PDMS Membranes Drilled by Proton Microbeam Writing: A Customizable Platform for the Investigation of Endothelial Cell–Substrate Interactions in Transwell-like Devices
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
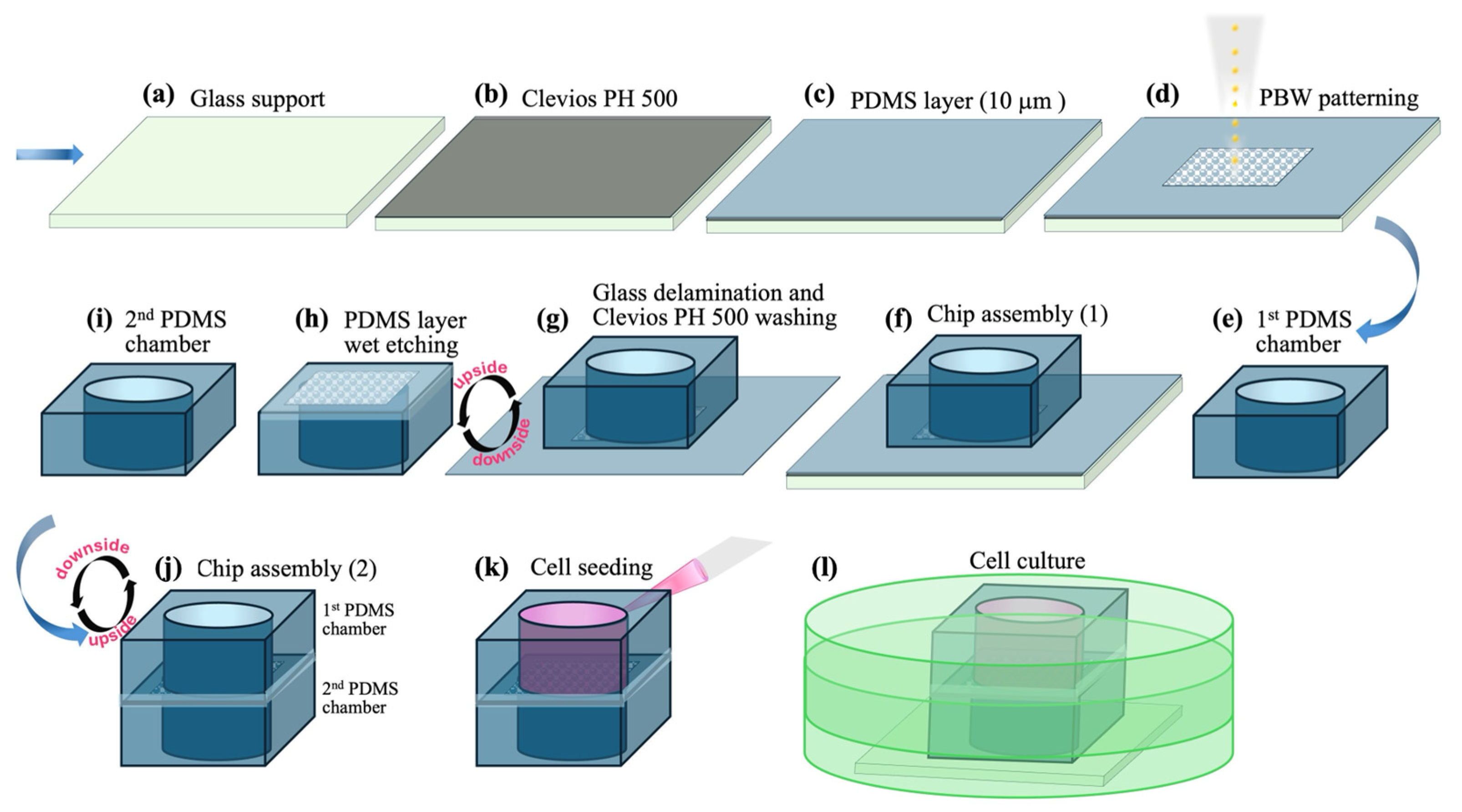
2.2. Preparation of the PDMS-Holed Membranes by PBW
2.2.1. Preparation of PDMS Films and PBW Experiments
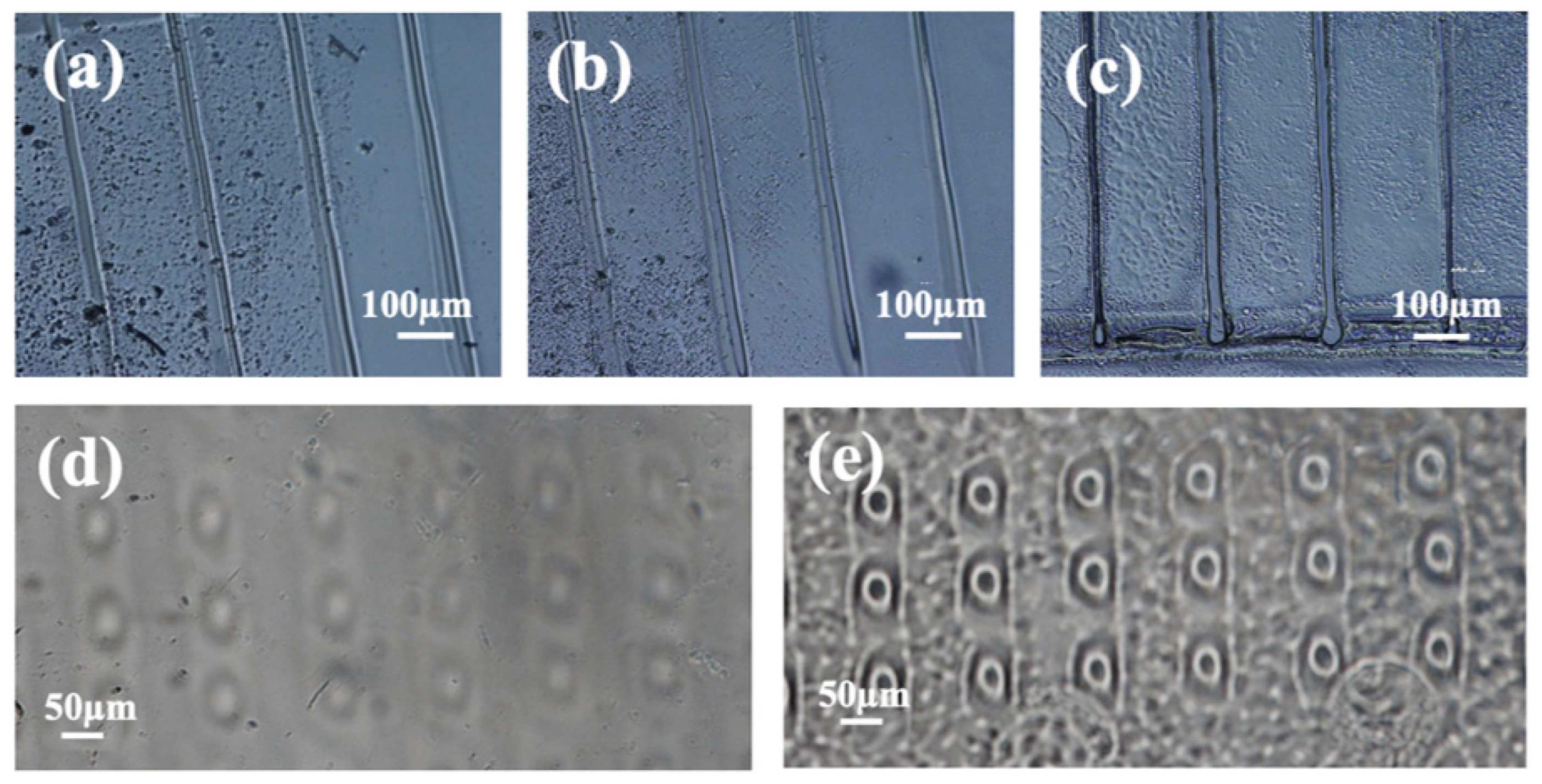
2.2.2. Etching Protocol for Removing the Proton Beam Irradiated PDMS
2.3. Fabrication and Assembly of a Transwell-like Device Embedding the Membrane Patterned by PBW
2.4. Cellular Growth in the Transwell Device and Cell Imaging
2.5. Image Processing and Statistical Analysis
3. Results and Discussion
3.1. Wet Etching Protocol to Remove the Proton Beam Irradiated PDMS
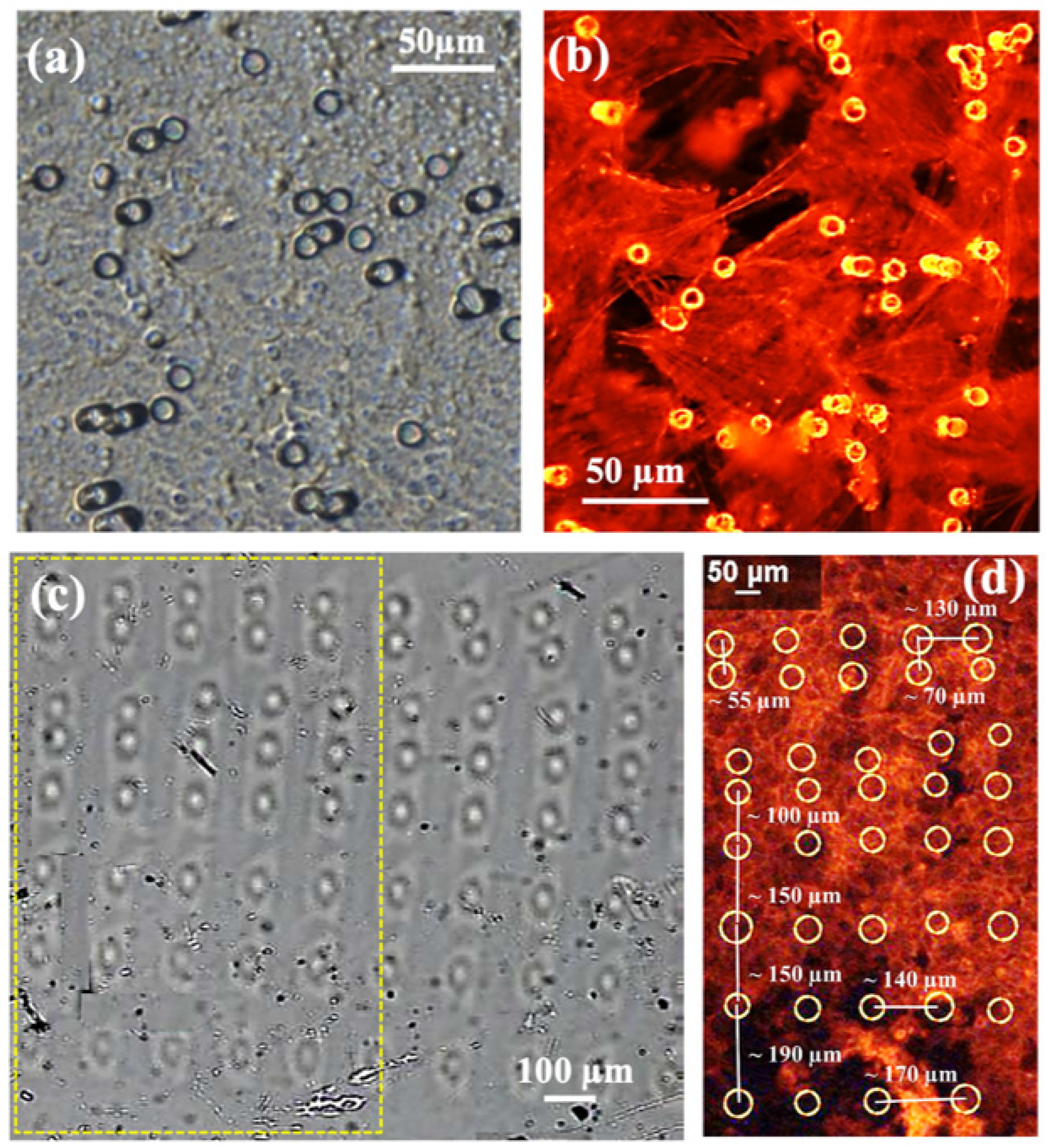
3.2. Cell Growth, Arrangement, and Migration Across the Designed Transwell-like Devices
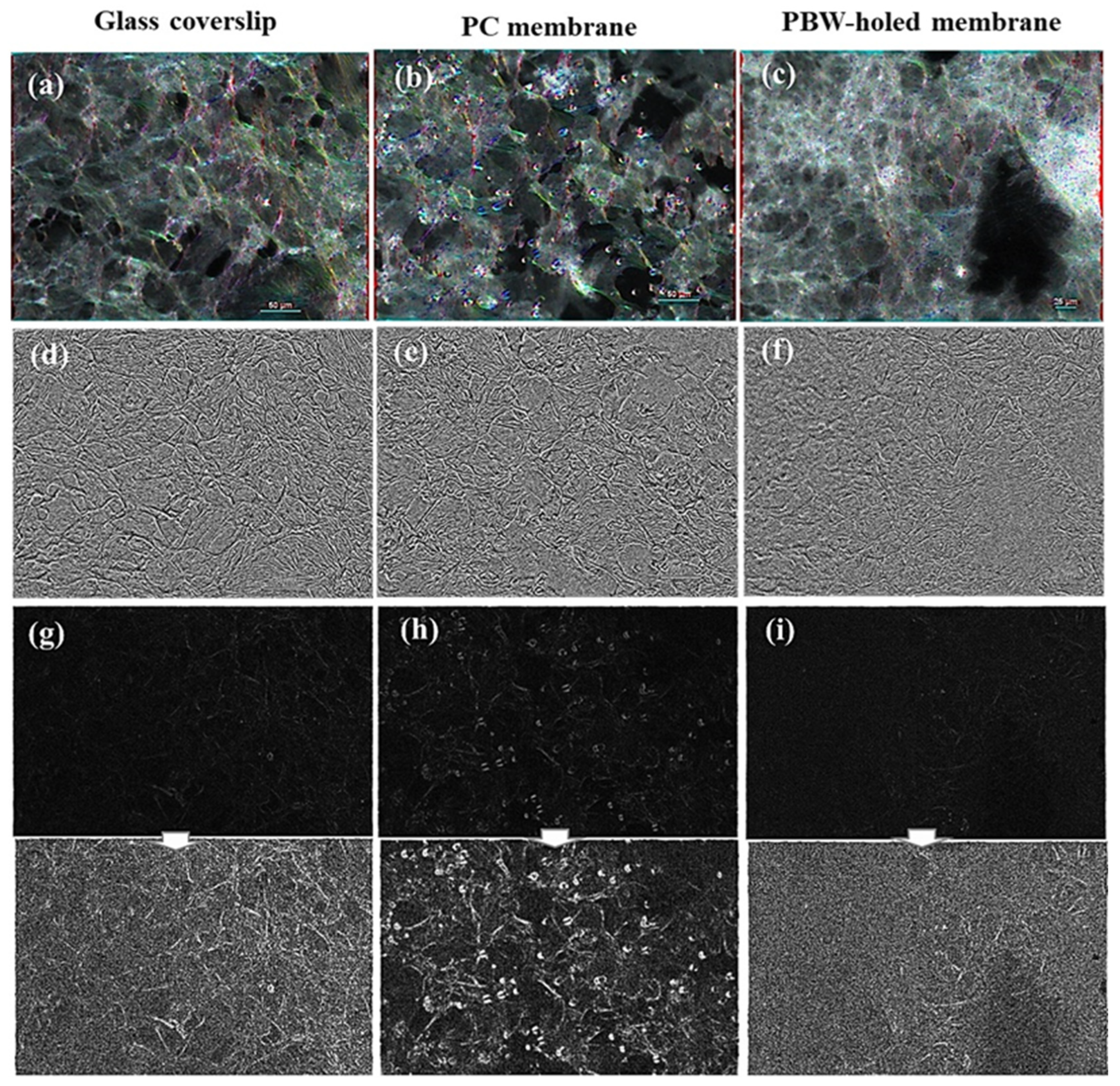
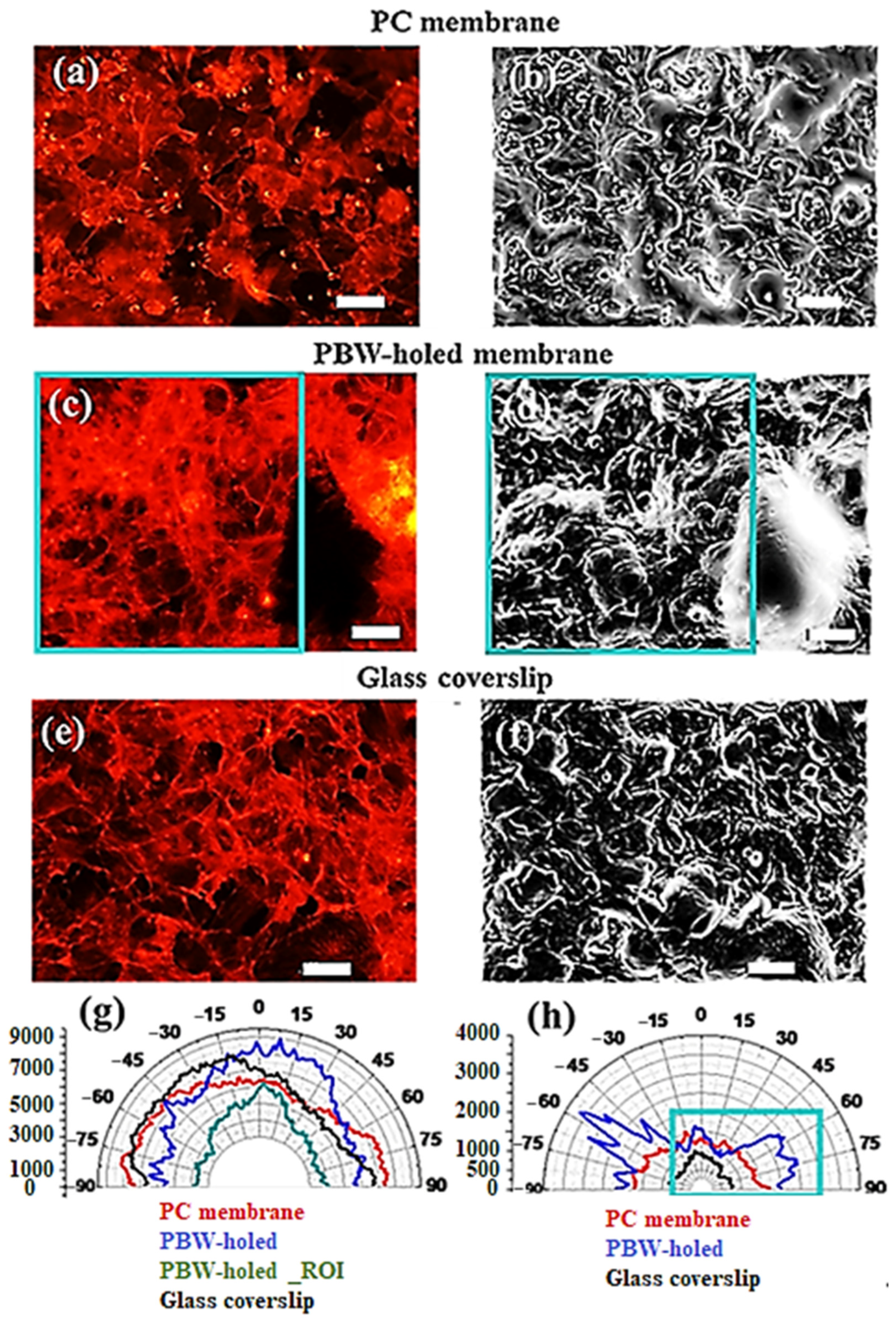
3.2.1. Impact of the Material Membrane on the Arrangement of HCMEC/D3 Cells
3.2.2. Deeper Insight in Cell Arrangement Around and in Between the PBW Holes
3.3. Remarks and Future Perspectives
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CEDAD | Centre of Applied Physics, Dating, and Diagnostics |
| DAPI | 4′,6-diamidino-2-phenylindole |
| GLCM | Gray Level Co-Occurrence Matrix |
| HUE | Hue–saturation–brightness |
| HCMEC | Human Cerebral Microvascular Endothelial Cells |
| OoC | Organ-on-chip |
| PBS | Phosphate Buffered Saline |
| PC | Polycarbonate |
| PDMS | Polydimethylsiloxane |
| PET | Polyethylene terephthalate |
| PCA | Principal Component Analysis |
| PBW | Proton beam writing |
| ROI | Region of interest |
| SEM | Scanning Electron Microscopy |
| TRITC | Tetramethylrhodamine isothiocyanate |
| 3D | Three-dimensional |
| 2D | Two-dimensional |
References
- Ridley, A.J.; Schwartz, M.A.; Burridge, K.; Firtel, R.A.; Ginsberg, M.H.; Borisy, G.; Parsons, J.T.; Horwitz, A.R. Cell Migration: Integrating Signals from Front to Back. Science 2003, 302, 1704–1709. [Google Scholar] [CrossRef]
- Trepat, X.; Chen, Z.; Jacobson, K. Cell Migration. Compr. Physiol. 2012, 2, 2369. [Google Scholar] [CrossRef] [PubMed]
- van Helvert, S.; Storm, C.; Friedl, P. Mechanoreciprocity in Cell Migration. Nat. Cell Biol. 2018, 20, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Förster, C. Tight Junctions and the Modulation of Barrier Function in Disease. Histochem. Cell Biol. 2008, 130, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Olson, M.F.; Sahai, E. The Actin Cytoskeleton in Cancer Cell Motility. Clin. Exp. Metastasis 2009, 26, 273–287. [Google Scholar] [CrossRef]
- Hulkower, K.I.; Herber, R.L. Cell Migration and Invasion Assays as Tools for Drug Discovery. Pharmaceutics 2011, 3, 107–124. [Google Scholar] [CrossRef]
- Friedl, P.; Sahai, E.; Weiss, S.; Yamada, K.M. New Dimensions in Cell Migration. Nat. Rev. Mol. Cell Biol. 2012, 13, 743–747. [Google Scholar] [CrossRef]
- Merino-Casallo, F.; Gomez-Benito, M.J.; Hervas-Raluy, S.; Garcia-Aznar, J.M. Unravelling Cell Migration: Defining Movement from the Cell Surface. Cell Adhes. Migr. 2022, 16, 25–64. [Google Scholar] [CrossRef]
- Svensson, C.-M.; Medyukhina, A.; Belyaev, I.; Al-Zaben, N.; Figge, M.T. Untangling Cell Tracks: Quantifying Cell Migration by Time Lapse Image Data Analysis. Cytom. Part A 2018, 93, 357–370. [Google Scholar] [CrossRef]
- Kramer, N.; Walzl, A.; Unger, C.; Rosner, M.; Krupitza, G.; Hengstschläger, M.; Dolznig, H. In Vitro Cell Migration and Invasion Assays. Mutat. Res./Rev. Mutat. Res. 2013, 752, 10–24. [Google Scholar] [CrossRef]
- Yeste, J.; Illa, X.; Alvarez, M.; Villa, R. Engineering and Monitoring Cellular Barrier Models. J. Biol. Eng. 2018, 12, 18. [Google Scholar] [CrossRef] [PubMed]
- Justus, C.R.; Marie, M.A.; Sanderlin, E.J.; Yang, L.V. Transwell In Vitro Cell Migration and Invasion Assays. In Cell Viability Assays: Methods and Protocols; Friedrich, O., Gilbert, D.F., Eds.; Springer: New York, NY, USA, 2023; pp. 349–359. ISBN 978-1-07-163052-5. [Google Scholar]
- Yarrow, J.C.; Perlman, Z.E.; Westwood, N.J.; Mitchison, T.J. A High-Throughput Cell Migration Assay Using Scratch Wound Healing, a Comparison of Image-Based Readout Methods. BMC Biotechnol. 2004, 4, 21. [Google Scholar] [CrossRef]
- Liang, C.-C.; Park, A.Y.; Guan, J.-L. In Vitro Scratch Assay: A Convenient and Inexpensive Method for Analysis of Cell Migration In Vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef]
- Valster, A.; Tran, N.L.; Nakada, M.; Berens, M.E.; Chan, A.Y.; Symons, M. Cell Migration and Invasion Assays. Methods 2005, 37, 208–215. [Google Scholar] [CrossRef]
- Nie, F.-Q.; Yamada, M.; Kobayashi, J.; Yamato, M.; Kikuchi, A.; Okano, T. On-Chip Cell Migration Assay Using Microfluidic Channels. Biomaterials 2007, 28, 4017–4022. [Google Scholar] [CrossRef]
- Ren, X.; Levin, D.; Lin, F. Cell Migration Research Based on Organ-on-Chip-Related Approaches. Micromachines 2017, 8, 324. [Google Scholar] [CrossRef]
- Ren, J.; Wang, N.; Guo, P.; Fan, Y.; Lin, F.; Wu, J. Recent Advances in Microfluidics-Based Cell Migration Research. Lab Chip 2022, 22, 3361–3376. [Google Scholar] [CrossRef]
- Chung, H.H.; Mireles, M.; Kwarta, B.J.; Gaborski, T.R. Use of Porous Membranes in Tissue Barrier and Co-Culture Models. Lab Chip 2018, 18, 1671–1689. [Google Scholar] [CrossRef] [PubMed]
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Hsin, H.Y.; Ingber, D.E. Reconstituting Organ-Level Lung Functions on a Chip. Science 2010, 328, 1662–1668. [Google Scholar] [CrossRef] [PubMed]
- Jerka, D.; Bonowicz, K.; Piekarska, K.; Gokyer, S.; Derici, U.S.; Hindy, O.A.; Altunay, B.B.; Yazgan, I.; Steinbrink, K.; Kleszczyński, K.; et al. Unraveling Endothelial Cell Migration: Insights into Fundamental Forces, Inflammation, Biomaterial Applications, and Tissue Regeneration Strategies. ACS Appl. Bio Mater. 2024, 7, 2054–2069. [Google Scholar] [CrossRef]
- Salminen, A.T.; Zhang, J.; Madejski, G.R.; Khire, T.S.; Waugh, R.E.; McGrath, J.L.; Gaborski, T.R. Ultrathin Dual-Scale Nano- and Micro-Porous Membranes for Vascular Transmigration Models. Small 2019, 15, e1804111. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.M.; de Haan, P.; Ronaldson-Bouchard, K.; Kim, G.-A.; Ko, J.; Rho, H.S.; Chen, Z.; Habibovic, P.; Jeon, N.L.; Takayama, S.; et al. A Guide to the Organ-on-a-Chip. Nat. Rev. Methods Primers 2022, 2, 33. [Google Scholar] [CrossRef]
- Aran, K.; Sasso, L.A.; Kamdar, N.; Zahn, J.D. Irreversible, Direct Bonding of Nanoporous Polymer Membranes to PDMS or Glass Microdevices. Lab Chip 2010, 10, 548. [Google Scholar] [CrossRef]
- Huh, D.; Leslie, D.C.; Matthews, B.D.; Fraser, J.P.; Jurek, S.; Hamilton, G.A.; Thorneloe, K.S.; McAlexander, M.A.; Ingber, D.E. A Human Disease Model of Drug Toxicity-Induced Pulmonary Edema in a Lung-on-a-Chip Microdevice. Sci. Transl. Med. 2012, 4, 159ra147. [Google Scholar] [CrossRef]
- Quirós-Solano, W.F.; Gaio, N.; Stassen, O.M.J.A.; Arik, Y.B.; Silvestri, C.; Van Engeland, N.C.A.; Van der Meer, A.; Passier, R.; Sahlgren, C.M.; Bouten, C.V.C.; et al. Microfabricated Tuneable and Transferable Porous PDMS Membranes for Organs-on-Chips. Sci. Rep. 2018, 8, 13524. [Google Scholar] [CrossRef]
- Özkan, A.; LoGrande, N.T.; Feitor, J.F.; Goyal, G.; Ingber, D.E. Intestinal Organ Chips for Disease Modelling and Personalized Medicine. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 751–773. [Google Scholar] [CrossRef]
- Zhao, Y.; Landau, S.; Okhovatian, S.; Liu, C.; Lu, R.X.Z.; Lai, B.F.L.; Wu, Q.; Kieda, J.; Cheung, K.; Rajasekar, S.; et al. Integrating Organoids and Organ-on-a-Chip Devices. Nat. Rev. Bioeng. 2024, 2, 588–608. [Google Scholar] [CrossRef]
- Guarino, V.; Perrone, E.; De Luca, E.; Rainer, A.; Cesaria, M.; Zizzari, A.; Bianco, M.; Gigli, G.; Moroni, L.; Arima, V. Pericyte-Assisted Vascular Lumen Organization in a Novel Dynamic Human Blood-Brain Barrier-on-Chip Model. Adv. Healthc. Mater. 2025, 14, 2401804. [Google Scholar] [CrossRef]
- Baek, D.; Lee, S.H.; Jun, B.-H.; Lee, S.H. Lithography Technology for Micro- and Nanofabrication. In Nanotechnology for Bioapplications; Jun, B.-H., Ed.; Springer: Singapore, 2021; pp. 217–233. ISBN 978-981-336-158-4. [Google Scholar]
- Vasco, G.; Arima, V.; Boudjelida, S.; Carraro, M.; Bianco, M.; Zizzari, A.; Perrone, E.; Galiano, F.; Figoli, A.; Cesaria, M. Polymeric Membranes Doped with Halloysite Nanotubes Imaged Using Proton Microbeam Microscopy. Nanomaterials 2023, 13, 2970. [Google Scholar] [CrossRef]
- van Kan, J.A.; Malar, P.; Baysic de Vera, A. The Second Generation Singapore High Resolution Proton Beam Writing Facility. Rev. Sci. Instrum. 2012, 83, 02B902. [Google Scholar] [CrossRef] [PubMed]
- Van Kan, J.A.; Shao, P.G.; Wang, Y.H.; Malar, P. Proton Beam Writing a Platform Technology for High Quality Three-Dimensional Metal Mold Fabrication for Nanofluidic Applications. Microsyst. Technol. 2011, 17, 1519–1527. [Google Scholar] [CrossRef]
- Huszank, R.; Rajta, I.; Cserháti, C. Proton Beam Lithography in Negative Tone Liquid Phase PDMS Polymer Resist. Nucl. Instrum. Methods Phys. Res. B 2015, 348, 213–217. [Google Scholar] [CrossRef]
- Szilasi, S.Z.; Cserháti, C. Selective Etching of PDMS: Etching Technique for Application as a Positive Tone Resist. Appl. Surf. Sci. 2018, 457, 662–669. [Google Scholar] [CrossRef]
- Lamalice, L.; Le Boeuf, F.; Huot, J. Endothelial Cell Migration During Angiogenesis. Circ. Res. 2007, 100, 782–794. [Google Scholar] [CrossRef]
- Calcagnile, L.; Maruccio, L.; Scrimieri, L.; delle Side, D.; Braione, E.; D’Elia, M.; Quarta, G. Development and Application of Facilities at the Centre for Applied Physics, Dating and Diagnostics (CEDAD) at the University of Salento during the Last 15 years. Nucl. Instrum. Methods Phys. Res. Sect. B 2019, 456, 252–256. [Google Scholar] [CrossRef]
- Narayan, D.; Venkatraman, S.S. Effect of Pore Size and Interpore Distance on Endothelial Cell Growth on Polymers. J. Biomed. Mater. Res. Part A 2008, 87, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Zizzari, A.; Bianco, M.; del Mercato, L.L.; Sorarù, A.; Carraro, M.; Pellegrino, P.; Perrone, E.; Monteduro, A.G.; Bonchio, M.; Rinaldi, R.; et al. Highly Sensitive Membrane-Based Pressure Sensors (MePS) for Real-Time Monitoring of Catalytic Reactions. Anal. Chem. 2018, 90, 7659–7665. [Google Scholar] [CrossRef]
- Guarino, V.; Perrone, E.; Zizzari, A.; Bianco, M.; Giancane, G.; Rella, R.; Manera, M.G.; Arima, V. Controlling Endotoxin Contamination in PDMS-Based Microfluidic Systems for Organ-on-Chip Technologies. Polym. Test. 2025, 147, 108795. [Google Scholar] [CrossRef]
- Bianco, M.; Zizzari, A.; Priore, P.; Moroni, L.; Metrangolo, P.; Frigione, M.; Rella, R.; Gaballo, A.; Arima, V. Lab-on-a-Brane for Spheroid Formation. Biofabrication 2019, 11, 021002. [Google Scholar] [CrossRef]
- Sage, D. OrientationJ. Available online: https://bigwww.epfl.ch/demo/orientation/ (accessed on 7 May 2025).
- Schmidt, U.; Weigert, M.; Broaddus, C.; Myers, G. Cell Detection with Star-Convex Polygons. In Medical Image Computing and Computer Assisted Intervention—MICCAI 2018; Lecture Notes in Computer Science; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 265–273. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Jähne, B. (Ed.) Spatio-Temporal Image Processing: Theory and Scientific Applications; Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 1993; Volume 751, ISBN 978-3-540-57418-7. [Google Scholar]
- Püspöki, Z.; Storath, M.; Sage, D.; Unser, M. Transforms and Operators for Directional Bioimage Analysis: A Survey. Adv. Anat. Embryol. Cell Biol. 2016, 219, 69–93. [Google Scholar] [CrossRef]
- MonogenicJ: A ImageJ Plugin for Wavelet-Based Monogenic Analysis of Images. Available online: https://bigwww.epfl.ch/demo/monogenic/ (accessed on 15 January 2025).
- Unser, M.; Sage, D.; Van De Ville, D. Multiresolution Monogenic Signal Analysis Using the Riesz–Laplace Wavelet Transform. IEEE Trans. Image Process. 2009, 18, 2402–2418. [Google Scholar] [CrossRef]
- Soulard, R.; Carre, P.; Fernandez-Maloigne, C. Vector Extension of Monogenic Wavelets for Geometric Representation of Color Images. IEEE Trans. Image Process. 2013, 22, 1070–1083. [Google Scholar] [CrossRef]
- Oppenheim, A.V.; Lim, J.S. The Importance of Phase in Signals. Proc. IEEE 1981, 69, 529–541. [Google Scholar] [CrossRef]
- Caicedo, J.C.; Goodman, A.; Karhohs, K.W.; Cimini, B.A.; Ackerman, J.; Haghighi, M.; Heng, C.; Becker, T.; Doan, M.; McQuin, C.; et al. Nucleus segmentation across imaging experiments: The 2018 Data Science Bowl. Nat. Methods 2019, 16, 1247–1253. [Google Scholar] [CrossRef]
- Jolliffe, I.T.; Cadima, J. Principal Component Analysis: A Review and Recent Developments. Philos. Trans. R. Soc. A 2016, 374, 20150202. [Google Scholar] [CrossRef]
- Haralick, R.M.; Shanmugam, K.; Dinstein, I. Textural Features for Image Classification. IEEE Trans. Syst. Man Cybern. 1973, SMC-3, 610–621. [Google Scholar] [CrossRef]
- Uppuluri, A. GLCM Texture Features. Available online: https://www.mathworks.com/matlabcentral/fileexchange/22187-glcm-texture-features (accessed on 20 November 2008).
- Nanni, L.; Brahnam, S.; Ghidoni, S.; Menegatti, E.; Barrier, T. Different Approaches for Extracting Information from the Co-Occurrence Matrix. PLoS ONE 2013, 8, e83554. [Google Scholar] [CrossRef] [PubMed]
- Borjanović, V.; Bistričić, L.; Vlasov, I.; Furić, K.; Zamboni, I.; Jakšić, M.; Shenderova, O. Influence of Proton Irradiation on the Structure and Stability of Poly(Dimethylsiloxane) and Poly(Dimethylsiloxane)-Nanodiamond Composite. J. Vac. Sci. Technol. B 2009, 27, 2396–2403. [Google Scholar] [CrossRef]
- Szilasi, S.Z.; Kokavecz, J.; Huszank, R.; Rajta, I. Compaction of Poly(Dimethylsiloxane) (PDMS) Due to Proton Beam Irradiation. Appl. Surf. Sci. 2011, 257, 4612–4615. [Google Scholar] [CrossRef]
- Zizzari, A.; Cesaria, M.; Bianco, M.; del Mercato, L.L.; Carraro, M.; Bonchio, M.; Rella, R.; Arima, V. Mixing Enhancement Induced by Viscoelastic Micromotors in Microfluidic Platforms. Chem. Eng. J. 2020, 391, 123572. [Google Scholar] [CrossRef]
- Chiriacò, M.S.; Bianco, M.; Amato, F.; Primiceri, E.; Ferrara, F.; Arima, V.; Maruccio, G. Fabrication of Interconnected Multilevel Channels in a Monolithic SU-8 Structure Using a LOR Sacrificial Layer. Microelectron. Eng. 2016, 164, 30–35. [Google Scholar] [CrossRef]
- Perrone, E.; Cesaria, M.; Zizzari, A.; Bianco, M.; Ferrara, F.; Raia, L.; Guarino, V.; Cuscunà, M.; Mazzeo, M.; Gigli, G.; et al. Potential of CO2-Laser Processing of Quartz for Fast Prototyping of Microfluidic Reactors and Templates for 3D Cell Assembly over Large Scale. Mater. Today Bio 2021, 12, 100163. [Google Scholar] [CrossRef]
- Guarino, V.; Zizzari, A.; Bianco, M.; Gigli, G.; Moroni, L.; Arima, V. Advancements in Modelling Human Blood Brain-Barrier on a Chip. Biofabrication 2023, 15, 022003. [Google Scholar] [CrossRef]
- Majhy, B.; Priyadarshini, P.; Sen, A.K. Effect of Surface Energy and Roughness on Cell Adhesion and Growth—Facile Surface Modification for Enhanced Cell Culture. RSC Adv. 2021, 11, 15467–15476. [Google Scholar] [CrossRef]
- Lee, K.S.; Kwon, T.-H.; Park, T.; Jeong, M.S. Chapter 3—Theory and Experiments. In Theory and Practice in Microbial Enhanced Oil Recovery; Lee, K.S., Kwon, T.-H., Park, T., Jeong, M.S., Eds.; Gulf Professional Publishing: Houston, TX, USA, 2020; pp. 67–108. ISBN 978-0-12-819983-1. [Google Scholar]
- Kimber, A.C. Exploratory Data Analysis for Possibly Censored Data from Skewed Distributions. J. R. Stat. Soc. Ser. C 1990, 39, 21–30. [Google Scholar] [CrossRef]
- Gupta, M.; Sarangi, B.R.; Deschamps, J.; Nematbakhsh, Y.; Callan-Jones, A.; Margadant, F.; Mège, R.-M.; Lim, C.T.; Voituriez, R.; Ladoux, B. Adaptive Rheology and Ordering of Cell Cytoskeleton Govern Matrix Rigidity Sensing. Nat. Commun. 2015, 6, 7525. [Google Scholar] [CrossRef]
- Bayless, K.J.; Johnson, G.A. Role of the Cytoskeleton in Formation and Maintenance of Angiogenic Sprouts. J. Vasc. Res. 2011, 48, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Chung, M.; Lee, S.-R.; Jeon, N.L. 3D Brain Angiogenesis Model to Reconstitute Functional Human Blood–Brain Barrier In Vitro. Biotechnol. Bioeng. 2020, 117, 748–762. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Haase, K.; Roberts, J.J.; Hoffmann, J.; Nguyen, H.T.; Wan, Z.; Zhang, S.; Sarker, B.; Friedman, N.; Ristić-Lehmann, Č.; et al. A Novel 3D Vascular Assay for Evaluating Angiogenesis across Porous Membranes. Biomaterials 2021, 268, 120592. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Sathe, S.R.; Yim, E.K.F. From Nano to Micro: Topographical Scale and Its Impact on Cell Adhesion, Morphology and Contact Guidance. J. Phys. 2016, 28, 183001. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, J.; Roy, A.; Chanda, A.; Gouripriya, D.A.; Thomas, S.; Ghosh, M.; Kim, J.; Saha, P. Effects of Surface Patterning and Topography on the Cellular Functions of Tissue Engineered Scaffolds with Special Reference to 3D Bioprinting. Biomater. Sci. 2023, 11, 1236–1269. [Google Scholar] [CrossRef]
- Chi, J.; Wang, M.; Chen, J.; Hu, L.; Chen, Z.; Backman, L.J.; Zhang, W. Topographic Orientation of Scaffolds for Tissue Regeneration: Recent Advances in Biomaterial Design and Applications. Biomimetics 2022, 7, 131. [Google Scholar] [CrossRef]
- Xiao, X.; Wang, W.; Liu, D.; Zhang, H.; Gao, P.; Geng, L.; Yuan, Y.; Lu, J.; Wang, Z. The Promotion of Angiogenesis Induced by Three-Dimensional Porous Beta-Tricalcium Phosphate Scaffold with Different Interconnection Sizes via Activation of PI3K/Akt Pathways. Sci. Rep. 2015, 5, 9409. [Google Scholar] [CrossRef] [PubMed]
- Van Kan, J.A.; Bettiol, A.A.; Watt, F. Three-dimensional nanolithography using proton beam writing. Appl. Phys. Lett. 2003, 83, 1629–1631. [Google Scholar] [CrossRef]
- Furuta, Y.; Nishikawa, H.; Satoh, T.; Ishii, Y.; Kamiya, T.; Nakao, R.; Uchida, S. Applications of microstructures fabricated by proton beam writing to electric-micro filters. Nucl. Instrum. Methods Phys. Res. Sect. B 2009, 267, 2285–2288. [Google Scholar] [CrossRef]
- Udalagama, C.N.B.; Bettiol, A.A.; Watt, F. A Monte Carlo study of the extent of proximity effects in e-beam and p-beam writing of PMMA. Nucl. Instrum. Methods Phys. Res. Sect. B 2007, 260, 384–389. [Google Scholar] [CrossRef]
- Watt, F.; Breese, M.B.H.; Bettiol, A.A.; van Kan, J.A. Proton beam writing. Mater. Today 2007, 10, 20–29. [Google Scholar] [CrossRef]
- Mistry, P.; Gomez-Morilla, I.; Grime, G.W.; Webb, R.P.; Gwilliam, R.; Cansell, A.; Merchant, M.; Kirkby, K.J.; Teo, E.J.; Breese, M.B.H.; et al. New developments in the applications of proton beam writing. Nucl. Instrum. Methods Phys. Res. Sect. B 2005, 237, 188–192. [Google Scholar] [CrossRef]
- Cutroneo, M.; Havranek, V.; Torrisi, L.; Svecova, B. Ion Micro Beam, promising methods for interdisciplinary research. J. Inst. 2016, 11, C05001. [Google Scholar] [CrossRef]
- Schmidt, B.; Wetzig, K. Ion Beams in Materials Processing and Analysis; Springer: Vienna, Austria, 2013. [Google Scholar] [CrossRef]
- Calcagnile, L.; Quarta, G.; D’Elia, M.; Muscogiuri, D.; Maruccio, L.; Butalag, K.; Gianfrate, G.; Sanapo, C.; Toma, U. Instrumental developments at the IBA-AMS dating facility at the University of Lecce. Nucl. Instrum. Methods Phys. Res. Sect. B 2005, 240, 22–25. [Google Scholar] [CrossRef]
- Podaru, N.C.; Mous, D.J.W. Recent developments and upgrades in ion source technology and ion beam systems at HVE. Nucl. Instrum. Methods Phys. Res. Sect. B 2016, 371, 137–141. [Google Scholar] [CrossRef]
- Satti, A.J.; Andreucetti, N.A.; Ciolino, A.E.; Vitale, C.; Sarmoria, C.; Vallés, E.M. Molecular weight changes induced in an anionic polydimethylsiloxane by gamma irradiation in vacuum. Radiat. Phys. Chem. 2010, 79, 1137–1143. [Google Scholar] [CrossRef]
- Huszank, R.; Szilasi, S.Z.; Szikra, D. Ion-Energy Dependency in Proton Irradiation Induced Chemical Processes of Poly(dimethylsiloxane). J. Phys. Chem. C 2013, 117, 25884–25889. [Google Scholar] [CrossRef]
- Szilasi, S.Z.; Huszank, R.; Csik, A.; Cserháti, C.; Rajta, I. PDMS patterning by proton beam. Nucl. Instrum. Methods Phys. Res. B 2009, 267, 2296. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guarino, V.; Vasco, G.; Arima, V.; Cataldo, R.; Zizzari, A.; Perrone, E.; Gigli, G.; Cesaria, M. PDMS Membranes Drilled by Proton Microbeam Writing: A Customizable Platform for the Investigation of Endothelial Cell–Substrate Interactions in Transwell-like Devices. J. Funct. Biomater. 2025, 16, 274. https://doi.org/10.3390/jfb16080274
Guarino V, Vasco G, Arima V, Cataldo R, Zizzari A, Perrone E, Gigli G, Cesaria M. PDMS Membranes Drilled by Proton Microbeam Writing: A Customizable Platform for the Investigation of Endothelial Cell–Substrate Interactions in Transwell-like Devices. Journal of Functional Biomaterials. 2025; 16(8):274. https://doi.org/10.3390/jfb16080274
Chicago/Turabian StyleGuarino, Vita, Giovanna Vasco, Valentina Arima, Rosella Cataldo, Alessandra Zizzari, Elisabetta Perrone, Giuseppe Gigli, and Maura Cesaria. 2025. "PDMS Membranes Drilled by Proton Microbeam Writing: A Customizable Platform for the Investigation of Endothelial Cell–Substrate Interactions in Transwell-like Devices" Journal of Functional Biomaterials 16, no. 8: 274. https://doi.org/10.3390/jfb16080274
APA StyleGuarino, V., Vasco, G., Arima, V., Cataldo, R., Zizzari, A., Perrone, E., Gigli, G., & Cesaria, M. (2025). PDMS Membranes Drilled by Proton Microbeam Writing: A Customizable Platform for the Investigation of Endothelial Cell–Substrate Interactions in Transwell-like Devices. Journal of Functional Biomaterials, 16(8), 274. https://doi.org/10.3390/jfb16080274





