An Insight into Advanced Approaches for Photosensitizer Optimization in Endodontics—A Critical Review
Abstract
1. Introduction
2. Bacteriophages
3. Drug Delivery Systems
3.1. Cellulose
3.2. Chitosan
3.3. Alginate Foam
3.4. Electrolyzed Water
3.5. Hydrogels
3.6. Lipid Delivery Systems
3.6.1. Invasomes
3.6.2. Liposomes
3.6.3. Micelles
3.7. Oil-Based Emulsions
4. Metal–Organic Frameworks
5. Nanoparticles
5.1. Carbon
5.1.1. Carbon Nanotubes
5.1.2. Nano-Graphene Oxide
5.1.3. Fullerenes
5.2. Gold
5.3. Platinum
5.4. Silica
5.5. Silver
5.6. Superparamagnetic Iron Oxide Nanoparticles
6. Efflux Pump Inhibitors
7. Light Delivery Systems
7.1. Light with or with No Fiber
7.2. Light Irradiation through Periapical Bone
8. Negative Pressure Systems
9. Peptides
9.1. Oligopeptides
9.2. Polypeptides
10. Other Approaches for Improving aPDT
10.1. PS Structural Features
10.2. Incubation Period
10.3. Solubilizers
11. Sonodynamic Therapy
11.1. Ultrasonic Activation
11.2. Ultrasound Sonication
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Santajit, S.; Indrawattana, N. Mechanisms of Antimicrobial Resistance in ESKAPE Pathogens. BioMed Res. Int. 2016, 2475067. [Google Scholar] [CrossRef]
- Aas, J.A.; Paster, B.J.; Stokes, L.N.; Olsen, I.; Dewhirst, F.E. Defining the Normal Bacterial Flora of the Oral Cavity. J. Clin. Microbiol. 2005, 43, 5721–5732. [Google Scholar] [CrossRef]
- Pessi, T.; Karhunen, V.; Karjalainen, P.P.; Ylitalo, A.; Airaksinen, J.K.; Niemi, M.; Pietila, M.; Lounatmaa, K.; Haapaniemi, T.; Lehtimäki, T.; et al. Bacterial signatures in thrombus aspirates of patients with myocardial infarction. Circulation 2013, 127, 1219–1228. [Google Scholar] [CrossRef]
- Siqueira, J.F., Jr. Aetiology of root canal treatment failure: Why well-treated teeth can fail. Int. Endod. J. 2001, 34, 1–10. [Google Scholar] [CrossRef]
- Tennert, C.; Feldmann, K.; Haamann, E.; Al-ahmad, A.; Follo, M.; Wrbas, K.; Hellwig, E.; Altenburger, M.J. Effect of photodynamic therapy (PDT) on Enterococcus faecalis biofilm in experimental primary and secondary endodontic infections. BMC Oral Health 2014, 14, 132. [Google Scholar] [CrossRef]
- Sjögren, U.; Figdor, D.; Persson, S.; Sundqvist, G. Influence of infection at the time of root filling on the outcome of endodontic treatment of teeth with apical periodontitis. Int. Endod. J. 1997, 30, 297–306. [Google Scholar] [CrossRef]
- Molander, A.; Reit, C.; Dahlen, G.; Kvist, T. Microbiological status of root-filled teeth with apical periodontitis. Int. Endod. J. 1998, 31, 1–7. [Google Scholar] [CrossRef]
- Căpută, P.E.; Retsas, A.; Kuijk, L.; Chávez de Paz, L.E.; Boutsioukis, C. Ultrasonic Irrigant Activation during Root Canal Treatment: A Systematic Review. Int. Endod. J. 2019, 45, 31–44. [Google Scholar] [CrossRef]
- Swimberghe, R.C.D.; Coenye, T.; De Moor, R.J.G.; Meire, M.A. Biofilm model systems for root canal disinfection: A literature review. Int. Endod. J. 2018, 52, 604–628. [Google Scholar] [CrossRef]
- Diogo, P.; Fernandes, C.; Caramelo, F.; Mota, M.; Miranda, I.M.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Uliana, M.P.; de Oliveira, K.T.; Santos, J.M.; et al. Antimicrobial photodynamic therapy against endodontic Enterococcus faecalis and Candida albicans mono and mixed biofilms in the presence of photosensitizers: A comparative study with classical endodontic irrigants. Front. Microbiol. 2017, 8, 498. [Google Scholar] [CrossRef]
- Alves, E.; Faustimo, M.A.; Neves, M.G.; Cunha, A.; Tome, J.; Almeida, A. An insight on bacterial cellular targets of photodynamic inactivation. Future Med. Chem. 2014, 6, 141–164. [Google Scholar] [CrossRef] [PubMed]
- Diogo, P.; Gonçalves, T.; Palma, P.; Santos, J.M. Photodynamic antimicrobial chemotherapy for root canal system asepsis: A narrative literature review. Int. J. Dent. 2015, 269205. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.; Tomé, J.P.; Neves, M.G.; Tomé, A.C.; Cavaleiro, J.A.; Cunha, Â.; Costa, L.; Faustino, M.A.; Almeida, A. Photodynamic inactivation of multidrug-resistant bacteria in hospital wastewaters: Influence of residual antibiotics. Photochem. Photobiol. Sci. 2014, 13, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Branco, T.M.; Valério, N.C.; Jesus, V.I.R.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Almeida, A. Single and combined effects of photodynamic therapy and antibiotics to inactivate Staphylococcus aureus on skin. Photodiagnosis Photodyn. Ther. 2018, 21, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Lauro, F.M.; Pretto, P.; Covolo, L.; Jori, G.; Bertoloni, G. Photoinactivation of bacterial strains involved in periodontal diseases sensitized by porphycene—Polylysine conjugates. Photochem. Photobiol. Sci. 2002, 1, 468–470. [Google Scholar] [CrossRef] [PubMed]
- Diogo, P.; Mota, M.; Fernandes, C.; Sequeira, D.; Palma, P.; Caramelo, F.; Neves, M.G.M.P.S.; Faustino, M.A.F.; Gonçalves, T.; Santos, J.M. Is the chlorophyll derivative Zn(II)e6Me a good photosensitizer to be used in root canal disinfection? Photodiagnosis Photodyn. Ther. 2018, 22, 205–211. [Google Scholar] [CrossRef]
- St Denis, T.G.; Dai, T.; Izikson, L.; Astrakas, C.; Anderson, R.R.; Hamblin, M.R.; Tegos, G.P. All you need is light, antimicrobial photoinactivation as an evolving and emerging discovery strategy against infectious disease. Virulence 2011, 2, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Gandra, N.; Abbineni, G.; Qu, X.; Huai, Y.; Wang, L.; Mao, C. Bacteriophage Bionanowire as a Carrier for Both Cancer-Targeting Peptides and Photosensitizers and its use in Selective Cancer Cell Killing by Photodynamic Therapy. Small 2013, 9, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Shi, H.; Zhang, X.; Chen, X.; Cao, D.; Mao, C.; Gao, X.; Wang, L. Difunctional bacteriophage conjugated with photosensitizers for Candida albicans-targeting photodynamic inactivation. Int. J. Nanomed. 2018, 13, 2199–2216. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Drug Delivery Systems: Entering the Mainstream. Drug Discov. 2004, 303, 1818–1823. [Google Scholar] [CrossRef]
- Tiwari, G.; Tiwari, R.; Sriwastawa, B.; Bhati, L.; Pandey, S.; Pandey, P.; Bannerjee, S. Drug delivery systems: An updated review. Int. J. Pharm. Investig. 2012, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Park, K. Controlled drug delivery systems: Past forward and future back. J. Control. Release 2014, 190, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Hu, S. “Smart” Materials Based on Cellulose: A Review of the Preparations, Properties, and Applications. Materials 2013, 6, 738–781. [Google Scholar] [CrossRef] [PubMed]
- Decraene, V.; Pratten, J.; Wilson, M. Cellulose Acetate Containing Toluidine Blue and Rose Bengal Is an Effective Antimicrobial Coating when Exposed to White Light. Appl. Environ. Microbiol. 2006, 72, 4436–4439. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, R.; Fayyaz, F.; Rassa, M. The study of cellulosic fabrics impregnated with porphyrin compounds for use as photo-bactericidal polymers. Mater. Sci. Eng. C 2016, 59, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Hamblin, M.R.; Kishen, A. Photoactivated rose bengal functionalized chitosan nanoparticles produce antibacterial/biofilm activity and stabilize dentin-collagen. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Hegge, A.B.; Andersen, T.; Melvik, J.E.; Kristensen, S.; Tønnesen, H.H. Evaluation of Novel Alginate Foams as Drug Delivery Systems in Antimicrobial Photodynamic Therapy (aPDT) of Infected Wounds—An In Vitro Study: Studies on Curcumin and Curcuminoides XL. J. Pharm. Sci. 2010, 99, 3499–3513. [Google Scholar] [CrossRef]
- Hegge, A.B.; Andersen, T.; Melvik, J.E.; Bruzell, E.; Kristensen, S.; Tønnesen, H.H. Formulation and bacterial phototoxicity of curcumin loaded alginate foams for wound treatment application: Studies on curcumin and Curcuminoides XL. J. Pharm. Sci. 2011, 100, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.M.E.; Khan, I.; Oh, D.H. Electrolyzed Water as a Novel Sanitizer in the Food Industry: Current Trends and Future Perspectives. Compr. Rev. Food Sci. Food Saf. 2016, 15, 471–490. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Cassidy, C.M.; Loughlin, R.G.; Brown, A.; Tunney, M.M.; Jenkins, M.G.; Mccarron, P.A. Delivery of Methylene Blue and meso-tetra (N-methyl-4-pyridyl) porphine tetra tosylate from cross-linked poly(vinyl alcohol) hydrogels: A potential means of photodynamic therapy of infected wounds. J. Photochem. Photobiol. B 2009, 96, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Ossmann, A.; Kranz, S.; Andre, G.; Völpel, A.; Albrecht, V.; Fahr, A.; Sigusch, B.W. Photodynamic killing of Enterococcus faecalis in dentinal tubules using mTHPC incorporated in liposomes and invasomes. Clin. Oral Investig. 2015, 19, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Ferro, S.; Ricchelli, F.; Monti, D.; Macini, G.; Jori, G. Efficient photoinactivation of methicillin-resistant Staphylococcus aureus by a novel porphyrin incorporated into a poly-cationic liposome. Int. J. Biochem. Cell Biol. 2007, 39, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.; Yang, Y.T.; Wang, T.H.; Chien, H.F.; Chen, C.T. Improved photodynamie inactivation of gram-positive bacteria using hematoporphyrin encapsulated in liposomes and micelles. Lasers Surg. Med. 2009, 41, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Rout, B.; Liu, C.H.; Wu, W.C. Enhancement of photodynamic inactivation against Pseudomonas aeruginosa by a nano-carrier approach. Colloids Surf. B Biointerfaces 2016, 140, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.P.; Andrade, M.C.; Bagnato, V.S.; Vergani, C.E.; Primo, F.L.; Tedesco, A.C.; Pavarina, A.C. Antimicrobial photodynamic therapy against pathogenic bacterial suspensions and biofilms using chloro-aluminum phthalocyanine encapsulated in nanoemulsions. Lasers Med. Sci. 2015, 30, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Rabea, E.I.; Badawy, M.E.T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Moczek, Ł.; Nowakowska, M. Novel water-soluble photosensitizers from chitosan. Biomacromolecules 2007, 8, 433–438. [Google Scholar] [CrossRef]
- Shrestha, A.; Kishen, A. Polycationic chitosan-conjugated photosensitizer for antibacterial photodynamic therapy. Photochem. Photobiol. 2012, 88, 577–583. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Connors, K.A. The Stability of Cyclodextrin Complexes in Solution. Chem. Rev. 1997, 97, 1325–1358. [Google Scholar] [CrossRef]
- Ishiyama, K.; Nakamura, K.; Kanno, T.; Niwano, Y. Bactericidal Action of Photodynamic Antimicrobial Chemotherapy (PACT) with Photosensitizers Used as Plaque-Disclosing Agents against Experimental Biofilm. Biocontrol Sci. 2016, 21, 187–191. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chen, J.; Deng, C.; Suuronen, E.J.; Zhong, Z. Click hydrogels, microgels and nanogels: Emerging platforms for drug delivery and tissue engineering. Biomaterials 2014, 35, 4969–4985. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xia, L.Y.; Chen, X.; Chen, Z.; Wu, F.G. Hydrogel-based phototherapy for fighting cancer and bacterial infection. Sci. China Mater. 2017, 60, 487–503. [Google Scholar] [CrossRef]
- Mesquita, M.Q.; Dias, C.J.; Neves, M.G.P.M.S.; Almeida, A.; Faustino, M.A.F. Revisiting current photoactive materials for antimicrobial photodynamic therapy. Molecules 2018, 23, 2424. [Google Scholar] [CrossRef]
- Chen, C.; Chen, C.; Yang, J.; Tsai, T. Liposome-Encapsulated Photosensitizers Against Bacteria. Recent Pat. Antiinfect. Drug Discov. 2013, 8, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, P.K.; Kalpana, B.; Prasanthi, D. Invasomes-novel Vesicular Carriers for Enhanced Skin Permeation. Syst. Rev. Pharm. 2013, 4, 26. [Google Scholar] [CrossRef]
- Shrestha, H.; Bala, R.; Arora, S. Lipid-Based Drug Delivery Systems. J. Pharm. 2014, 801820. [Google Scholar] [CrossRef]
- Deshmukh, A.S.; Chauhan, P.N.; Noolvi, M.N.; Chaturvedi, K.; Ganguly, K.; Shukla, S.S.; Nadagouda, M.N.; Aminabhavi, T.M. Polymeric micelles: Basic research to clinical practice. Int. J. Pharm. 2017, 532, 249–268. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter. 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Peng, J.; Wu, R. Metal-organic frameworks in proteomics/petidomics—A review. Anal. Chim. Acta 2018, 16, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Faraji, A.H.; Wipf, P. Nanoparticles in cellular drug delivery. Bioorg. Med. Chem. 2009, 17, 2950–2962. [Google Scholar] [CrossRef] [PubMed]
- Golmohamadpour, A.; Bahramian, B.; Khoobi, M.; Pourhajibagher, M.; Barikani, H.R.; Bahador, A. Antimicrobial photodynamic therapy assessment of three indocyanine green-loaded metal-organic frameworks against Enterococcus faecalis. Photodiagnosis Photodyn. Ther. 2018, 23, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Sah, U.; Sharma, K.; Chaudhri, N.; Sankar, M.; Gopinath, P. Antimicrobial photodynamic therapy: Single-walled carbon nanotube (SWCNT)-Porphyrin conjugate for visible light mediated inactivation of Staphylococcus aureus. Colloids Surf. B Biointerfaces 2018, 162, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, I.; Mondal, D.; Martin, J.; Kane, R.S. Photoactivated Antimicrobial Activity of Carbon Nanotube-Porphyrin Conjugates. Langmuir ACS J. 2010, 26, 17369–17374. [Google Scholar] [CrossRef] [PubMed]
- Akbari, T.; Pourhajibagher, M.; Hosseini, F.; Chiniforush, N.; Gholibegloo, E.; Khoobi, M.; Shahabi, S.; Bahador, A. The effect of indocyanine green loaded on a novel nano-graphene oxide for high performance of photodynamic therapy against Enterococcus faecalis. Photodiagnosis Photodyn. Ther. 2017, 20, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Tegos, G.P.; Demidova, T.N.; Arcila-Lopez, D.; Lee, H.; Wharton, T.; Gali, H.; Hamblin, M.R. Cationic fullerenes are effective and selective antimicrobial photosensitizers. Chem. Biol. 2005, 12, 1127–1135. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, T.; Wang, M.; Vecchio, D.; Chiang, L.Y.; Hamblin, M.R. Potentiation of antimicrobial photodynamic inactivation mediated by a cationic fullerene by added iodide: In vitro and in vivo studies. Nanomedicine 2015, 10, 603–614. [Google Scholar] [CrossRef]
- Pagonis, T.C.; Chen, J.; Fontana, C.R.; Devalapally, H.; Ruggiero, K.; Song, X.; Foschi, F.; Dunham, J.; Skobe, Z.; Yamazaki, H.; et al. Nanoparticle-based endodontic antimicrobial photodynamic therapy. J. Endod. 2010, 36, 322–328. [Google Scholar] [CrossRef]
- Managa, M.; Antunes, E.; Nyokong, T. Conjugates of platinum nanoparticles with gallium tetra—(4-Carboxyphenyl) porphyrin and their use in photodynamic antimicrobial chemotherapy when in solution or embedded in electrospun fiber. Polyhedron 2014, 76, 94–101. [Google Scholar] [CrossRef]
- Guo, Y.; Rogelj, S.; Zhang, P. Rose Bengal-decorated silica nanopartciles as photosensitizers for inactivation of gram-positive bacteria. Nanotechnology 2016, 21, 065102. [Google Scholar] [CrossRef] [PubMed]
- Lyutakov, O.; Hejna, O.; Solovyev, A.; Kalachyova, Y.; Svorcik, V. Polymethylmethacrylate doped with porphyrin and silver nanoparticles as light-activated antimicrobial material. RSC Adv. 2014, 4, 50624–50630. [Google Scholar] [CrossRef]
- Thandu, M.M.; Cavalli, S.; Rossi, G.; Rizzardini, C.B.; Goi, D.; Comuzzi, C. Biological evaluation of a Porphyrin-SPION nanoconjugate as an antimicrobial magnetic photosensitizer. J. Porphyr. Phthalocyanines 2017, 21, 581–588. [Google Scholar] [CrossRef]
- Allaker, R.P.; Memarzadeh, K. Nanoparticles and the control of oral infections. Int. J. Antimicrob. Agents 2014, 43, 95–104. [Google Scholar] [CrossRef]
- Samiei, M.; Farjami, A.; Dizaj, S.M.; Lotfipour, F. Nanoparticles for antimicrobial purposes in Endodontics: A systematic review of in vitro studies. Mater. Sci. Eng. C Biol. Appl. 2016, 58, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Lucky, S.S.; Soo, K.C.; Zhang, Y. Nanoparticles in photodynamic therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef]
- Hu, C.; Hu, S. Carbon Nanotube-Based Electrochemical Sensors: Principles and Applications in Biomedical Systems. Sensors 2009, 187615. [Google Scholar] [CrossRef]
- Kang, S.; Herzberg, M.; Rodrigues, D.F.; Elimelech, M. Antibacterial Effects of Carbon Nanotubes: Size Does Matter! Langmuir 2008, 24, 6409–6413. [Google Scholar] [CrossRef]
- Yin, R.; Agrawal, T.; Khan, U.; Gupta, G.K.; Rai, V.; Huang, Y.Y.; Hamblin, M.R. Antimicrobial photodynamic inactivation in nanomedicine: Small light strides against bad bugs. Nanomedicine 2015, 10, 2379–2404. [Google Scholar] [CrossRef]
- Mroz, P.; Tegos, G.P.; Gali, H.; Wharton, T.; Sarna, T.; Hamblin, M.R. Photodynamic therapy with fullerenes. Photochem. Photobiol. Sci. 2007, 6, 1139–1149. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Foote, M.; Prow, T.W. Therapeutic gold, silver, and platinum nanoparticles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 428–445. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.J.; Kim, H.S.; Park, Y.K.; Park, Y.K.; Hwang, C.Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.O.; Ahmad, M.F.; Shadab, G.G.H.A.; Siddique, H.R. Superparamagnetic iron oxide nanoparticles based cancer theranostics: A double edge sword to fight against cancer. J. Drug Deliv. Sci. Technol. 2018, 45, 177–183. [Google Scholar] [CrossRef]
- Kandasamy, G.; Maity, D. Recent advances in superparamagnetic iron oxide nanopartciles (SPIONs) for in vitro and in vivo cancer nanotheranostics. Int. J. Pharm. 2015, 496, 191–218. [Google Scholar] [CrossRef]
- Sun, J.; Deng, Z.; Yan, A. Biochemical and Biophysical Research Communications Bacterial multidrug efflux pumps: Mechanisms, physiology and pharmacological exploitations. Biochem. Biophys. Res. Commun. 2014, 453, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Poole, K. Efflux pumps as antimicrobial resistance mechanisms. Ann. Med. 2007, 39, 162–176. [Google Scholar] [CrossRef]
- Tegos, G.P.; Hamblin, M.R. Phenothiazinium Antimicrobial Photosensitizers Are Substrates of Bacterial Multidrug Resistance Pumps. Antimicrob. Agents Chemother. 2006, 50, 196–203. [Google Scholar] [CrossRef]
- Kishen, A.; Upadya, M.; Tegos, G.P.; Hamblin, M.R. Efflux pump inhibitor potentiates antimicrobial photodynamic inactivation of Enterococcus faecalis biofilm. Photochem. Photobiol. 2010, 86, 1343–1349. [Google Scholar] [CrossRef]
- Nunes, M.R.; Mello, I.; Franco, G.C.N.; de Medeiros, J.M.F.; dos Santos, S.S.F.; Habitante, S.M.; Lage-Marques, J.L.; Raldi, D.P. Effectiveness of Photodynamic Therapy Against Enterococcus faecalis, With and Without the Use of an Intracanal Optical Fiber: An In vitro Study. Photomed. Laser Surg. 2011, 29, 803–808. [Google Scholar] [CrossRef]
- Sabino, C.P.; Garcez, A.S.; Núñez, S.C.; Ribeiro, M.S.; Hamblin, M.R. Real-time evaluation of two light delivery systems for photodynamic disinfection of Candida albicans biofilm in curved root canals. Lasers Med. Sci. 2015, 30, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Cieplik, F.; Pummer, A.; Leibl, C.; Regensburger, J.; Schmalz, G.; Buchalla, W.; Hiller, K.A.; Maisch, T. Photodynamic Inactivation of Root Canal Bacteria by Light Activation through Human Dental Hard and Simulated Surrounding Tissue. Front. Microbiol. 2016, 7, 929. [Google Scholar] [CrossRef] [PubMed]
- Miranda, R.G.; Santos, E.B.; Souto, R.M.; Gusman, H.; Colombo, A.P. Ex vivo antimicrobial efficacy of the EndoVac® system plus photodynamic therapy associated with calcium hydroxide against intracanal Enterococcus faecalis. Int. Endod. J. 2013, 46, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Cohen, K.A.; Winglee, K.; Maiga, M.; Diarra, B.; Bishai, W.R. Efflux Inhibition with Verapamil Potentiates Bedaquiline in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2014, 58, 574–576. [Google Scholar] [CrossRef] [PubMed]
- Garcez, A.S.; Fregnani, E.R.; Rodriguez, H.M.; Nunez, S.C.; Sabino, C.P.; Suzuki, H.; Ribeiro, M.S. The use of optical fiber in endodontic photodynamic therapy. Is it really relevant? Lasers Med. Sci. 2013, 28, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Rödig, T.; Endres, S.; Konietschke, F.; Zimmermann, O.; Sydow, H.G.; Wiegand, A. Effect of fiber insertion depth on antibacterial efficacy of photodynamic therapy against Enterococcus faecalis in root canals. Clin. Oral Investig. 2017, 21, 1753–1759. [Google Scholar] [CrossRef] [PubMed]
- Odor, T.M.; Chandler, N.P.; Watson, T.F.; Ford, T.R.; McDonald, F. Laser light transmission in teeth: A study of the patterns in different species. Int. Endod. J. 1999, 32, 296–302. [Google Scholar] [CrossRef]
- Seal, G.J.; Ng, Y.; Spratt, D.; Bhatti, M.; Gulabivala, K. An in vitro comparison of the bactericidal efficacy of lethal photosensitization or sodium hyphochlorite irrigation on Streptococcus intermedius biofilms in root canals. Int. Endod. J. 2002, 35, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Soukos, N.S.; Chen, P.S.; Morris, J.T.; Ruggiero, K.; Abernethy, A.D.; Som, S.; Foschi, F.; Doucette, S.; Bammann, L.L.; Fontana, C.R.; et al. Photodynamic therapy for endodontic disinfection. J. Endod. 2006, 32, 979–984. [Google Scholar] [CrossRef]
- Fonseca, M.B.; Júnior, P.O.; Pallota, R.C.; Filho, H.F.; Denardin, O.V.; Rapoport, A.; Dedivitis, R.A.; Veronezi, J.F.; Genovese, W.J.; Ricardo, A.L. Photodynamic therapy for root canals infected with Enterococcus faecalis. Photomed. Laser Surg. 2008, 26, 209–213. [Google Scholar] [CrossRef]
- Meire, M.A.; De Prijck, K.; Coenye, T.; Nelis, H.J.; De Moor, R.J. Effectiveness of different laser systems to kill Enterococcus faecalis in aqueous suspension and in an infected tooth model. Int. Endod. J. 2009, 42, 351–359. [Google Scholar] [CrossRef]
- Soares, J.A.; Santos Soares, S.M.S.; Santos César, C.A.; de Carvalho, M.A.R.; Brito-Júnior, M.; de Sousa, G.R.; Soares, B.M.; De Macêdo Farias, L. Monitoring the effectiveness of photodynamic therapy with periodoc renewal of the photosensitizer on intracanal Enterococcus faecalis biofilms. Photodiagnosis Photodyn. Ther. 2016, 13, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part one—Photosensitizers, photochemistry and cellular localization. Photodiagnosis Photodyn. Ther. 2004, 1, 279–293. [Google Scholar] [CrossRef]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part two—Cellular signaling, cell metabolism and modes of cell death. Photodiagnosis Photodyn. Ther. 2005, 2, 1–23. [Google Scholar] [CrossRef]
- Nielsen, B.A.; Baumgartner, J.C. Comparison of the EndoVac System to Needle Irrigation of Root Canals. J. Endod. 2007, 33, 611–615. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, C.; Estevez, R.; Cisneros, R.; Heilborn, C.; Cohenca, N. Effect of EDTA, Sonic, and Ultrasonic Activation on the Penetration of Sodium Hypochlorite into Simulated Lateral Canals: An In vitro Study. J. Endod. 2009, 35, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Bago, I.; Plečko, V.; Gabrić Pandurić, D.; Schauperl, Z.; Baraba, A.; Anić, I. Antimicrobial efficacy of a high-power diode laser, photo-activated disinfection, conventional and sonic activated irrigation during root canal treatment. Int. Endod. J. 2013, 46, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Levison, M.E.; Levison, J.H.; Phil, M. Pharmacokinetics and Pharmacodynamics of Antibacterial Agents. Infect. Dis. Clin. N. Am. 2009, 23, 791–815. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, D.I.; Le Brun, A.P.; Whitwell, T.C.; Sani, M.A.; James, M.; Separovic, F. The antimicrobial peptide aurein 1.2 disrupts model membranes via the carpet mechanism. Phys. Chem. Chem. Phys. 2012, 14, 15739–15751. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, L.M.; Lorenzón, E.N.; Santos-Filho, N.A.; Zago, L.H.P.; Uliana, M.P.; de Oliveira, K.T.; Cilli, E.M.; Fontana, C.R. Antimicrobial Photodynamic therapy enhanced by the peptide aurein 1.2. Sci. Rep. 2018, 8, 4212. [Google Scholar] [CrossRef]
- Polo, L.; Segalla, A.; Bertoloni, G.; Jori, G.; Schaffner, K.; Reddi, E. Polylysine-porphycene conjugates as efficient photosensitizeers for the inactivation of microbial pathogens. J. Photochem. Photobiol. B Biol. 2000, 59, 152–158. [Google Scholar] [CrossRef]
- Chen, J.; Wang, W.; Hu, P.; Wang, D.; Lin, F.; Xue, J.; Chen, Z.; Iqbal, Z.; Huang, M. Dual antimicrobial actions on modified fabric leads to inactivation of drug-resistant bacteria. Dye. Pigment. 2017, 140, 236–243. [Google Scholar] [CrossRef]
- Soukos, N.S.; Hamblin, M.R.; Hasant, T. The Effect of Charge on Cellular Uptake and Phototoxicity of Polylysine Chlorin e6 Conjugates. Photochem. Photobiol. 1997, 65, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival Mechanisms of Clinically Relevant Microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef]
- Fang, F.; Lu, W.T.; Shan, Q.; Cao, J.S. Characteristics of extracellular polymeric substances of phototrophic biofilms at different aquatic habitats. Carbohydr. Polym. 2014, 106, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.M.B.; Gomes, A.T.P.C.; Fernandes, S.C.D.; Prata, A.C.B.; Almeida, M.A.; Cunha, M.A.; Tomé, J.P.C.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Tomé, A.C.; et al. Photoinactivation of bacteria in wastewater by porphyrins: Bacterial β-galactosidade activity and leucine-uptake as methods to monitor the process. J. Photochem. Photobiol. B Biol. 2007, 88, 112–118. [Google Scholar] [CrossRef]
- Lazzeri, D.; Rovera, M.; Pascua, L.; Durantini, E.N. Photodynamic Studies and Photo inactivation of Escherichia coli using meso-substituted cationic Porphyrin Derivatives with Asymmetric Charge Distribution. Photochem. Photobiol. 2004, 80, 286–293. [Google Scholar] [CrossRef]
- Spesia, M.B.; Lazzeri, D.; Pascual, L.; Rovera, M.; Durantini, E.N. Photoinactivation of Escherichia coli using porphyrin derivatives with different number of cationic charges. FEMS Immunol. Med. Microbiol. 2005, 44, 289–295. [Google Scholar] [CrossRef]
- Alves, E.; Costa, L.; Carvalho, C.M.; Tomé, J.P.; Faustino, M.A.; Neves, M.G.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, A.; Almeida, A. Charge effect on the photoinactivation of Gram-negative and Gram-positive bacteria by cationic meso-substituted porphyrins. BMC Microbiol. 2009, 13, 70. [Google Scholar] [CrossRef]
- Simões, C.; Gomes, M.C.; Neves, M.G.P.M.S.; Cunha, A.; Tomé, J.P.C.; Tomé, A.C.; Cavaleiro, J.A.S.; Almeida, A.; Faustino, M.A.F. Photodynamic inactivation of Escheria coli with cationic meso-teraarylporphyrins—The carge number and charge distribution effects. Catal. Today 2016, 266, 197–204. [Google Scholar] [CrossRef]
- Mesquita, M.Q.; Menezes, J.C.J.M.D.S.; Pires, S.M.G.; Neves, M.G.P.M.S.; Simões, M.M.Q.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, A.; Daniel-Da-Silva, A.L.; Almeida, A.; et al. Pyrrolidine-fused chlorin photosensitizer immobilized on solid supports for the photoinactivation of Gram negative bacteria. Dye. Pigment. 2014, 110, 123–133. [Google Scholar] [CrossRef]
- Gsponer, N.S.; Spesia, M.B.; Durantini, E.N. Effects of divalent cations, EDTA and chitosan on the uptake and photoinactivation of Escherichia coli mediated by cationic and anionic porphyrins. Photodiagnosis Photodyn. Ther. 2015, 12, 67–75. [Google Scholar] [CrossRef] [PubMed]
- George, S.; Kishen, A. Photophysical, photochemical, and photobiological characterization of methylene blue formulations for light-activated root canal disinfection. J. Biomed. Opt. 2007, 12, 034029. [Google Scholar] [CrossRef] [PubMed]
- George, S.; Kishen, A. Influence of photosensitizer solvent on the mechanisms of photoactivated killing of Enterococcus faecalis. Photochem. Photobiol. 2008, 84, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Maisch, T.; Wagner, J.; Papastamou, V.; Nerl, H.J.; Hiller, K.A.; Szeimies, R.M.; Schmalz, G. Combination of 10% EDTA, Photosan, and a blue light hand-held photopolymerizer to inactivate leading oral bacteria in dentistry in vitro. J. Appl. Microbiol. 2009, 107, 5–1569. [Google Scholar] [CrossRef]
- Weller, R.N.; Brady, J.M.; Bernier, W.E. Efficacy of Ultrasonic Cleaning. J. Endod. 1980, 6, 740–743. [Google Scholar] [CrossRef]
- Huang, L.; St Denis, T.G.; Xuan, Y.; Huang, Y.Y.; Tanaka, M.; Zadlo, A.; Sarna, T.; Hamblin, M.R. Paradoxical potentiation of methylene blue-mediated antimicrobial photodynamic inactivation by sodium azide: Role of ambient oxygen and azide radicals. Free Radic. Biol. Med. 2012, 53, 2062–2071. [Google Scholar] [CrossRef] [PubMed]
- St Denis, T.G.; Vecchio, D.; Zadlo, A.; Rineh, A.; Sadasivam, M.; Avci, P.; Huang, L.; Kozinska, A.; Chandran, R.; Sarna, T.; et al. Thiocyanate potentiates antimicrobial photodynamic therapy: In situ generation of the sulfur trioxide radical anion by singlet oxygen. Free Radic. Biol. Med. 2013, 65, 800–810. [Google Scholar] [CrossRef]
- Huang, L.; Szewczyk, G.; Sarna, T.; Hamblin, M.R. Potassium Iodide Potentiates Broad-Spectrum Antimicrobial Photodynamic Inactivation Using Photofrin. ACS Infect. Dis. 2017, 3, 320–328. [Google Scholar] [CrossRef]
- Caminos, D.A.; Spesia, M.B.; Durantini, E.N. Photodynamic inactivation of Escherichia coli by novel meso-substituted porphyrins by 4-(3-N,N,N-trimethylammoniumpropoxy)phenyl and 4-(trifluoromethyl)phenyl groups. Photochem. Photobiol. Sci. 2006, 5, 56–65. [Google Scholar] [CrossRef]
- George, S.; Hamblin, M.R.; Kishen, A. Uptake pathways of anionic photosensitizers into bacteria. Photochem Photobiol Sci. 2009, 8, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Eckl, D.B.; Dengler, L.; Nemmert, M.; Eichner, A.; Baumler, W.; Huber, H. A closer look at dark toxicity of the photosensitizer TMPyP in bacteria. Photochem. Photobiol. Sci. 2018, 94, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R.; Abrahamse, H. Inorganic Salts and Antimicrobial Photodynamic Therapy: Mechanistic Conundrums? Molecules 2018, 23, 3190. [Google Scholar] [CrossRef] [PubMed]
- Van Straten, D.; Mashayekhi, V.; de Bruijn, H.S.; Oliveira, S.; Robinson, D.J. Oncologic Photodynamic Therapy: Basic Principles, Current clinical status and future directions. Cancers 2017, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Plotino, G.; Grande, N.M.; Mercade, M. Photodynamic therapy in endodontics. Int. Endod. J. 2018, 52, 760–774. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, M.; Phoenix, D.A.; Marland, J.; Wareing, D.R.; Bolton, F.J. A study of photobactericidal activity in the phenothiazinium series. FEMS Immunol. Med. Microbiol. 1997, 19, 75–80. [Google Scholar] [CrossRef]
- Carvalho, E.S.; Mello, I.; Albergaria, S.J.; Habitante, S.M.; Lage-Marques, J.L.; Raldi, D.P. Effect of Chemical Substances in Removing Methylene Blue After Photodynamic Therapy in Root Canal Treatment. Photomed. Laser Surg. 2011, 29, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, R.A.; Anami, L.C.; Mello, I.; Carvalho, E.S.; Habitante, S.M.; Raldi, D.P. Tooth Discoloration Induced by Endodontic Phenothiazine Dyes in Photodynamic Therapy. Photomed. Laser Surg. 2014, 32, 458–462. [Google Scholar] [CrossRef]
- Upadya, M.H.; Kishen, A. Influence of bacterial growth modes on the susceptibility to light-activated disinfection. Int. Endod. J. 2010, 43, 978–987. [Google Scholar] [CrossRef]
- Garcez, A.S.; Núñez, S.C.; Azambuja, N., Jr.; Fregnani, E.R.; Rodriguez, H.M.; Hamblin, M.R.; Suzuki, H.; Ribeiro, M.S. Effects of photodynamic therapy on Gram-positive and Gram-negative bacterial biofilms by bioluminescence imaging and scanning electron microscopic analysis. Photomed. Laser Surg. 2013, 31, 519–525. [Google Scholar] [CrossRef]
- Kang, S.M.; Jung, H.I.; Kim, B.I. Susceptibility of oral bacteria to antibacterial photodynamic therapy. J. Oral Microbiol. 2019, 11, 1644111. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Zhang, X.; Guo, W.; Li, F.; Yu, M.; Kong, X.; Wu, W.; Hong, Z. Highly water-soluble and tumor-targeted photosensitizers for photodynamic therapy. Org. Biomol. Chem. 2015, 13, 7681–7694. [Google Scholar] [CrossRef] [PubMed]
- Ng, R.; Singh, F.; Papamanou, D.A.; Song, X.; Patel, X.; Holewa, C.; Patel, N.; Klepac-Ceraj, V.; Fontana, C.R.; Kent, R.; et al. Endodontic photodynamic therapy ex vivo. J. Endod. 2011, 37, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Hoedke, D.; Enseleit, C.; Gruner, D.; Dommisch, H.; Schlafer, S.; Dige, I.; Bitter, K. Effect of photodynamic therapy in combination with various irrigation protocols on an endodontic multispecies biofilm ex vivo. Int. Endod. J. 2018, 51, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Vaid, D.; Shah, N.; Kothari, D.; Bilgi, P. Additive effect of photoactivated disinfection on the antibacterial activity of QMix 2in1 against 6-week Enterococcus faecalis biofilms: An in vitro study. J. Conserv. Dent. 2017, 20, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Y.; Cline, C.S.; Koker, E.B.; Carmichael, H.H.; Chignell, C.F.; Bilski, P. Quenching of Singlet Molecular Oxygen (1O2) by Azide Anion in Solvent Mixtures. Photochem. Photobiol. 2001, 74, 760–764. [Google Scholar] [CrossRef]
- Vieira, C.; Santos, A.; Mesquita, M.Q.; Gomes, A.T.P.C.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Almeida, A. Quenching of Singlet Molecular Oxygen (1O2) by Azide Anion in Solvent Mixtures. Photochem. Photobiol. 2001, 74, 760–764. [Google Scholar]
- Yumita, N.; Nishigaki, R.; Umemura, K.; Umemura, S.I. Synergistic Effect of Ultrasound and Hematoporphyrin on Sarcomes 180. Jpn. J. Cancer Res. 1990, 81, 304–308. [Google Scholar] [CrossRef]
- Rosenthal, I.; Sostaric, J.Z.; Riesz, P. Sonodynamic therapy—A review of the synergistic effects of drugs and ultrasound. Ultrason. Sonochem. 2004, 11, 349–363. [Google Scholar] [CrossRef]
- Misik, V.; Miyoshi, N.; Riesz, P. EPR spin trapping study of the decompostition of azo compounds solutions by ultrasound: Potential for use as sonodynamic sensitizers for cell killing. Free Radic Res. 1996, 25, 13–22. [Google Scholar] [CrossRef]
- Miyoshi, N.; Misik, V.; Fukudat, M.; Riesz, P. Effect of Gallium-Porphyrin Analogue ATX-70 on Nitroxide from a Cyclic Secondary Amine by Ultrasound: Formation On the Mechanism of Sonodynamic Activation. Radiat. Res. 1995, 143, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Song, J.; Chen, X.; Yang, H. Ultrasound Activated Sensitizers and Applications. Angew. Chem. Int. Ed. Engl. 2019. [Google Scholar] [CrossRef] [PubMed]
- Alves, F.; Pavarina, A.C.; Garcia, E.; Mima, D.O.; Mchale, A.P.; Callan, J.F. Antimicrobial sonodynamic and photodynamic therapies against Candida Albicans. Biofouling 2018, 34, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Yumita, N.; Nishigaki, R.; Umemura, K.; Umemura, S. Hematophorphyrin as a Sensitizer of Cell-damaging effect of Ultrasound. Jpn. J. Cancer Res. 1989, 80, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Vanerio, N.; Stijnem, M.; de Mol, B.A.J.M.; Kock, L.M. Biomedical Applications of Photo- and Sono-Activated Rose Bengal: A Review. Photobiomodul. Photomed. Laser Surg. 2019, 37, 383–394. [Google Scholar] [CrossRef]
- Nakonechny, F.; Nisnevitch, M.; Nitzan, Y.; Nisnevitch, M. Sonodynamic Excitation of Rose Bengal for Eradication of Gram-Positive and Gram-Negative Bacteria. BioMed Res. Int. 2013, 684930, 1–7. [Google Scholar] [CrossRef]
- Hatanaka, S.; Mitome, H.; Yasui, K.; Hayashi, S. Single-Bubble Sonochemiluminescence in Aqueous Luminol Solutions. JACS Commun. 2002, 124, 10250–10251. [Google Scholar] [CrossRef]
- Van der Sluis, L.W.; Versluis, M.; Wu, M.K.; Wesselink, P.R. Passive ultrasonic irrigation of the root canal: A review of the literature. Int. Endod. J. 2007, 40, 415–426. [Google Scholar] [CrossRef]
- Wang, X.; Ip, M.; Wingnang, A.; Xu, C. Sonodynamic inactivation of methicillin-resistant Staphylococcus aureus in planktonic condition by curcumin under ultrasound sonication. Ultrasonics 2014, 54, 2109–2114. [Google Scholar] [CrossRef]
- Ghinzelli, G.C.; Souza, M.A.; Cecchin, D.; Farina, A.; de Figueiredo, J.A. Influence of ultrasonic activation on photodynamic therapy over root canal system infected with Enterococcus faecalis—An in vitro study. Photodiagnosis Photodyn. Ther. 2014, 11, 472–478. [Google Scholar] [CrossRef]
- Tennert, C.; Drews, A.M.; Walther, V.; Altenburger, M.J.; Karygianni, L.; Wrbas, K.T.; Hellwig, E.; Al-Ahmad, A. Ultrasonic activation and chemical modification of photosensitizers enhances the effects of photodynamic therapy against Enterococcus faecalis root-canal isolates. Photodiagnosis Photodyn. Ther. 2015, 12, 244–251. [Google Scholar] [CrossRef] [PubMed]
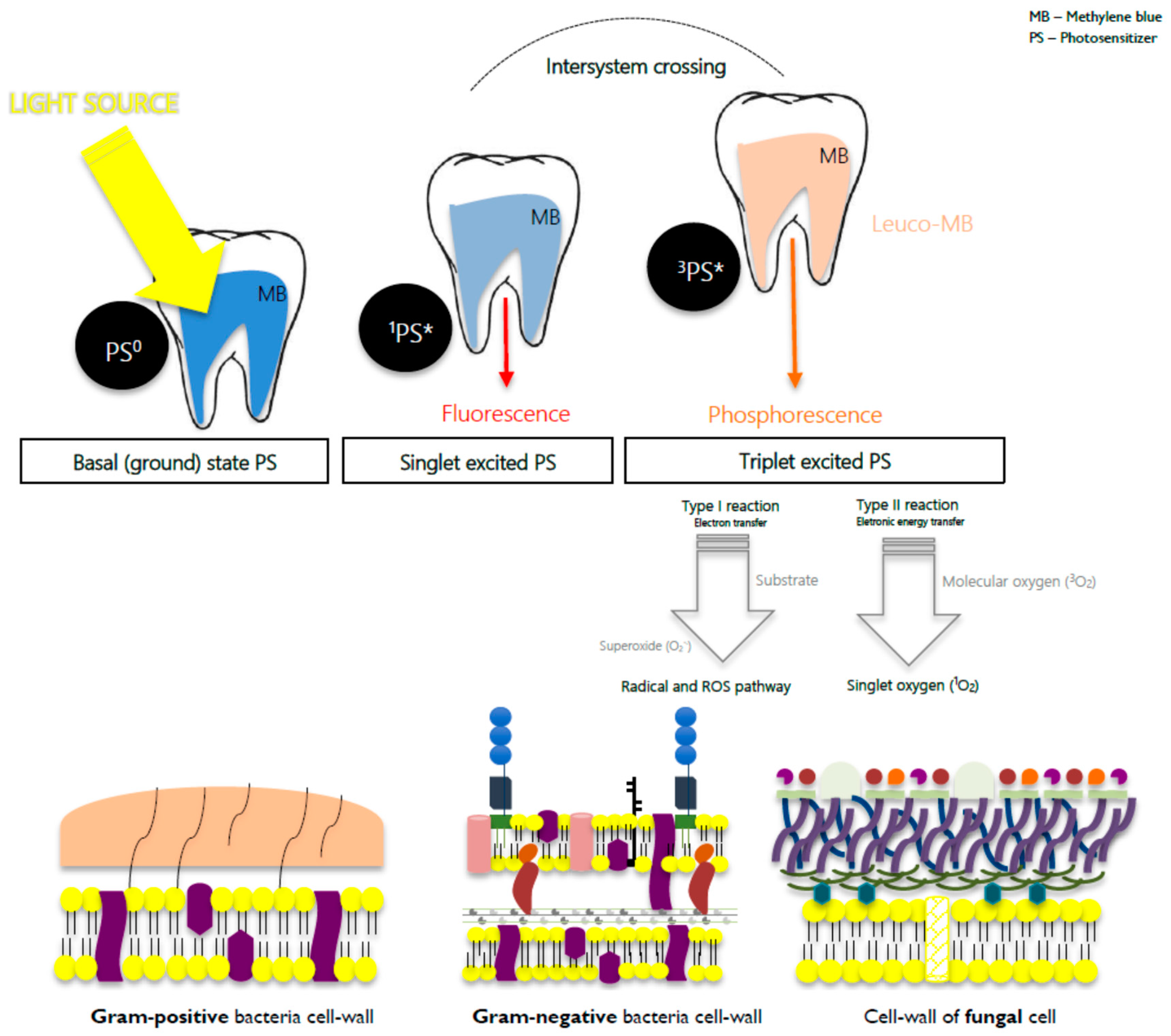
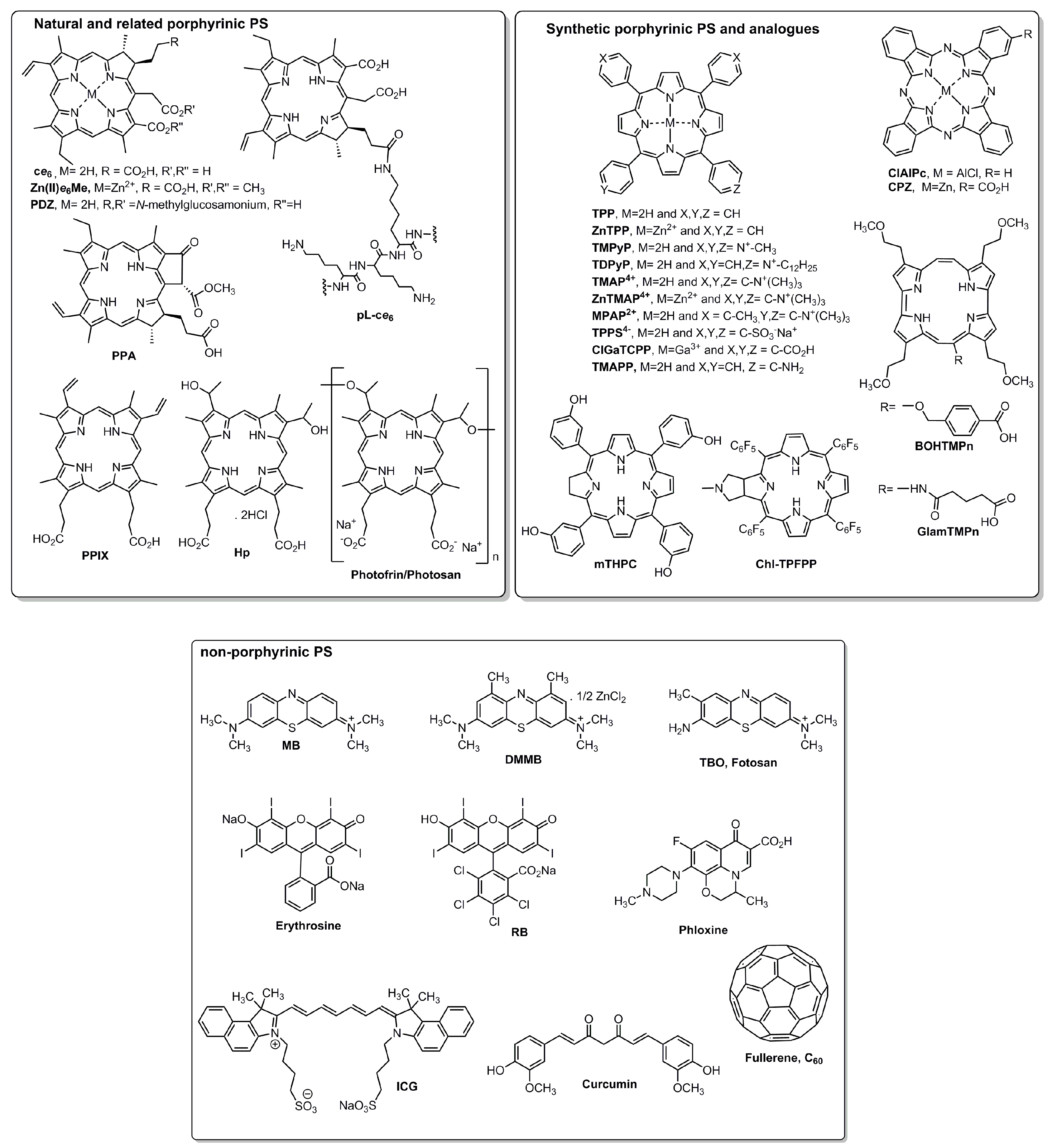
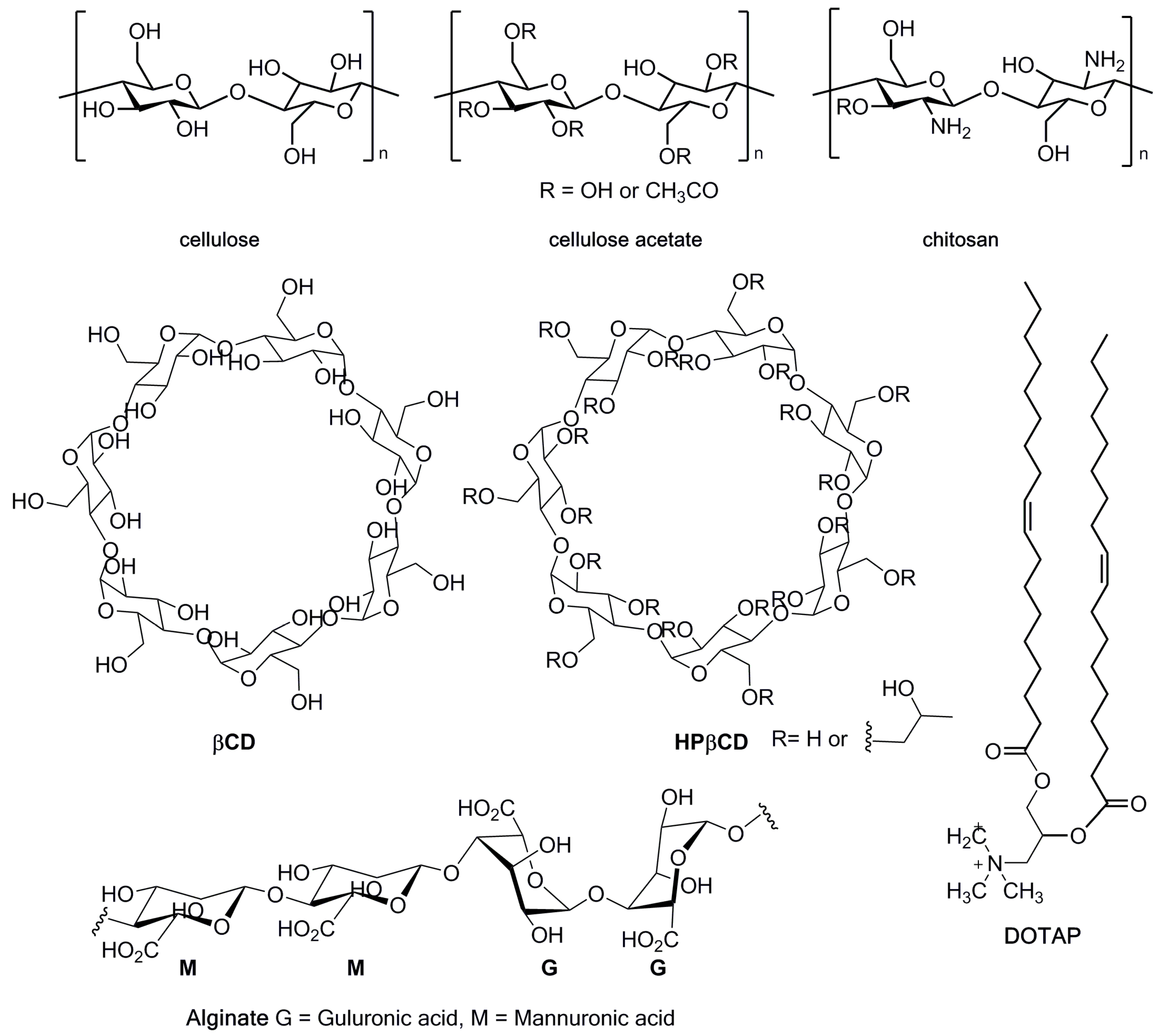
| System | Approach | PS/λ(nm) | Microorganism(s) | |
|---|---|---|---|---|
| Bacteriophage [19] | Supramacromolecule of DNA and Coated Proteins | PPA 658 | Candida albicans | |
| Drug Delivery Systems | Cellulose [23,24,25] | Cellulose acetate dissolved in acetone | TBO RB 610/545 | MRSA, Escherichia coli, Clostridium difficile, Bacteriophage, and C. albicans |
| Cellulosic fabric of β(1,4)-d-glucopyranose chains | TMAP 4+ ZnTMAP 4+ | Staphylococcus aureus, E. coli, and Pseudomonas aeruginosa | ||
| Chitosan [26] | Poly-β(1,4)-d-glucopyranosamine | RB MB 540 | Enterococcus faecalis P. aeruginosa | |
| Alginate Foam/ Cyclodextrins [27,28] | Foam formulations constituted by the gel-forming polymer sodium alginate, the gelling agent calcium carbonate, the plasticizers sorbitol and glycerol, the foaming agent hydroxypropylMethylcellulose, and as PS solubilizer agents β- and γ-cyclodextrins and polyethylene glycol 400 | Curcumin | Infected wounds | |
| Electrolyzed Water (EW) [29] | Water and salt (sodium chloride). Acid–EW and Alkaline–EW | RB Erythrosine Phloxine | Streptococcus mutans | |
| Hydrogel [30] | Cross-linked poly (vinyl alcohol) (PVA)–borate complexes | MB TMP 635 | MRSA | |
| Lipid Delivery System [31,32,33] | Invasome | mTHPC652 | E. faecalis | |
| Liposomes | TDPyP | MRSA | ||
| Micelles | Hp 635 | MRSA Staphylococcus epidermis Streptococcus pyogenes | ||
| Oil Based Emulsions [34,35] | Microemulsions | TBO 600 | P. aeruginosa | |
| Nanoemulsions | ClAlPc 660 | Methicillin-susceptible S. aureus and MRSA | ||
| System | Approach | PS/λ(nm) | Microorganism(s) | ||
|---|---|---|---|---|---|
| Metal–Organic Frameworks (MOFs) [54] | Metal ions coordinated to organic ligands with one-, two-, or three-dimensional structure | ICG 810 | E. faecalis | ||
| Nanoparticles (NPs) | Carbon [55,56,57,58,59] | Carbon Nanotubes | SWCNTs | H2TriMAPP 419 | S. aureus |
| MWCNTs | PPIX | ||||
| Nano-graphene oxide (NGO) | ICG 810 | E. faecalis | |||
| Fullerenes (C60, C70, and C84) in a closed sphere of carbon molecules | BF4-6 | S. aureus, E. coli, C. albicans, P. aeruginosa | |||
| LC16 | Acinetobacter baumannii, MRSA, C. albicans | ||||
| Chitosan [26] | Poly(d-glucosamine) | MB RB 540 | E. faecalis | ||
| Gold [60] | Colloidal gold particles complexed with poly lactic-co-glycolic acid (PLGA) | MB 665 | |||
| Platinum [61] | Platinum hexagonal nanoparticles | ClGaTCPP | S. aureus | ||
| Silica [62] | Pure SiO2 nanoparticles synthesized by hydrolysis of tetraethyl orthosilicate in reverse microemulsion | RB 525 | MRSA, S. epidermis | ||
| Silver [63] | Silver nitrate was dissolved in n-methylpyrrolidone and mixed with solution of PMMA in dichloroethane | TPP 405/470 | P. aeruginosa, S. aureus | ||
| Superparamagnetic Iron Oxide (SPIONs) [64] | Hematite (α-Fe2O3), maghemite (γ-Fe2O3), and magnetite (Fe3O4) | S. aureus, S. mutans, E. coli | |||
| System | Approach | PS/λ(nm) | Microorganism(s) | ||
|---|---|---|---|---|---|
| Efflux Pump Inhibitors (EPIs) [78,79] | NorA | Deficient mutants of Gram + | TBO MB DMMB pL–ce6 660 | S. aureus | |
| TolC | Deficient mutants of Gram − | E. coli | |||
| MexAB | P. aeruginosa | ||||
| Verapamil | MB 660 | E. faecalis | |||
| Light Delivery Systems | Optical fiber [80,81] | Optical fiber inside the root canal at the established working length (WL) with spiral movements for apical to cervical | MB 660 | ||
| Optical diffuser fiber within the canal | C. albicans | ||||
| Through Periapical Bone [82] | Experimental model with human premolars and molars in an acrylic resin bloc simulating the optical properties of a porcine jaw | TMPyPb MB 430 | E. faecalis | ||
| Negative Pressure System [83] | EndoVac® system (Discus Dental, Culver City, CA, USA) | MB 660 | E. faecalis | ||
| Systems | Approach | PS/λ(nm) | Microorganisms | |
|---|---|---|---|---|
| Peptides | Oligopeptides [100] | Aurein 1.2 (AU1.2) peptide with 13 amino acid residues | MB Chlorin e6 Curcumin 660 | E. faecalis, S. aureus, A. baumannii, E. coli, Enterococcus faecium, VRE |
| Polypeptides [101,102] | Poly-l-lysine hydrochloride added to porphycenes | BOHTMPn GlamTMPn 650 | E. coli, MRSA, C. albicans | |
| ε-Polylysine acquired from a commercial department | CPZ 630 | E. coli, S. aureus (two strains of non-resistant and one resistant to methicillin) | ||
| Approach | Materials or Methodologies | PS/λ(nm) | Microorganism(s) | ||
|---|---|---|---|---|---|
| OTHER APPROACHES FOR IMPROVING aPDT | PS Structural Features [59,102,111,112] | 3-Bromopropyl functionalized silica and Merrifield resin positively charged with 1-methylimidazole and pyridine | Chl-TPFPP | E. coli | |
| Amine groups and chains as coupling chemistry | LC16 | Acinetobacter baumannii, MRSA, C. albicans | |||
| Divalent cations such as Ca2+ and Mg2+ from CaCl2 and MgCl2 | TMAP4+ MPAP2+ TPPS4− | E. coli | |||
| Incubation Period [10] | Incubation periods of 5–15 min accordingly to several studies. | TBO RB TMPyP Zn(II)e6Me 557/627 | C. albicans, E. faecalis, dual-species biofilms (Ca:Ef) | ||
| Solubilizers [86,113,114,115,116] | 20% Citric Acid | TBO | E. faecalis | ||
| 70% Glycerol, 70% PEG, MIX (glycerol:ethanol:water 30:20:50), and H2O | MB | E. faecalis, Aggregatibacter actinomycetemcomitans | |||
| BHI Broth | MB | Porphyromonas gingivalis, Peptostreptococcus micros, Prevotella intermedia; E. faecalis, Fusobacterium nucleatum, Porphyromonas endodontalis | |||
| EDTA | 10% | Photosan | S. mutans, E. faecalis, A. actinomycetemcomitans | ||
| 20% | TBO | E. faecalis | |||
| H2O2 and Perfluorodecahydronaphthalene | MB | ||||
| Inorganic Salts [81,117,118,119] | Potassium iodide (KI) | LC16 | A. baumannii, MRSA, C. albicans | ||
| Photofrin | E. coli, P. aeruginosa, Klebsiella pneumoniae, Proteus mirabilis, A. baumannii, MRSA, C. albicans | ||||
| Potassium thiocyanate (KSCN) | MB | S. aureus, E. coli | |||
| Sodium azide (NaN3) | MBce6 660 | ||||
| Approach | Conditions | PS/λ(nm) | Microorganism(s) | |
|---|---|---|---|---|
| Sonodynamic Therapy (SDT) | Ultrasonic Activation [116,147,148] | 28 kHz | RB MB | S. aureus, E. coli |
| 28–36 kHz VDW Ultra-Device | TBO | E. faecalis | ||
| Passive ultrasonic irrigation (PUI) | MB 660–690 | |||
| Ultrasound Sonication [143,149] | 1 MHz | Curcumin | MRSA | |
| PDZ RB 660 | C. albicans | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diogo, P.; F. Faustino, M.A.; P. M. S. Neves, M.G.; Palma, P.J.; P. Baptista, I.; Gonçalves, T.; Santos, J.M. An Insight into Advanced Approaches for Photosensitizer Optimization in Endodontics—A Critical Review. J. Funct. Biomater. 2019, 10, 44. https://doi.org/10.3390/jfb10040044
Diogo P, F. Faustino MA, P. M. S. Neves MG, Palma PJ, P. Baptista I, Gonçalves T, Santos JM. An Insight into Advanced Approaches for Photosensitizer Optimization in Endodontics—A Critical Review. Journal of Functional Biomaterials. 2019; 10(4):44. https://doi.org/10.3390/jfb10040044
Chicago/Turabian StyleDiogo, Patrícia, M. Amparo F. Faustino, M. Graça P. M. S. Neves, Paulo J. Palma, Isabel P. Baptista, Teresa Gonçalves, and João Miguel Santos. 2019. "An Insight into Advanced Approaches for Photosensitizer Optimization in Endodontics—A Critical Review" Journal of Functional Biomaterials 10, no. 4: 44. https://doi.org/10.3390/jfb10040044
APA StyleDiogo, P., F. Faustino, M. A., P. M. S. Neves, M. G., Palma, P. J., P. Baptista, I., Gonçalves, T., & Santos, J. M. (2019). An Insight into Advanced Approaches for Photosensitizer Optimization in Endodontics—A Critical Review. Journal of Functional Biomaterials, 10(4), 44. https://doi.org/10.3390/jfb10040044











