Engineering Stepped Structures on Hydroxyapatite Surfaces: A Potential Strategy to Modulate Bone Marrow Mesenchymal Stem Adhesion, Spreading, and Proliferation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of HA Dishes
2.3. Characterization
2.4. Protein Adsorption Measurement
2.5. Culture and Identification of BMSCs
2.6. Cytotoxicity Assay HA Dish Extracts
2.7. Immunofluorescence Assay
2.8. RNA Sequencing
2.9. Statistical Analysis
3. Results and Discussion
3.1. Fabrication and Characterization of HA Dishes with Stepped Structures
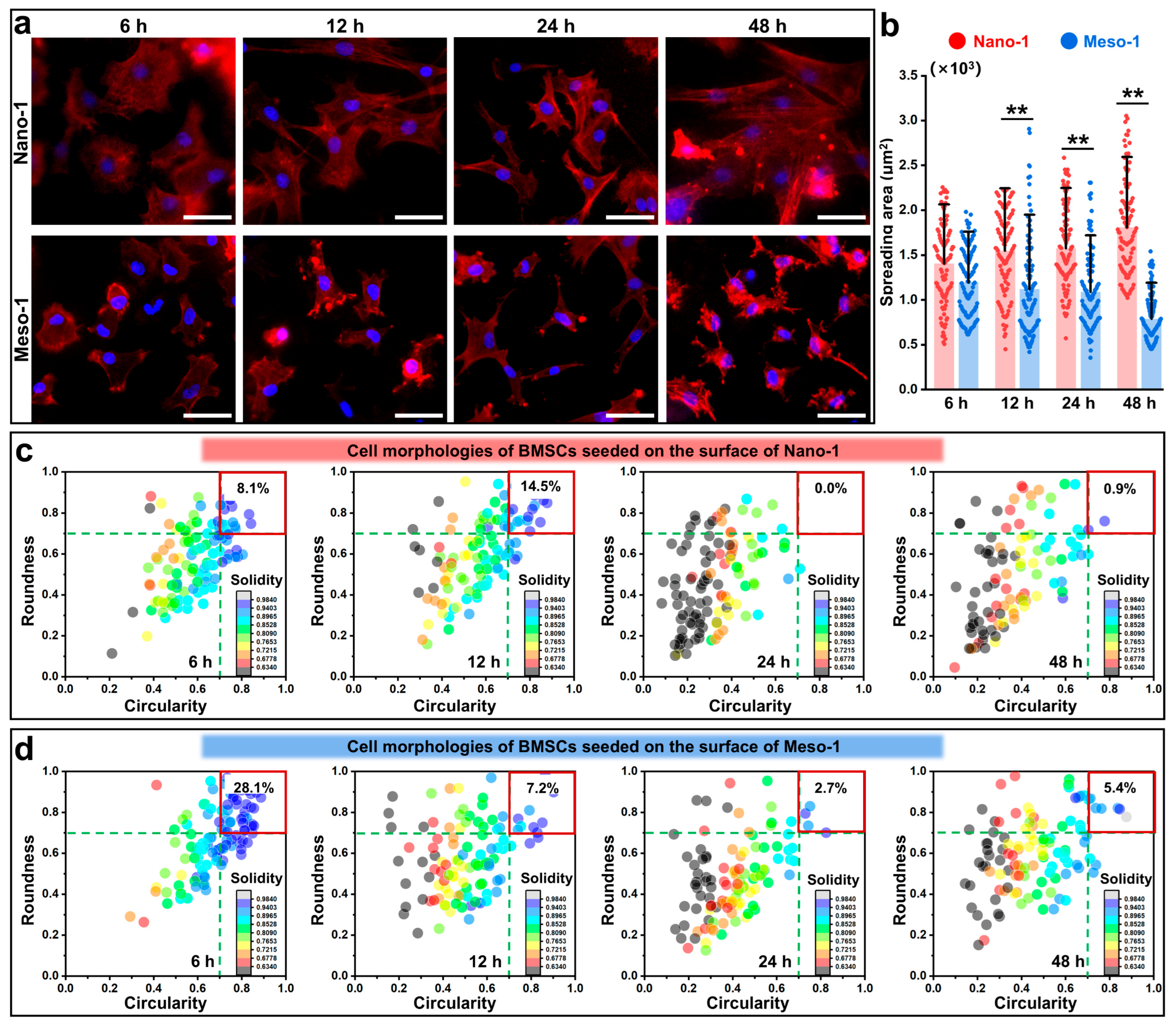
3.2. The Effects of Stepped Structures on the Cell Morphology and Cytoskeleton of BMSCs
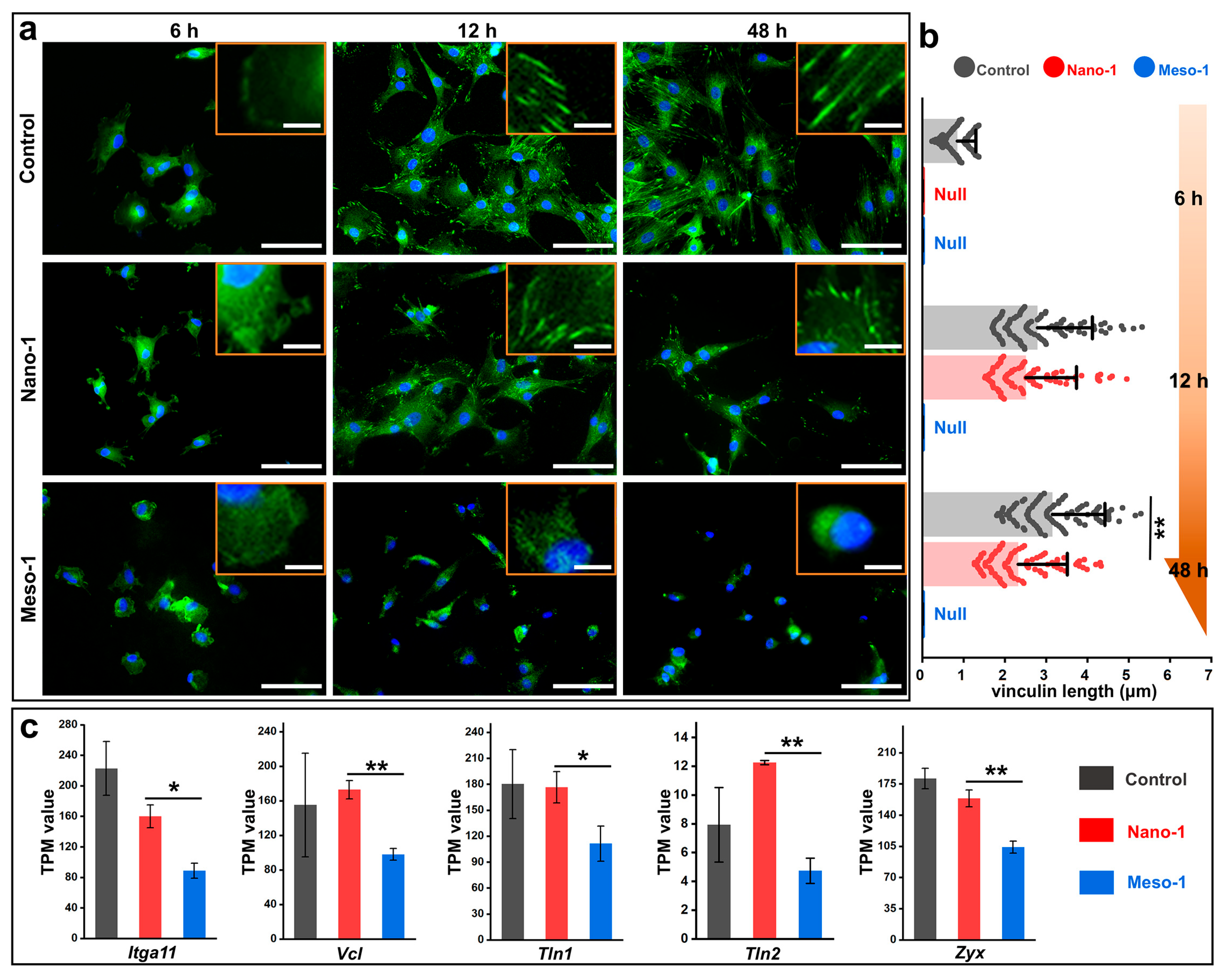
3.3. The Effects of Stepped Structures on the FAs of BMSCs
3.4. The Effects of Stepped Structures on the Localization of YAP and MRTF-A
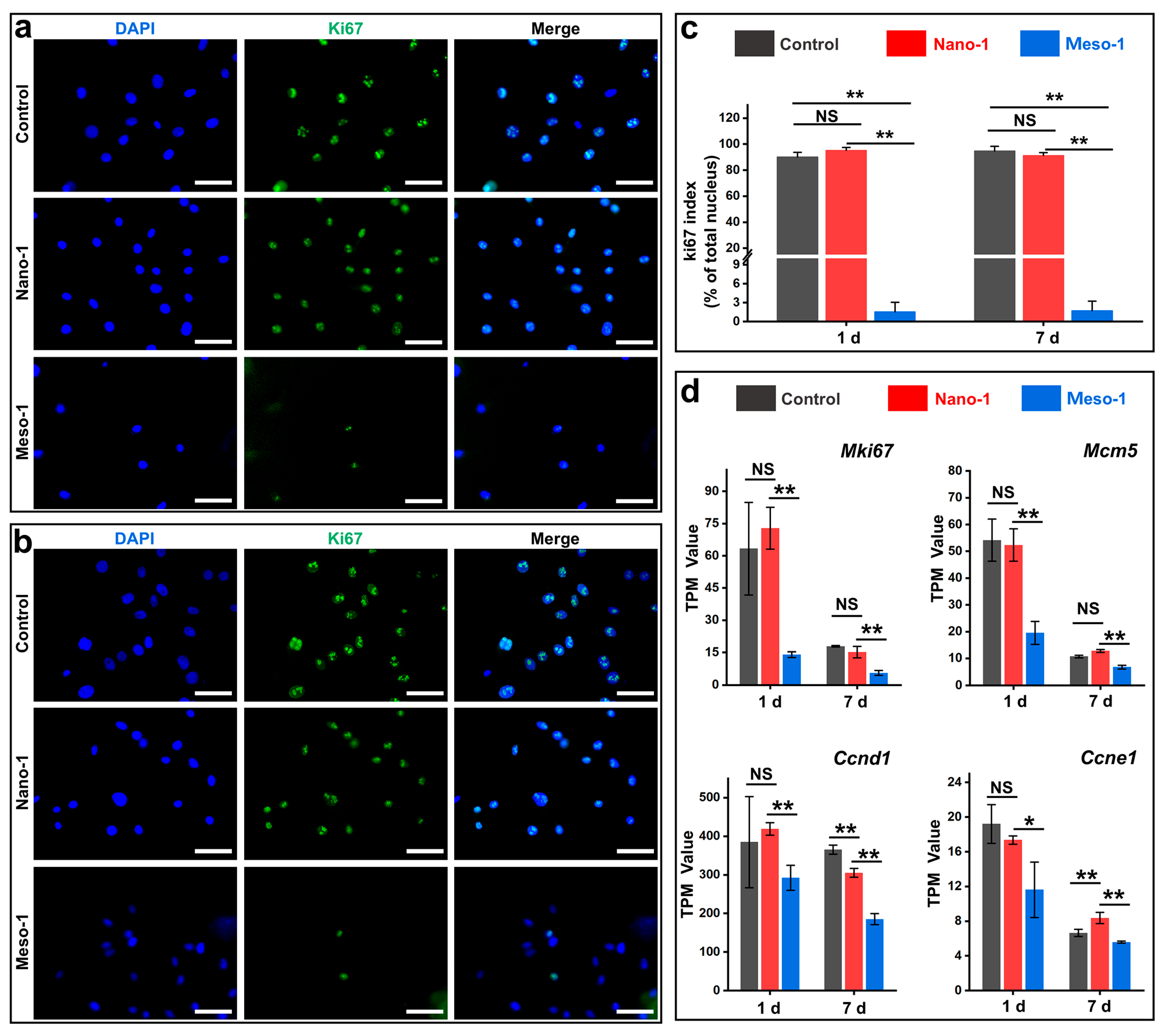
3.5. The Effects of Stepped Structures on the Cell Proliferation of BMSCs
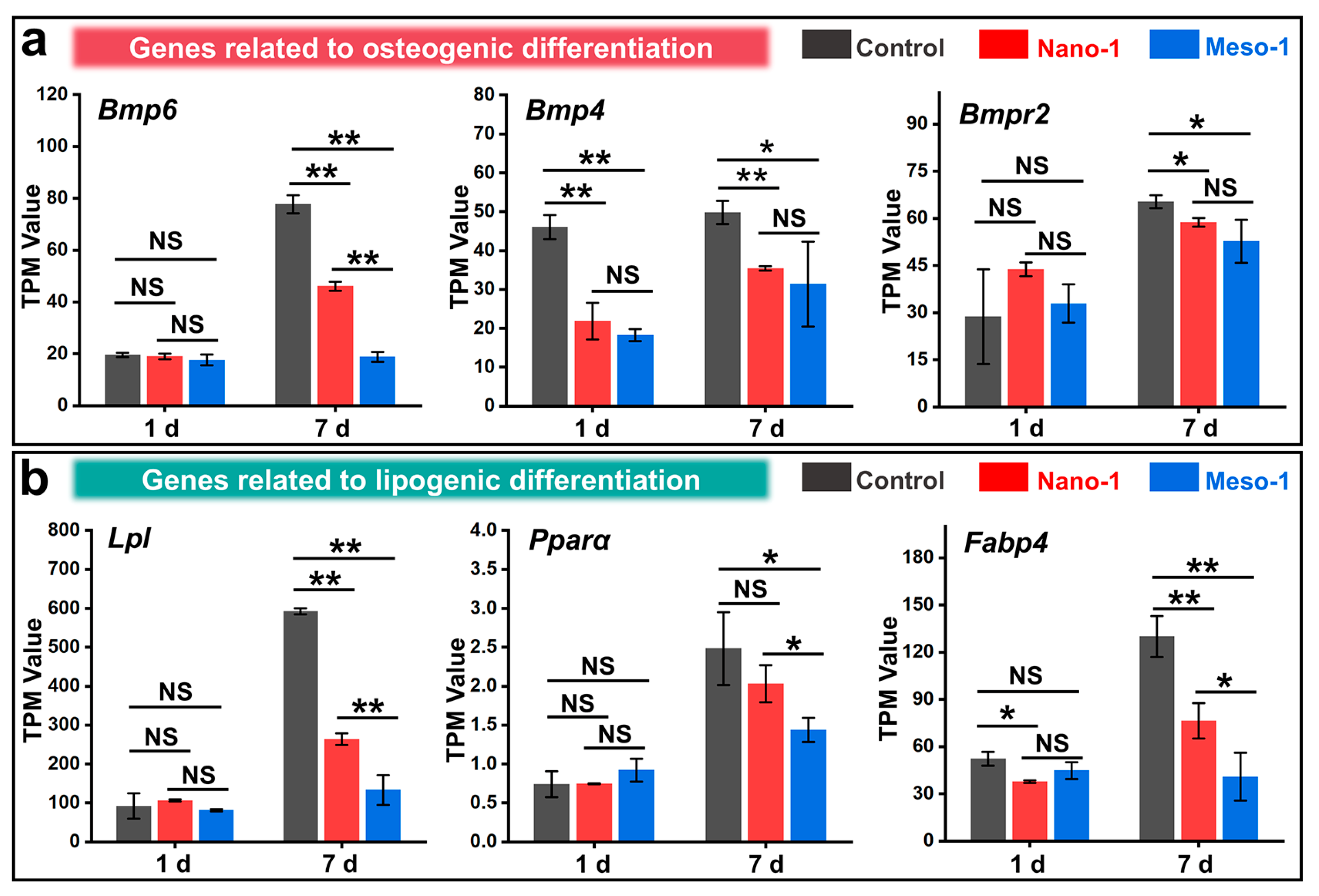
3.6. The Effects of Stepped Structures on the Cell Differentiation of BMSCs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, H.; Sohn, J.; Shen, H.; Langhans, M.T.; Tuan, R.S. Bone Marrow Mesenchymal Stem Cells: Aging and Tissue Engineering Applications to Enhance Bone Healing. Biomaterials 2019, 203, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Pajarinen, J.; Lin, T.; Gibon, E.; Kohno, Y.; Maruyama, M.; Nathan, K.; Lu, L.; Yao, Z.; Goodman, S.B. Mesenchymal Stem Cell-Macrophage Crosstalk and Bone Healing. Biomaterials 2019, 196, 80–89. [Google Scholar] [CrossRef]
- Huang, G.; Li, F.; Zhao, X.; Ma, Y.; Li, Y.; Lin, M.; Jin, G.; Lu, T.J.; Genin, G.M.; Xu, F. Functional and Biomimetic Materials for Engineering of the Three-Dimensional Cell Microenvironment. Chem. Rev. 2017, 117, 12764–12850. [Google Scholar] [CrossRef]
- Dalby, M.J.; Gadegaard, N.; Oreffo, R.O.C. Harnessing Nanotopography and Integrin-Matrix Interactions to Influence Stem Cell Fate. Nat. Mater. 2014, 13, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Ozboyaci, M.; Kokh, D.B.; Corni, S.; Wade, R.C. Modeling and Simulation of Protein-Surface Interactions: Achievements and Challenges. Q. Rev. Biophys. 2016, 49, e4. [Google Scholar] [CrossRef] [PubMed]
- Saraswathibhatla, A.; Indana, D.; Chaudhuri, O. Cell–Extracellular Matrix Mechanotransduction in 3D. Nat. Rev. Mol. Cell Biol. 2023, 24, 495–516. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, C.; Chang, J. Bioceramics to Regulate Stem Cells and Their Microenvironment for Tissue Regeneration. Mater. Today 2019, 24, 41–56. [Google Scholar] [CrossRef]
- Kulkarni, M.; Mazare, A.; Park, J.; Gongadze, E.; Killian, M.S.; Kralj, S.; von der Mark, K.; Iglič, A.; Schmuki, P. Protein Interactions with Layers of TiO2 Nanotube and Nanopore Arrays: Morphology and Surface Charge Influence. Acta Biomater. 2016, 45, 357–366. [Google Scholar] [CrossRef]
- Pedrosa, C.R.; Arl, D.; Grysan, P.; Khan, I.; Durrieu, S.; Krishnamoorthy, S.; Durrieu, M.-C. Controlled Nanoscale Topographies for Osteogenic Differentiation of Mesenchymal Stem Cells. ACS Appl. Mater. Interfaces 2019, 11, 8858–8866. [Google Scholar] [CrossRef]
- Feng, C.; Ma, B.; Xu, M.; Zhai, D.; Liu, Y.; Xue, J.; Chang, J.; Wu, C. Three-Dimensional Printing of Scaffolds with Synergistic Effects of Micro–Nano Surfaces and Hollow Channels for Bone Regeneration. ACS Biomater. Sci. Eng. 2020, 7, 872–880. [Google Scholar] [CrossRef]
- Vining, K.H.; Mooney, D.J. Mechanical Forces Direct Stem Cell Behaviour in Development and Regeneration. Nat. Rev. Mol. Cell Biol. 2017, 18, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Martino, F.; Perestrelo, A.R.; Vinarský, V.; Pagliari, S.; Forte, G. Cellular Mechanotransduction: From Tension to Function. Front. Physiol. 2018, 9, 824. [Google Scholar] [CrossRef]
- Kanchanawong, P.; Calderwood, D.A. Organization, Dynamics and Mechanoregulation of Integrin-Mediated Cell–ECM Adhesions. Nat. Rev. Mol. Cell Biol. 2023, 24, 142–161. [Google Scholar] [CrossRef] [PubMed]
- Kanoldt, V.; Kluger, C.; Barz, C.; Schweizer, A.L.; Ramanujam, D.; Windgasse, L.; Engelhardt, S.; Chrostek-Grashoff, A.; Grashoff, C. Metavinculin modulates force transduction in cell adhesion sites. Nat. Commun. 2020, 17, 6403. [Google Scholar] [CrossRef]
- Labouesse, C.; Tan, B.X.; Agley, C.C.; Hofer, M.; Winkel, A.K.; Stirparo, G.G.; Stuart, H.T.; Verstreken, C.M.; Mulas, C.; Mansfield, W.; et al. StemBond hydrogels control the mechanical microenvironment for pluripotent stem cells. Nat. Commun. 2021, 12, 6132. [Google Scholar] [CrossRef]
- Gegenfurtner, F.A.; Jahn, B.; Wagner, H.; Ziegenhain, C.; Enard, W.; Geistlinger, L.; Rädler, J.O.; Vollmar, A.M.; Zahler, S. Micropatterning as a tool to identify regulatory triggers and kinetics of actin-mediated endothelial mechanosensing. J. Cell Sci. 2018, 29, jcs212886. [Google Scholar] [CrossRef]
- Strzelecka-Kiliszek, A.; Romiszewska, M.; Bozycki, L.; Mebarek, S.; Bandorowicz-Pikula, J.; Buchet, R.; Pikula, S. Src and ROCK Kinases Differentially Regulate Mineralization of Human Osteosarcoma Saos-2 Cells. Int. J. Mol. Sci. 2019, 20, 2872. [Google Scholar] [CrossRef] [PubMed]
- Zhukova, Y.; Hiepen, C.; Knaus, P.; Osterland, M.; Prohaska, S.; Dunlop, J.W.C.; Fratzl, P.; Skorb, E.V. The Role of Titanium Surface Nanostructuring on Preosteoblast Morphology, Adhesion, and Migration. Adv. Healthc. Mater. 2017, 6, 1601244. [Google Scholar] [CrossRef]
- Lee, L.C.Y.; Gadegaard, N.; de Andrés, M.C.; Turner, L.A.; Burgess, K.V.; Yarwood, S.J.; Wells, J.; Salmeron-Sanchez, M.; Meek, D.; Oreffo, R.O.C.; et al. Nanotopography Controls Cell Cycle Changes Involved with Skeletal Stem Cell Self-Renewal and Multipotency. Biomaterials 2017, 116, 10–20. [Google Scholar] [CrossRef]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials Design for Bone-Tissue Engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium Phosphates in Biomedical Applications: Materials for the Future? Mater. Today 2016, 19, 69–87. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, C.; Wang, X.; Shi, M.; Zhu, Y.; Jing, L.; Wu, C.; Chang, J. Stimulation of Osteogenesis and Angiogenesis by Micro/Nano Hierarchical Hydroxyapatite: Via Macrophage Immunomodulation. Nanoscale 2019, 11, 17699–17708. [Google Scholar] [CrossRef]
- Zhao, C.; Xia, L.; Zhai, D.; Zhang, N.; Liu, J.; Fang, B.; Chang, J.; Lin, K. Designing Ordered Micropatterned Hydroxyapatite Bioceramics to Promote the Growth and Osteogenic Differentiation of Bone Marrow Stromal Cells. J. Mater. Chem. B 2015, 3, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Han, W.; He, W.; Li, J.; Wang, J.; Feng, H.; Qian, Y. Surface Topography of Hydroxyapatite Promotes Osteogenic Differentiation of Human Bone Marrow Mesenchymal Stem Cells. Mater. Sci. Eng. C 2016, 60, 45–53. [Google Scholar] [CrossRef]
- Nasiri, N.; Mukherjee, S.; Panneerselvan, A.; Nisbet, D.R.; Tricoli, A. Optimally Hierarchical Nanostructured Hydroxyapatite Coatings for Superior Prosthesis Biointegration. ACS Appl. Mater. Interfaces 2018, 10, 24840–24849. [Google Scholar] [CrossRef] [PubMed]
- Nada, H. Difference in the Conformation and Dynamics of Aspartic Acid on the Flat Regions, Step Edges, and Kinks of a Calcite Surface: A Molecular Dynamics Study. J. Phys. Chem. C 2014, 118, 14335–14345. [Google Scholar] [CrossRef]
- Liao, C.; Zhou, J. Replica-Exchange Molecular Dynamics Simulation of Basic Fibroblast Growth Factor Adsorption on Hydroxyapatite. J. Phys. Chem. B 2014, 118, 5843–5852. [Google Scholar] [CrossRef]
- Friddle, R.W.; Battle, K.; Trubetskoy, V.; Tao, J.; Salter, E.A.; Moradian-Oldak, J.; De Yoreo, J.J.; Wierzbicki, A. Single-Molecule Determination of the Face-Specific Adsorption of Amelogenin’s C-Terminus on Hydroxyapatite. Angew. Chem. 2011, 123, 7683–7687. [Google Scholar] [CrossRef]
- Makrodimitris, K.; Masica, D.L.; Kim, E.T.; Gray, J.J. Structure Prediction of Protein-Solid Surface Interactions Reveals a Molecular Recognition Motif of Statherin for Hydroxyapatite. J. Am. Chem. Soc. 2007, 129, 13713–13722. [Google Scholar] [CrossRef]
- Wu, C.; Chen, M.; Xing, C. Molecular Understanding of Conformational Dynamics of a Fibronectin Module on Rutile (110) Surface. Langmuir 2010, 26, 15972–15981. [Google Scholar] [CrossRef]
- Lin, K.; Xia, L.; Gan, J.; Zhang, Z.; Chen, H.; Jiang, X.; Chang, J. Tailoring the Nanostructured Surfaces of Hydroxyapatite Bioceramics to Promote Protein Adsorption, Osteoblast Growth, and Osteogenic Differentiation. ACS Appl. Mater. Interfaces 2013, 5, 8008–8017. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Xie, Y.; Zhou, J. Computer Simulations of Fibronectin Adsorption on Hydroxyapatite Surfaces. RSC Adv. 2014, 4, 15759–15769. [Google Scholar] [CrossRef]
- De Yoreo, J.J.; Gilbert, P.U.P.A.; Sommerdijk, N.A.J.M.; Penn, R.L.; Whitelam, S.; Joester, D.; Zhang, H.; Rimer, J.D.; Navrotsky, A.; Banfield, J.F.; et al. Crystallization by Particle Attachment in Synthetic, Biogenic, and Geologic Environments. Science 2015, 349, aaa6760. [Google Scholar] [CrossRef] [PubMed]
- Dandekar, P.; Kuvadia, Z.B.; Doherty, M.F. Engineering Crystal Morphology. Annu. Rev. Mater. Res. 2013, 43, 359–386. [Google Scholar] [CrossRef]
- Chen, J.; Lei, B.; Xie, H.; Zhou, L.; Wang, S. Easy Way to Create Stepped Surface: A Thought from Oriented Attachment. Chem. Mater. 2017, 29, 7653–7657. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Z.; Wang, F.; Gong, M.; Zhang, X.; Wang, Y.; Hu, Z.; Zeng, Z.; Wang, Y. The Restricted Adhesion of Bone Marrow Mesenchymal Stem Cells by Stepped Structures on Surfaces of Hydroxyapatite. RSC Adv. 2022, 12, 12002–12010. [Google Scholar] [CrossRef]
- Ohka, T.; Akiyama, T.; Pradipto, A.M.; Nakamura, K.; Ito, T. Effect of Step Edges on Adsorption Behavior for GaN(0001) Surfaces during Metalorganic Vapor Phase Epitaxy: An Ab Initio Study. Cryst. Growth Des. 2020, 20, 4358–4365. [Google Scholar] [CrossRef]
- Yin, X.; Geng, D.; Wang, X. Inverted Wedding Cake Growth Operated by the Ehrlich-Schwoebel Barrier in Two-Dimensional Nanocrystal Evolution. Angew. Chem. Int. Ed. 2016, 55, 2217–2221. [Google Scholar] [CrossRef]
- Morin, S.A.; Forticaux, A.; Bierman, M.J.; Jin, S. Screw Dislocation-Driven Growth of Two-Dimensional Nanoplates. Nano Lett. 2011, 11, 4449–4455. [Google Scholar] [CrossRef]
- Yin, X.; Shi, J.; Niu, X.; Huang, H.; Wang, X. Wedding Cake Growth Mechanism in One-Dimensional and Two-Dimensional Nanostructure Evolution. Nano Lett. 2015, 15, 7766–7772. [Google Scholar] [CrossRef]
- Dai, J.; Lu, J.; Wang, F.; Guo, J.; Gu, N.; Xu, C. Optical and Exciton Dynamical Properties of a Screw-Dislocation-Driven ZnO:Sn Microstructure. ACS Appl. Mater. Interfaces 2015, 7, 12655–12662. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Liu, X.; Chen, L.; Lin, K.; Chang, J. Dental Enamel-like Hydroxyapatite Transformed Directly from Monetite. J. Mater. Chem. 2012, 22, 22637–22641. [Google Scholar] [CrossRef]
- Chen, J.; Li, X.; Lei, B.; Zhou, L.; Wang, S. Visible Light Photocatalysis of Pristine Anatase TiO2 Mesocrystals Induced by Largely Exposed and Stepped {001} Surface. Green. Chem. 2019, 21, 483–490. [Google Scholar] [CrossRef]
- Surmeneva, M.A.; Surmenev, R.A.; Tyurin, A.I.; Mukhametkaliyev, T.M.; Teresov, A.D.; Koval, N.N.; Pirozhkova, T.S.; Shuvarin, I.A.; Oehr, C. Comparative Study of the Radio-Frequency Magnetron Sputter Deposited CaP Films Fabricated onto Acid-Etched or Pulsed Electron Beam-Treated Titanium. Thin Solid. Film. 2014, 571, 218–224. [Google Scholar] [CrossRef]
- Saroj, S.; Vijayalakshmi, U. Structural, morphological and biological assessment of magnetic hydroxyapatite with superior hyperthermia potential for orthopedic applications. Sci. Rep. 2025, 15, 3234. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, M.; Jiang, F.; Yin, S.; Lin, S.; Yang, G.; Lu, Y.; Zhang, W.; Jiang, X. Marginal Sealing around Integral Bilayer Scaffolds for Repairing Osteochondral Defects Based on Photocurable Silk Hydrogels. Bioact. Mater. 2021, 6, 3976–3986. [Google Scholar] [CrossRef]
- Uynuk-Ool, T.; Rothdiener, M.; Walters, B.; Hegemann, M.; Palm, J.; Nguyen, P.; Seeger, T.; Stöckle, U.; Stegemann, J.P.; Aicher, W.K.; et al. The Geometrical Shape of Mesenchymal Stromal Cells Measured by Quantitative Shape Descriptors Is Determined by the Stiffness of the Biomaterial and by Cyclic Tensile Forces. J. Tissue Eng. Regen. Med. 2017, 11, 3508–3522. [Google Scholar] [CrossRef]
- Amann, E.; Amirall, A.; Franco, A.R.; Poh, P.S.P.; Sola Dueñas, F.J.; Fuentes Estévez, G.; Leonor, I.B.; Reis, R.L.; van Griensven, M.; Balmayor, E.R. A Graded, Porous Composite of Natural Biopolymers and Octacalcium Phosphate Guides Osteochondral Differentiation of Stem Cells. Adv. Healthc. Mater. 2021, 10, 2001692. [Google Scholar] [CrossRef]
- Chen, Y.; Zhai, M.J.; Mehwish, N.; Xu, M.D.; Wang, Y.; Gong, Y.X.; Ren, M.M.; Deng, H.; Lee, B.H. Comparison of Globular Albumin Methacryloyl and Random-Coil Gelatin Methacryloyl: Preparation, Hydrogel Properties, Cell Behaviors, and Mineralization. Int. J. Biol. Macromol. 2022, 204, 692–708. [Google Scholar] [CrossRef]
- Özkale, B.; Sakar, M.S.; Mooney, D.J. Active Biomaterials for Mechanobiology. Biomaterials 2021, 267, 120497. [Google Scholar] [CrossRef]
- Chandorkar, Y.; Castro Nava, A.; Schweizerhof, S.; Van Dongen, M.; Haraszti, T.; Köhler, J.; Zhang, H.; Windoffer, R.; Mourran, A.; Möller, M.; et al. Cellular Responses to Beating Hydrogels to Investigate Mechanotransduction. Nat. Commun. 2019, 10, 4027. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, L.; Xu, H.; Du, Q.; Li, L.; Wang, L.; Zhang, E.S.; Chen, G.; Wang, Y. Heterogeneous Responses to Mechanical Force of Prostate Cancer Cells Inducing Different Metastasis Patterns. Adv. Sci. 2020, 7, 1903583. [Google Scholar] [CrossRef] [PubMed]
- Kalukula, Y.; Stephens, A.D.; Lammerding, J.; Gabriele, S. Mechanics and Functional Consequences of Nuclear Deformations. Nat. Rev. Mol. Cell Biol. 2022, 23, 583–602. [Google Scholar] [CrossRef] [PubMed]
- Hyväri, L.; Vanhatupa, S.; Halonen, H.T.; Kääriäinen, M.; Miettinen, S. Myocardin-Related Transcription Factor A (MRTF-A) Regulates the Balance between Adipogenesis and Osteogenesis of Human Adipose Stem Cells. Stem Cells Int. 2020, 2020, 8853541. [Google Scholar] [CrossRef]
- Ferreira, S.A.; Faull, P.A.; Seymour, A.J.; Yu, T.T.L.; Loaiza, S.; Auner, H.W.; Snijders, A.P.; Gentleman, E. Neighboring Cells Override 3D Hydrogel Matrix Cues to Drive Human MSC Quiescence. Biomaterials 2018, 176, 13–23. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, X.; Gao, L.; Jing, L.; Zhou, Q.; Chang, J. The Role of the Micro-Pattern and Nano-Topography of Hydroxyapatite Bioceramics on Stimulating Osteogenic Differentiation of Mesenchymal Stem Cells. Acta Biomater. 2018, 73, 509–521. [Google Scholar] [CrossRef]
- Chen, Z.; Bachhuka, A.; Wei, F.; Wang, X.; Liu, G.; Vasilev, K.; Xiao, Y. Nanotopography-Based Strategy for the Precise Manipulation of Osteoimmunomodulation in Bone Regeneration. Nanoscale 2017, 9, 18129–18152. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, F.; Gong, M.; Chen, L.; Wang, Y.; Xu, P.; Zeng, Z.; Hu, Z.; Chen, J. Engineering Stepped Structures on Hydroxyapatite Surfaces: A Potential Strategy to Modulate Bone Marrow Mesenchymal Stem Adhesion, Spreading, and Proliferation. J. Funct. Biomater. 2025, 16, 165. https://doi.org/10.3390/jfb16050165
Wang Y, Wang F, Gong M, Chen L, Wang Y, Xu P, Zeng Z, Hu Z, Chen J. Engineering Stepped Structures on Hydroxyapatite Surfaces: A Potential Strategy to Modulate Bone Marrow Mesenchymal Stem Adhesion, Spreading, and Proliferation. Journal of Functional Biomaterials. 2025; 16(5):165. https://doi.org/10.3390/jfb16050165
Chicago/Turabian StyleWang, Yongmei, Fang Wang, Min Gong, Lidan Chen, Yun Wang, Pu Xu, Zhu Zeng, Zuquan Hu, and Jin Chen. 2025. "Engineering Stepped Structures on Hydroxyapatite Surfaces: A Potential Strategy to Modulate Bone Marrow Mesenchymal Stem Adhesion, Spreading, and Proliferation" Journal of Functional Biomaterials 16, no. 5: 165. https://doi.org/10.3390/jfb16050165
APA StyleWang, Y., Wang, F., Gong, M., Chen, L., Wang, Y., Xu, P., Zeng, Z., Hu, Z., & Chen, J. (2025). Engineering Stepped Structures on Hydroxyapatite Surfaces: A Potential Strategy to Modulate Bone Marrow Mesenchymal Stem Adhesion, Spreading, and Proliferation. Journal of Functional Biomaterials, 16(5), 165. https://doi.org/10.3390/jfb16050165






