Electrosprayed Chitin Nanofibril/Electrospun Polyhydroxyalkanoate Fiber Mesh as Functional Nonwoven for Skin Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. P(3HO-co-3HD) and P(3HB) Production
2.3. Preparation of CN and CLA Solutions and Electrospraying Protocols
2.4. PHB/PHOHD Solution Preparation
2.5. Production of PHB/PHOHD Films and Fiber Meshes
2.6. Electrospray of CN and CLA on PHB/PHOHD Film and Fiber Mesh
2.7. Morphological Characterization
2.8. Chemical Structure Characterization
2.9. Evaluation of HaCaT Cell Line Viability
2.10. Anti-Inflammatory and Immune Responses Evaluation of HaCaT Cells
3. Results
3.1. Morphological Characterization
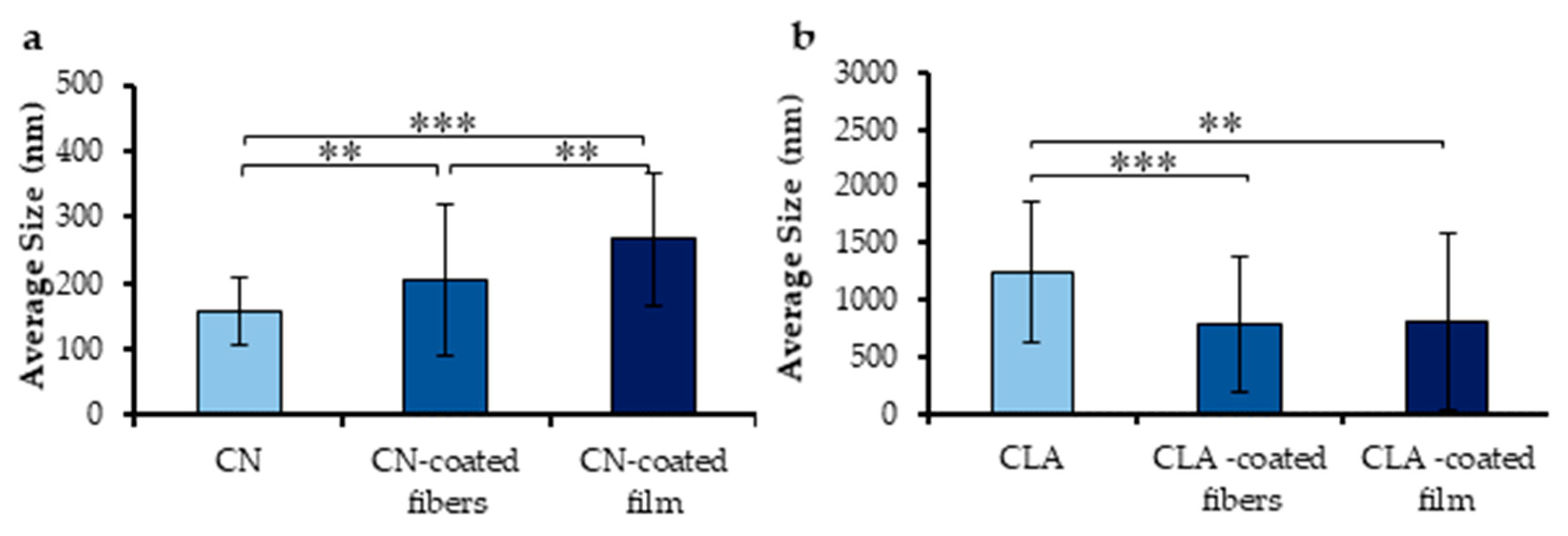
3.1.1. Morphological Characterization of Electrosprayed CN and CLA
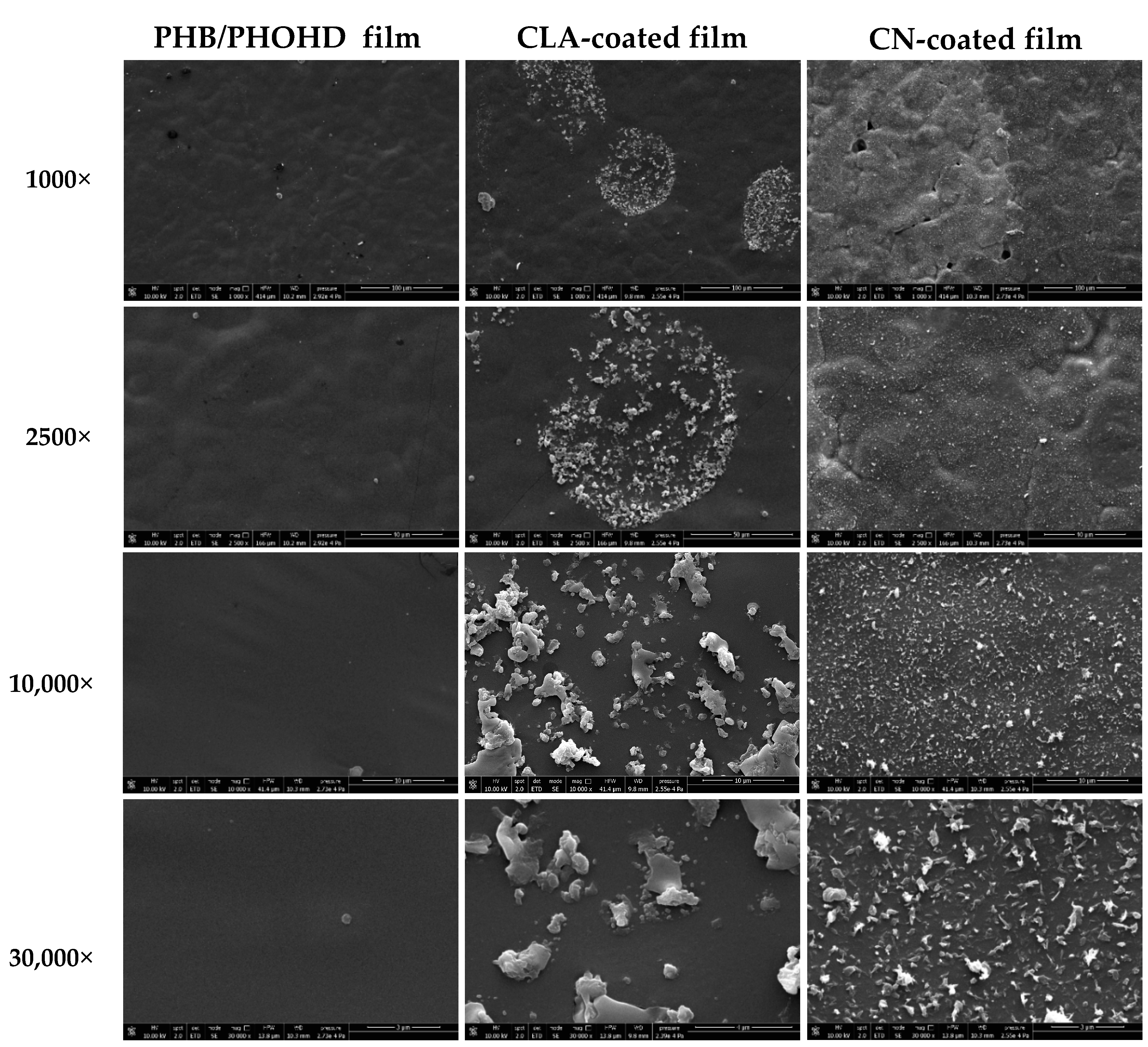
3.1.2. Morphological Analysis of Functionalized PHB/PHOHD Films
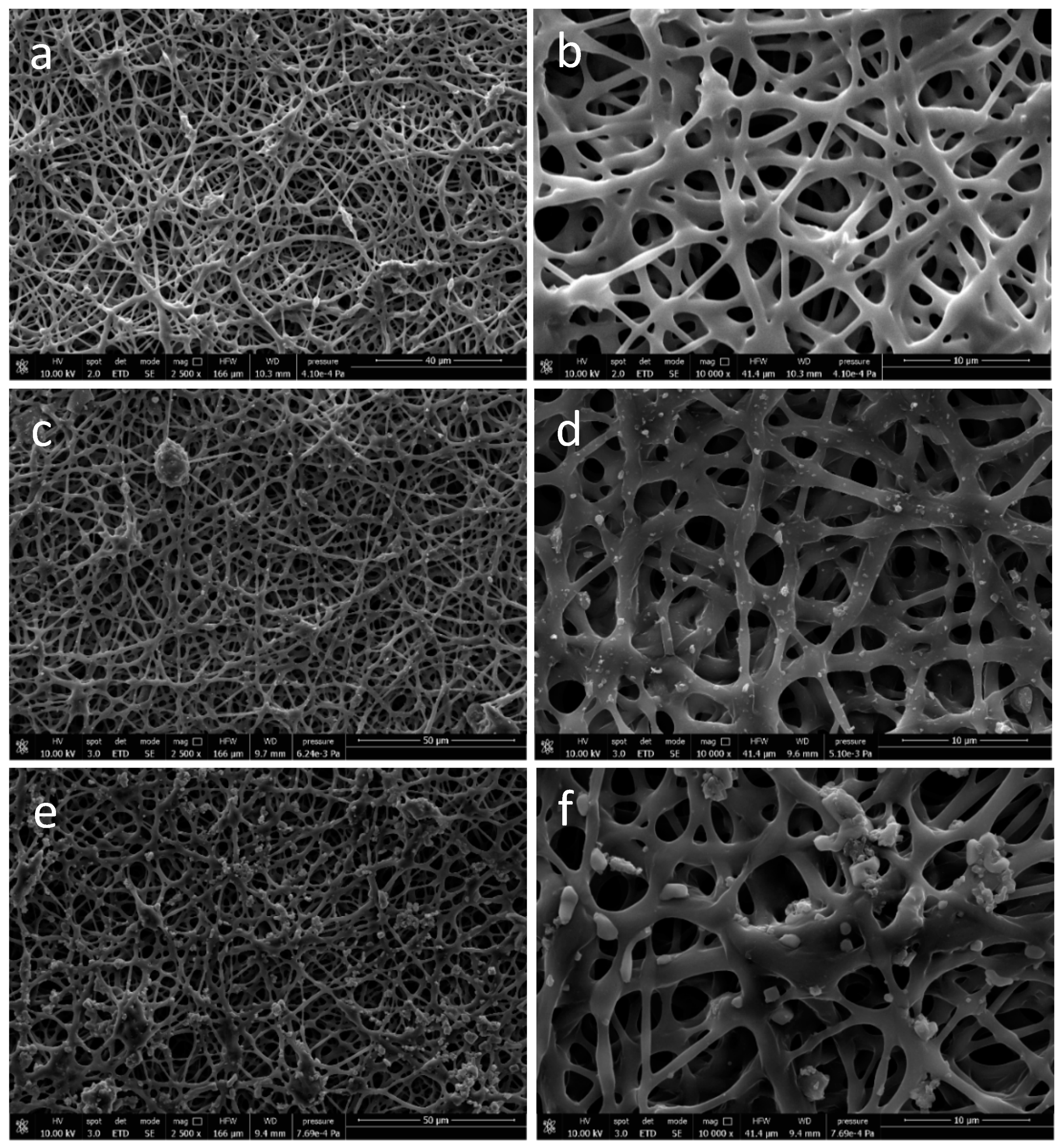
3.1.3. Morphological Analysis of Functionalized PHB/PHOHD-Electrospun Fiber Meshes
3.2. Chemical Characterization
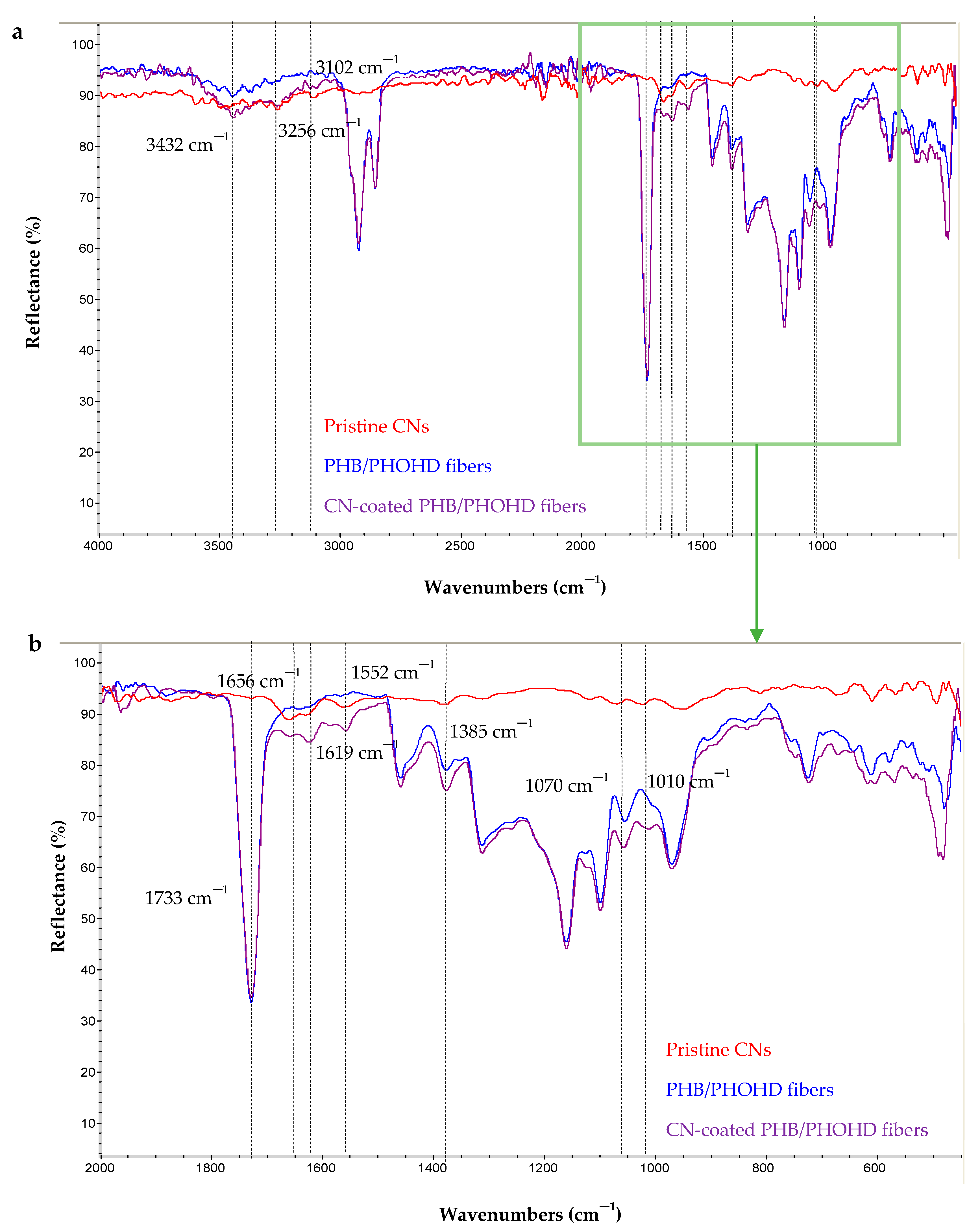
3.2.1. Chemical Characterization of (PHB/PHOHD)-Electrospun Fiber Mesh and Film
3.2.2. Chemical Characterization of CN-Coated PHB/PHOHD Fibers
3.2.3. Chemical Characterization of CLA-Coated PHB/PHOHD Fibres
3.3. HaCaT Cell Metabolic Activity
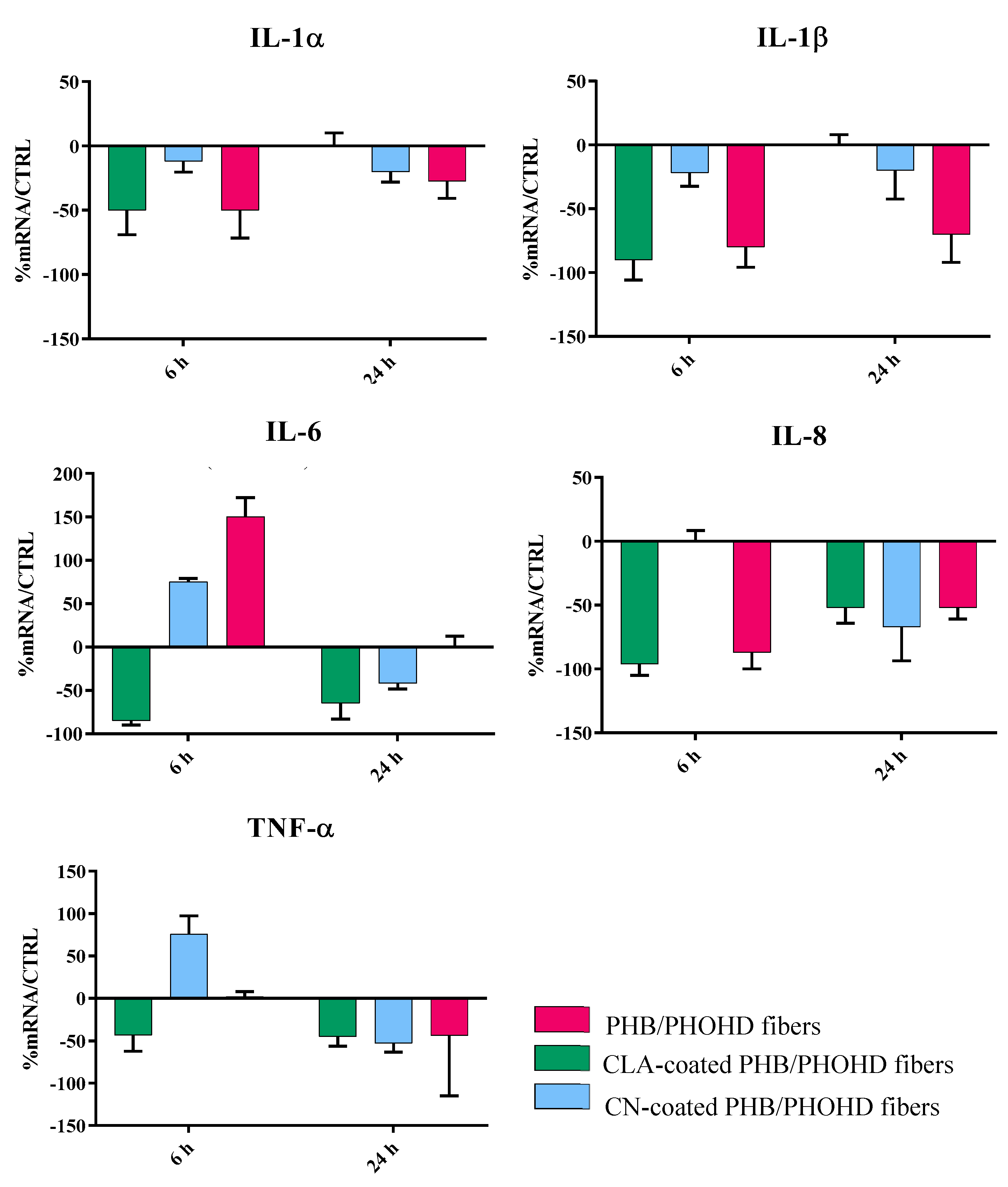
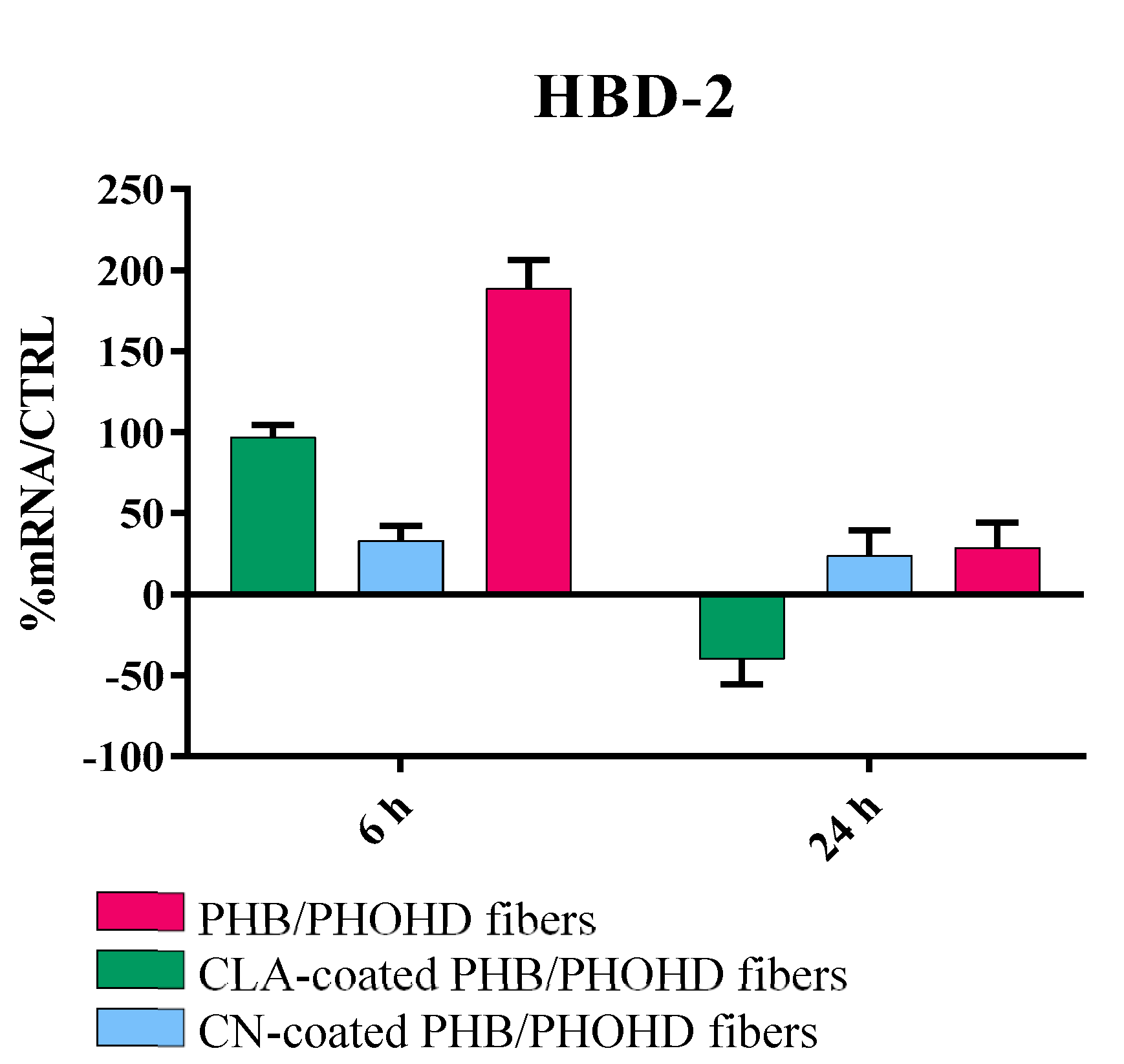
3.4. Immunomodulatory Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CLA | Chitin nanofibril/nanolignin-glycyrrhetinic acid |
| CN | Chitin nanofibril |
| DMEM | Dulbecco’s modified essential medium |
| DPBS | Dulbecco’s phosphate-buffered saline |
| EtOH | Ethanol |
| GA | Glycyrrhizin acid |
| HBD-2 | Human beta-defensin 2 |
| IL | Interleukin |
| NL | Nanolignin |
| P(3HB) | Poly(3-hydroxybutyrate) |
| P(3HO-co-3HD) | Poly(3-h ydroxyoctanoate-co-3-hydroxydecanoate) |
| PEG | Poly(ethylene glycol) |
| PHAs | Polyhydroxyalkanoates |
| TGF-β | Transforming growth factor beta |
| TNF-α | Tumor necrosis factor alpha |
References
- Ying, T.; Ishii, D.; Mahara Murakami, S.; Yamaok, T.; Kumar, S.; Samian, R.; Fujita, M.; Maeda, M.; Iwata, T. Scaffolds from electrospun polyhydroxyalkanoate copolymers: Fabrication, characterization, bioabsorption and tissue response. Biomaterials 2008, 29, 1307–1317. [Google Scholar] [CrossRef] [PubMed]
- Sombatmankhong, K.; Suwantong, O.; Waleetorncheepsawat, S.; Supaphol, P. Electrospun fiber mats of poly(3-hydroxybutyrate), poly(3-hydroxybutyrate-co-3-hydroxyvalerate), and their blends. J. Polym. Sci. Polym. Phys. 2006, 44, 2923–2933. [Google Scholar] [CrossRef]
- Dinjaski, N.; Fernández-Gutiérrez, M.; Selvam, S.; Parra-Ruiz, F.J.; Lehman, S.M.; San Román, J.; García, E.; García, J.L.; García, A.J.; Prieto, M.A. PHACOS, a functionalized bacterial polyester with bactericidal activity against methicillin-resistant Staphylococcus aureus. Biomaterials 2014, 35, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Coltelli, M.B.; Panariello, L.; Morganti, P.; Danti, S.; Baroni, A.; Lazzeri, A.; Fusco, A.; Donnarumma, G. Skin-compatible biobased beauty masks prepared by extrusion. J. Funct. Biomater 2020, 11, 23. [Google Scholar] [CrossRef]
- Coltelli, M.B.; Danti, S.; Trombi, L.; Morganti, P.; Donnarumma, G.; Baroni, A.; Fusco, A.; Lazzeri, A. Preparation of innovative skin compatible films to release polysaccharides for biobased beauty masks. Cosmetics 2020, 5, 70. [Google Scholar] [CrossRef]
- Piarali, S.; Marlinghaus, L.; Viebahn, R.; Lewis, H.; Ryadnov, M.J.; Groll, J.; Salber, J.; Roy, I. Activated Polyhydroxyalkanoate Meshes Prevent Bacterial Adhesion and Biofilm Development in Regenerative Medicine Applications. Front. Bioeng. Biotech. 2020, 8, 442. [Google Scholar] [CrossRef]
- Basnett, P.; Marcello, E.; Lukasiewicz, B.; Nigmatullin, R.; Paxinou, A.; Haseeb Ahmad, M.; Gurumayum, B.; Roy, I. Antimicrobial Materials with Lime Oil and a Poly(3-hydroxyalkanoate) Produced via Valorisation of Sugar Cane Molasses. J. Funct. Biomater. 2020, 11, 24. [Google Scholar] [CrossRef]
- Ramos Avilez, H.; Castilla Casadiego, D.; Vega Avila, A.; Perales Perez, O.; Almodovar, J. Production of chitosan coatings on metal and ceramic biomaterials. Chitosan Based Biomater. 2017, 1, 255–293. [Google Scholar] [CrossRef]
- Azimi, B.; Millazo, M.; Lazzeri, A.; Berrettini, S.; Uddin, Z.J.; Qin, M.; Buehler, M.J.; Danti, S. Electrospinning Piezoelectric Fibers for Biocompatible Devices. Adv. Healthcare Mater. 2020, 9, 1901287. [Google Scholar] [CrossRef]
- Dietrich, K.; Dumont, M.; Del Rio, L.; Orsat, V. Producing PHAs in the bioeconomy—Towards a sustainable bioplastic. SPAC 2017, 9, 58–70. [Google Scholar] [CrossRef]
- Kavadiya, S.; Biswas, P. Electrospray deposition of biomolecules: Applications, challenges, and recommendations. J. Aerosol Sci. 2018, 125, 182–207. [Google Scholar] [CrossRef]
- Morganti, P.; Morganti, G. Chitin nanofibrils for advanced cosmeceuticals. Clin. Dermatol. 2008, 26, 334–340. [Google Scholar] [CrossRef]
- Kowalska, A.; Kalinowska-Lis, U. Beta-Glycyrrhetinic acid: Its core biological properties and dermatological applications. Int. J. Cosmet. Sci. 2019, 41, 325–331. [Google Scholar]
- Danti, S.; Trombi, L.; Fusco, A.; Azimi, B.; Lazzeri, A.; Morganti, P.; Coltelli, M.; Donnarumma, G. Chitin Nanofibrils and Nanolignin as Functional Agents in Skin Regeneration. Int. J. Mol. Sci. 2019, 20, 2669. [Google Scholar] [CrossRef]
- Obisesan, K.A.; Neri, S.; Bugnicourt, E.; Campos, I.; Rodriguez-Turienzo, L. Determination and Quantification of the Distribution of CN-NL Nanoparticles Encapsulating Glycyrrhetic Acid on Novel Textile Surfaces with Hyperspectral Imaging. J. Funct. Biomater. 2020, 11, 32. [Google Scholar] [CrossRef]
- Morganti, P.; Muzzarelli, C. Spray-Dried Chitin Nanofibrils, Method for Production and Uses. Thereof. Patent WO2007060628, 5 May 2007. [Google Scholar]
- Morganti, P. Method of Preparation of Chitin and Active Principles Complexes and the so Obtained. Complexes. Patent WO2012143875, 26 October 2012. [Google Scholar]
- Boeriu, C.G.; Bravo, D.; Gosselink, R.J.A.; Van Dam, J.E.G. Characterisation of structure-dependent functional properties of lignin with infrared spectroscopy. Ind. Crops Prod. 2004, 20, 205–218. [Google Scholar] [CrossRef]
- Danti, S.; D’Alessandro, D.; Mota, C.; Bruschini, L.; Berrettini, S. Applications of bioresorbable polymers in skin and eardrum. In Bioresorbable Polymers for Biomedical Applications: From Fundamentals to Translational Medicine; Perale, G., Hilborn, J., Eds.; Woodhead Publishing, Elsevier: Cambridge, UK, 2017; pp. 423–444. [Google Scholar]
- Lebeaux, D.; Ghigo, J.M.; Beloin, C. Biofilm-related infections: Bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol. Mol. Biol. Rev. 2014, 78, 510–543. [Google Scholar] [CrossRef]
- Li, Z.; Yang, J.; Loh, X.J. Polyhydroxyalkanoates: Opening doors for a sustainable future. NPG Asia Mater. 2016, 8, e265. [Google Scholar] [CrossRef]
- Reis, R.L.; Neves, N.M.; Mano, J.F.; Gomes, M.E.; Marques, A.P.; Azevedo, H.S. Natural-Based Polymers for Biomedical Applications; Elsevier: Amsterdam, The Netherland, 2008. [Google Scholar]
- Roy, I.; Visakh, P.M. (Eds.) Polyhydroxyalkanoate (PHA) Based Blends, Composites and Nanocomposites; Royal Society of Chemistry: London, UK, 2014. [Google Scholar]
- Ding, J.; Zhang, J.; Li, J.; Li, D.; Xiao, C.; Xiao, H.; Yang, H.; Zhuang, X.; Chen, X. Electrospun polymer biomaterials. Prog. Polym. Sci. 2019, 90, 1–34. [Google Scholar] [CrossRef]
- Danti, S.; Azimi, B.; Candito, M.; Fusco, A.; Sorayani Bafqi, M.S.; Ricci, C.; Milazzo, M.; Cristallini, C.; Latifi, M.; Donnarumma, G.; et al. Lithium niobate nanoparticles as biofunctional interface material for inner ear devices. Biointerphases 2020, 15, 031004. [Google Scholar] [CrossRef]
- Repanas, A.; Andriopoulou, S.; Glasmacher, B. The significance of electrospinning as a method to create fibrous scaffolds for biomedical engineering and drug delivery applications. J. Drug Deliv. Sci. Technol. 2016, 31, 137–146. [Google Scholar] [CrossRef]
- Salvatore, L.; Carofiglio, V.E.; Stufano, P.; Bonfrate, V.; Calò, E.; Scarlino, S.; Nitti, P.; Centrone, D.; Cascione, M.; Leporatti, S.; et al. Potential of Electrospun Poly(3-hydroxybutyrate)/Collagen Blends for Tissue Engineering Applications. J. Healthcare Eng. 2018, 6573947. [Google Scholar] [CrossRef]
- Coltelli, M.-B.; Aliotta, L.; Vannozzi, A.; Morganti, P.; Panariello, L.; Danti, S.; Neri, S.; Fernandez-Avila, C.; Fusco, A.; Donnarumma, G.; et al. Properties and Skin Compatibility of Films Based on Poly(Lactic Acid) (PLA) Bionanocomposites Incorporating Chitin Nanofibrils (CN). J. Funct. Biomater. 2020, 11, 21. [Google Scholar] [CrossRef]
- Mirjalili, M.; Zohoori, S. Review for application of electrospinning and electrospun nanofibers technology in textile industry. J. Nanostructure Chem. 2016, 6, 207–213. [Google Scholar] [CrossRef]
- Merrill, M.H.; Pogue, W.R.; Baucom, J.N. Electrospray Ionization of Polymers: Evaporation, Drop Fission, and Deposited Particle Morphology. ASME. J. Micro Nano-Manuf. 2015, 3, 011003. [Google Scholar] [CrossRef]
- Zamani, M.; Prabhakaran, M.P.; Ramakrishna, S. Advances in drug delivery via electrospun and electrosprayed nanomaterials. Int. J. Nanomed. 2013, 8, 2997–3017. [Google Scholar] [CrossRef]
- Morganti, P.; Palombo, M.; Tishchenko, G.; Yudin, V.E.; Guarneri, F.; Cardillo, M.; Del Ciotto, P.; Carezzi, F.; Morganti, G.; Fabrizi, G. Chitin-hyaluronan nanoparticles: A multifunctional carrier to deliver anti-aging active ingredients through the skin. Cosmetics 2014, 1, 140–158. [Google Scholar] [CrossRef]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef]
- Dinarello, C.A. Interleukin-1. Cytokine Growth Factor Rev. 1997, 8, 253–265. [Google Scholar] [CrossRef]
- Koch, A.E.; Polverini, P.J.; Kunkel, S.L.; Harlow, L.A.; DiPietro, L.A.; Elner, V.M.; Elner, S.G.; Strieter, R.M. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science 1992, 258, 1798–1801. [Google Scholar] [CrossRef]
- Esposito, E.; Cuzzocrea, S. TNF-alpha as a therapeutic target in inflammatory diseases, ischemia-reperfusion injury and trauma. Curr. Med. Chem. 2009, 16, 3152–3167. [Google Scholar] [CrossRef]
- Donnarumma, G.; Paoletti, I.; Fusco, A.; Perfetto, B.; Buommino, E.; de Gregorio, V.; Baroni, A. β-Defensins: Work in Progress. Adv. Exp. Med. Biol. 2016, 901, 59–76. [Google Scholar] [CrossRef]
- Nunan, R.; Harding, K.G.; Martin, P. Clinical challenges of chronic wounds: Searching for an optimal animal model to recapitulate their complexity. Dis. Models Mech. 2014, 7, 1205–1213. [Google Scholar] [CrossRef]
- Zhang, X.; Shu, W.; Yu, Q.; Qu, W.; Wang, Y.; Li, R. Functional Biomaterials for Treatment of Chronic Wound. Front. Bioeng. Biotechnol. 2020, 8, 516. [Google Scholar] [CrossRef]
- Lazzeri, L.; Cascone, M.G.; Danti, S.; Serino, L.P.; Moscato, S.; Bernardini, N. Gelatine/PLLA sponge-like scaffolds: Morphological and biological characterization. J. Mater. Sci. Mater Med. 2007, 18, 1399–1405. [Google Scholar] [CrossRef]
- Milazzo, M.; Gallone, G.; Marcello, E.; Mariniello, M.D.; Bruschini, L.; Roy, I.; Danti, S. Biodegradable polymeric micro/nano-structures with intrinsic antifouling/antimicrobial properties: Relevance in damaged skin and other biomedical applications. J. Funct. Biomater. 2020, 11, 60. [Google Scholar] [CrossRef]



| Gene | Primers Sequence | Conditions | Product Size (bp) |
|---|---|---|---|
| IL-1α | 5′-CATGTCAAATTTCACTGCTTCATCC-3′ | 5 s at 95 °C, 8 s at 55 °C, 17 s at 72 °C for 45 cycles | 421 |
| 5′-GTCTCTGAATCAGAAATCCTTCTATC-3′ | |||
| IL-1β | 5′-GCATCCAGCTACGAATCTCC-3′ | 5 s at 95 °C, 14 s at 58 °C, 28 s at 72 °C for 40 cycles | 708 |
| 5′-CCACATTCAGCACAGGACTC-3′ | |||
| IL-6 | 5′-ATGAACTCCTTCTCCACAAGCGC-3′ | 5 s at 95 °C, 13 s at 56 °C, 25 at 72 °C for 40 cycles | 628 |
| 5′-GAAGAGCCCTCAGGCTGGACTG-3′ | |||
| IL-8 | 5′-ATGACTTCCAAGCTGGCCGTG-3′ | 5 s at 94 °C, 6 s at 55 °C, 12 s at 72 °C for 40 cycles | 297 |
| 5′-TGAATTCTCAGCCCTCTTCAAAAACTTCTC-3′ | |||
| TNF-α | 5′-CAGAGGGAAGAGTTCCCCAG-3′ | 5 s at 95 °C, 6 s at 57 °C, 13 s at 72 °C for 40 cycles | 324 |
| 5′-CCTTGGTCTGGTAGGAGACG-3′ | |||
| TGF-β | 5′-CCGACTACTACGCCAAGGAGGTCAC-3′ | 5 s at 94 °C, 9 s at 60 °C, 18 s at 72 °C for 40 cycles | 439 |
| 5′-AGGCCGGTTCATGCCATGAATGGTG-3′ | |||
| HBD-2 | 5′-GGATCCATGGGTATAGGCGATCCTGTTA-3′ | 5 s at 94 °C, 6 s at 63 °C, 10 s at 72 °C for 50 cycles | 198 |
| 5′-AAGCTTCTCTGATGAGGGAGCCCTTTCT-3′ |
| Sample | %ABRED |
|---|---|
| PHB/PHOHD fiber mesh | 76 |
| CLA-coated PHB/PHOHD fiber mesh | 64 |
| CN-coated PHB/PHOHD fiber mesh | 69 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azimi, B.; Thomas, L.; Fusco, A.; Kalaoglu-Altan, O.I.; Basnett, P.; Cinelli, P.; De Clerck, K.; Roy, I.; Donnarumma, G.; Coltelli, M.-B.; et al. Electrosprayed Chitin Nanofibril/Electrospun Polyhydroxyalkanoate Fiber Mesh as Functional Nonwoven for Skin Application. J. Funct. Biomater. 2020, 11, 62. https://doi.org/10.3390/jfb11030062
Azimi B, Thomas L, Fusco A, Kalaoglu-Altan OI, Basnett P, Cinelli P, De Clerck K, Roy I, Donnarumma G, Coltelli M-B, et al. Electrosprayed Chitin Nanofibril/Electrospun Polyhydroxyalkanoate Fiber Mesh as Functional Nonwoven for Skin Application. Journal of Functional Biomaterials. 2020; 11(3):62. https://doi.org/10.3390/jfb11030062
Chicago/Turabian StyleAzimi, Bahareh, Lily Thomas, Alessandra Fusco, Ozlem Ipek Kalaoglu-Altan, Pooja Basnett, Patrizia Cinelli, Karen De Clerck, Ipsita Roy, Giovanna Donnarumma, Maria-Beatrice Coltelli, and et al. 2020. "Electrosprayed Chitin Nanofibril/Electrospun Polyhydroxyalkanoate Fiber Mesh as Functional Nonwoven for Skin Application" Journal of Functional Biomaterials 11, no. 3: 62. https://doi.org/10.3390/jfb11030062
APA StyleAzimi, B., Thomas, L., Fusco, A., Kalaoglu-Altan, O. I., Basnett, P., Cinelli, P., De Clerck, K., Roy, I., Donnarumma, G., Coltelli, M.-B., Danti, S., & Lazzeri, A. (2020). Electrosprayed Chitin Nanofibril/Electrospun Polyhydroxyalkanoate Fiber Mesh as Functional Nonwoven for Skin Application. Journal of Functional Biomaterials, 11(3), 62. https://doi.org/10.3390/jfb11030062












