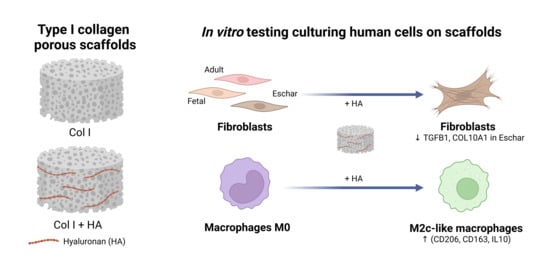
Effect of Hyaluronan in Collagen Biomaterials on Human Macrophages and Fibroblasts In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of Type I Collagen Scaffolds with Hyaluronan
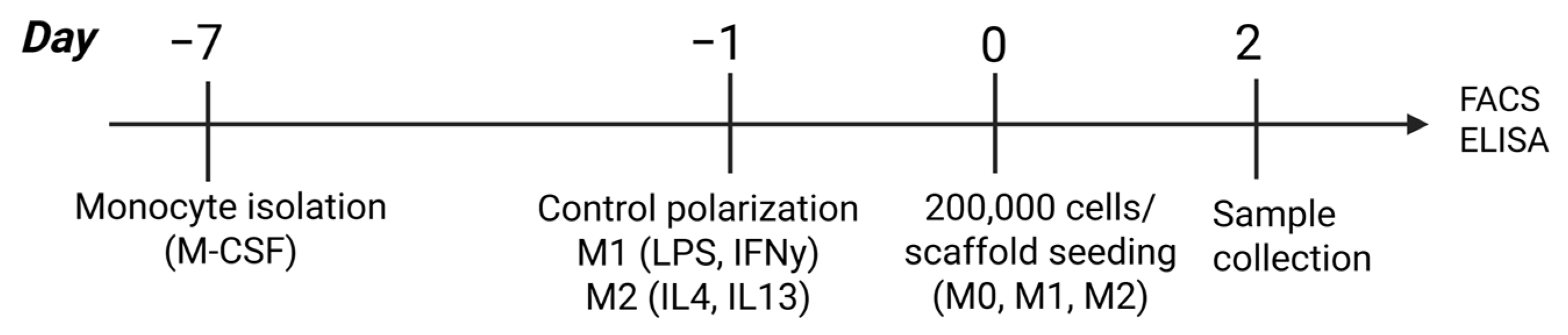
2.2. Macrophage Culture and Analysis
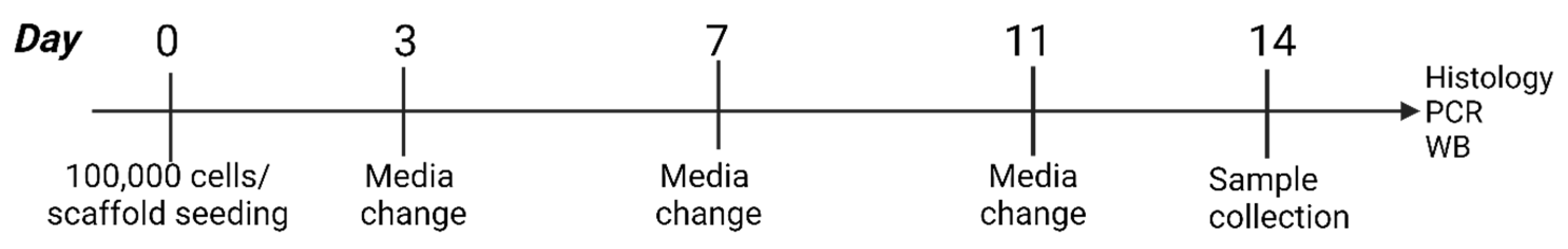
2.3. Fibroblast Culture and Analysis
2.4. Statistical Analysis
3. Results
3.1. Incorporation of HA in Collagen Scaffolds
3.2. Effect of Hyaluronan-Containing Scaffolds on Macrophage Polarization
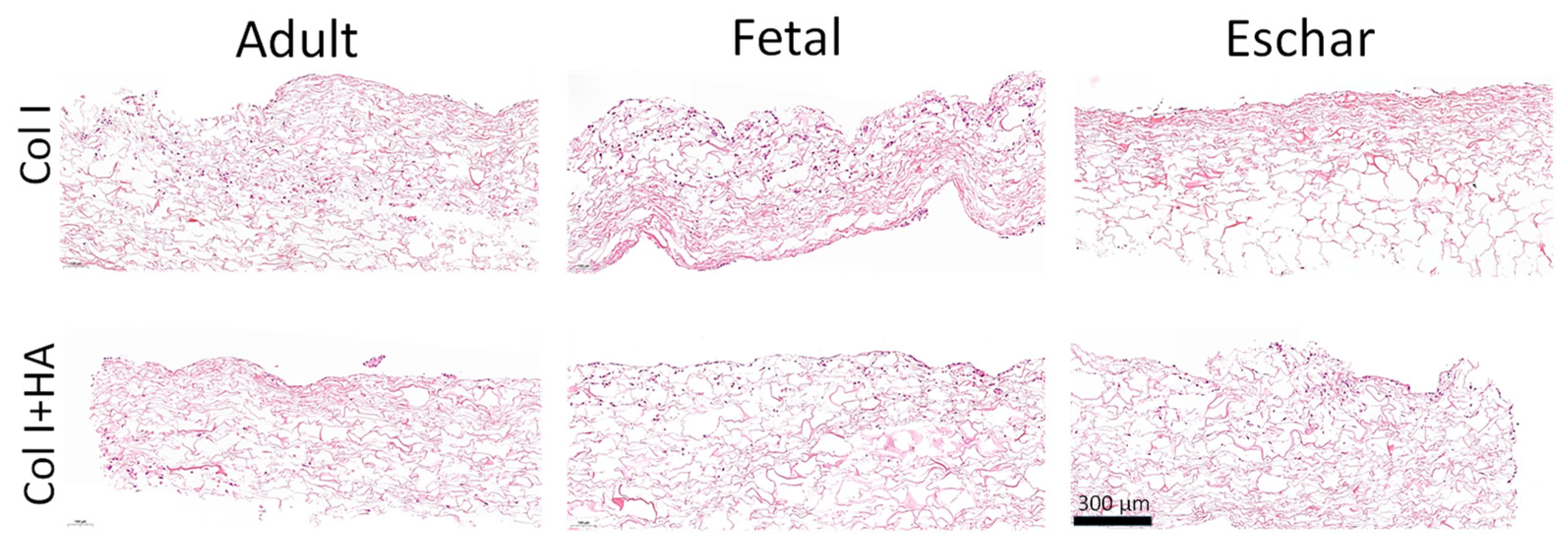
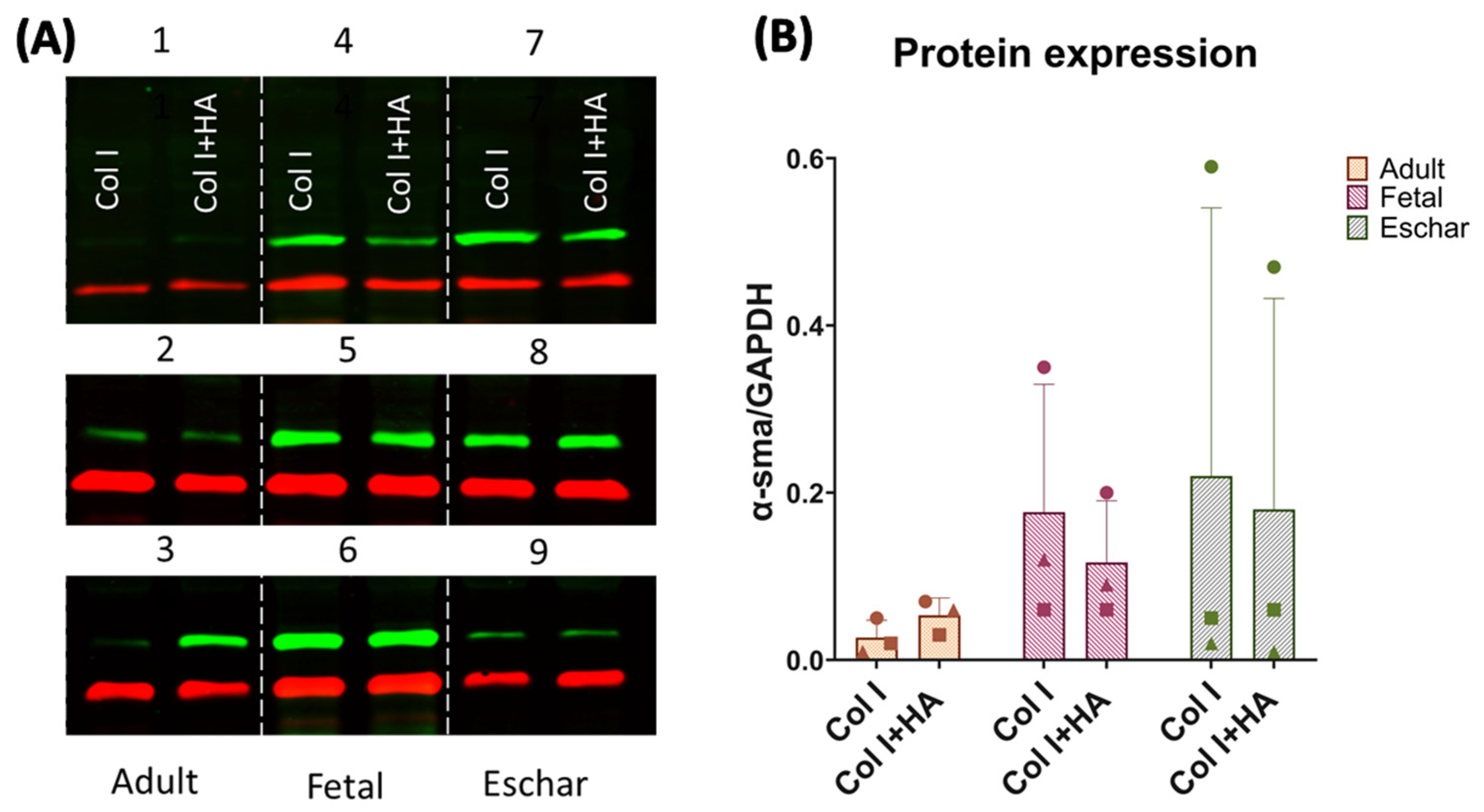
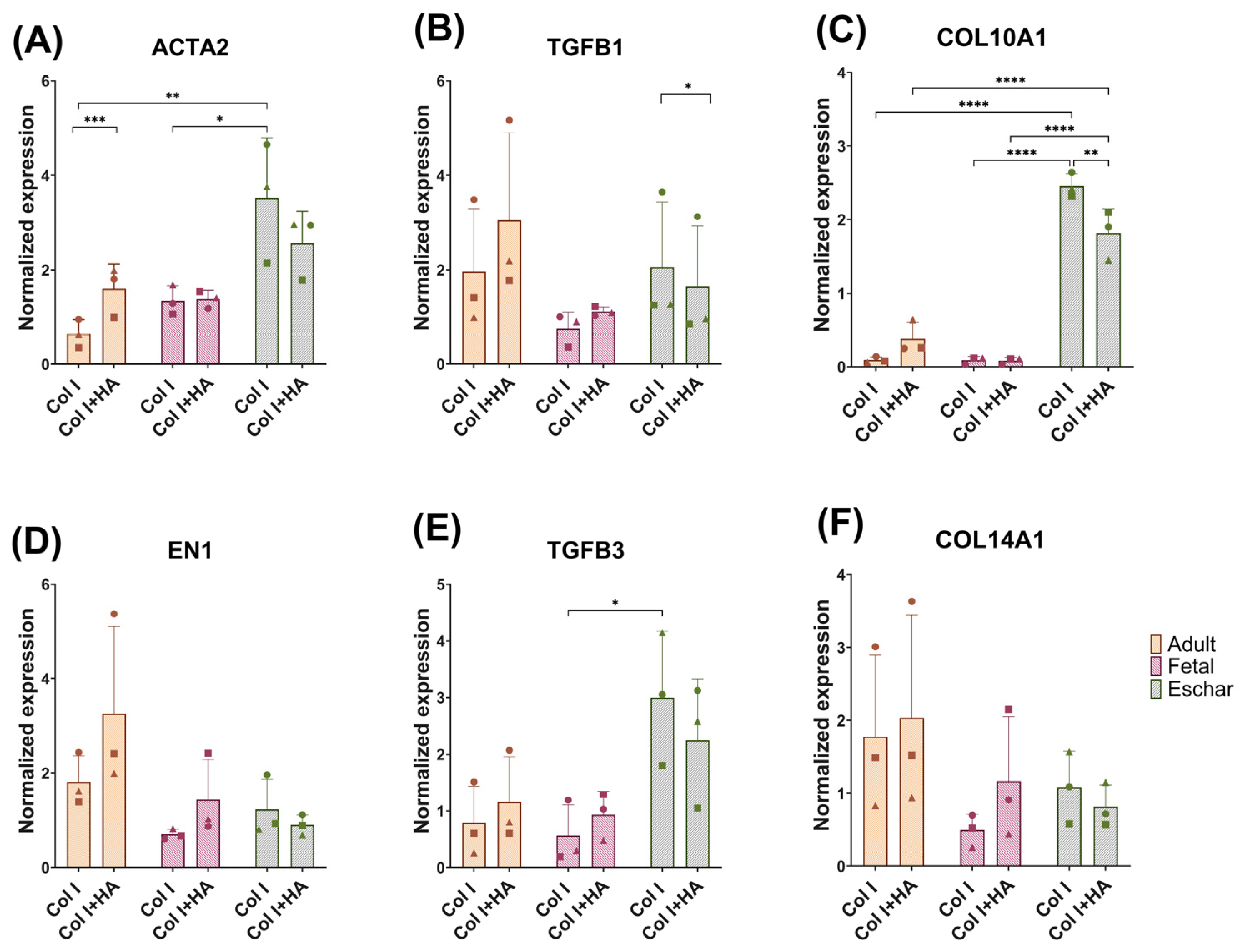
3.3. Effect of Hyaluronan on Different Fibroblast Types
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mamun, A.; Shao, C.; Geng, P.; Wang, S.; Xiao, J. Recent advances in molecular mechanisms of skin wound healing and its treatments. Front. Immunol. 2024, 15, 1395479. [Google Scholar] [CrossRef] [PubMed]
- Chacon, M.A.; Haas, J.; Hansen, T.C.; Mushin, O.P.; Bell, D.E. Thin and Ultra-Thin Split-Thickness Skin Grafts Are Safe and Efficacious in the Burn Population. J. Burn Care Res. 2019, 41, 849–852. [Google Scholar] [CrossRef] [PubMed]
- Uijtdewilligen, P.J.E.; Versteeg, E.M.M.; Gilissen, C.; van Reijmersdal, S.V.; Schoppmeyer, R.; Wismans, R.G.; Daamen, W.F.; van Kuppevelt, T.H. Towards embryonic-like scaffolds for skin tissue engineering: Identification of effector molecules and construction of scaffolds. J. Tissue Eng. Regen. Med. 2016, 10, E34–E44. [Google Scholar] [CrossRef] [PubMed]
- Estes, J.; Adzick, N.; Harrison, M.; Longaker, M.; Stern, R. Hyaluronate metabolism undergoes an ontogenic transition during fetal development—Implications for scar-free wound-healing. J. Pediatr. Surg. 1993, 28, 1227–1231. [Google Scholar] [CrossRef]
- Gomes, M.; Krijnen, P.A.J.; Middelkoop, E.; Niessen, H.W.M.; Boekema, B. Fetal Skin Wound Healing: Key Extracellular Matrix Components and Regulators in Scarless Healing. J. Investig. Dermatol. 2025, 145, 280–302. [Google Scholar] [CrossRef]
- Dovedytis, M.; Liu, Z.J.; Bartlett, S. Hyaluronic acid and its biomedical applications: A review. Eng. Regen. 2020, 1, 102–113. [Google Scholar] [CrossRef]
- Acharya, P.S.; Majumdar, S.; Jacob, M.; Hayden, J.; Mrass, P.; Weninger, W.; Assoian, R.K.; Puré, E. Fibroblast migration is mediated by CD44-dependent TGFβ activation. J. Cell Sci. 2008, 121, 1393–1402. [Google Scholar] [CrossRef]
- Potter, D.A.; Veitch, D.; Johnston, G.A. Scarring and wound healing. Br. J. Hosp. Med. 2019, 80, C166–C171. [Google Scholar] [CrossRef]
- Zheng, H.; Cheng, X.; Jin, L.; Shan, S.; Yang, J.; Zhou, J. Recent advances in strategies to target the behavior of macrophages in wound healing. Biomed. Pharmacother. 2023, 165, 115199. [Google Scholar] [CrossRef]
- Rios de la Rosa, J.M.; Tirella, A.; Gennari, A.; Stratford, I.J.; Tirelli, N. The CD44-Mediated Uptake of Hyaluronic Acid-Based Carriers in Macrophages. Adv. Healthc. Mater. 2017, 6, 1601012. [Google Scholar] [CrossRef]
- Alaish, S.M.; Yager, D.; Diegelmann, R.F.; Cohen, I.K. Biology of fetal wound healing: Hyaluronate receptor expression in fetal fibroblasts. J. Pediatr. Surg. 1994, 29, 1040–1043. [Google Scholar] [CrossRef] [PubMed]
- Soo, C.; Hu, F.-Y.; Zhang, X.; Wang, Y.; Beanes, S.R.; Lorenz, H.P.; Hedrick, M.H.; Mackool, R.J.; Plaas, A.; Kim, S.-J.; et al. Differential Expression of Fibromodulin, a Transforming Growth Factor-β Modulator, in Fetal Skin Development and Scarless Repair. Am. J. Pathol. 2000, 157, 423–433. [Google Scholar] [CrossRef]
- Van Der Veen, V.C.; Vlig, M.; Van Milligen, F.J.; De Vries, S.I.; Middelkoop, E.; Ulrich, M.M.W. Stem Cells in Burn Eschar. Cell Transplant. 2012, 21, 933–942. [Google Scholar] [CrossRef]
- David-Raoudi, M.; Tranchepain, F.; Deschrevel, B.; Vincent, J.; Bogdanowicz, P.; Boumediene, K.; Pujol, J. Differential effects of hyaluronan and its fragments on fibroblasts: Relation to wound healing. Wound Repair Regen. 2008, 16, 274–287. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Song, L.; Sun, B.; Chu, D.; Yang, L.; Li, M.; Li, H.; Dai, Y.; Yu, Z.; Guo, J. Modulation of macrophages by a paeoniflorin-loaded hyaluronic acid-based hydrogel promotes diabetic wound healing. Mater. Today Bio 2021, 12, 100139. [Google Scholar] [CrossRef]
- O’Brien, F.; Harley, B.; Yannas, I.; Gibson, L. The effect of pore size on cell adhesion in collagen-GAG scaffolds. Biomaterials 2005, 26, 433–441. [Google Scholar] [CrossRef]
- Liu, J.-Y.; Li, Y.; Hu, Y.; Cheng, G.; Ye, E.; Shen, C.; Xu, F.-J. Hemostatic porous sponges of cross-linked hyaluronic acid/cationized dextran by one self-foaming process. Mater. Sci. Eng. C 2018, 83, 160–168. [Google Scholar] [CrossRef]
- Buttafoco, L.; Engbers-Buijtenhuijs, P.; Poot, A.A.; Dijkstra, P.J.; Daamen, W.F.; van Kuppevelt, T.H.; Vermes, I.; Feijen, J. First steps towards tissue engineering of small-diameter blood vessels: Preparation of flat scaffolds of collagen and elastin by means of freeze drying. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 77B, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Schroepfer, M.; Meyer, M. DSC investigation of bovine hide collagen at varying degrees of crosslinking and humidities. Int. J. Biol. Macromol. 2017, 103, 120–128. [Google Scholar] [CrossRef]
- van den Bogaerdt, A.J.; van Zuijlen, P.P.; van Galen, M.; Lamme, E.N.; Middelkoop, E. The suitability of cells from different tissues for use in tissue-engineered skin substitutes. Arch. Dermatol. Res. 2002, 294, 135–142. [Google Scholar] [CrossRef]
- Akershoek, J.J.; Vlig, M.; Talhout, W.; Boekema, B.K.H.L.; Richters, C.D.; Beelen, R.H.J.; Brouwer, K.M.; Middelkoop, E.; Ulrich, M.M.W. Cell therapy for full-thickness wounds: Are fetal dermal cells a potential source? Cell Tissue Res. 2016, 364, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Oostendorp, C.; Geutjes, P.J.; Smit, F.; Tiemessen, D.M.; Polman, S.; Abbawi, A.; Brouwer, K.M.; Eggink, A.J.; Feitz, W.F.J.; Daamen, W.F.; et al. Sustained Postnatal Skin Regeneration Upon Prenatal Application of Functionalized Collagen Scaffolds. Tissue Eng. Part A 2021, 27, 10–25. [Google Scholar] [CrossRef]
- Kim, K.K.; Sheppard, D.; Chapman, H.A. TGF-β1 Signaling and Tissue Fibrosis. Cold Spring Harb. Perspect. Biol. 2018, 10, a022293. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Huang, H.; Shi, G.; Zhao, L.; Li, T.; Zhang, Z.; Liu, R.; Hu, Y.; Liu, H.; Yu, J.; et al. TGF-β1-SOX9 axis-inducible COL10A1 promotes invasion and metastasis in gastric cancer via epithelial-to-mesenchymal transition. Cell Death Dis. 2018, 9, 849. [Google Scholar] [CrossRef] [PubMed]
- Kutluoğlu, G.C.; Vlig, M.; Elgersma, A.; Boekema, B.; Daamen, W.F.; Doberenz, C.; Manikowski, D. Comparison of dermal and eschar fibroblasts in full skin equivalents. Wound Repair Regen. 2025, 33, e70001. [Google Scholar] [CrossRef]
- Györfi, A.; Matei, A.; Fuchs, M.; Liang, C.; Rigau, A.; Hong, X.; Zhu, H.; Luber, M.; Bergmann, C.; Dees, C.; et al. Engrailed 1 coordinates cytoskeletal reorganization to induce myofibroblast differentiation. J. Exp. Med. 2021, 218, e20201916. [Google Scholar] [CrossRef]
- Rinkevich, Y.; Walmsley, G.G.; Hu, M.S.; Maan, Z.N.; Newman, A.M.; Drukker, M.; Januszyk, M.; Krampitz, G.W.; Gurtner, G.C.; Lorenz, H.P.; et al. Skin fibrosis. Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science 2015, 348, aaa2151. [Google Scholar] [CrossRef]
- Mascharak, S.; DesJardins-Park, H.E.; Davitt, M.F.; Griffin, M.; Borrelli, M.R.; Moore, A.L.; Chen, K.; Duoto, B.; Chinta, M.; Foster, D.S.; et al. Preventing Engrailed-1 activation in fibroblasts yields wound regeneration without scarring. Science 2021, 372, eaba2374. [Google Scholar] [CrossRef]
- Lichtman, M.K.; Otero-Vinas, M.; Falanga, V. Transforming growth factor beta (TGF-β) isoforms in wound healing and fibrosis. Wound Repair Regen. 2016, 24, 215–222. [Google Scholar] [CrossRef]
- Correia, C.R.; Moreira-Teixeira, L.S.; Moroni, L.; Reis, R.L.; van Blitterswijk, C.A.; Karperien, M.; Mano, J.F. Chitosan Scaffolds Containing Hyaluronic Acid for Cartilage Tissue Engineering. Tissue Eng. Part C Methods 2011, 17, 717–730. [Google Scholar] [CrossRef]
- Yu, L.; Liu, T.; Ma, Y.; Zhang, F.; Huang, Y.; Fan, Z. Fabrication of Silk-Hyaluronan Composite as a Potential Scaffold for Tissue Repair. Front. Bioeng. Biotech. 2020, 8, 578988. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Slusarewicz, P.; Hedman, T. Thermal analysis reveals differential effects of various crosslinkers on bovine annulus fibrosis. J. Orthop. Res. 2011, 29, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Mast, B.; Flood, L.; Haynes, J.; Depalma, R.; Cohen, I.; Diegelmann, R.; Krummel, T. Hyaluronic-acid is a major component of the matrix of fetal rabbit skin and wounds—Implications for healing by regeneration. Matrix 1991, 11, 63–68. [Google Scholar] [CrossRef]
- Ulrich, M.M.W. Fetal Wound Healing. In Textbook on Scar Management: State of the Art Management and Emerging Technologies; Téot, L., Mustoe, T.A., Middelkoop, E., Gauglitz, G.G., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 3–9. [Google Scholar]
- Hesketh, M.; Sahin, K.B.; West, Z.E.; Murray, R.Z. Macrophage Phenotypes Regulate Scar Formation and Chronic Wound Healing. Int. J. Mol. Sci. 2017, 18, 1545. [Google Scholar] [CrossRef]
- Mondadori, C.; Chandrakar, A.; Lopa, S.; Wieringa, P.; Talò, G.; Perego, S.; Lombardi, G.; Colombini, A.; Moretti, M.; Moroni, L. Assessing the response of human primary macrophages to defined fibrous architectures fabricated by melt electrowriting. Bioact. Mater. 2023, 21, 209–222. [Google Scholar] [CrossRef]
- Spiller, K.L.; Anfang, R.R.; Spiller, K.J.; Ng, J.; Nakazawa, K.R.; Daulton, J.W.; Vunjak-Novakovic, G. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials 2014, 35, 4477–4488. [Google Scholar] [CrossRef]
- Lu, J.; Cao, Q.; Zheng, D.; Sun, Y.; Wang, C.; Yu, X.; Wang, Y.; Lee, V.W.S.; Zheng, G.; Tan, T.K.; et al. Discrete functions of M2a and M2c macrophage subsets determine their relative efficacy in treating chronic kidney disease. Kidney Int. 2013, 84, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Liechty, K.W.; Kim, H.B.; Adzick, N.S.; Crombleholme, T.M. Fetal wound repair results in scar formation in interleukin-10–deficient mice in a syngeneic murine model of scarless fetal wound repair. J. Pediatr. Surg. 2000, 35, 866–873. [Google Scholar] [CrossRef]
- King, A.; Balaji, S.; Le, L.D.; Marsh, E.; Crombleholme, T.M.; Keswani, S.G. Interleukin-10 regulates fetal extracellular matrix hyaluronan production. J. Pediatr. Surg. 2013, 48, 1211–1217. [Google Scholar] [CrossRef]
- Yang, P.; Lu, Y.; Gou, W.; Qin, Y.; Tan, J.; Luo, G.; Zhang, Q. Glycosaminoglycans’ Ability to Promote Wound Healing: From Native Living Macromolecules to Artificial Biomaterials. Adv. Sci. 2024, 11, 2305918. [Google Scholar] [CrossRef]
- Oates, T.C.; Boyd, J.; Dolan, L.; Kergariou, C.d.; Toye, A.; Perriman, A.W.; Boussahel, A. The Immobilization of Hyaluronic Acid in 3D Hydrogel Scaffolds Modulates Macrophage Polarization. bioRxiv 2024. [Google Scholar] [CrossRef]
- Sapudom, J.; Ullm, F.; Martin, S.; Kalbitzer, L.; Naab, J.; Möller, S.; Schnabelrauch, M.; Anderegg, U.; Schmidt, S.; Pompe, T. Molecular weight specific impact of soluble and immobilized hyaluronan on CD44 expressing melanoma cells in 3D collagen matrices. Acta Biomater. 2017, 50, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, X.; Liu, Y.; Xu, H.; Ye, C. Pan-cancer and single-cell analyses identify CD44 as an immunotherapy response predictor and regulating macrophage polarization and tumor progression in colorectal cancer. Front. Oncol. 2024, 14, 1380821. [Google Scholar] [CrossRef]
- Sapudom, J.; Karaman, S.; Mohamed, W.K.E.; Garcia-Sabaté, A.; Quartey, B.C.; Teo, J.C.M. 3D in vitro M2 macrophage model to mimic modulation of tissue repair. NPJ Regen. Med. 2021, 6, 83. [Google Scholar] [CrossRef]
- Rooney, P.; Wang, M.; Kumar, P.; Kumar, S. Angiogenic oligosaccharides of hyaluronan enhance the production of collagens by endothelial cells. J. Cell Sci. 1993, 105, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Schulz, J.; Plomann, M.; Sengle, G.; Gullberg, D.; Krieg, T.; Eckes, B. New developments on skin fibrosis—Essential signals emanating from the extracellular matrix for the control of myofibroblasts. Matrix Biol. 2018, 68–69, 522–532. [Google Scholar] [CrossRef]
- Walraven, M.; Akershoek, J.; Beelen, R.; Ulrich, M. In vitro cultured fetal fibroblasts have myofibroblast-associated characteristics and produce a fibrotic-like environment upon stimulation with TGF-β1: Is there a thin line between fetal scarless healing and fibrosis? Arch. Dermatol. Res. 2017, 309, 111–121. [Google Scholar] [CrossRef]
- Nakamura, M.; Yoshida, H.; Moriyama, Y.; Kawakita, I.; Wlizla, M.; Takebayashi-Suzuki, K.; Horb, M.E.; Suzuki, A. TGF-β1 signaling is essential for tissue regeneration in the Xenopus tadpole tail. Biochem. Biophys. Res. Commun. 2021, 565, 91–96. [Google Scholar] [CrossRef]
- McAndrews, K.; Miyake, T.; Ehsanipour, E.; Kelly, P.; Becker, L.; McGrail, D.; Sugimoto, H.; LeBleu, V.; Ge, Y.; Kalluri, R. Dermal αSMA+ myofibroblasts orchestrate skin wound repair via β1 integrin and independent of type I collagen production. EMBO J. 2022, 41, e109470. [Google Scholar] [CrossRef]
- Huettl, R.E.; Luxenhofer, G.; Bianchi, E.; Haupt, C.; Joshi, R.; Prochiantz, A.; Huber, A.B. Engrailed 1 mediates correct formation of limb innervation through two distinct mechanisms. PLoS ONE 2015, 10, e0118505. [Google Scholar] [CrossRef]
- Tabib, T.; Huang, M.; Morse, N.; Papazoglou, A.; Behera, R.; Jia, M.; Bulik, M.; Monier, D.; Benos, P.; Chen, W.; et al. Myofibroblast transcriptome indicates SFRP2hi fibroblast progenitors in systemic sclerosis skin. Nat. Commun. 2021, 12, 4384. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, J.; Cheng, W.; Diao, T.; Liu, H.; Bo, Y.; Liu, C.; Zhou, W.; Chen, M.; Zhang, Y.; et al. Cross-tissue human fibroblast atlas reveals myofibroblast subtypes with distinct roles in immune modulation. Cancer Cell 2024, 42, 1764–1783.e10. [Google Scholar] [CrossRef]
- Lorenz, H.P.; Lin, R.Y.; Longaker, M.T.; Whitby, D.J.; Adzick, N.S. The fetal fibroblast—The effector cell of scarless fetal skin repair. Plast. Reconstr. Surg. 1995, 96, 1251–1259. [Google Scholar] [CrossRef] [PubMed]
- Ansorge, H.L.; Meng, X.; Zhang, G.; Veit, G.; Sun, M.; Klement, J.F.; Beason, D.P.; Soslowsky, L.J.; Koch, M.; Birk, D.E. Type XIV Collagen Regulates Fibrillogenesis. J. Biol. Chem. 2009, 284, 8427–8438. [Google Scholar] [CrossRef]
- Smithmyer, M.E.; Cassel, S.E.; Kloxin, A.M. Bridging 2D and 3D culture: Probing impact of extracellular environment on fibroblast activation in layered hydrogels. AIChE J. 2019, 65, e16837. [Google Scholar] [CrossRef] [PubMed]
- Xue, K.; Zhang, J.; Li, C.; Li, J.; Wang, C.; Zhang, Q.; Chen, X.; Yu, X.; Sun, L.; Yu, X. The role and mechanism of transforming growth factor beta 3 in human myocardial infarction-induced myocardial fibrosis. J. Cell. Mol. Med. 2019, 23, 4229–4243. [Google Scholar] [CrossRef]
- Pipiya, V.V.; Gilazieva, Z.E.; Issa, S.S.; Rizvanov, A.A.; Solovyeva, V.V. Comparison of primary and passaged tumor cell cultures and their application in personalized medicine. Explor. Target. Antitumor Ther. 2024, 5, 581–599. [Google Scholar] [CrossRef]
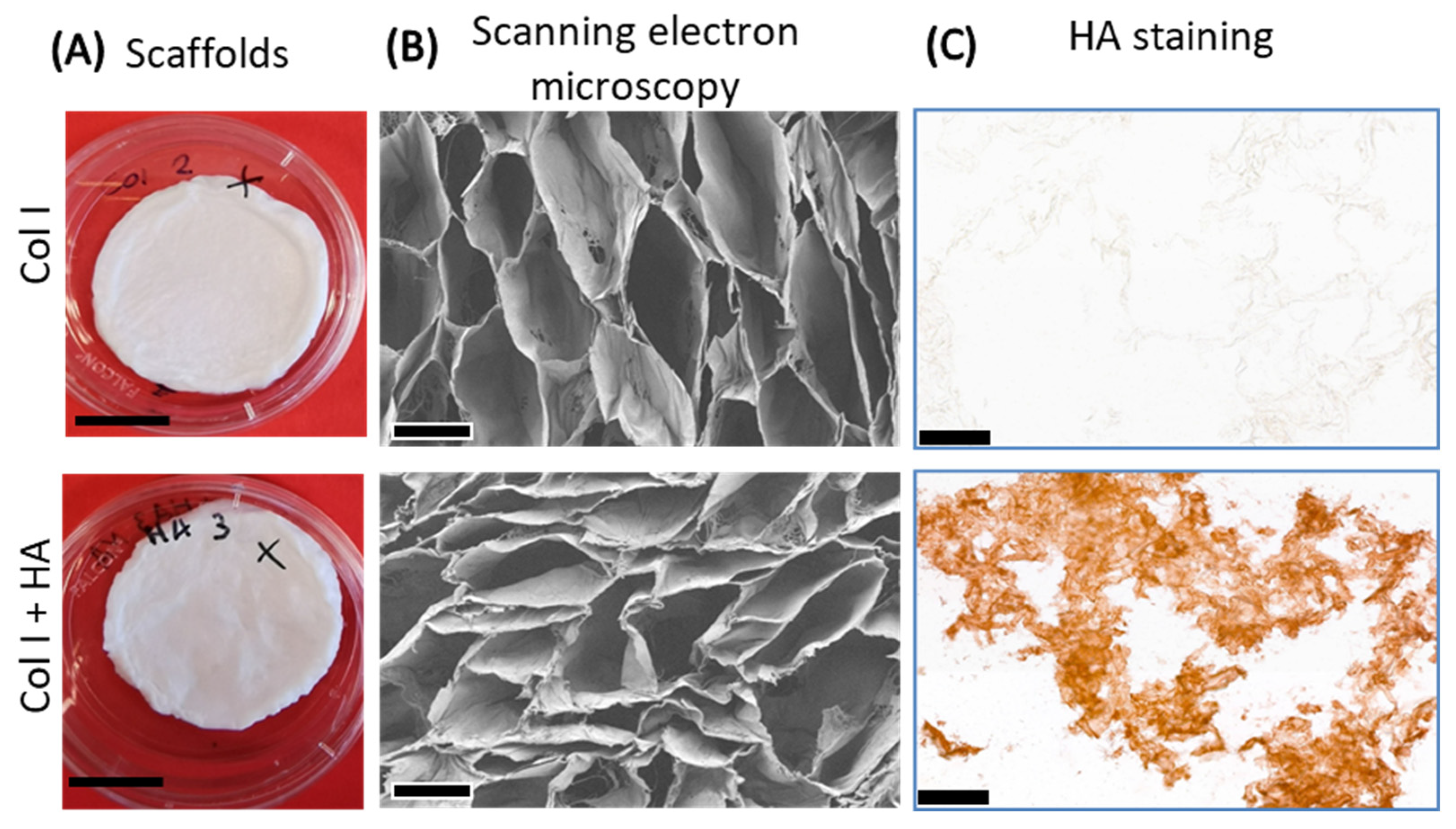

| Scaffold Type | Amine Group Content (nmol/mg Scaffold) | Denaturation Temperature Td (°C) | Hyaluronan Content (µg/mg Scaffold) | |||
|---|---|---|---|---|---|---|
| Crosslinked | No | Yes | No | Yes | No | Yes |
| Col I | 361 ± 49 | 206 ± 19 | 59 ± 1 | 78 ± 0 | 1 ± 1 | 1 ± 1 |
| Col I+HA | 341 ± 36 | 197 ± 23 | 58 ± 1 | 76 ± 0 | 19 ± 4 | 43 ± 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avila-Martinez, N.; Pfirrmann, M.; Gomes, M.L.N.P.; Krymchenko, R.; Versteeg, E.M.M.; Vlig, M.; Verdoes, M.; van Kuppevelt, T.H.; Boekema, B.K.H.L.; Daamen, W.F. Effect of Hyaluronan in Collagen Biomaterials on Human Macrophages and Fibroblasts In Vitro. J. Funct. Biomater. 2025, 16, 167. https://doi.org/10.3390/jfb16050167
Avila-Martinez N, Pfirrmann M, Gomes MLNP, Krymchenko R, Versteeg EMM, Vlig M, Verdoes M, van Kuppevelt TH, Boekema BKHL, Daamen WF. Effect of Hyaluronan in Collagen Biomaterials on Human Macrophages and Fibroblasts In Vitro. Journal of Functional Biomaterials. 2025; 16(5):167. https://doi.org/10.3390/jfb16050167
Chicago/Turabian StyleAvila-Martinez, Nancy, Maren Pfirrmann, Madalena L. N. P. Gomes, Roman Krymchenko, Elly M. M. Versteeg, Marcel Vlig, Martijn Verdoes, Toin H. van Kuppevelt, Bouke K. H. L. Boekema, and Willeke F. Daamen. 2025. "Effect of Hyaluronan in Collagen Biomaterials on Human Macrophages and Fibroblasts In Vitro" Journal of Functional Biomaterials 16, no. 5: 167. https://doi.org/10.3390/jfb16050167
APA StyleAvila-Martinez, N., Pfirrmann, M., Gomes, M. L. N. P., Krymchenko, R., Versteeg, E. M. M., Vlig, M., Verdoes, M., van Kuppevelt, T. H., Boekema, B. K. H. L., & Daamen, W. F. (2025). Effect of Hyaluronan in Collagen Biomaterials on Human Macrophages and Fibroblasts In Vitro. Journal of Functional Biomaterials, 16(5), 167. https://doi.org/10.3390/jfb16050167