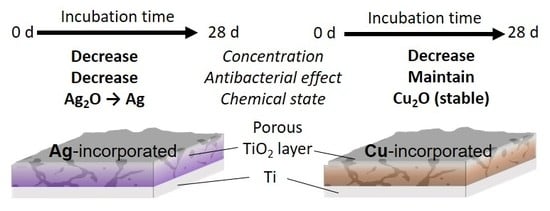
Time-Transient Effects of Silver and Copper in the Porous Titanium Dioxide Layer on Antibacterial Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
2.2. Surface Characterization
2.3. Evaluation of Antibacterial Activity
2.4. Statistical Analysis
3. Results
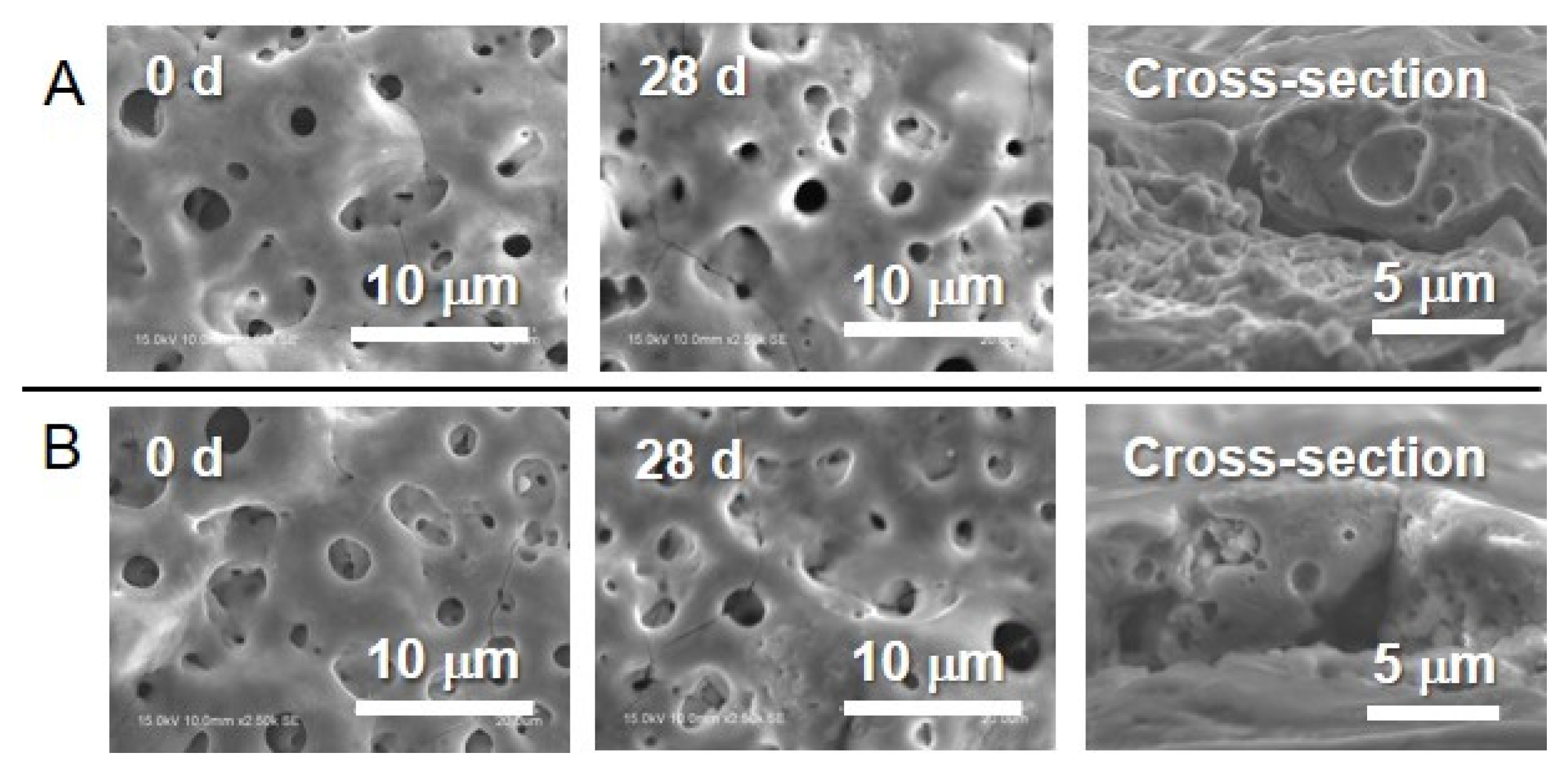
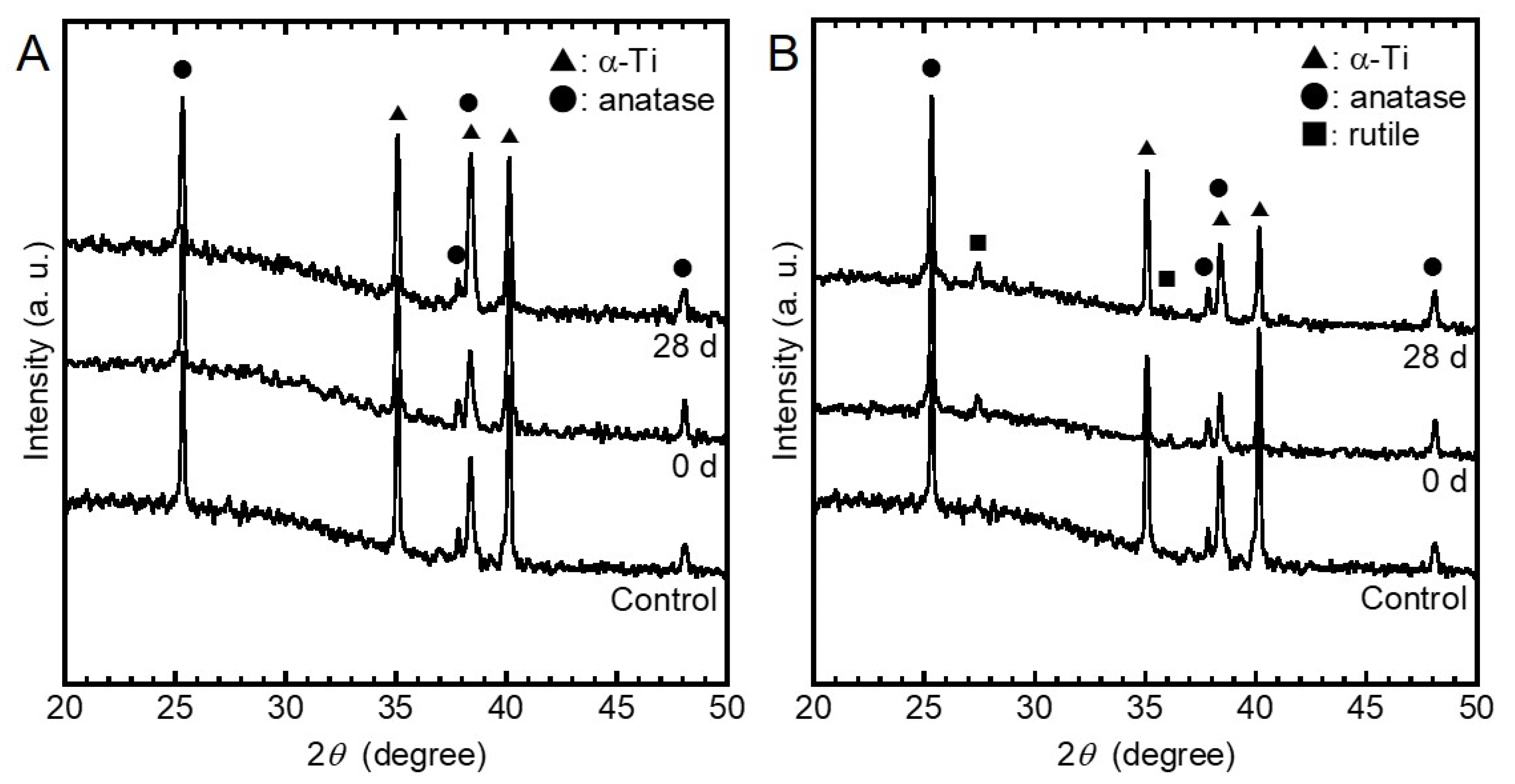
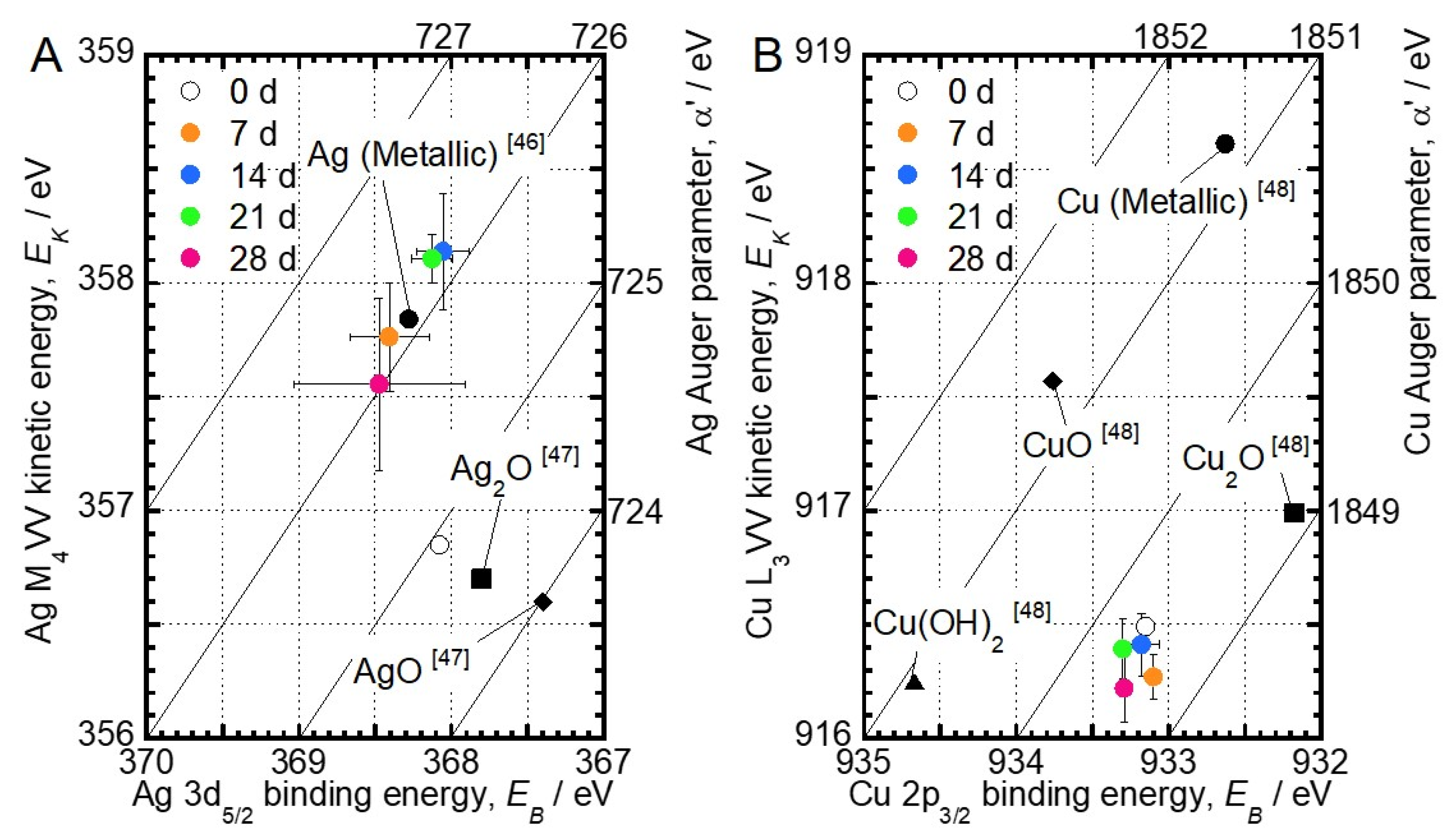
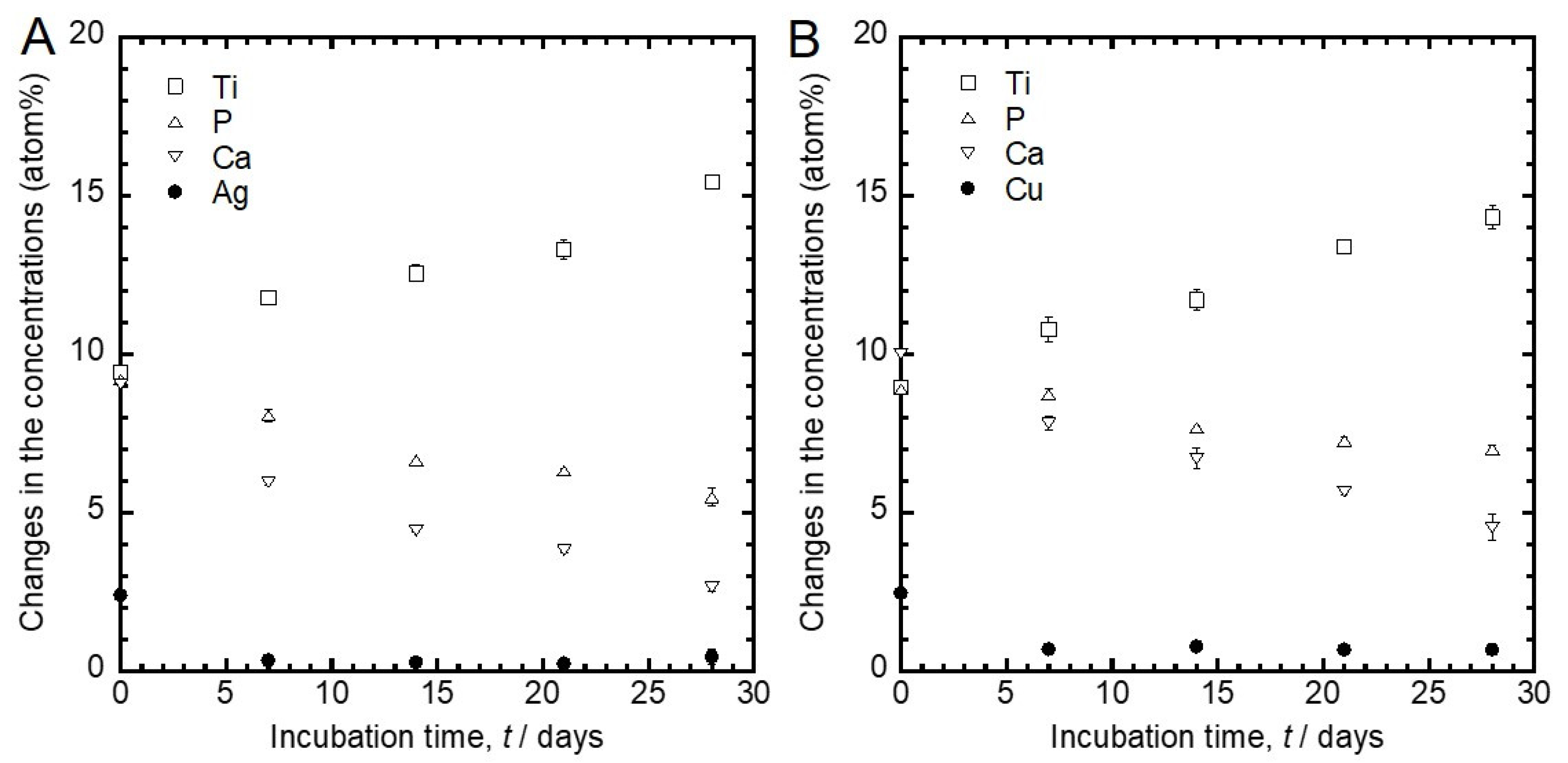
3.1. Surface Characterization
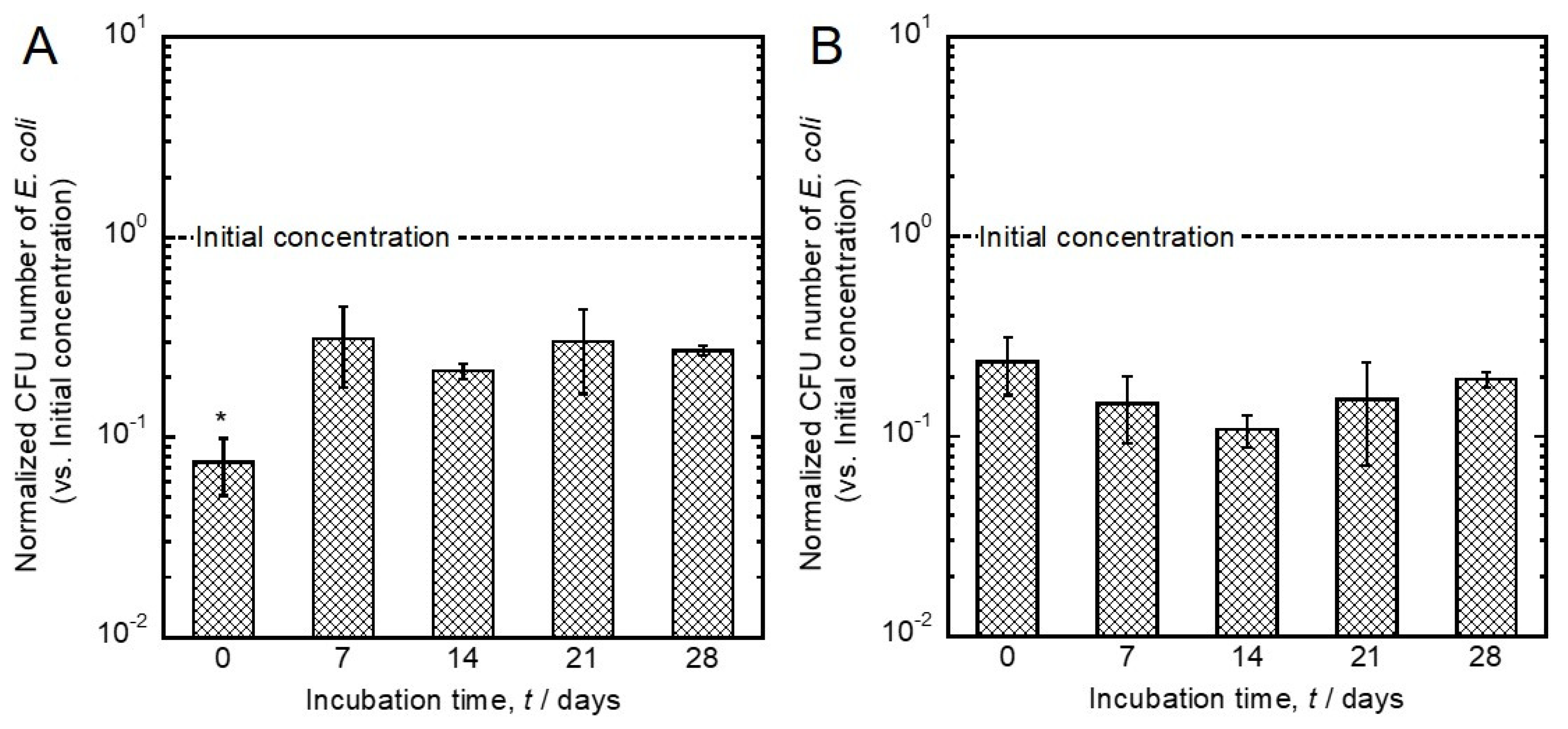
3.2. Evaluation of Antibacterial Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tande, J.; Patel, R. Prosthetic Joint Infection. Clin. Microbiol. Rev. 2014, 27, 302–345. [Google Scholar] [CrossRef] [PubMed]
- Osmon, D.R.; Berbari, E.F.; Berendt, A.R.; Lew, D.; Zimmerli, W.; Steckelberg, J.M.; Rao, N.; Hanssen, A.; Wilson, W.R. Diagnosis and Management of Prosthetic Joint Infection: Clinical Practice Guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 2013, 56, e1–e25. [Google Scholar] [CrossRef] [PubMed]
- Hobley, L.; Harkins, C.; MacPhee, C.E.; Stanley-Wall, N.R. Giving structure to the biofilm matrix: An overview of individual strategies and emerging common themes. FEMS Microbiol. Rev. 2015, 39, 649–669. [Google Scholar] [CrossRef] [PubMed]
- Hoiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 34, 322–332. [Google Scholar] [CrossRef]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Stoodley, P.; Hall-Stoodley, L. Targeting microbial biofilms: Current and prospective therapeutic strategies. Nat. Rev. Microbiol. 2017, 15, 740–755. [Google Scholar] [CrossRef]
- Lindsay, D.; von Holy, A. Bacterial biofilms within the clinical setting: What healthcare professionals should know. J. Hosp. Infect. 2006, 64, 313–325. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Van Acker, H.; Van Dijck, P.; Coenye, T. Molecular mechanisms of antimicrobial tolerance and resistance in bacterial and fungal biofilms. Trends Microbiol. 2014, 22, 326–333. [Google Scholar] [CrossRef]
- Lebeaux, D.; Ghigo, J.M.; Beloin, C. Biofilm-related infections: Bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol. Mol. Biol. Rev. 2014, 78, 510–543. [Google Scholar] [CrossRef]
- Shimabukuro, M.; Ito, H.; Tsutsumi, Y.; Nozaki, K.; Chen, P.; Yamada, R.; Ashida, M.; Nagai, A.; Hanawa, T. The Effects of Various Metallic Surfaces on Cellular and Bacterial Adhesion. Metals 2019, 9, 1145. [Google Scholar] [CrossRef]
- Rai, M.K.; Deshmukh, S.D.; Ingle, A.P.; Gade, A.K. Silver nanoparticles: The powerful nanoweapon against multidrug-resistant bacteria. J. Appl. Microbiol. 2012, 112, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Lara, H.H.; Ayala-Nunez, N.V.; Turrent, L.D.I.; Padilla, C.R. Bactericidal effect of silver nanoparticles against multidrug-resistant bacteria. World J. Microbiol. Biotechnol. 2010, 26, 615–621. [Google Scholar] [CrossRef]
- Prakash, P.; Gnanaprakasam, P.; Emmanuel, R.; Arokiyaraj, S.; Saravanan, M. Green synthesis of silver nanoparticles from leaf extract of Mimusops elengi, Linn. for enhanced antibacterial activity against multi drug resistant clinical isolates. Colloids Surf. B Biointerfaces 2013, 108, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Pant, J.; Goudie, M.J.; Hopkins, S.P.; Brisbois, E.J.; Handa, H. Tunable Nitric Oxide Release from S-Nitroso-N-acetylpenicillamine via Catalytic Copper Nanoparticles for Biomedical Applications. ACS Appl. Mater. Interfaces 2017, 9, 15254–15264. [Google Scholar] [CrossRef] [PubMed]
- Gasqueres, C.; Schneider, G.; Nusko, R.; Maier, G.; Dingeldein, E.; Eliezer, A. Innovative antibacterial coating by anodic spark deposition. Surf. Coat. Technol. 2012, 206, 3410–3414. [Google Scholar] [CrossRef]
- Ferraris, S.; Spriano, S. Antibacterial titanium surfaces for medical implants. Mater. Sci. Eng. C 2016, 61, 965–978. [Google Scholar] [CrossRef]
- Chai, H.; Guo, L.; Wang, X.; Fu, Y.; Guan, J.; Tan, L.; Ren, L.; Yang, K. Antibacterial effect of 317L stainless steel contained copper in prevention of implant-related infection in vitro and in vivo. J. Mater. Sci. Mater. Med. 2011, 22, 2525–2535. [Google Scholar] [CrossRef]
- Tilmaciu, C.M.; Mathieu, M.; Lavigne, J.P.; Toupet, K.; Guerrero, G.; Ponche, A.; Amalric, J.; Noel, D.; Mutin, P.H. In vitro and in vivo characterization of antibacterial activity and biocompatibility: A study on silver-containing phosphonate monolayers on titanium. Acta Biomater. 2015, 15, 266–277. [Google Scholar] [CrossRef]
- Ha, J.Y.; Tsutsumi, Y.; Doi, Y.; Nomura, N.; Kim, K.H.; Hanawa, T. Enhancement of calcium phosphate formation on zirconium by micro-arc oxidation and chemical treatments. Surf. Coat. Technol. 2011, 205, 4948–4955. [Google Scholar] [CrossRef]
- Song, W.H.; Ryu, H.S.; Hong, S.H. Antibacterial properties of Ag (or Pt)-containing calcium phosphate coatings formed by micro-arc oxidation. J. Biomed. Mater. Res. A 2009, 88, 246–254. [Google Scholar] [CrossRef]
- Li, L.H.; Kong, Y.M.; Kim, H.W.; Kim, Y.W.; Kim, H.E.; Heo, S.J.; Koak, J.Y. Improved biological performance of Ti implants due to surface modification by micro-arc oxidation. Biomaterials 2004, 25, 2867–2875. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lee, I.S.; Cui, F.Z.; Choi, S.H. The biocompatibility of nanostructured calcium phosphate coated on micro-arc oxidized titanium. Biomaterials 2008, 29, 2025–2032. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.Y.; Janga, B.C.; Zhu, X.; Ong, J.L.; Kim, K.H. Effect of hydrothermally treated anodic oxide films on osteoblast attachment and proliferation. Biomaterials 2003, 24, 347–355. [Google Scholar] [CrossRef]
- Son, W.W.; Zhu, X.; Shin, H.I.; Ong, J.L.; Kim, K.H. In vivo histological response to anodized and anodized/hydrothermally treated titanium implants. J. Biomed. Mater. Res. B 2003, 66, 520–525. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kim, M.; Kim, H.E.; Koh, Y.H.; Kim, H.W.; Jang, J.H. Formation of hydroxyapatite within porous TiO2 layer by micro-arc oxidation coupled with electrophoretic deposition. Acta Biomater. 2009, 5, 2196–2205. [Google Scholar] [CrossRef]
- Li, L.H.; Kim, H.W.; Lee, S.H.; Kong, Y.M.; Kim, H.E. Biocompatibility of titanium implants modified by microarc oxidation and hydroxyapatite coating. J. Biomed. Mater. Res. A 2005, 73, 48–54. [Google Scholar] [CrossRef]
- Shimabukuro, M.; Tsutsumi, Y.; Yamada, R.; Ashida, M.; Chen, P.; Doi, H.; Nozaki, K.; Nagai, A.; Hanawa, T. Investigation of realizing both antibacterial property and osteogenic cell compatibility on titanium surface by simple electrochemical treatment. ACS Biomater. Sci. Eng. 2019, 5, 5623–5630. [Google Scholar] [CrossRef]
- Shimabukuro, M.; Tsutsumi, Y.; Nozali, K.; Chen, P.; Yamada, R.; Aahida, M.; Doi, H.; Nagai, A.; Hanawa, T. Investigation of antibacterial effect of copper introduced titanium surface by electrochemical treatment against facultative anaerobic bacteria. Dent. Mater. J. 2020. [Google Scholar] [CrossRef]
- Cochis, A.; Azzimonti, B.; Della Valle, C.; De Giglio, E.; Bloise, N.; Visai, L.; Cometa, S.; Rimondini, L.; Chiesa, R. The effect of silver or gallium doped titanium against the multidrug resistant Acinetobacter baumannii. Biomaterials 2016, 80, 80–95. [Google Scholar] [CrossRef]
- Tsutsumi, Y.; Niinomi, M.; Nakai, M.; Shimabukuro, M.; Ashida, M.; Chen, P.; Doi, H.; Hanawa, T. Electrochemical Surface Treatment of a b-titanium Alloy to Realize an Antibacterial Property and Bioactivity. Metals 2016, 6, 76. [Google Scholar] [CrossRef]
- He, X.; Zhang, X.; Bai, L.; Hang, R.; Huang, X.; Qin, L.; Yao, X.; Tang, B. Antibacterial ability and osteogenic activity of porous Sr/Ag-containing TiO2 coatings. Biomed. Mater. 2016, 11, 045008. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.H.; Zhang, X.Y.; Wu, H.B.; Tian, L.H.; Ma, Y.; Tang, B. Microstructure and antibacterial properties of Cu-doped TiO2 coating on titanium by micro-arc oxidation. Appl. Surf. Sci. 2014, 292, 944–947. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, X.; Geng, Z.; Yin, Y.; Hang, R.; Huang, X.; Yao, X.; Tang, B. Preparation, antibacterial effects and corrosion resistant of porous Cu-TiO2 coatings. Appl. Surf. Sci. 2014, 308, 43–49. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, Z.; Gu, B.; Sun, J.; Zhu, L. Biological activity and antibacterial property of nano-structured TiO2 coating incorporated with Cu prepared by micro-arc oxidation. J. Mater. Sci. Technol. 2013, 29, 237–244. [Google Scholar] [CrossRef]
- He, X.; Zhang, X.; Wang, X.; Qin, L. Review of antibacterial activity of titanium-based implants’ surfaces fabricated by micro-arc oxidation. Coatings 2017, 7, 45. [Google Scholar] [CrossRef]
- Tande, A.J.; Palraj, B.R.; Osmon, D.R.; Berbari, E.F.; Baddour, L.M.; Lohse, C.M.; Steckelberg, J.M.; Wilson, W.R.; Sohail, M.R. Clinical Presentation, Risk Factors, and Outcomes of Hematogenous Prosthetic Joint Infection in Patients with Staphylococcus aureus Bacteremia. Am. J. Med. 2016, 129, 221.e11–221.e20. [Google Scholar] [CrossRef]
- Conventry, M.B. Treatment of infections occurring in total hip surgery. Orthop. Clin. N. Am. 1975, 6, 991–1003. [Google Scholar]
- Rodríguez, D.; Pigrau, C.; Euba, G.; Cobo, J.; García-Lechuz, J.; Palomino, J.; Riera, M.; del Toro, M.D.; Granados, A.; Ariza, X. Acute haematogenous prosthetic joint infection: Prospective evaluation of medical and surgical management. Clin. Microbiol. Infect. 2010, 16, 1789–1795. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kobayashi, E.; Hiromoto, S.; Asami, K.; Imai, H.; Hanawa, T. Calcium phosphate formation on Ti by low-voltage electrolytic treatments. J. Mater. Sci. Mater. Med. 2007, 18, 797–806. [Google Scholar] [CrossRef]
- Shimabukuro, M.; Tsutsumi, Y.; Nozaki, K.; Chen, P.; Yamada, R.; Ashida, M.; Doi, H.; Nagai, A.; Hanawa, T. Chemical and Biological Roles of Zinc in a Porous Titanium Dioxide Layer Formed by Micro-Arc Oxidation. Coatings 2019, 9, 705. [Google Scholar] [CrossRef]
- Shirley, D.A. High-Resolution X-ray Photoemission Spectrum of the Valence Bands of Gold. Phys. Rev. B 1972, 5, 552–556. [Google Scholar] [CrossRef]
- Asami, K.; Hashimoto, K.; Shimodaira, S. XPS determination of compositions of alloy surfaces and surface oxides on mechanically polished iron–chromium alloys. Corros. Sci. 1977, 17, 713–723. [Google Scholar] [CrossRef]
- Asami, K.; Chen, S.C.; Habazaki, H.; Kawashima, A.; Hashimoto, K. A photoelectrochemical and ESCA study of passivity of amorphous nickel-valve metal alloys. Corros. Sci. 1990, 31, 727–732. [Google Scholar] [CrossRef]
- Hashimoto, K.; Kasaya, M.; Asami, K.; Masumoto, T. Electrochemical and XPS studies on corrosion behavior of amorphous Ni–Cr–P–B alloys. Corros. Eng. 1977, 26, 445–452. [Google Scholar] [CrossRef]
- Scofield, J.H. Hartree-Slater subshell photoionization cross-sections at 1254 and 1487 eV. J. Electron. Spectrosc. Relat. Phenom. 1976, 8, 129–137. [Google Scholar] [CrossRef]
- Anthony, M.T.; Seah, M.P. XPS: Energy calibration of electron spectrometers. 1—An absolute, traceable energy calibration and the provision of atomic reference line energies. Surf. Interface Anal. 1984, 6, 95–106. [Google Scholar] [CrossRef]
- Gaarenstroom, S.W.; Winograd, N. Initial and final state effects in the ESCA spectra of cadmium and silver oxides. J. Chem. Phys. 1977, 67, 3500–3506. [Google Scholar] [CrossRef]
- Biesinger, M.C. Advanced analysis of copper X-ray photoelectron spectra. Surf. Interface Anal. 2017, 49, 1325–1334. [Google Scholar] [CrossRef]
- Henglein, A.; Giersig, M. Formation of Colloidal Silver Nanoparticles: Capping Action of Citrate. J. Phys. Chem. B 1999, 103, 9533–9539. [Google Scholar] [CrossRef]
- Hans, M.; Erbe, A.; Mathews, S.; Chen, Y.; Solioz, M.; Mucklich, F. Role of copper oxides in contact killing of bacteria. Langmuir 2013, 29, 16160–16166. [Google Scholar] [CrossRef]
- Shimabukuro, M.; Manaka, T.; Tsutsumi, Y.; Nozaki, K.; Chen, P.; Ashida, M.; Nagai, A.; Hanawa, T. Corrosion Behavior and Bacterial Viability on Different Surface States of Copper. Mater. Trans. 2020. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimabukuro, M.; Hiji, A.; Manaka, T.; Nozaki, K.; Chen, P.; Ashida, M.; Tsutsumi, Y.; Nagai, A.; Hanawa, T. Time-Transient Effects of Silver and Copper in the Porous Titanium Dioxide Layer on Antibacterial Properties. J. Funct. Biomater. 2020, 11, 44. https://doi.org/10.3390/jfb11020044
Shimabukuro M, Hiji A, Manaka T, Nozaki K, Chen P, Ashida M, Tsutsumi Y, Nagai A, Hanawa T. Time-Transient Effects of Silver and Copper in the Porous Titanium Dioxide Layer on Antibacterial Properties. Journal of Functional Biomaterials. 2020; 11(2):44. https://doi.org/10.3390/jfb11020044
Chicago/Turabian StyleShimabukuro, Masaya, Akari Hiji, Tomoyo Manaka, Kosuke Nozaki, Peng Chen, Maki Ashida, Yusuke Tsutsumi, Akiko Nagai, and Takao Hanawa. 2020. "Time-Transient Effects of Silver and Copper in the Porous Titanium Dioxide Layer on Antibacterial Properties" Journal of Functional Biomaterials 11, no. 2: 44. https://doi.org/10.3390/jfb11020044
APA StyleShimabukuro, M., Hiji, A., Manaka, T., Nozaki, K., Chen, P., Ashida, M., Tsutsumi, Y., Nagai, A., & Hanawa, T. (2020). Time-Transient Effects of Silver and Copper in the Porous Titanium Dioxide Layer on Antibacterial Properties. Journal of Functional Biomaterials, 11(2), 44. https://doi.org/10.3390/jfb11020044