Impact of Bi2O3 and ZrO2 Radiopacifiers on the Early Hydration and C–S–H Gel Structure of White Portland Cement
Abstract
1. Introduction
2. Results
2.1. Initial and Final Setting Times
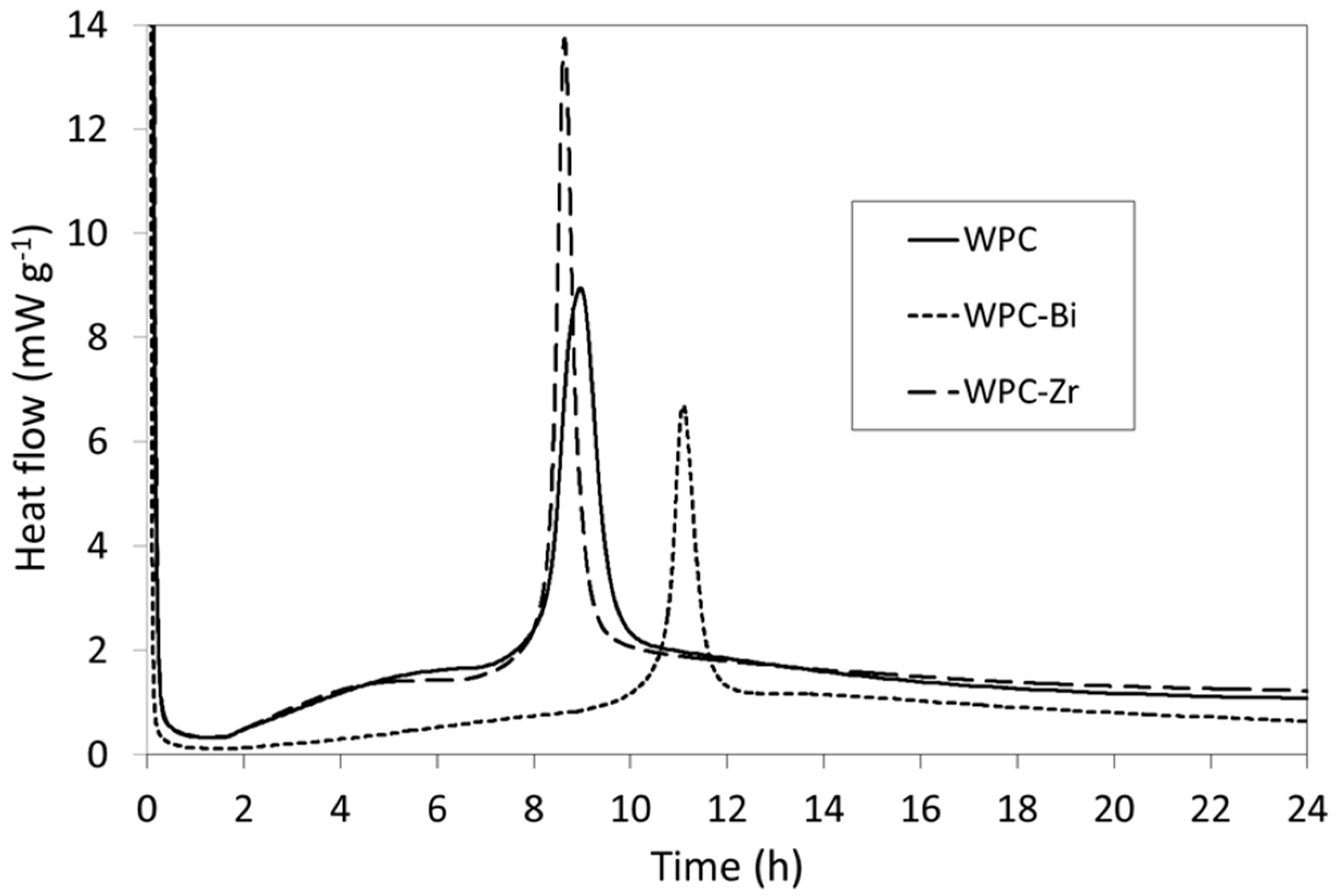
2.2. Isothermal Conduction Calorimetry
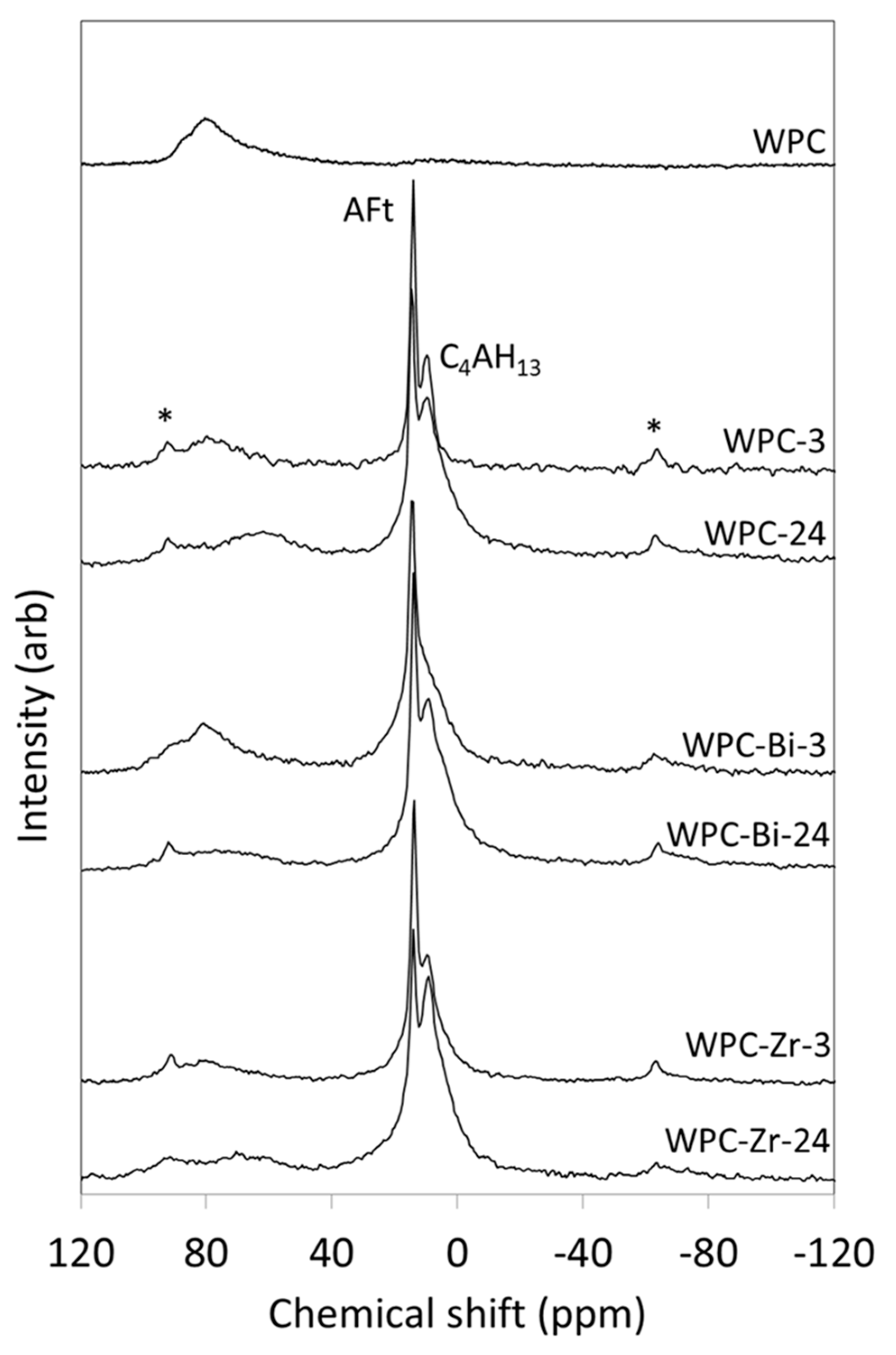
2.3. 27Al MAS NMR Spectroscopy
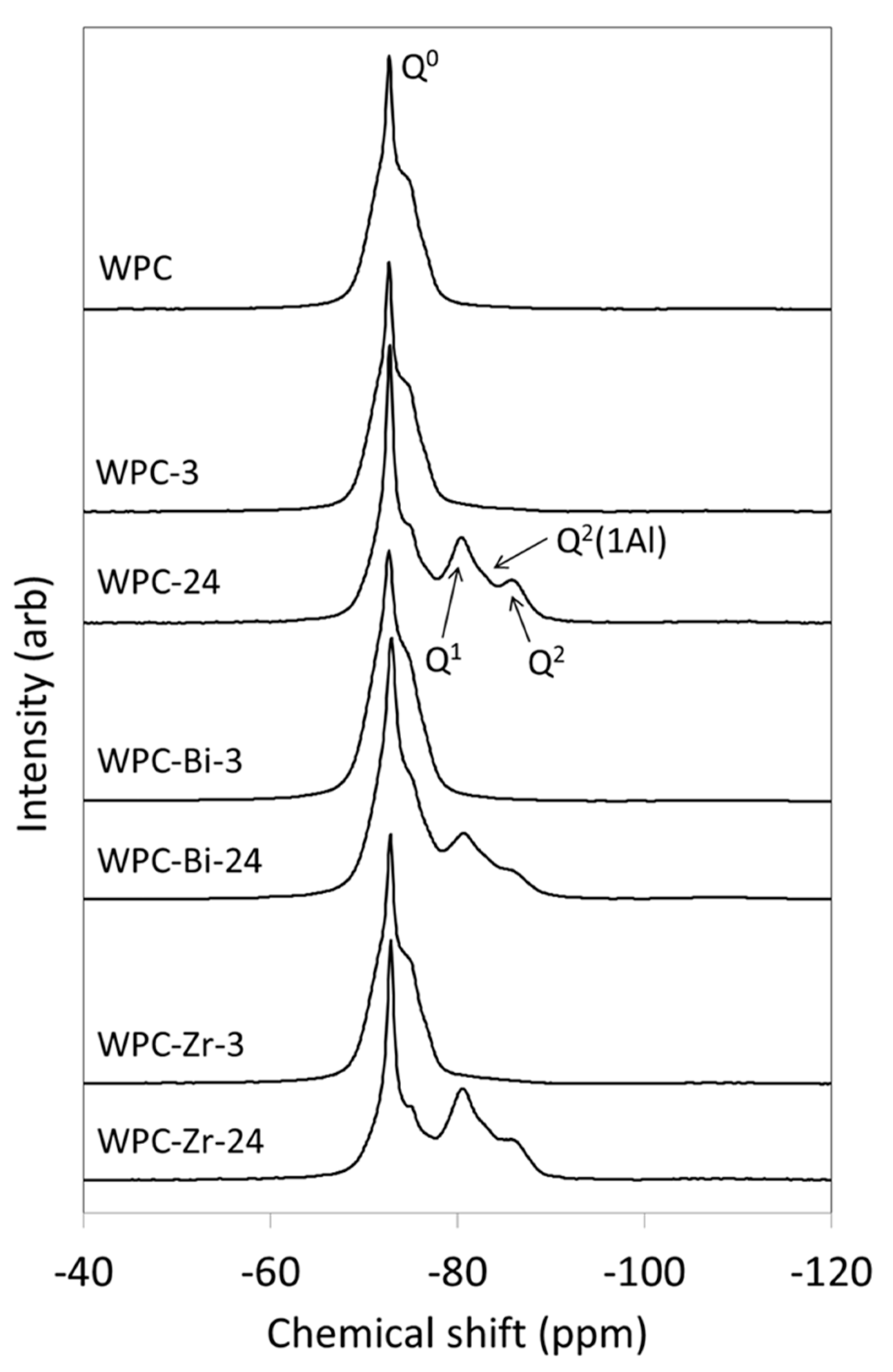
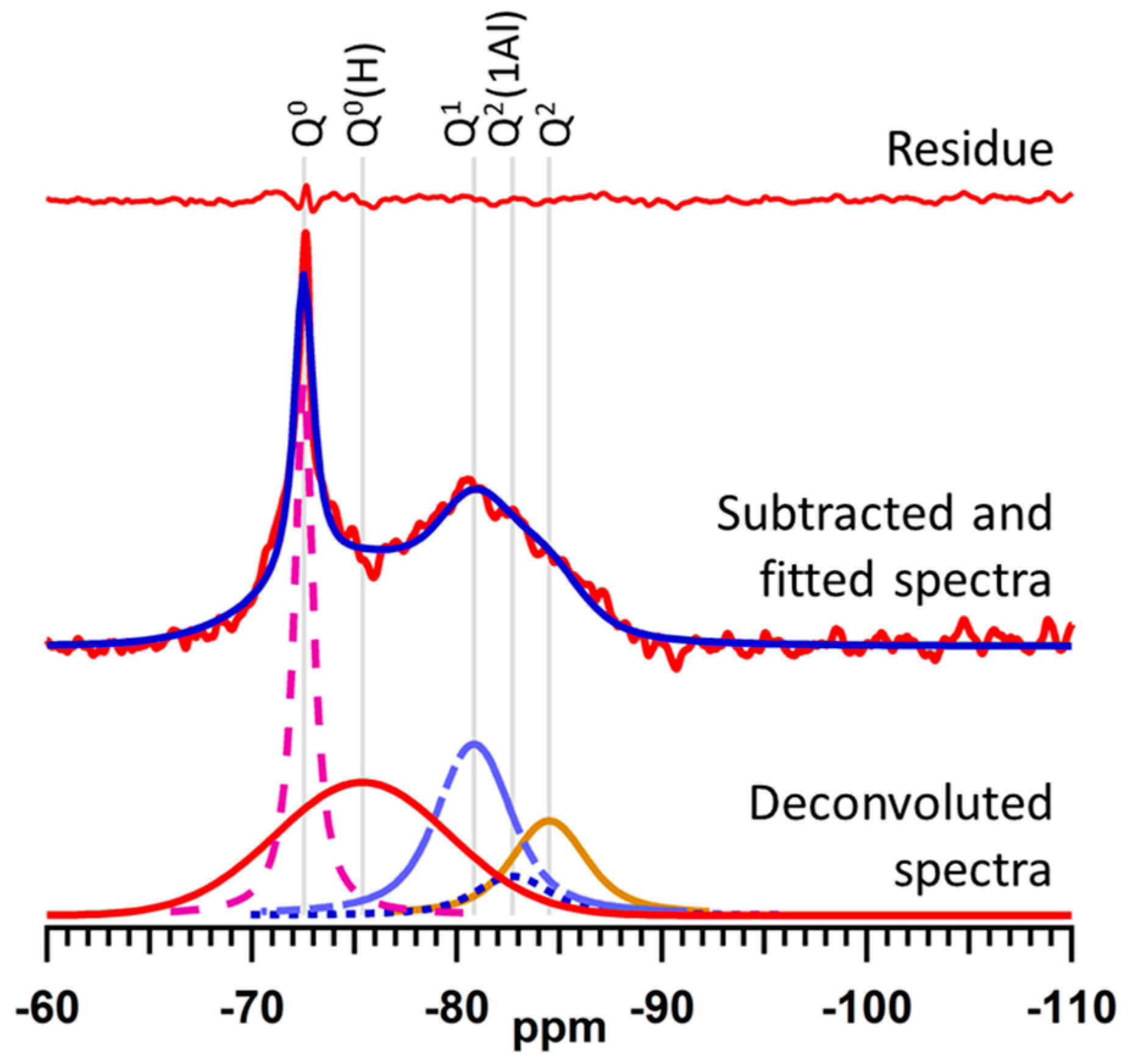
2.4. 29Si MAS NMR Spectroscopy
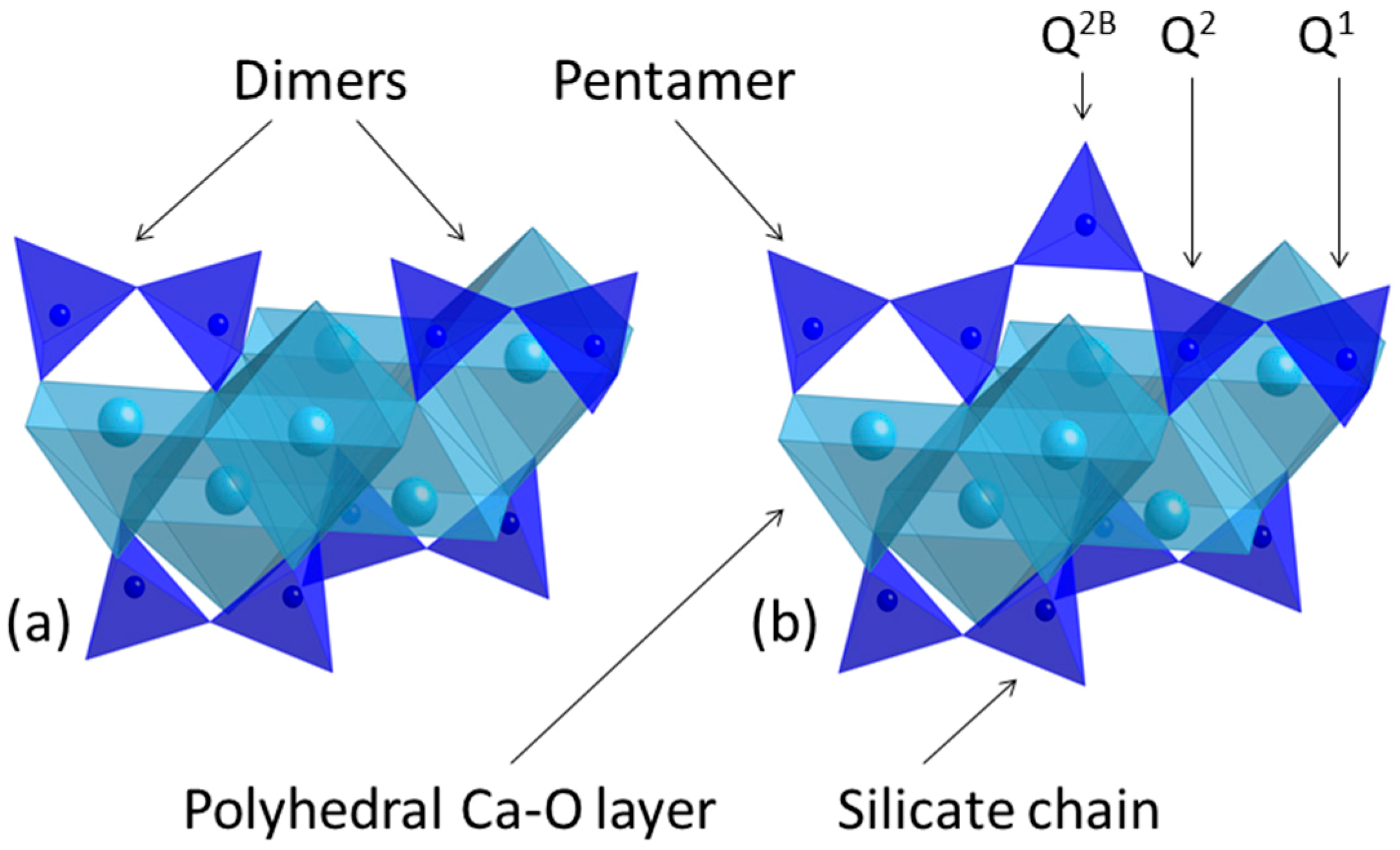
2.5. Structure of C–S–H Gel
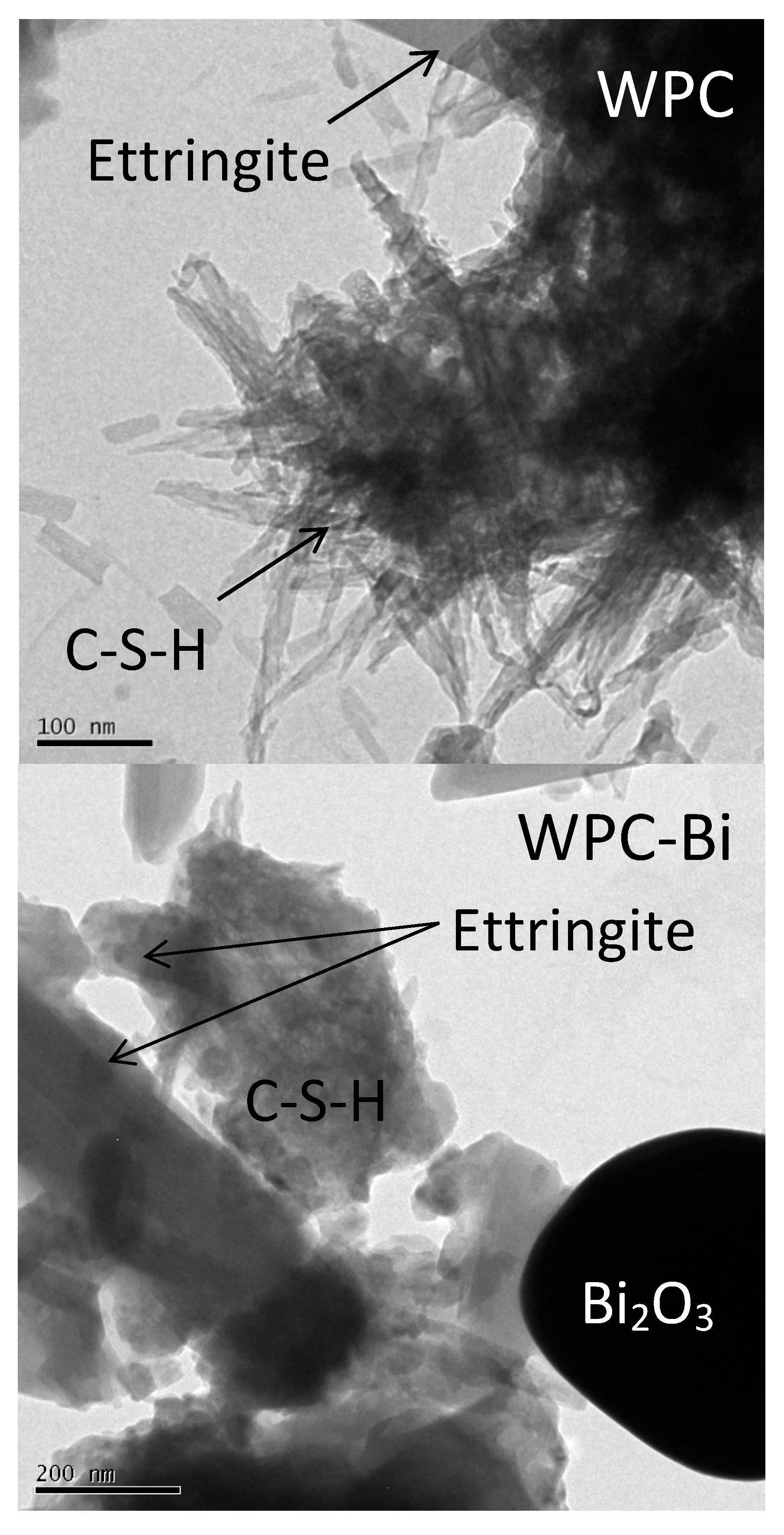
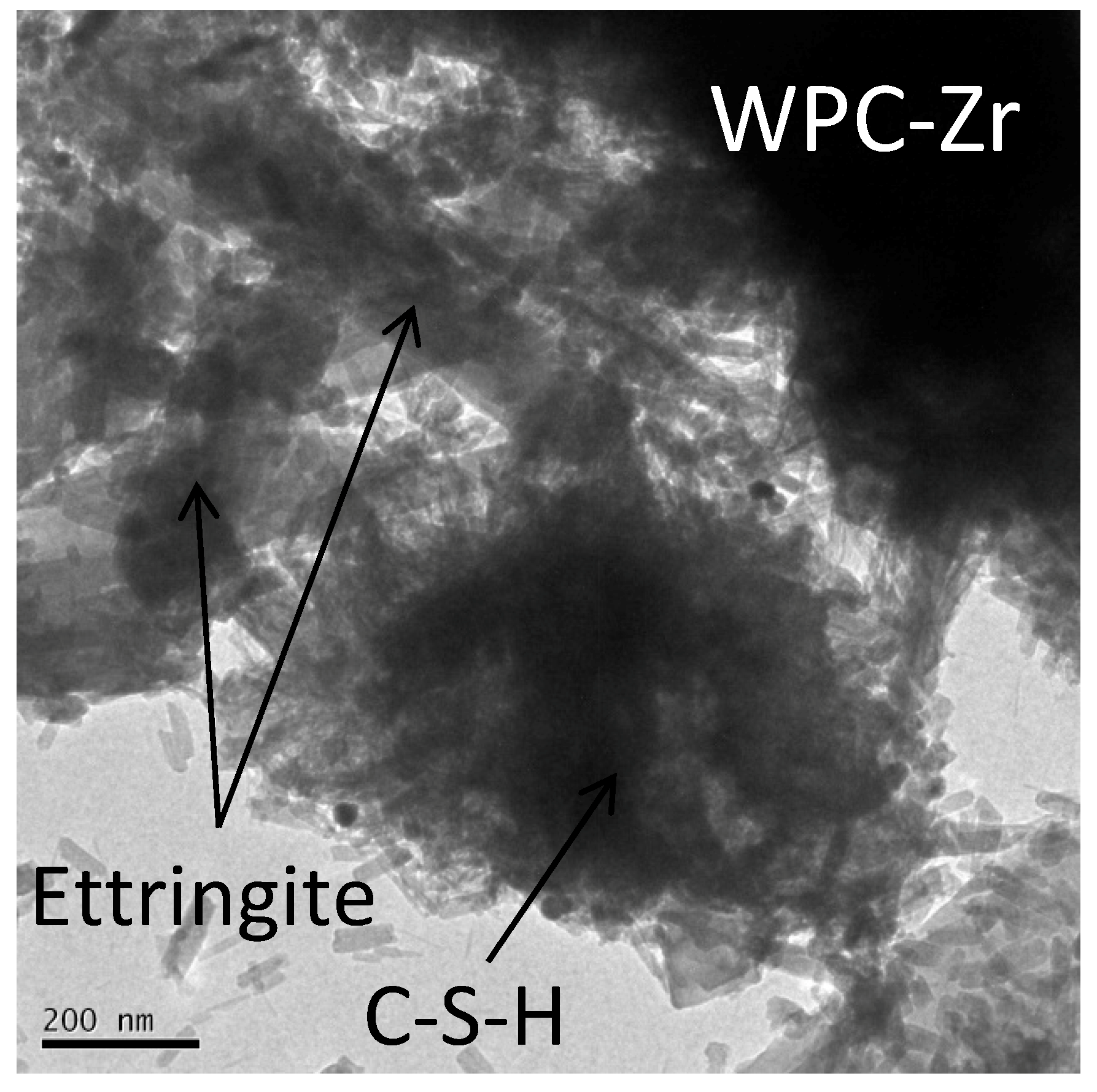
2.6. Transmission Electron Microscopy (TEM)
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Initial and Final Setting Times
4.3. Isothermal Conduction Calorimetry
4.4. 27Al and 29Si Magic Angle Spinning Nuclear Magnetic Resonance Spectroscopy
4.5. Deconvolution and Quantitative Analysis of 29Si MAS NMR Spectra
4.6. Transmission Electron Microscopy
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Parirokh, M.; Torabinejad, M.; Dummer, P.M.H. Mineral trioxide aggregate and other bioactive endodontic cements: An updated overview—Part I: Vital pulp therapy. Int. Endod. J. 2018, 51, 177–205. [Google Scholar] [CrossRef] [PubMed]
- Torabinejad, M.; Parirokh, M.; Dummer, P.M.H. Mineral trioxide aggregate and other bioactive endodontic cements: An updated overview—Part II: Other clinical applications and complications. Int. Endod. J. 2018, 51, 284–317. [Google Scholar] [CrossRef] [PubMed]
- Dawood, A.E.; Parashos, P.; Wong, R.H.K.; Reynolds, E.C.; Manton, D.J. Calcium silicate-based cements: Composition, properties, and clinical applications. J. Investig. Clin. Dent. 2017, 8, e12195. [Google Scholar] [CrossRef] [PubMed]
- Emara, R.; Elhennawy, K.; Schwendicke, F. Effects of calcium silicate cements on dental pulp cells: A systematic review. J. Dent. 2018, 77, 18–36. [Google Scholar] [CrossRef]
- Donnermeyer, D.; Burklein, S.; Dammaschke, T.; Schäfer, E. Endodontic sealers based on calcium silicates: A systematic review. Odontology 2019, 107, 421–436. [Google Scholar] [CrossRef]
- Prati, C.; Gandolfi, M.G. Calcium silicate bioactive cements: Biological perspectives and clinical applications. Dent. Mater. 2015, 31, 351–370. [Google Scholar] [CrossRef]
- Ha, W.N.; Nicholson, T.; Kahler, B.; Walsh, L.J. Mineral trioxide aggregate—A review of properties and testing methodologies. Materials 2017, 10, 1261. [Google Scholar] [CrossRef]
- ISO 6876:2012 Dental Root Canal Sealing Materials, 3rd ed.; International Organization for Standardization: Geneva, Switzerland, 2012.
- ANSI/ADA Specification 57: Endodontic Sealing Material; American National Standards Institute/American Dental Association: Chicago, IL, USA, 2000.
- Coleman, N.J.; Li, Q. The impact of iodoform on the hydration, bioactivity and antimicrobial properties of white Portland cement. MATEC Web Conf. 2017, 109, 04002. [Google Scholar] [CrossRef]
- Coleman, N.J.; Hanarasinghe, R.; Güçlü, Z.A.; Booth, S.E. In vitro bioactivity and setting times of white Portland cement combined with different radio pacifying agents. MATEC Web Conf. 2017, 109, 03003. [Google Scholar] [CrossRef]
- Elsaka, S.E.; Elnaghy, A.M.; Mandorah, A.; Elshazli, A.H. Effect of titanium tetrafluoride addition on the physicochemical and antibacterial properties of Biodentine as intraorfice barrier. Dent. Mater. 2019, 35, 185–193. [Google Scholar] [CrossRef]
- Ochoa-Rodríguez, V.M.; Tanomaru-Filho, M.; Rodrigues, E.M.; Guerreiro-Tanomaru, J.M.; Spin-Neto, R.; Faria, G. Addition of zirconium oxide to Biodentine increases radiopacity and does not alter its physicochemical and biological properties. J. Appl. Oral Sci. 2019, 27, e20180429. [Google Scholar] [CrossRef] [PubMed]
- Cost, B.C.; Guerreiro-Tanomaru, J.M.; Bosso-Martelo, R.; Rodrigues, E.M.; Bonetti-Filho, I.; Tanomaru-Filho, M. Ytterbium oxide as radiopacifier of calcium silicate-based cements. Physicochemical and biological properties. Braz. Dent. J. 2018, 29, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Antonijevic, D.; Medigovic, I.; Zrilic, M.; Jokic, B.; Vukovic, Z.; Todorovic, L. The influence of different radiopacifying agents on the radiopacity, compressive strength, setting time, and porosity of Portland cement. Clin. Oral Investig. 2014, 18, 1597–1604. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Coleman, N.J. Early hydration of white Portland cement in the presence of bismuth oxide. Adv. Appl. Ceram. 2013, 112, 207–212. [Google Scholar] [CrossRef]
- Coleman, N.J.; Li, Q. The impact of zirconium oxide radiopacifier on the early hydration behaviour of white Portland cement. Mater. Sci. Eng. C 2013, 33, 427–433. [Google Scholar] [CrossRef]
- Wang, J.; Han, B.; Li, Z.; Yu, X.; Dong, X. Effect investigation of nanofillers on C-S-H gel structure with Si NMR. J. Mater. Civ. Eng. 2019, 31, 04018352. [Google Scholar] [CrossRef]
- Silva, G.F.; Bosso, R.; Ferino, R.V.; Tanomaru-Filho, M.; Bernardi, M.I.B.; Guerreiro-Tanomaru, J.M.; Cerri, P.S. Microparticulated and nanoparticulated zirconium oxide added to calcium silicate cement: Evaluation of physicochemical and biological properties. J. Biomed. Mater. Res. Part. A 2014, 102, 4336–4345. [Google Scholar] [CrossRef]
- Li, Q.; Coleman, N.J. The hydration chemistry of ProRoot MTA. Dent. Mater. J. 2015, 34, 458–465. [Google Scholar] [CrossRef]
- Li, Q.; Hurt, A.P.; Coleman, N.J. The Application of 29Si NMR Spectroscopy to the Analysis of Calcium Silicate-Based Cement Using Biodentine as an Example. J. Funct. Biomater. 2019, 10, 25. [Google Scholar] [CrossRef]
- Hou, D.; Ma, H.; Li, Z. Morphology of calcium silicate hydrate (C-S-H) gel: A molecular dynamic study. Adv. Cem. Res. 2015, 27, 135–146. [Google Scholar] [CrossRef]
- Pustovgar, E.; Sangodkar, R.P.; Andreev, A.S.; Palacios, M.; Chmelka, B.F.; Flatt, R.J.; d’Espinose de Lacaillerie, J.-B. Understanding silicate hydration from quantitative analyses of hydrating tricalcium silicates. Nat. Commun. 2016, 7, 10952. [Google Scholar] [CrossRef] [PubMed]
- Ayuela, A.; Dolado, J.S.; Campillo, I.; de Miguel, Y.R.; Erkizia, E.; Sánchez-Portal, D.; Echenique, P.M. Silicate chain formation in the nanostructure of cement-based materials. J. Chem. Phys. 2007, 127, 164710. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.; Dai, Z.; Enemark-Rasmussen, K.; Skibsted, J. Pozzolanic reactivity of thermally activated kaolinite and montmorillonite in Portland cement blends and their impact on the formed C-S-H phase. In Proceedings of the 14th International Congress on the Chemistry of Cement, Beijing, China, 13–16 October 2015. [Google Scholar]
- ASTM C191-08, Standard Test Methods for Time of Setting of Hydraulic Cement by Vicat Needle; ASTM International: West Conshohocken, PA, USA, 2008. [CrossRef]
- Gartner, E.M.; Young, J.F.; Damidot, D.A.; Jawed, I. Hydration of Portland cement. In Structure and Performance of Cements, 2nd ed.; Bensted, J., Barnes, P., Eds.; Spon Press: London, UK, 2002; pp. 57–113. [Google Scholar]
- Taylor, H.F.W. Cement Chemistry; Academic Press: London, UK, 1990. [Google Scholar]
- Love, C.A.; Richardson, I.G.; Brough, A.R. Composition and structure of C-S-H in white Portland cement-20% metakaolin pastes hydrated at 25 °C. Cem. Concr. Res. 2007, 37, 109–117. [Google Scholar] [CrossRef]
- Justnes, H.; Meland, I.; Bjoergum, O.; Krane, J.; Skjetne, T. Nuclear magnetic resonance—A powerful tool in cement and concrete research. Adv. Cem. Res. 1990, 3, 105–110. [Google Scholar] [CrossRef]
- Andersen, M.D.; Jakobsen, H.J.; Skibsted, J. Characterization of white Portland cement hydration and the C-S-H structure in the presence of sodium aluminate by 27Al and 29Si MAS NMR spectroscopy. Cem. Concr. Res. 2004, 34, 857–868. [Google Scholar] [CrossRef]
- Skibsted, J.; Jakobsen, H.J.; Hall, C. Direct observations of aluminium guest ions in the silicate phases of cement minerals by 27Al MAS NMR spectroscopy. J. Chem. Soc. Faraday. Trans. 1994, 90, 2095–2098. [Google Scholar] [CrossRef]
- Engelhardt, G.; Michel, D. High-Resolution Solid State NMR of Silicates and Zeolites; John Wiley & Sons: Chichester, UK, 1987. [Google Scholar]
- Sáez del Bosque, I.F.; Martínez-Ramírez, S.; Martín-Pastor, M.; Blanco-Varela, M.T. Effect of temperature on C-S-H gel nanostructure in white cement. Mater. Struct. 2014, 47, 1867–1878. [Google Scholar] [CrossRef]
- Ruiz-Clavijo, A.; Hurt, A.P.; Kotha, A.K.; Coleman, N.J. Effect of calcium precursor on the bioactivity and biocompatibility of sol-gel-derived glasses. J. Funct. Biomater. 2019, 10, 13. [Google Scholar] [CrossRef]
- Ouyang, X.; Koleva, D.A.; Ye, G.; van Breugel, K. Insights into the mechanisms of nucleation and growth of C-S-H on fillers. Mater. Struct. 2017, 50, 213. [Google Scholar] [CrossRef]
- Kadri, E.H.; Aggoun, S.; De Schutter, G.; Ezziane, K. Combined effect of chemical nature and fineness of mineral powders on Portland cement hydration. Mater. Struct. 2010, 43, 665–673. [Google Scholar] [CrossRef]
- Lai, Y.-W.; Wei, W.-C.J. Synthesis and study on ionic conductive (Bi1−x,Vx)O1.5−δ materials with a dual-phase microstructure. Materials 2016, 9, 863. [Google Scholar]
- Hincapié, C.M.B.; Cárdenas, M.J.P.; Orjuela, J.E.A.; Parra, E.R.; Florez, J.J.O. Physical-chemical properties of bismuth and bismuth oxides: Synthesis, characterization and applications. Dyna 2012, 79, 139–148. [Google Scholar]
- Coomaraswamy, K.S.; Lumley, P.J.; Hofmann, M.P. Effect of bismuth oxide radioopacifier content on the material properties of an endodontic Portland cement-based (MTA-like) system. J. Endod. 2007, 33, 295–298. [Google Scholar] [CrossRef] [PubMed]
| Cement | WPC | WPC-Bi | WPC-Zr |
|---|---|---|---|
| Initial set (min) | 143 ± 11 a | 255 ± 21 b | 163 ± 4 a |
| Final set (min) | 190 ± 1 c | 360 ± 14 d | 195 ± 10 c |
| Sample | Time (h) | Q0(H) (%) | Q1 (%) | Q2(1Al) (%) | Q2 (%) | Hydration (%) | MCL | Al/Si |
|---|---|---|---|---|---|---|---|---|
| WPC | 3 | 0.58 | 0.47 | 0.18 | 0.20 | 1.4 | 4.0 | 0.062 |
| 24 | 1.41 | 23.54 | 5.87 | 11.44 | 42.2 | 3.7 | 0.069 | |
| WPC-Bi | 3 | 2.05 | 0.15 | 0.00 | 0.00 | 2.2 | 2.0 | 0.000 |
| 24 | 2.54 | 12.83 | 7.11 | 6.13 | 28.6 | 4.6 | 0.124 | |
| WPC-Zr | 3 | 0.94 | 0.66 | 0.15 | 0.37 | 2.1 | 3.8 | 0.036 |
| 24 | 3.57 | 26.62 | 10.49 | 11.19 | 51.9 | 4.0 | 0.101 |
| Major Oxide Components | Minor Oxide Components | Major Crystalline Phases | |||
|---|---|---|---|---|---|
| Formula | Mass (%) | Formula | Mass (%) | Formula | Mass (%) |
| CaO | 69.2 | MgO | 0.49 | Ca3SiO5 | 65 |
| SiO2 | 25.0 | P2O5 | 0.43 | Ca2SiO4 | 22 |
| Al2O3 | 1.76 | Fe2O3 | 0.33 | Ca3Al2O6 | 4.1 |
| SO3 | 2.00 | SrO | 0.14 | Ca2(Al/Fe)O5 | 1.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Coleman, N.J. Impact of Bi2O3 and ZrO2 Radiopacifiers on the Early Hydration and C–S–H Gel Structure of White Portland Cement. J. Funct. Biomater. 2019, 10, 46. https://doi.org/10.3390/jfb10040046
Li Q, Coleman NJ. Impact of Bi2O3 and ZrO2 Radiopacifiers on the Early Hydration and C–S–H Gel Structure of White Portland Cement. Journal of Functional Biomaterials. 2019; 10(4):46. https://doi.org/10.3390/jfb10040046
Chicago/Turabian StyleLi, Qiu, and Nichola J. Coleman. 2019. "Impact of Bi2O3 and ZrO2 Radiopacifiers on the Early Hydration and C–S–H Gel Structure of White Portland Cement" Journal of Functional Biomaterials 10, no. 4: 46. https://doi.org/10.3390/jfb10040046
APA StyleLi, Q., & Coleman, N. J. (2019). Impact of Bi2O3 and ZrO2 Radiopacifiers on the Early Hydration and C–S–H Gel Structure of White Portland Cement. Journal of Functional Biomaterials, 10(4), 46. https://doi.org/10.3390/jfb10040046






