Green Version]
- Duong, H.T.; Dong, Z.; Su, L.; Boyer, C.; George, J.; Davis, T.P.; Wang, J. The use of nanoparticles to deliver nitric oxide to hepatic stellate cells for treating liver fibrosis and portal hypertension. Small Weinh. Der Bergstr. Ger. 2015, 11, 2291–2304. [Google Scholar] [CrossRef]
- Calvente, C.J.; Sehgal, A.; Popov, Y.; Kim, Y.O.; Zevallos, V.; Sahin, U.; Diken, M.; Schuppan, D. Specific hepatic delivery of procollagen α1(I) small interfering RNA in lipid-like nanoparticles resolves liver fibrosis. Hepatology 2015, 62, 1285–1297. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Wang, H.; Ong, Z.Y.; Xu, K.; Ee, P.L.R.; Zheng, S.; Hedrick, J.L.; Yang, Y.-Y. Polymer- and lipid-based nanoparticle therapeutics for the treatment of liver diseases. Nano Today 2010, 5, 296–312. [Google Scholar] [CrossRef]
- Melgert, B.N.; Olinga, P.; Jack, V.K.; Molema, G.; Meijer, D.K.F.; Poelstra, K. Dexamethasone coupled to albumin is selectively taken up by rat nonparenchymal liver cells and attenuates LPS-induced activation of hepatic cells. J. Hepatol. 2000, 32, 603–611. [Google Scholar] [CrossRef]
- Petros, R.A.; DeSimone, J.M. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010, 9, 615–627. [Google Scholar] [CrossRef]
- Nagórniewicz, B.; Mardhian, D.F.; Booijink, R.; Storm, G.; Prakash, J.; Bansal, R. Engineered Relaxin as Theranostic nanomedicine to diagnose and ameliorate liver cirrhosis. Nanomed. Nanotechnol. Biol. Med. 2019, 17, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Evason, K.J.; Asahina, K.; Stainier, D.Y.R. Hepatic stellate cells in liver development, regeneration, and cancer. J. Clin. Investig. 2013, 123, 1902–1910. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Gea, V.; Friedman, S.L. Pathogenesis of Liver Fibrosis. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 425–456. [Google Scholar] [CrossRef]
- Tsuchida, T.; Friedman, S.L. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 397–411. [Google Scholar] [CrossRef]
- Meng, X.-M.; Nikolic-Paterson, D.J.; Lan, H.Y. TGF-β: The master regulator of fibrosis. Nat. Rev. Nephrol. 2016, 12, 325–338. [Google Scholar] [CrossRef]
- Kocabayoglu, P.; Lade, A.; Lee, Y.A.; Dragomir, A.-C.; Sun, X.; Fiel, M.I.; Thung, S.; Aloman, C.; Soriano, P.; Hoshida, Y.; et al. β-PDGF receptor expressed by hepatic stellate cells regulates fibrosis in murine liver injury, but not carcinogenesis. J. Hepatol. 2015, 63, 141–147. [Google Scholar] [CrossRef] [Green Version]
- Okuno, M.; Moriwaki, H.; Muto, Y.; Kojima, S. Protease inhibitors suppress TGF-β generation by hepatic stellate cells. J. Hepatol. 1998, 29, 1031–1032. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, J.; Zhang, D.; Zhang, J.; Ma, J.; Jiang, H. New insights into the antifibrotic effects of sorafenib on hepatic stellate cells and liver fibrosis. J. Hepatol. 2010, 53, 132–144. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Ikeda, K.; Ikebe, T.; Hirakawa, K.; Sowa, M.; Nakatani, K.; Kawada, N.; Kaneda, K. Inhibition of hepatic stellate cell proliferation and activation by the semisynthetic analogue of fumagillin TNP-470 in rats. Hepatology 2000, 32, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, H.; Ueno, T.; Torimura, T.; Inuzuka, S.; Tanikawa, K. Inhibitory effect of OPC-15161, a component of fungus Thielavia minor, on proliferation and extracellular matrix production of rat cultured hepatic stellate cells. J. Cell. Physiol. 1998, 174, 398–406. [Google Scholar] [CrossRef]
- Morry, J.; Ngamcherdtrakul, W.; Yantasee, W. Oxidative stress in cancer and fibrosis: Opportunity for therapeutic intervention with antioxidant compounds, enzymes, and nanoparticles. Redox Biol. 2017, 11, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Hickman, I.; Macdonald, G. Is vitamin E beneficial in chronic liver disease? Hepatology 2007, 46, 288–290. [Google Scholar] [CrossRef] [PubMed]
- Sukalingam, K.; Ganesan, K.; Xu, B. Protective Effect of Aqueous Extract from the Leaves of Justicia tranquebariesis against Thioacetamide-Induced Oxidative Stress and Hepatic Fibrosis in Rats. Antioxidants 2018, 7, 78. [Google Scholar] [CrossRef] [Green Version]
- Di Pascoli, M.; Diví, M.; Rodríguez-Vilarrupla, A.; Rosado, E.; Gracia-Sancho, J.; Vilaseca, M.; Bosch, J.; García-Pagán, J.C. Resveratrol improves intrahepatic endothelial dysfunction and reduces hepatic fibrosis and portal pressure in cirrhotic rats. J. Hepatol. 2013, 58, 904–910. [Google Scholar] [CrossRef]
- Mezey, E. Prevention of alcohol-induced hepatic fibrosis by phosphatidylcholine. Gastroenterology 1994, 106, 257–259. [Google Scholar] [CrossRef]
- Hirano, A.; Kaplowitz, N.; Tsukamoto, H.; Kamimura, S.; Fernandez-Checa, J.C. Hepatic mitochondrial glutathione depletion and progression of experimental alcoholic liver disease in rats. Hepatology 1992, 16, 1423–1427. [Google Scholar] [CrossRef]
- Ankoma-Sey, V.; Wang, Y.; Dai, Z. Hypoxic stimulation of vascular endothelial growth factor expression in activated rat hepatic stellate cells. Hepatology 2000, 31, 141–148. [Google Scholar] [CrossRef]
- Aleffi, S.; Petrai, I.; Bertolani, C.; Parola, M.; Colombatto, S.; Novo, E.; Vizzutti, F.; Anania, F.A.; Milani, S.; Rombouts, K.; et al. Upregulation of proinflammatory and proangiogenic cytokines by leptin in human hepatic stellate cells. Hepatology 2005, 42, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.; Semela, D.; Bruix, J.; Colle, I.; Pinzani, M.; Bosch, J. Angiogenesis in liver disease. J. Hepatol. 2009, 50, 604–620. [Google Scholar] [CrossRef] [PubMed]
- Taura, K.; De Minicis, S.; Seki, E.; Hatano, E.; Iwaisako, K.; Osterreicher, C.H.; Kodama, Y.; Miura, K.; Ikai, I.; Uemoto, S.; et al. Hepatic Stellate Cells Secrete Angiopoietin 1 That Induces Angiogenesis in Liver Fibrosis. Gastroenterology 2008, 135, 1729–1738. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.-O.; Welch, T.P.; Gonzalez, F.J.; Copple, B.L. Reduced liver fibrosis in hypoxia-inducible factor-1α-deficient mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G582–G592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Valle, V.; Chávez-Tapia, N.C.; Uribe, M.; Méndez-Sánchez, N. Role of oxidative stress and molecular changes in liver fibrosis: A review. Curr. Med. Chem. 2012, 19, 4850–4860. [Google Scholar] [CrossRef]
- Seki, E.; Schwabe, R.F. Hepatic inflammation and fibrosis: Functional links and key pathways. Hepatology 2015, 61, 1066–1079. [Google Scholar] [CrossRef] [PubMed]
- Anand, L.; Choudhury, A.; Bihari, C.; Sharma, B.C.; Kumar, M.; Maiwall, R.; Tan, S.S.; Shah, S.R.; Hamid, S.; Butt, A.S.; et al. Flare of Autoimmune Hepatitis Causing Acute on Chronic Liver Failure: Diagnosis and Response to Corticosteroid Therapy. Hepatology 2019, 70, 587–596. [Google Scholar] [CrossRef]
- Morgan, T.R.; Weiss, D.G.; Nemchausky, B.; Schiff, E.R.; Anand, B.; Simon, F.; Kidao, J.; Cecil, B.; Mendenhall, C.L.; Nelson, D.; et al. Colchicine treatment of alcoholic cirrhosis: A randomized, placebo-controlled clinical trial of patient survival. Gastroenterology 2005, 128, 882–890. [Google Scholar] [CrossRef]
- Cheng, K.; Ashby, D.; Smyth, R.L. Ursodeoxycholic acid for cystic fibrosis-related liver disease. Cochrane Database Syst. Rev. 2017, 2017, CD000222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, D.R.; Lauwers, G.Y.; Lau, J.Y.N.; Davis, G.L. Interleukin 10 treatment reduces fibrosis in patients with chronic hepatitis C: A pilot trial of interferon nonresponders. Gastroenterology 2000, 118, 655–660. [Google Scholar] [CrossRef]
- Heide, D.v.d.; Weiskirchen, R.; Bansal, R. Therapeutic Targeting of Hepatic Macrophages for the Treatment of Liver Diseases. Front. Immunol. 2019, 10, 2852. [Google Scholar] [CrossRef] [Green Version]
- Van Bergen, T.; Spangler, R.; Marshall, D.; Hollanders, K.; Van de Veire, S.; Vandewalle, E.; Moons, L.; Herman, J.; Smith, V.; Stalmans, I. The Role of LOX and LOXL2 in the Pathogenesis of an Experimental Model of Choroidal Neovascularization. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5280–5289. [Google Scholar] [CrossRef] [Green Version]
- Yoshio, S.; Atsushi, N.; Yoshito, I. Limitations of liver biopsy and non-invasive diagnostic tests for the diagnosis of nonalcoholic fatty liver disease/ nonalcoholic steatohepatitis. World J. Gastroenterol 2014, 20, 475–485. [Google Scholar]
- Dulai, P.S.; Sirlin, C.B.; Loomba, R. MRI and MRE for non-invasive quantitative assessment of hepatic steatosis and fibrosis in NAFLD and NASH: Clinical trials to clinical practice. J. Hepatol. 2016, 65, 1006–1016. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Lee, J.S.H.; Zhang, M. Magnetic nanoparticles in MR imaging and drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1252–1265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saraswathy, A.; Nazeer, S.S.; Nimi, N.; Arumugam, S.; Shenoy, S.J.; Jayasree, R.S. Synthesis and characterization of dextran stabilized superparamagnetic iron oxide nanoparticles for in vivo MR imaging of liver fibrosis. Carbohydr. Polym. 2014, 101, 760–768. [Google Scholar] [CrossRef]
- Saraswathy, A.; Nazeer, S.S.; Jeevan, M.; Nimi, N.; Arumugam, S.; Harikrishnan, V.S.; Varma, P.R.; Jayasree, R.S. Citrate coated iron oxide nanoparticles with enhanced relaxivity for in vivo magnetic resonance imaging of liver fibrosis. Colloids Surf. B Biointerfaces 2014, 117, 216–224. [Google Scholar] [CrossRef]
- Nimi, N.; Saraswathy, A.; Nazeer, S.S.; Francis, N.; Shenoy, S.J.; Jayasree, R.S. Multifunctional hybrid nanoconstruct of zerovalent iron and carbon dots for Magnetic Resonance Angiography and Optical Imaging: An In vivo study. Biomaterials 2018, 171, 46–56. [Google Scholar] [CrossRef]
- Xuan, J.; Chen, Y.; Zhu, L.; Guo, Y.; Ao, M. Ultrasound molecular imaging with cRGD-PLGA-PFOB nanoparticles for liver fibrosis staging in a rat model. Oncotarget 2017, 8, 108676–108691. [Google Scholar] [CrossRef] [Green Version]
- Anselmo, A.C.; Mitragotri, S. A Review of Clinical Translation of Inorganic Nanoparticles. AAPS J. 2015, 17, 1041–1054. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.P.; Zeng, Q.H.; Lu, G.Q.; Yu, A.B. Inorganic nanoparticles as carriers for efficient cellular delivery. Chem. Eng. Sci. 2006, 61, 1027–1040. [Google Scholar] [CrossRef]
- Tee, J.K.; Peng, F.; Ho, H.K. Effects of inorganic nanoparticles on liver fibrosis: Optimizing a double-edged sword for therapeutics. Biochem. Pharmacol. 2019, 160, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Tee, J.K.; Setyawati, M.I.; Ding, X.; Yeo, H.L.A.; Tan, Y.L.; Leong, D.T.; Ho, H.K. Inorganic Nanomaterials as Highly Efficient Inhibitors of Cellular Hepatic Fibrosis. ACS Appl. Mater. Interfaces 2018, 10, 31938–31946. [Google Scholar] [CrossRef] [PubMed]
- Oró, D.; Yudina, T.; Fernández-Varo, G.; Casals, E.; Reichenbach, V.; Casals, G.; González de la Presa, B.; Sandalinas, S.; Carvajal, S.; Puntes, V.; et al. Cerium oxide nanoparticles reduce steatosis, portal hypertension and display anti-inflammatory properties in rats with liver fibrosis. J. Hepatol. 2016, 64, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, A.; Polley, N.; Darbar, S.; Bagchi, D.; Pal, S.K. Citrate functionalized Mn3O4 in nanotherapy of hepatic fibrosis by oral administration. Future Sci. OA 2016, 2, 2056–5623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rani, V.; Verma, Y.; Rana, K.; Rana, S.V.S. Zinc oxide nanoparticles inhibit dimethylnitrosamine induced liver injury in rat. Chem. Biol. Interact. 2018, 295, 84–92. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, T.G.; Garcia, V.B.; de Araújo, A.A.; da Silva Gasparotto, L.H.; Silva, H.; Guerra, G.C.B.; de Castro Miguel, E.; de Carvalho Leitão, R.F.; da Silva Costa, D.V.; Cruz, L.J.; et al. Spherical neutral gold nanoparticles improve anti-inflammatory response, oxidative stress and fibrosis in alcohol-methamphetamine-induced liver injury in rats. Int. J. Pharm. 2018, 548, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hamza, R.Z.; EL-Megharbel, S.M.; Altalhi, T.; Gobouri, A.A.; Alrogi, A.A. Hypolipidemic and hepatoprotective synergistic effects of selenium nanoparticles and vitamin. E against acrylamide-induced hepatic alterations in male albino mice. Appl. Organomet. Chem. 2020, 34, e5458. [Google Scholar] [CrossRef]
- Böttger, R.; Pauli, G.; Chao, P.-H.; Al Fayez, N.; Hohenwarter, L.; Li, S.-D. Lipid-based nanoparticle technologies for liver targeting. Adv. Drug Deliv. Rev. 2020, in press. [Google Scholar]
- Reebye, V.; Huang, K.-W.; Lin, V.; Jarvis, S.; Cutilas, P.; Dorman, S.; Ciriello, S.; Andrikakou, P.; Voutila, J.; Saetrom, P.; et al. Gene activation of CEBPA using saRNA: Preclinical studies of the first in human saRNA drug candidate for liver cancer. Oncogene 2018, 37, 3216–3228. [Google Scholar] [CrossRef]
- Bartneck, M.; Scheyda, K.M.; Warzecha, K.T.; Rizzo, L.Y.; Hittatiya, K.; Luedde, T.; Storm, G.; Trautwein, C.; Lammers, T.; Tacke, F. Fluorescent cell-traceable dexamethasone-loaded liposomes for the treatment of inflammatory liver diseases. Biomaterials 2015, 37, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Kaps, L.; Nuhn, L.; Aslam, M.; Brose, A.; Foerster, F.; Rosigkeit, S.; Renz, P.; Heck, R.; Kim, Y.O.; Lieberwirth, I.; et al. In Vivo Gene-Silencing in Fibrotic Liver by siRNA-Loaded Cationic Nanohydrogel Particles. Adv. Healthc. Mater. 2015, 4, 2809–2815. [Google Scholar] [CrossRef]
- Leber, N.; Kaps, L.; Aslam, M.; Schupp, J.; Brose, A.; Schäffel, D.; Fischer, K.; Diken, M.; Strand, D.; Koynov, K.; et al. SiRNA-mediated in vivo gene knockdown by acid-degradable cationic nanohydrogel particles. J. Control. Release 2017, 248, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Krithika, R.; Vhora, I.; Verma, R.J. Preparation, toxicity analysis and in vivo protective effect of phyllanthin-loaded PLGA nanoparticles against CCl4-induced hepatic fibrosis. J. Drug Deliv. Sci. Technol. 2019, 51, 364–371. [Google Scholar] [CrossRef]
- Lin, T.-T.; Gao, D.-Y.; Liu, Y.-C.; Sung, Y.-C.; Wan, D.; Liu, J.-Y.; Chiang, T.; Wang, L.; Chen, Y. Development and characterization of sorafenib-loaded PLGA nanoparticles for the systemic treatment of liver fibrosis. J. Control. Release 2016, 221, 62–70. [Google Scholar] [CrossRef]
- Younis, N.; Shaheen, M.A.; Abdallah, M.H. Silymarin-loaded Eudragit® RS100 nanoparticles improved the ability of silymarin to resolve hepatic fibrosis in bile duct ligated rats. Biomed. Pharmacother. 2016, 81, 93–103. [Google Scholar] [CrossRef]
- He, Q.; Zhang, J.; Chen, F.; Guo, L.; Zhu, Z.; Shi, J. An anti-ROS/hepatic fibrosis drug delivery system based on salvianolic acid B loaded mesoporous silica nanoparticles. Biomaterials 2010, 31, 7785–7796. [Google Scholar] [CrossRef]
- Vivero-Escoto, J.L.; Vadarevu, H.; Juneja, R.; Schrum, L.W.; Benbow, J.H. Nanoparticle mediated silencing of tenascin C in hepatic stellate cells: Effect on inflammatory gene expression and cell migration. J. Mater. Chem. B 2019, 7, 7396–7405. [Google Scholar] [CrossRef]
- Krishnan, G.; Subramaniyan, J.; Chengalvarayan Subramani, P.; Muralidharan, B.; Thiruvengadam, D. Hesperetin conjugated PEGylated gold nanoparticles exploring the potential role in anti-inflammation and anti-proliferation during diethylnitrosamine-induced hepatocarcinogenesis in rats. Asian J. Pharm. Sci. 2017, 12, 442–455. [Google Scholar] [CrossRef]
- Das, A.; Mukherjee, P.; Singla, S.K.; Guturu, P.; Frost, M.C.; Mukhopadhyay, D.; Shah, V.H.; Patra, C.R. Fabrication and characterization of an inorganic gold and silica nanoparticle mediated drug delivery system for nitric oxide. Nanotechnology 2010, 21, 305102. [Google Scholar] [CrossRef]
- Yu, X.; Yuan, L.; Zhu, N.; Wang, K.; Xia, Y. Fabrication of antimicrobial curcumin stabilized platinum nanoparticles and their anti-liver fibrosis activity for potential use in nursing care. J. Photochem. Photobiol. B Biol. 2019, 195, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, M.; Fu, L.; Lin, J.; Zhou, X.; Zhou, P.; Huang, P.; Hu, H.; Han, Y. Liver-targeted delivery of TSG-6 by calcium phosphate nanoparticles for the management of liver fibrosis. Theranostics 2020, 10, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Melgar-Lesmes, P.; Luquero, A.; Parra-Robert, M.; Mora, A.; Ribera, J.; Edelman, E.R.; Jiménez, W. Graphene–Dendrimer Nanostars for Targeted Macrophage Overexpression of Metalloproteinase 9 and Hepatic Fibrosis Precision Therapy. Nano Lett. 2018, 18, 5839–5845. [Google Scholar] [CrossRef] [PubMed]
- Algandaby, M.M.; Al-Sawahli, M.M.; Oaa, A.; Fahmy, U.A.; Abdallah, H.M.; Hattori, M.; Ashour, O.M.; Abdel-Naim, A.B. Curcumin-Zein Nanospheres Improve Liver Targeting and Antifibrotic Activity of Curcumin in Carbon Tetrachloride-Induced Mice Liver Fibrosis. J. Biomed. Nanotechnol. 2016, 12, 1746–1757. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.L.; Kok, S.H.L.; Gambari, R.; Kok, T.W.; Leung, H.Y.; Choi, K.L.; Wong, C.S.; Hau, D.K.P.; Wong, W.Y.; Lam, K.H.; et al. Evaluation of berberine/bovine serum albumin nanoparticles for liver fibrosis therapy. Green Chem. 2015, 17, 1640–1646. [Google Scholar] [CrossRef]
- Violatto, M.B.; Casarin, E.; Talamini, L.; Russo, L.; Baldan, S.; Tondello, C.; Messmer, M.; Hintermann, E.; Rossi, A.; Passoni, A.; et al. Dexamethasone Conjugation to Biodegradable Avidin-Nucleic-Acid-Nano-Assemblies Promotes Selective Liver Targeting and Improves Therapeutic Efficacy in an Autoimmune Hepatitis Murine Model. ACS Nano 2019, 13, 4410–4423. [Google Scholar] [CrossRef]
- Hawkins, M.J.; Soon-Shiong, P.; Desai, N. Protein nanoparticles as drug carriers in clinical medicine. Adv. Drug Deliv. Rev. 2008, 60, 876–885. [Google Scholar] [CrossRef]
- El-Mezayen, N.S.; El-Hadidy, W.F.; El-Refaie, W.M.; Shalaby, T.I.; Khattab, M.M.; El-Khatib, A.S. Hepatic stellate cell-targeted imatinib nanomedicine versus conventional imatinib: A novel strategy with potent efficacy in experimental liver fibrosis. J. Control. Release 2017, 266, 226–237. [Google Scholar] [CrossRef]
- El-Mezayen, N.S.; El-Hadidy, W.F.; El-Refaie, W.M.; Shalaby, T.I.; Khattab, M.M.; El-Khatib, A.S. Oral vitamin-A-coupled valsartan nanomedicine: High hepatic stellate cell receptors accessibility and prolonged enterohepatic residence. J. Control. Release 2018, 283, 32–44. [Google Scholar] [CrossRef]
- Li, Q.; Ding, Y.; Guo, X.; Luo, S.; Zhuang, H.; Zhou, J.; Xu, N.; Yan, Z. Chemically modified liposomes carrying TRAIL target activated hepatic stellate cells and ameliorate hepatic fibrosis in vitro and in vivo. J. Cell. Mol. Med. 2019, 23, 1951–1962. [Google Scholar] [CrossRef]
- Liu, C.-H.; Chan, K.-M.; Chiang, T.; Liu, J.-Y.; Chern, G.-G.; Hsu, F.-F.; Wu, Y.-H.; Liu, Y.-C.; Chen, Y. Dual-Functional Nanoparticles Targeting CXCR4 and Delivering Antiangiogenic siRNA Ameliorate Liver Fibrosis. Mol. Pharm. 2016, 13, 2253–2262. [Google Scholar] [CrossRef] [PubMed]
- Morsy, M.A.; Nair, A.B. Prevention of rat liver fibrosis by selective targeting of hepatic stellate cells using hesperidin carriers. Int. J. Pharm. 2018, 552, 241–250. [Google Scholar] [CrossRef]
- Omar, R.; Yang, J.; Alrushaid, S.; Burczynski, F.J.; Minuk, G.Y.; Gong, Y. Inhibition of BMP4 and Alpha Smooth Muscle Actin Expression in LX-2 Hepatic Stellate Cells by BMP4-siRNA Lipid Based Nanoparticle. J. Pharm. Pharm. Sci. 2018, 21, 119–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, D.; Gao, Y.H.; Tan, K.B.; Zuo, Z.X.; Yang, W.X.; Hua, X.; Li, P.J.; Zhang, Y.; Wang, G. Inhibition of hepatic fibrosis with artificial microRNA using ultrasound and cationic liposome-bearing microbubbles. Gene Ther. 2013, 20, 1140–1148. [Google Scholar] [CrossRef]
- Qiao, J.-B.; Fan, Q.-Q.; Zhang, C.-L.; Lee, J.; Byun, J.; Xing, L.; Gao, X.-D.; Oh, Y.-K.; Jiang, H.-L. Hyperbranched lipoid-based lipid nanoparticles for bidirectional regulation of collagen accumulation in liver fibrosis. J. Control. Release 2020, 321, 629–640. [Google Scholar] [CrossRef]
- Jia, Z.; Gong, Y.; Pi, Y.; Liu, X.; Gao, L.; Kang, L.; Wang, J.; Yang, F.; Tang, J.; Lu, W.; et al. pPB Peptide-Mediated siRNA-Loaded Stable Nucleic Acid Lipid Nanoparticles on Targeting Therapy of Hepatic Fibrosis. Mol. Pharm. 2018, 15, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Khaja, F.; Jayawardena, D.; Kuzmis, A.; Önyüksel, H. Targeted Sterically Stabilized Phospholipid siRNA Nanomedicine for Hepatic and Renal Fibrosis. Nanomaterials 2016, 6, 8. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.-H.; Chern, G.-J.; Hsu, F.-F.; Huang, K.-W.; Sung, Y.-C.; Huang, H.-C.; Qiu, J.T.; Wang, S.-K.; Lin, C.-C.; Wu, C.-H.; et al. A multifunctional nanocarrier for efficient TRAIL-based gene therapy against hepatocellular carcinoma with desmoplasia in mice. Hepatology 2018, 67, 899–913. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Pan, W.; Wang, Y.; Lei, W.; Feng, B.; Du, C.; Wang, X.J. Enhanced efficacy of curcumin with phosphatidylserine-decorated nanoparticles in the treatment of hepatic fibrosis. Drug Deliv. 2018, 25, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Fan, Q.-Q.; Zhang, C.-L.; Qiao, J.-B.; Cui, P.-F.; Xing, L.; Oh, Y.-K.; Jiang, H.-L. Extracellular matrix-penetrating nanodrill micelles for liver fibrosis therapy. Biomaterials 2020, 230, 119616. [Google Scholar] [CrossRef]
- Qiao, J.-B.; Fan, Q.-Q.; Xing, L.; Cui, P.-F.; He, Y.-J.; Zhu, J.-C.; Wang, L.; Pang, T.; Oh, Y.-K.; Zhang, C.; et al. Vitamin A-decorated biocompatible micelles for chemogene therapy of liver fibrosis. J. Control. Release 2018, 283, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhang, P.; Zhao, T.; Jia, M.; Yin, P.; Li, W.; Zhang, Z.-R.; Fu, Y.; Gong, T. Golgi Apparatus-Targeted Chondroitin-Modified Nanomicelles Suppress Hepatic Stellate Cell Activation for the Management of Liver Fibrosis. ACS Nano 2019, 13, 3910–3923. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, C.; Zha, Y.; Hu, W.; Gao, Z.; Zang, Y.; Chen, J.; Zhang, J.; Dong, L. Corona-Directed Nucleic Acid Delivery into Hepatic Stellate Cells for Liver Fibrosis Therapy. ACS Nano 2015, 9, 2405–2419. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, D.W.; Jajoriya, A.K.; Dhawan, G.; Mishra, D.; Argemi, J.; Bataller, R.; Storm, G.; Mishra, D.P.; Prakash, J.; Bansal, R. Therapeutic inhibition of spleen tyrosine kinase in inflammatory macrophages using PLGA nanoparticles for the treatment of non-alcoholic steatohepatitis. J. Control. Release 2018, 288, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Hassan, R.; Tammam, S.N.; Safy, S.E.; Abdel-Halim, M.; Asimakopoulou, A.; Weiskirchen, R.; Mansour, S. Prevention of hepatic stellate cell activation using JQ1- and atorvastatin-loaded chitosan nanoparticles as a promising approach in therapy of liver fibrosis. Eur. J. Pharm. Biopharm. 2019, 134, 96–106. [Google Scholar] [CrossRef]
- Deshmukh, M.; Nakagawa, S.; Higashi, T.; Vincek, A.; Venkatesh, A.; Ruiz de Galarreta, M.; Koh, A.P.; Goossens, N.; Hirschfield, H.; Bian, C.B.; et al. Cell type-specific pharmacological kinase inhibition for cancer chemoprevention. Nanomedicine 2018, 14, 317–325. [Google Scholar] [CrossRef] [Green Version]
- Gou, Y.; Miao, D.D.; Zhou, M.; Wang, L.J.; Zhou, H.Y.; Su, G.X. Bio-Inspired Protein-Based Nanoformulations for Cancer Theranostics. Front. Pharmacol. 2018, 9, 421. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, D.; Guo, Q.; Shan, W.; Yang, J.; Lin, T.; Ye, S.; Zhou, X.; Ge, Y.; Bi, S.; et al. Theranostic Quercetin Nanoparticle for Treatment of Hepatic Fibrosis. Bioconjugate Chem. 2019, 30, 2939–2946. [Google Scholar] [CrossRef]
- Wu, J.; Huang, J.; Kuang, S.; Chen, J.; Li, X.; Chen, B.; Wang, J.; Cheng, D.; Shuai, X. Synergistic MicroRNA Therapy in Liver Fibrotic Rat Using MRI-Visible Nanocarrier Targeting Hepatic Stellate Cells. Adv. Sci. 2019, 6, 1801809. [Google Scholar] [CrossRef]
- Yu, Y.; Duan, J.; Li, Y.; Li, Y.; Sun, Z. Silica nanoparticles induce liver fibrosis via TGF-β1/Smad3 pathway in ICR mice. Int. J. Nanomed. 2017, 12, 6045–6057. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Chang, X.; Wang, H.; Liu, Y.; Wang, X.; Wu, M.; Zhan, H.; Li, S.; Sun, Y. TGF-β1 mediated Smad signaling pathway and EMT in hepatic fibrosis induced by Nano NiO in vivo and in vitro. Environ. Toxicol. 2020, 35, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Bo, L.; Zhang, X.; Yang, J.; Zhang, Y.; Li, W.; Fan, C.; Huang, Q. Influence of polyethylene glycol coating on biodistribution and toxicity of nanoscale graphene oxide in mice after intravenous injection. Int. J. Nanomed. 2014, 9, 4697–4707. [Google Scholar]
- Lee, I.-C.; Ko, J.-W.; Park, S.-H.; Shin, N.-R.; Shin, I.-S.; Moon, C.; Kim, S.-H.; Yun, W.-K.; Kim, H.-C.; Kim, J.-C. Copper nanoparticles induce early fibrotic changes in the liver via TGF-β/Smad signaling and cause immunosuppressive effects in rats. Nanotoxicology 2018, 12, 637–651. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.; Du, L.; Chen, B.; Yan, D.; Yang, A.; Liu, J.; Gu, N.; Meng, J.; Xu, H. Iron oxide nanoparticles induce reversible endothelial-to-mesenchymal transition in vascular endothelial cells at acutely non-cytotoxic concentrations. Part. Fibre Toxicol. 2019, 16, 30. [Google Scholar] [CrossRef] [Green Version]
- Bartneck, M.; Ritz, T.; Keul, H.A.; Wambach, M.; Bornemann, J.; Gbureck, U.; Ehling, J.; Lammers, T.; Heymann, F.; Gassler, N.; et al. Peptide-Functionalized Gold Nanorods Increase Liver Injury in Hepatitis. ACS Nano 2012, 6, 8767–8777. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Li, F.; Zhou, H.; Bai, Y.; Liu, S.; Jiang, Y.; Jiang, G.; Yan, B. Oral Exposure to Silver Nanoparticles or Silver Ions May Aggravate Fatty Liver Disease in Overweight Mice. Environ. Sci. Technol. 2017, 51, 9334–9343. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; He, X.; Yang, Y.; Li, M.; Xu, C.; Yu, R. Risk assessment of silica nanoparticles on liver injury in metabolic syndrome mice induced by fructose. Sci. Total Environ. 2018, 628, 366–374. [Google Scholar] [CrossRef]
- Soule, B.; Tirucherai, G.; Kavita, U.; Kundu, S.; Christian, R. Safety, tolerability, and pharmacokinetics of BMS-986263/ND-L02-s0201, a novel targeted lipid nanoparticle delivering HSP47 siRNA, in healthy participants: A randomised, placebo-controlled, double-blind, phase 1 study. J. Hepatol. 2018, 68, S112. [Google Scholar] [CrossRef]
- Sakamoto, N.; Ogawa, K.; Suda, G.; Morikawa, K.; Sho, T.; Nakai, M.; Suzuki, H.; Yamagata, N.; Tanaka, Y.; Ying, W. Clinical phase 1b study results for safety, pharmacokinetics and efficacy of ND-L02-s0201, a novel targeted lipid nanoparticle delivering HSP47 SIRNA for the treatment of Japanese patients with advanced liver fibrosis. J. Hepatol. 2018, 68, S242. [Google Scholar] [CrossRef]
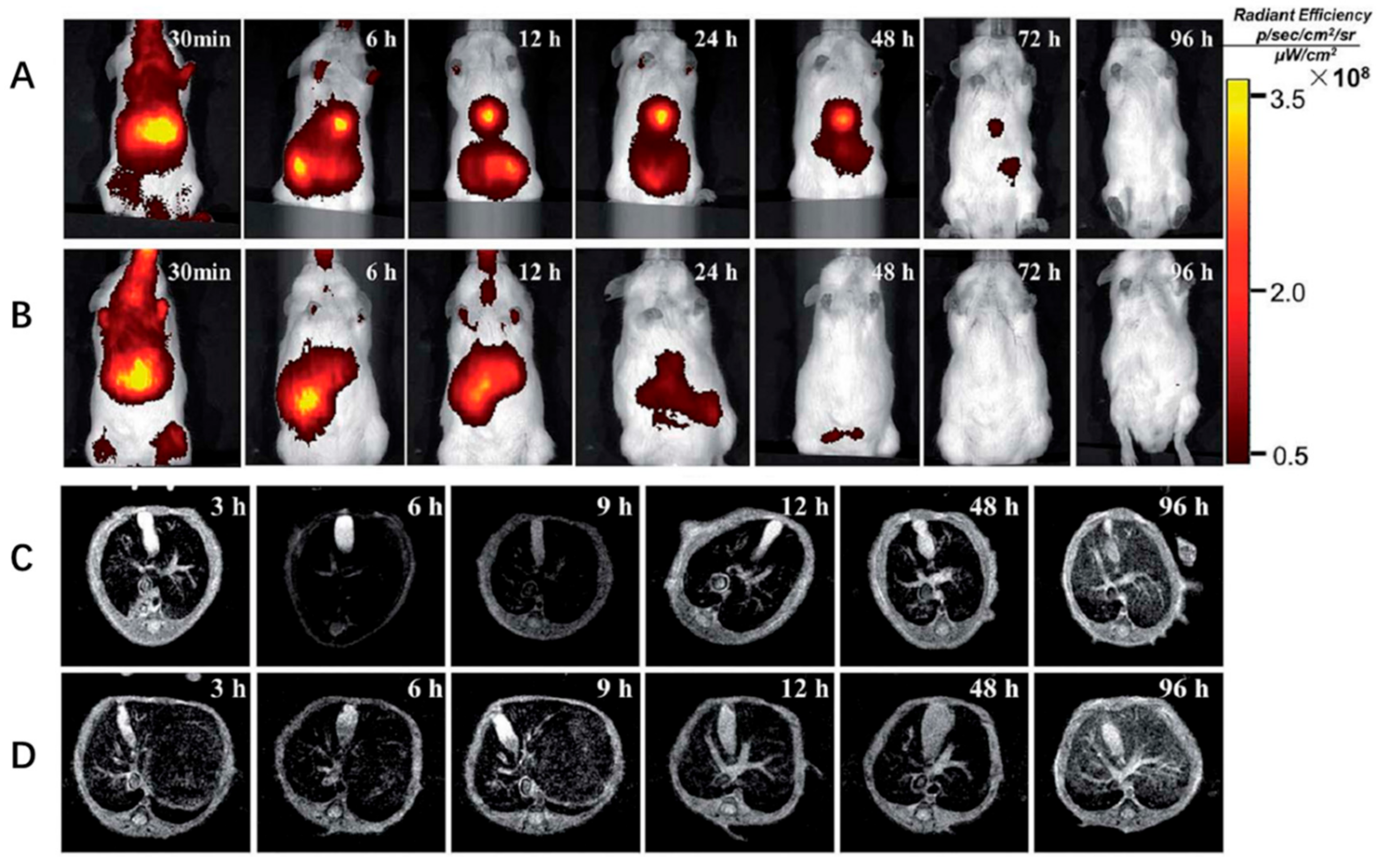
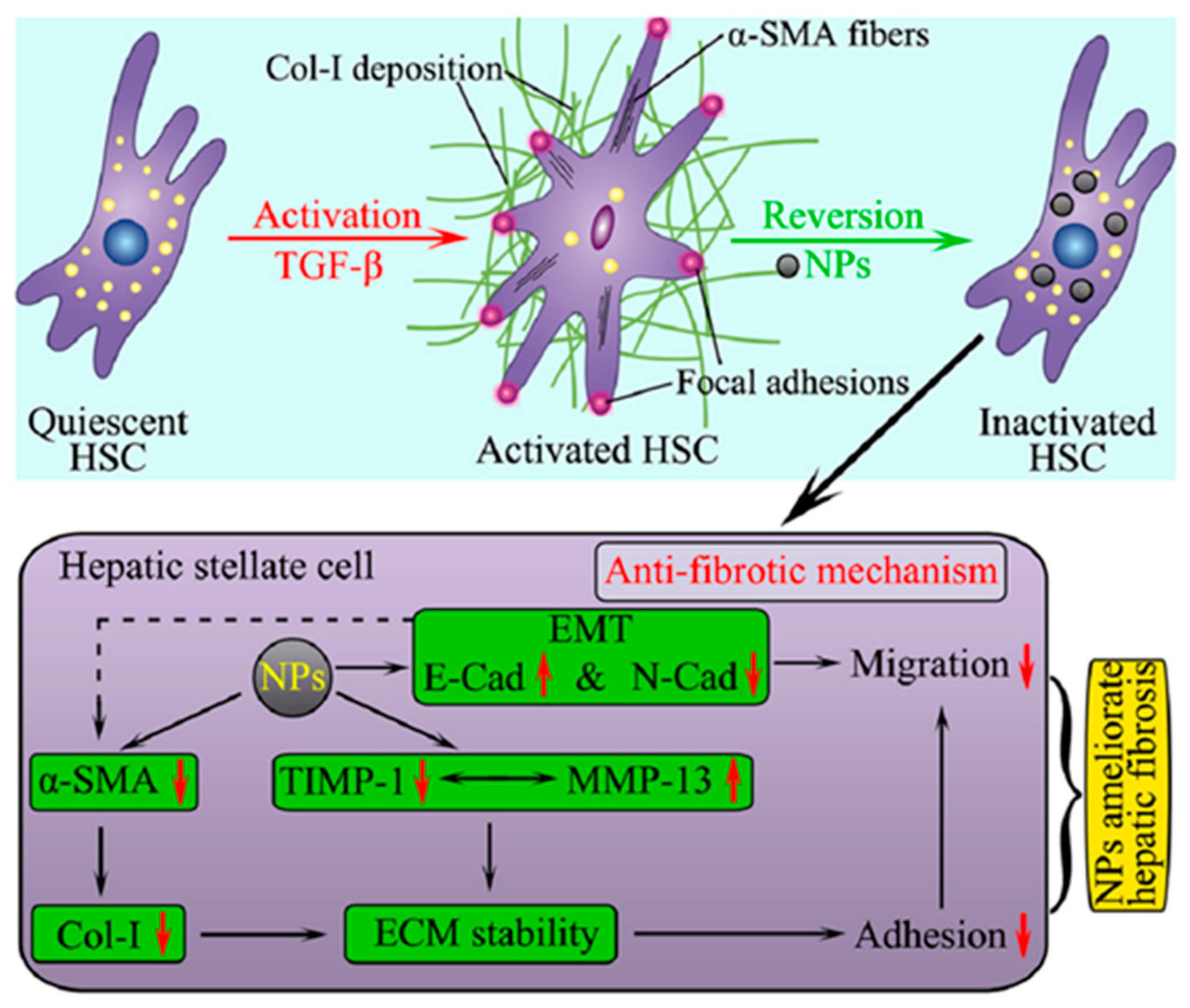
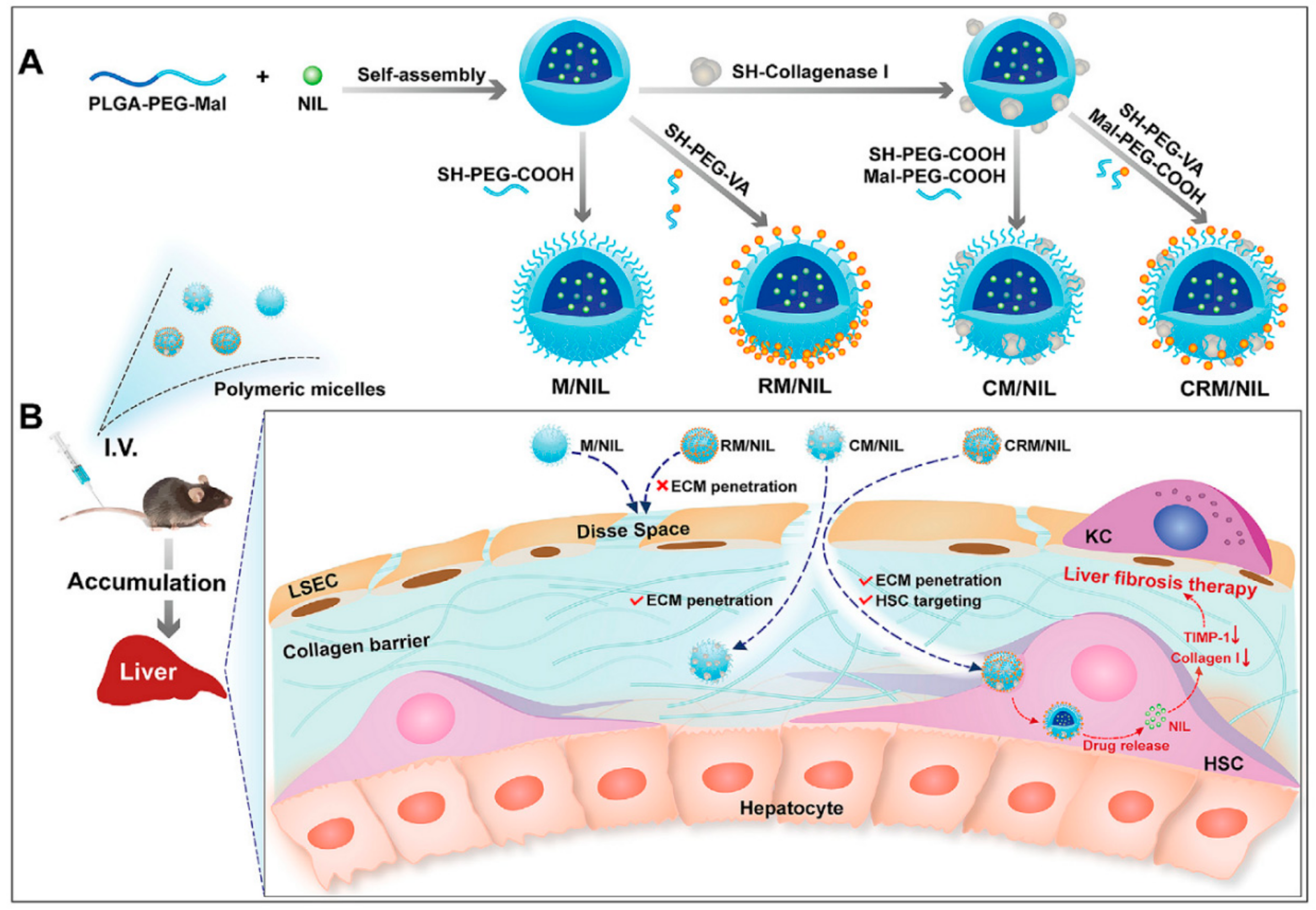

| Nanoparticle Systems | NPs Formulation | Delivered Drug | Reference |
|---|---|---|---|
| Lipid-based NPs | RNA oligonucleotide-liposomal | MTL-CEBPA | [70] |
| Cationic lipid NPs | small interfering RNA to the procollagen 1(I) gene | [20] | |
| Dexamethasone-liposomes | dexamethasone | [71] | |
| Polymer-based NPs | Cationic nanohydrogel particles | anti-Col1α1 siRNA | [72] |
| Ketal cross-linked cationic nanohydrogel | Cy5-labeled anti-col1α1 siRNA | [73] | |
| PLGA | phyllanthin | [74] | |
| PEG-PLGA or PEG-PLGA/PLGA NPs | sorafenib | [75] | |
| Eudragit(R) RS100 NPs (SMnps) | silymarin | [76] | |
| Inorganic NPs | Rhodamine B (RhB)-mesoporous silica NPs (MSNs-RhB | salvianolic acid B | [77] |
| Mesoporous silica NPs | siTnC | [78] | |
| PEG-AuNPs | hesperetin | [79] | |
| AuNPs and SiNPs | NO donors | [80] | |
| PtNPs | Curcumin | [81] | |
| Calcium phosphate NPs (CaP@BSA NPs) | TSG-6 | [82] | |
| Graphene nanostars linked to PAMAM-GS dendrimer | Plasmid | [83] | |
| Protein NPs | Zein nanospheres | Curcumin | [84] |
| Glucose modify albumin NPs | Berberine | [85] | |
| Albumin NPs | Bexamethasone | [22] | |
| Polyavidin-based NPs | Dexamethasone | [86] |
| Nanoparticle Systems | NPs Formulation | Delivered Drugs | Targeted Ligand | Targeted Structures | Reference |
|---|---|---|---|---|---|
| Lipid-based NPs | VA-liposomes | Imatinib | VA | HSC | [88] |
| VA-liposomes | Valsartan | VA | HSC | [89] | |
| pPB-modified liposomes | Recombinant human TRAIL | pPB | HSC | [90] | |
| AMD3100-liposomes | Antiangiogenic siRNA | VEGF siRNAs | HSC | [91] | |
| M6P-bovine serum albumin (BSA)-conjugated-liposomes | Hesperidin | M6P | HSC | [92] | |
| VA-coupled liposomes | BMP4-siRNA | VA | HSC | [93] | |
| Cationic liposomes | Artificial microRNA | microRNA | CTGF | [94] | |
| Chol-PEG-VA-amphiphilic cationic hyperbranched lipoid (C15-PA) | SiCol I α1 and siTIMP-1 | VA | HSC | [95] | |
| pPB-modified stable nucleic acid lipid | siRNAs against heat shock protein 47 | pPB | HSC | [96] | |
| Galactosamine-phospholipid NPs | siRNA targets CTGF | galactosamine | hepatocytes and renal tubular epithelial cells | [97] | |
| SP94-LCPP (lipid/calcium/phosphate/protamine) nanoparticle | TRAIL plasmid DNA | hepato-cellular carcinoma (HCC)-targeting peptide (SP94) | hepatocellular carcinoma (HCC) cells | [98] | |
| Phosphatidylserine-modified nanostructured lipid NPs | Curcumin | phosphatidylserine | macrophage | [99] | |
| Polymer-based NPs | VA-collagenase I-poly-(lactic-co-glycolic)-b-poly (ethylene glycol)-maleimide (PLGA-PEG-Mal) (named CRM) micelle | Nilotinib | VA | HSC | [100] |
| Poly (lactide-co-glycolide)-polyspermine-poly (ethylene glycol)-vitamin A (PLGA-PSPE-PEG-VA) self-assembled into core-shell polymeric micelles (PVMs) | Silibinin genetic (siCol1 alpha 1) drugs | VA | HSC | [101] | |
| Retinoic acid-chondroitin sulfate micells | Doxorubicin | VA | HSC | [102] | |
| POEGMA-b-PVDM -VA micelle | NO | VA | HSC | [19] | |
| Retinol-conjugated polyetherimine (RcP) nanoparticle | Antisense oligonucleotide (ASO) | RcP | HSC | [103] | |
| PLGA NPs | R406 | R406 | Macrophages | [104] | |
| retinol-chitosan NPs | JQ1 and atorvastatin | VA | HSC | [105] | |
| Inorganic NPs | pPB-MSNP | Erlotinib | pPB | PDGFRB | [106] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, X.; Su, G.; Zhai, S. Recent Advances in Nanomedicine for the Diagnosis and Therapy of Liver Fibrosis. Nanomaterials 2020, 10, 1945. https://doi.org/10.3390/nano10101945
Bai X, Su G, Zhai S. Recent Advances in Nanomedicine for the Diagnosis and Therapy of Liver Fibrosis. Nanomaterials. 2020; 10(10):1945. https://doi.org/10.3390/nano10101945
Chicago/Turabian StyleBai, Xue, Gaoxing Su, and Shumei Zhai. 2020. "Recent Advances in Nanomedicine for the Diagnosis and Therapy of Liver Fibrosis" Nanomaterials 10, no. 10: 1945. https://doi.org/10.3390/nano10101945
APA StyleBai, X., Su, G., & Zhai, S. (2020). Recent Advances in Nanomedicine for the Diagnosis and Therapy of Liver Fibrosis. Nanomaterials, 10(10), 1945. https://doi.org/10.3390/nano10101945





