Biomaterials, Current Strategies, and Novel Nano-Technological Approaches for Periodontal Regeneration
Abstract
1. Current Strategies in Periodontal Tissue Engineering
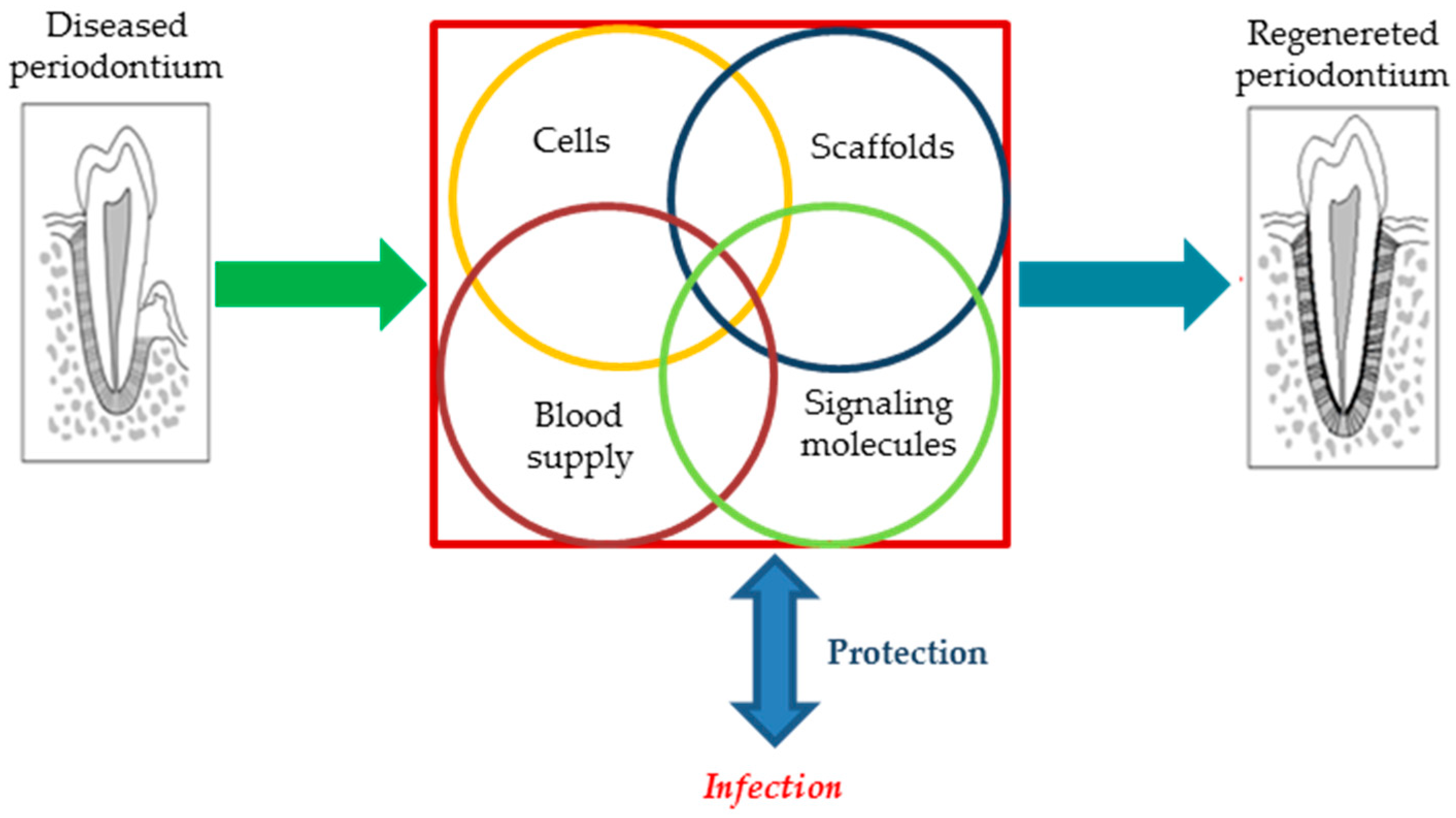
2. Inside Periodontal Regeneration—A More In-Depth Overview
3. Biomaterials for Periodontal Tissue Engineering
3.1. Bone Transplant Materials
3.1.1. Autografts
3.1.2. Allografts
3.1.3. Xenografts
3.2. Need for Alternatives to Bone Transplantation
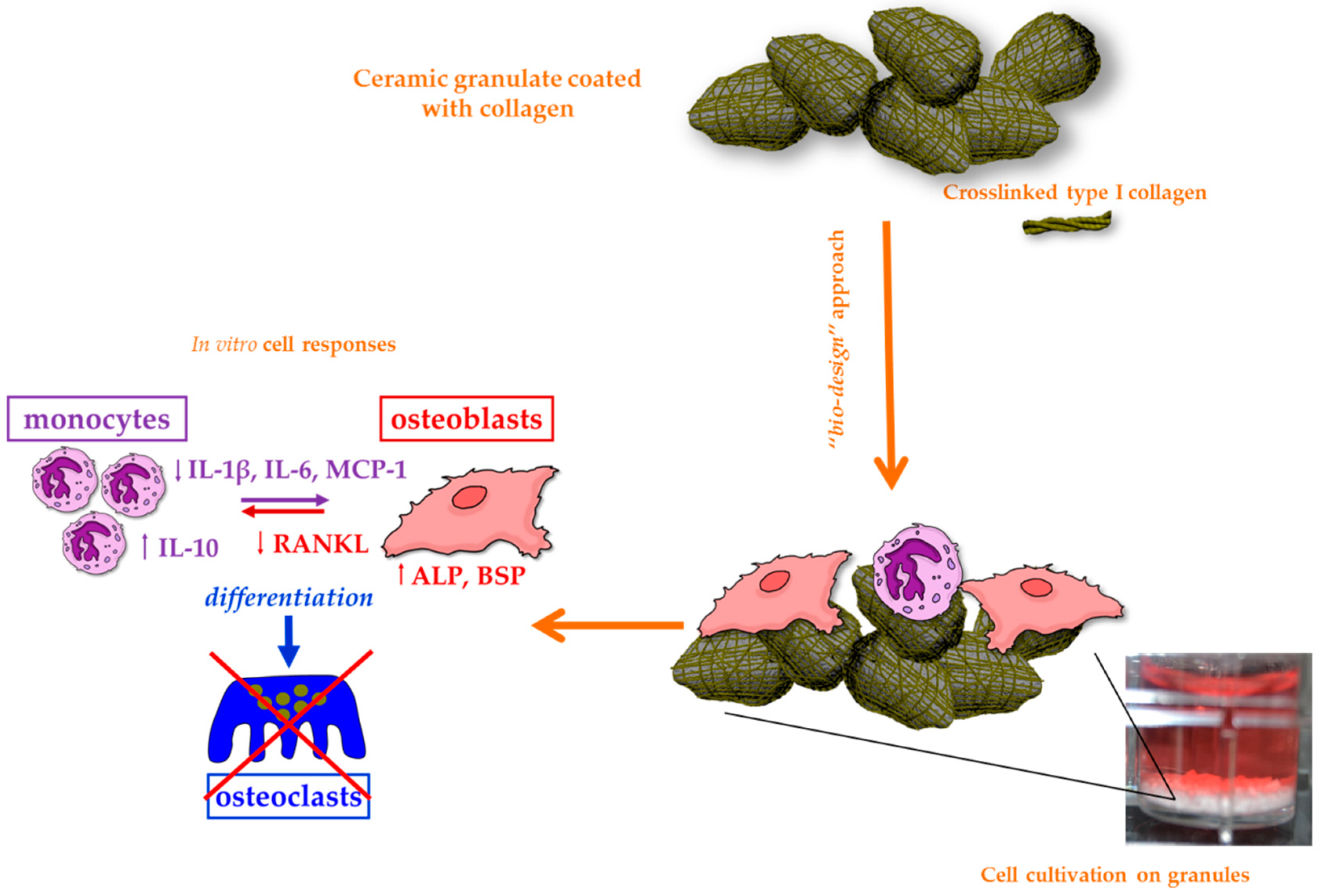
3.3. Bioceramics
3.3.1. Calcium Phosphates
3.3.2. Bioactive Glasses
3.3.3. Calcium Sulfate
3.4. Polymers
3.4.1. Collagen
3.4.2. Chitosan
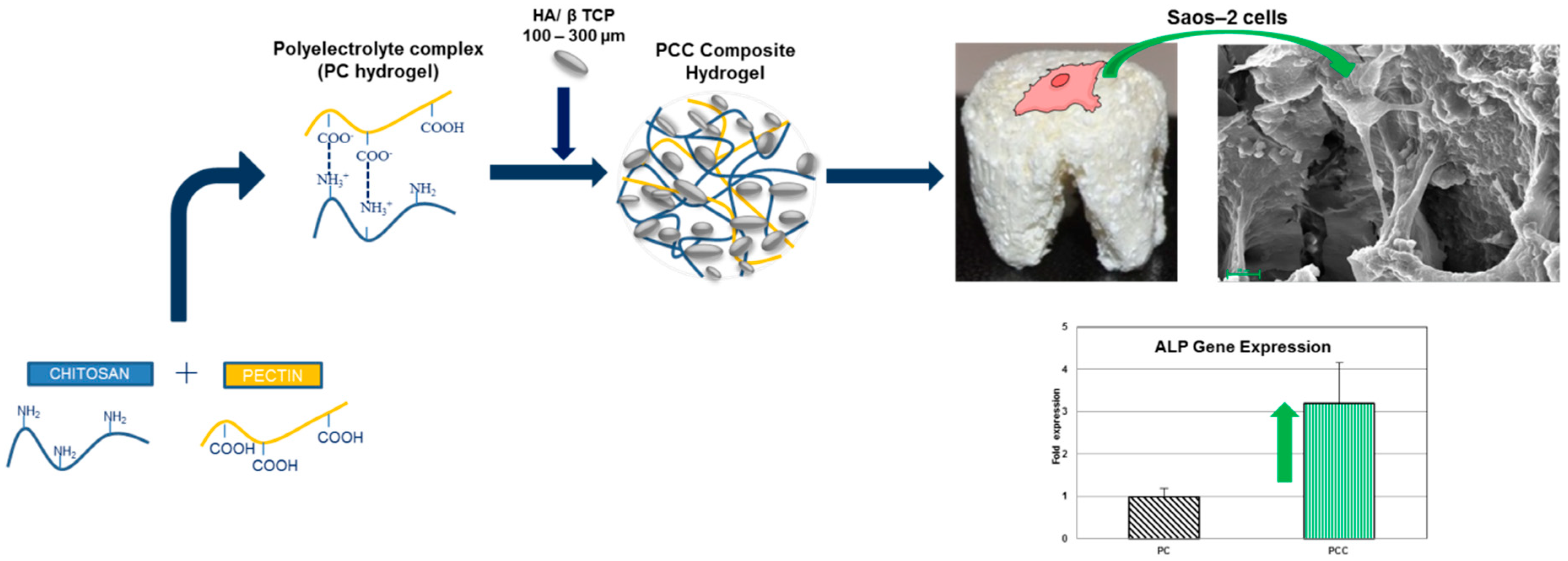
3.4.3. Pectin
3.4.4. Alginate
3.4.5. Hyaluronic Acid
4. Porous Scaffolds for Bone Grafting and Periodontal Tissue Engineering
5. Guided Tissue Regeneration (GTR) Membranes
5.1. Non-Resorbable Membranes
5.2. Resorbable Membranes
6. Growth Factors in Periodontal Tissue Engineering
6.1. Platelet-Rich Growth Factor (PDGF)
6.2. Bone Morphogenetic Proteins (BMPs)
6.3. Enamel Matrix Derivatives (EMDs)
7. Gene Therapy Approach in Periodontology
8. Nanobiomaterials and Functionally-Graded Implants: The Last Frontiers in Periodontal Tissue Engineering
9. Discussion and Conclusions
Funding
Conflicts of Interest
References
- Frencken, J.E.; Sharma, P.; Stenhouse, L.; Green, D.; Laverty, D.; Dietrich, T. Global epidemiology of dental caries and severe periodontitis—A comprehensive review. J. Clin. Periodontol. 2017, 44, S94–S105. [Google Scholar] [CrossRef] [PubMed]
- Snauwaert, K.; Duyck, J.; van Steenberghe, D.; Quirynen, M.; Naert, I. Time dependent failure rate and marginal bone loss of implant supported prostheses: A 15-year follow-up study. Clin. Oral Investig. 2000, 4, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Lekholm, U.; Gunne, J.; Henry, P.; Higuchi, K.; Lindén, U.; Bergström, C.; Van Steenberghe, D. Survival of the Brånemark implant in partially edentulous jaws: A 10-year prospective multicenter study. Int. J. Oral Maxillofac. Implants 1999, 14, 639–645. [Google Scholar] [PubMed]
- Gaviria, L.; Salcido, J.P.; Guda, T.; Ong, J.L. Current trends in dental implants. J. Korean Assoc. Oral Maxillofac. Surg. 2014, 40, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Simonis, P.; Dufour, T.; Tenenbaum, H. Long-term implant survival and success: A 10–16-year follow-up of non-submerged dental implants. Clin. Oral Implants Res. 2010, 21, 772–777. [Google Scholar] [CrossRef]
- Bashutski, J.D.; Wang, H.-L. Periodontal and endodontic regeneration. J. Endod. 2009, 35, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Shimauchi, H.; Nemoto, E.; Ishihata, H.; Shimomura, M. Possible functional scaffolds for periodontal regeneration. Jpn. Dent. Sci. Rev. 2013, 49, 118–130. [Google Scholar] [CrossRef]
- Illueca, F.; Vera, P.B.; Cabanilles, P.; Fernanades, V.; Loscos, F. Periodontal regeneration in clinical practice. Med. Oral Patol. Oral Cir. Bucal 2006, 11, 382–392. [Google Scholar]
- Rosling, B.; Nyman, S.; Lindhe, J.; Jern, B. The healing potential of the periodontal tissues following different techniques of periodontal surgery in plaque-free dentitions: A 2-year clinical study. J. Clin. Periodontol. 1976, 3, 233–250. [Google Scholar] [CrossRef] [PubMed]
- Prichard, J. The infrabony technique as a predictable procedure. J. Clin. Periodontol. 1957, 28, 202–216. [Google Scholar] [CrossRef]
- Proeslakis, G.; Soderholm, G.; Bratlhall, G.; Kullendorff, B.; Gröndah1, K.; Rohlin, M.; Attström, R. Gingivectomy versus flap surgery: The effect of the treatment of infrabony defects: A clinical and radio-graphic study. J. Clin. Periodontol. 1992, 19, 497–508. [Google Scholar] [CrossRef]
- Renvert, S.; Nilvéus, R.; Egelberg, J. Healing after treatment of periodontal intraosseous defects: V. Effect of root planing versus flap surgery. J. Clin. Periodontol. 1985, 12, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P. Focus on intrabony defects—Conservative therapy. Periodontology 2000 2000, 22, 51–58. [Google Scholar] [CrossRef]
- Listgarten, M. Structure of the microbial flora associated with periodontal health and disease in man: A light and electron microscopic study. J. Clin. Periodontol. 1976, 47, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Darhous, M.; Zahran, F.; Ragy, N. Bacteriological and clinical assessment of tetracycline as root conditioning in adjunct to periodontal surgery. Egypt. Dent. J. 1995, 41, 1167–1178. [Google Scholar] [PubMed]
- Mariotti, A. Efficacy of chemical root surface modifiers in the treatment of periodontal disease. A systematic review. Ann. Periodontol. 2003, 8, 205–226. [Google Scholar] [CrossRef] [PubMed]
- Hiatt, W.H.; Schallhorn, R.G.; Aaronian, A.J. The Induction of New Bone and Cementum Formation: IV. Microscopic Examination of the Periodontium Following Human Bone and Marrow Allograft, Autograft and Nongraft Periodontal Regenerative Procedures. J. Periodontol. 1978, 49, 495–512. [Google Scholar] [CrossRef]
- Hynes, K.; Menicanin, D.; Gronthos, S.; Bartold, P.M. Clinical utility of stem cells for periodontal regeneration. Periodontology 2000 2012, 59, 203–227. [Google Scholar] [CrossRef]
- Egusa, H.; Sonoyama, W.; Nishimura, M.; Atsuta, I.; Akiyama, K. Stem cells in dentistry—Part I: Stem cell sources. J. Prosthodont. Res. 2012, 56, 151–165. [Google Scholar] [CrossRef]
- Dabra, S.; Chhina, K.; Soni, N.; Bhatnagar, R. Tissue engineering in periodontal regeneration: A brief review. Dent. Res. J. 2012, 9, 671–680. [Google Scholar]
- Lindhe, J.; Westfelt, E.; Nyman, S.; Socransky, S.; Haffajee, A. Long-term effect of surgical/non-surgical treatment of periodontal disease. J. Clin. Periodontol. 1984, 11, 448–458. [Google Scholar] [CrossRef]
- Wikesjö, U.M.; Nilvéus, R.E.; Selvig, K.A. Significance of early healing events on periodontal repair: A review. J. Periodontol. 1992, 63, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Langer, R. Tissue engineering: A new field and its challenges. Pharm. Res. 1997, 14, 840. [Google Scholar] [CrossRef] [PubMed]
- Melcher, A. On the repair potential of periodontal tissues. J. Periodontol. 1976, 47, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A. The anatomy and physiology of the healthy periodontium. In Gingival Diseases-Their Aetiology, Prevention and Treatment; Intech, 2011. [Google Scholar]
- Nanci, A.; Bosshardt, D.D. Structure of periodontal tissues in health and disease. Periodontology 2000 2006, 40, 11–28. [Google Scholar] [CrossRef]
- Bei, M. Molecular genetics of tooth development. Curr. Opin. Genet. Dev. 2009, 19, 504–510. [Google Scholar] [CrossRef]
- Engler, W.; Ramfjord, S.; Hiniker, J. Healing following simple gingivectomy. A tritiated thymidine radioautographic study. I. Epithelialization. J. Periodontol. 1966, 37, 298–308. [Google Scholar] [CrossRef]
- Caton, J.; DeFuria, E.; Polson, A.; Nyman, S. Periodontal regeneration via selective cell repopulation. J. Periodontol. 1987, 58, 546–552. [Google Scholar] [CrossRef]
- Nyman, S.; Gottlow, J.; Lindhe, J.; Karring, T.; Wennstrom, J. New attachment formation by guided tissue regeneration. J. Periodontal Res. 1987, 22, 252–254. [Google Scholar] [CrossRef]
- Misch, C.E.; Dietsh, F. Bone-grafting materials in implant dentistry. Implant Dent. 1993, 2, 158–167. [Google Scholar] [CrossRef]
- Singh, A.K. GTR membranes: The barriers for periodontal regeneration. DHR Int. J. Med. Sci. 2013, 4, 31–38. [Google Scholar]
- Linde, A.; Alberius, P.; Dahlin, C.; Bjurstam, K.; Sundin, Y. Osteopromotion: A soft-tissue exclusion principle using a membrane for bone healing and bone neogenesis. J. Periodontol. 1993, 64, 1116–1128. [Google Scholar] [CrossRef] [PubMed]
- Melcher, A.; McCulloch, C.; Cheong, T.; Nemeth, E.; Shiga, A. Cells from bone synthesize cementum-like and bone-like tissue in vitro and may migrate into periodontal ligament in vivo. J. Periodontal Res. 1987, 22, 246–247. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10, S96–S101. [Google Scholar] [PubMed]
- Albrektsson, T.; Brånemark, P.-I.; Hansson, H.-A.; Lindström, J. Osseointegrated titanium implants: Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop. Scand. 1981, 52, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Burchardt, H. The biology of bone graft repair. Clin. Orthop. Relat. Res. 1983, 174, 28–42. [Google Scholar] [CrossRef]
- Sheikh, Z.; Sima, C.; Glogauer, M. Bone replacement materials and techniques used for achieving vertical alveolar bone augmentation. Materials 2015, 8, 2953–2993. [Google Scholar] [CrossRef]
- Miron, R.; Zhang, Y. Osteoinduction: A review of old concepts with new standards. J. Dent. Res. 2012, 91, 736–744. [Google Scholar] [CrossRef]
- Frost, H. The biology of fracture healing. An overview for clinicians. Part II. Clin. Orthop. Relat. Res. 1989, 248, 294–309. [Google Scholar]
- Frost, H.M. The biology of fracture healing. An overview for clinicians. Part I. Clin. Orthop. Relat. Res. 1989, 248, 283–293. [Google Scholar]
- Aurer, A.; Jorgić-Srdjak, K. Membranes for periodontal regeneration. Acta Stomatol. Croat. 2005, 39, 107–112. [Google Scholar]
- Koop, R.; Merheb, J.; Quirynen, M. Periodontal regeneration with enamel matrix derivative in reconstructive periodontal therapy: A systematic review. J. Periodontol. 2012, 83, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Vuong, C.; Otto, M. Staphylococcus epidermidis infections. Microbes Infect. 2002, 4, 481–489. [Google Scholar] [CrossRef]
- Pihlstrom, B.L.; Michalowicz, B.S.; Johnson, N.W. Periodontal diseases. Lancet 2005, 366, 1809–1820. [Google Scholar] [CrossRef]
- Zimmerli, W.; Trampuz, A.; Ochsner, P.E. Prosthetic-joint infections. N. Engl. J. Med. 2004, 351, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Hojo, K.; Nagaoka, S.; Ohshima, T.; Maeda, N. Bacterial interactions in dental biofilm development. J. Dent. Res. 2009, 88, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Fürst, M.M.; Salvi, G.E.; Lang, N.P.; Persson, G.R. Bacterial colonization immediately after installation on oral titanium implants. Clin. Oral Implants Res. 2007, 18, 501–508. [Google Scholar] [CrossRef]
- Quirynen, M.; De Soete, M.; Van Steenberghe, D. Infectious risks for oral implants: A review of the literature. Clin. Oral Implants Res. Rev. Artic. 2002, 13, 1–19. [Google Scholar] [CrossRef]
- Price, J.; Tencer, A.; Arm, D.; Bohach, G. Controlled release of antibiotics from coated orthopedic implants. J. Biomed. Mater. Res. 1996, 30, 281–286. [Google Scholar] [CrossRef]
- Adeli, B.; Parvizi, J. Strategies for the prevention of periprosthetic joint infection. J. Bone Jt. Surg. Br. Vol. 2012, 94, 42–46. [Google Scholar] [CrossRef]
- Shahi, A.; Parvizi, J. Prevention of periprosthetic joint infection. Arch. Bone Jt. Surg. 2015, 3, 72–81. [Google Scholar] [PubMed]
- Turgut, H.; Sacar, S.; Kaleli, I.; Sacar, M.; Goksin, I.; Toprak, S.; Asan, A.; Cevahir, N.; Tekin, K.; Baltalarli, A. Systemic and local antibiotic prophylaxis in the prevention of Staphylococcus epidermidis graft infection. BMC Infect. Dis. 2005, 5, 91. [Google Scholar] [CrossRef] [PubMed]
- Ketonis, C.; Adams, C.S.; Barr, S.; Aiyer, A.; Shapiro, I.M.; Parvizi, J.; Hickok, N.J. Antibiotic modification of native grafts: Improving upon nature’s scaffolds. Tissue Eng. Part A 2010, 16, 2041–2049. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chu, P.K.; Zhang, Y.; Wu, Z. Antibacterial coatings on titanium implants. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 91, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Zilberman, M.; Elsner, J.J. Antibiotic-eluting medical devices for various applications. J. Controll. Release 2008, 130, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Jose, B.; Antoci, V., Jr.; Zeiger, A.R.; Wickstrom, E.; Hickok, N.J. Vancomycin covalently bonded to titanium beads kills Staphylococcus aureus. Chem. Biol. 2005, 12, 1041–1048. [Google Scholar] [CrossRef]
- Gristina, A.G. Biomaterial-centered infection: Microbial adhesion versus tissue integration. Science 1987, 237, 1588–1595. [Google Scholar] [CrossRef]
- Springer, B.D.; Lee, G.-C.; Osmon, D.; Haidukewych, G.J.; Hanssen, A.D.; Jacofsky, D.J. Systemic safety of high-dose antibiotic-loaded cement spacers after resection of an infected total knee arthroplasty. Clin. Orthop. Relat. Res. 2004, 427, 47–51. [Google Scholar] [CrossRef]
- Stigter, M.; Bezemer, J.; De Groot, K.; Layrolle, P. Incorporation of different antibiotics into carbonated hydroxyapatite coatings on titanium implants, release and antibiotic efficacy. J. Controll. Release 2004, 99, 127–137. [Google Scholar] [CrossRef]
- Montali, A. Antibacterial coating systems. Injury 2006, 37, S81–S86. [Google Scholar] [CrossRef]
- Norowski, P.A., Jr.; Bumgardner, J.D. Biomaterial and antibiotic strategies for peri-implantitis: A review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 88, 530–543. [Google Scholar] [CrossRef] [PubMed]
- Winkler, H.; Kaudela, K.; Stoiber, A.; Menschik, F. Bone grafts impregnated with antibiotics as a tool for treating infected implants in orthopedic surgery—One stage revision results. Cell Tissue Bank. 2006, 7, 319–323. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, H.; Song, X.; Gu, X.; Sun, C. Biomaterials for periodontal tissue regeneration. Rev. Adv. Mater. Sci. 2015, 40, 209–214. [Google Scholar]
- Tonelli, P.; Duvina, M.; Barbato, L.; Biondi, E.; Nuti, N.; Brancato, L.; Delle Rose, G. Bone regeneration in dentistry. Clin. Cases Miner. Bone Metab. 2011, 8, 24. [Google Scholar]
- Rocchietta, I.; Fontana, F.; Simion, M. Clinical outcomes of vertical bone augmentation to enable dental implant placement: A systematic review. J. Clin. Periodontol. 2008, 35, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Chiapasco, M.; Casentini, P.; Zaniboni, M. Bone augmentation procedures in implant dentistry. Int. J. Oral Maxillofac. Implants 2009, 24, 237–259. [Google Scholar] [PubMed]
- Arrington, E.D.; Smith, W.J.; Chambers, H.G.; Bucknell, A.L.; Davino, N.A. Complications of iliac crest bone graft harvesting. Clin. Orthop. Relat. Res. 1996, 329, 300–309. [Google Scholar] [CrossRef]
- Proussaefs, P.; Lozada, J. The use of intraorally harvested autogenous block grafts for vertical alveolar ridge augmentation: A human study. Int. J. Periodontics Restor. Dent. 2005, 25, 351–363. [Google Scholar]
- Jensen, S.S.; Terheyden, H. Bone augmentation procedures in localized defects in the alveolar ridge: Clinical results with different bone grafts and bone-substitute materials. Int. J. Oral Maxillofac. Implants 2009, 24, 218–236. [Google Scholar]
- Schallhorn, R.G. The use of autogenous hip marrow biopsy implants for bony crater defects. J. Periodontol. 1968, 39, 145–147. [Google Scholar] [CrossRef]
- Schlickewei, W.; Schlickewei, C. The use of bone substitutes in the treatment of bone defects–the clinical view and history. In Proceedings of Macromolecular Symposia; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007; pp. 10–23. [Google Scholar]
- Boyce, T.; Edwards, J.; Scarborough, N. Allograft bone: The influence of processing on safety and performance. Orthopedic Clin. 1999, 30, 571–581. [Google Scholar]
- Tomford, W.W. Transmission of disease through transplantation of musculoskeletal allografts. JBJS 1995, 77, 1742–1754. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Update: Allograft-associated bacterial infections—United States, 2002. MMWR Morb. Mortal. Wkly. Rep. 2002, 51, 207. [Google Scholar]
- Urist, M.R. Bone: Formation by autoinduction. Science 1965, 150, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Yukna, R.A.; Vastardis, S. Comparative evaluation of decalcified and non-decalcified freeze-dried bone allografts in rhesus monkeys. I. Histologic findings. J. Periodontol. 2005, 76, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Altiere, E.T.; Reeve, C.M.; Sheridan, P.J. Lyophilized bone allografts in periodontal intraosseous defects. J. Periodontol. 1979, 50, 510–519. [Google Scholar] [CrossRef]
- Blumenthal, N.; Steinberg, J. The use of collagen membrane barriers in conjunction with combined demineralized bone-collagen gel implants in human infrabony defects. J. Periodontol. 1990, 61, 319–327. [Google Scholar] [CrossRef]
- Mellonig, J. Freeze-dried bone allografts in periodontal reconstructive surgery. Dent. Clin. N. Am. 1991, 35, 505–520. [Google Scholar]
- Mellonig, J.T. Decalcified freeze-dried bone allograft as an implant material in human periodontal defects. Int. J. Periodontics Restor. Dent. 1984, 4, 40–55. [Google Scholar]
- AlGhamdi, A.; Shibly, O.; Ciancio, S. Osseous grafting part II: Xenografts and alloplasts for periodontal regeneration—A literature review. J. Int. Acad. Periodontol. 2010, 12, 39–44. [Google Scholar]
- Spector, M. Anorganic bovine bone and ceramic analogs of bone mineral as implants to facilitate bone regeneration. Clin. Plast. Surg. 1994, 21, 437–444. [Google Scholar] [PubMed]
- Taschieri, S.; Del Fabbro, M.; Testori, T.; Saita, M.; Weinstein, R. Efficacy of guided tissue regeneration in the management of through-and-through lesions following surgical endodontics: A preliminary study. Int. J. Periodontics Restor. Dent. 2008, 28, 264–271. [Google Scholar]
- Sculean, A.; Stavropoulos, A.; Windisch, P.; Keglevich, T.; Karring, T.; Gera, I. Healing of human intrabony defects following regenerative periodontal therapy with a bovine-derived xenograft and guided tissue regeneration. Clin. Oral Investig. 2004, 8, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Shetty, V.; Han, T. Alloplastic materials in reconstructive periodontal surgery. Dent. Clin. N. Am. 1991, 35, 521–530. [Google Scholar]
- Hench, L.L. Bioceramics: From concept to clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Saffar, J.; Colombier, M.; Detienville, R. Bone formation in tricalcium phosphate-filled periodontal intrabony lesions. Histological observations in humans. J. Periodontol. 1990, 61, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Schepers, E.; Clercq, M.D.; Ducheyne, P.; Kempeneers, R. Bioactive glass particulate material as a filler for bone lesions. J. Oral Rehabil. 1991, 18, 439–452. [Google Scholar] [CrossRef]
- Shue, L.; Yufeng, Z.; Mony, U. Biomaterials for periodontal regeneration: A review of ceramics and polymers. Biomatter 2012, 2, 271–277. [Google Scholar] [CrossRef]
- Albee, F.H. Studies in bone growth: Triple calcium phosphate as a stimulus to osteogenesis. Ann. Surg. 1920, 71, 32–39. [Google Scholar] [CrossRef]
- Yuan, H.; Yang, Z.; Li, Y.; Zhang, X.; De Bruijn, J.; De Groot, K. Osteoinduction by calcium phosphate biomaterials. J. Mater. Sci. Mater. Med. 1998, 9, 723–726. [Google Scholar] [CrossRef]
- Samavedi, S.; Whittington, A.R.; Goldstein, A.S. Calcium phosphate ceramics in bone tissue engineering: A review of properties and their influence on cell behavior. Acta Biomater. 2013, 9, 8037–8045. [Google Scholar] [CrossRef] [PubMed]
- De Groot, K. Clinical applications of calcium phosphate biomaterials: A review. Ceram. Int. 1993, 19, 363–366. [Google Scholar] [CrossRef]
- Al-Sanabani, J.S.; Madfa, A.A.; Al-Sanabani, F.A. Application of calcium phosphate materials in dentistry. Int. J. Biomater. 2013, 2013, 876132. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Chow, K.L.; Leng, Y. Study of hydroxyapatite osteoinductivity with an osteogenic differentiation of mesenchymal stem cells. J. Biomed. Mater. Res. Part A 2009, 89, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Rouahi, M.; Champion, E.; Hardouin, P.; Anselme, K. Quantitative kinetic analysis of gene expression during human osteoblastic adhesion on orthopaedic materials. Biomaterials 2006, 27, 2829–2844. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.S.; Long, M.W.; Hankenson, K.D. Osteogenic differentiation of human mesenchymal stem cells is regulated by bone morphogenetic protein-6. J. Cell. Biochem. 2006, 98, 538–554. [Google Scholar] [CrossRef]
- Vohra, S.; Hennessy, K.M.; Sawyer, A.A.; Zhuo, Y.; Bellis, S.L. Comparison of mesenchymal stem cell and osteosarcoma cell adhesion to hydroxyapatite. J. Mater. Sci. Mater. Med. 2008, 19, 3567. [Google Scholar] [CrossRef]
- Alcaide, M.; Serrano, M.-C.; Pagani, R.; Sánchez-Salcedo, S.; Vallet-Regí, M.; Portolés, M.-T. Biocompatibility markers for the study of interactions between osteoblasts and composite biomaterials. Biomaterials 2009, 30, 45–51. [Google Scholar] [CrossRef]
- Bagambisa, F.B.; Joos, U.; Schilli, W. Mechanisms and structure of the bond between bone and hydroxyapatite ceramics. J. Biomed. Mater. Res. 1993, 27, 1047–1055. [Google Scholar] [CrossRef]
- Okumura, M.; Ohgushi, H.; Dohi, Y.; Katuda, T.; Tamai, S.; Koerten, H.K.; Tabata, S. Osteoblastic phenotype expression on the surface of hydroxyapatite ceramics. J. Biomed. Mater. Res. 1997, 37, 122–129. [Google Scholar] [CrossRef]
- Ogilvie, A.; Frank, R.; Benque, E.; Gineste, M.; Heughebaert, M.; Hemmerle, J. The biocompatibility of hydroxyapatite implanted in the human periodontium. J. Periodontal Res. 1987, 22, 270–283. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lee, J.K.; Moursi, A.; Lannutti, J.J. Ca/P ratio effects on the degradation of hydroxyapatite in vitro. J. Biomed. Mater. Res. Part A 2003, 67, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y.; Fiorellini, J.P.; Kim, D.M.; Weber, H.P. Regeneration of standardized mandibular bone defects using expanded polytetrafluoroethylene membrane and various bone fillers. Int. J. Periodontics Restor. Dent. 2007, 27, 151–159. [Google Scholar]
- Yamada, S.; Heymann, D.; Bouler, J.-M.; Daculsi, G. Osteoclastic resorption of calcium phosphate ceramics with different hydroxyapatite/β-tricalcium phosphate ratios. Biomaterials 1997, 18, 1037–1041. [Google Scholar] [CrossRef]
- Jarcho, M. Calcium phosphate ceramics as hard tissue prosthetics. Clin. Orthopaedics Relat. Res. 1981, 157, 259–278. [Google Scholar] [CrossRef]
- Lu, J.; Gallur, A.; Flautre, B.; Anselme, K.; Descamps, M.; Thierry, B.; Hardouin, P. Comparative study of tissue reactions to calcium phosphate ceramics among cancellous, cortical, and medullar bone sites in rabbits. J. Biomed. Mater. Res. 1998, 42, 357–367. [Google Scholar] [CrossRef]
- Lu, J.; Descamps, M.; Dejou, J.; Koubi, G.; Hardouin, P.; Lemaitre, J.; Proust, J.P. The biodegradation mechanism of calcium phosphate biomaterials in bone. J. Biomed. Mater. Res. 2002, 63, 408–412. [Google Scholar] [CrossRef]
- Wongwitwichot, P.; Kaewsrichan, J.; Chua, K.; Ruszymah, B. Comparison of TCP and TCP/HA hybrid scaffolds for osteoconductive activity. Open Biomed. Eng. J. 2010, 4, 279–285. [Google Scholar] [CrossRef]
- Schaefer, S.; Detsch, R.; Uhl, F.; Deisinger, U.; Ziegler, G. How degradation of calcium phosphate bone substitute materials is influenced by phase composition and porosity. Adv. Eng. Mater. 2011, 13, 342–350. [Google Scholar] [CrossRef]
- Bayerlein, T.; Mundt, T.; Mack, F.; Bienengräber, V.; Proff, P.; Gedrange, T. Bone graft substitutes in periodontal and peri-implant bone regeneration. Folia Morphol. 2006, 65, 66–69. [Google Scholar]
- Sharma, S.; Srivastava, D.; Grover, S.; Sharma, V. Biomaterials in tooth tissue engineering: A review. J. Clin. Diagn. Res. 2014, 8, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Ellinger, R.F.; Nery, E.B.; Lynch, K.L. Histological assessment of periodontal osseous defects following implantation of hydroxyapatite and biphasic calcium phosphate ceramics: A case report. Int. J. Periodontics Restor. Dent. 1986, 6, 22–33. [Google Scholar]
- Vahabi, S.; Amirizadeh, N.; Shokrgozar, M.; Mofeed, R.; Mashhadi, A.; Aghaloo, M.; Sharifi, D.; Jabbareh, L. A comparison between the efficacy of Bio-Oss, hydroxyapatite tricalcium phosphate and combination of mesenchymal stem cells in inducing bone regeneration. Chang Gung Med. J. 2012, 35, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Morra, M.; Giavaresi, G.; Sartori, M.; Ferrari, A.; Parrilli, A.; Bollati, D.; Baena, R.R.Y.; Cassinelli, C.; Fini, M. Surface chemistry and effects on bone regeneration of a novel biomimetic synthetic bone filler. J. Mater. Sci. Mater. Med. 2015, 26, 159. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Hamzehlou, S.; Baino, F. Potential of Bioactive Glasses for Cardiac and Pulmonary Tissue Engineering. Materials 2017, 10, 1429. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Hamzehlou, S.; Kargozar, S. Bioactive Glasses: Where Are We and Where Are We Going? J. Funct. Biomater. 2018, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Schepers, E.; Ducheyne, P. Bioactive glass particles of narrow size range for the treatment of oral bone defects: A 1–24 month experiment with several materials and particle sizes and size ranges. J. Oral Rehabil. 1997, 24, 171–181. [Google Scholar] [CrossRef]
- Hench, L.L. Genetic design of bioactive glass. J. Eur. Ceram. Soc. 2009, 29, 1257–1265. [Google Scholar] [CrossRef]
- Kargozar, S.; Lotfibakhshaiesh, N.; Ai, J.; Mozafari, M.; Brouki Milan, P.; Hamzehlou, S.; Barati, M.; Baino, F.; Hill, R.G.; Joghataei, M.T. Strontium- and cobalt-substituted bioactive glasses seeded with human umbilical cord perivascular cells to promote bone regeneration via enhanced osteogenic and angiogenic activities. Acta Biomater. 2017, 58, 502–514. [Google Scholar] [CrossRef]
- Kargozar, S.; Baino, F.; Hamzehlou, S.; Hill, R.G.; Mozafari, M. Bioactive glasses: Sprouting angiogenesis in tissue engineering. Trends Biotechnol. 2018, 36, 430–444. [Google Scholar] [CrossRef]
- Kargozar, S.; Baino, F.; Hamzehlou, S.; Hill, R.G.; Mozafari, M. Bioactive glasses entering the mainstream. Drug Discov. Today 2018, 23, 1700–1704. [Google Scholar] [CrossRef] [PubMed]
- Baino, F. Bioactive glasses–when glass science and technology meet regenerative medicine. Ceram. Int. 2018, 44, 14953–14966. [Google Scholar] [CrossRef]
- Vitale-Brovarone, C.; Baino, F.; Verné, E. High strength bioactive glass-ceramic scaffolds for bone regeneration. J. Mater. Sci. Mater. Med. 2009, 20, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Varanasi, V.G.; Owyoung, J.B.; Saiz, E.; Marshall, S.J.; Marshall, G.W.; Loomer, P.M. The ionic products of bioactive glass particle dissolution enhance periodontal ligament fibroblast osteocalcin expression and enhance early mineralized tissue development. J. Biomed. Mater. Res. Part A 2011, 98, 177–184. [Google Scholar] [CrossRef]
- Xynos, I.D.; Edgar, A.J.; Buttery, L.D.; Hench, L.L.; Polak, J.M. Gene-expression profiling of human osteoblasts following treatment with the ionic products of Bioglass® 45S5 dissolution. J. Biomed. Mater. Res. 2001, 55, 151–157. [Google Scholar] [CrossRef]
- Subbaiah, R.; Thomas, B. Efficacy of a bioactive alloplast, in the treatment of human periodontal osseous defects-a clinical study. Med. Oral Patol. Oral Cir. Bucal 2011, 16, e239–e244. [Google Scholar] [CrossRef]
- Mengel, R.; Soffner, M.; Flores-de-Jacoby, L. Bioabsorbable membrane and bioactive glass in the treatment of intrabony defects in patients with generalized aggressive periodontitis: Results of a 12-month clinical and radiological study. J. Periodontol. 2003, 74, 899–908. [Google Scholar] [CrossRef]
- Yadav, V.S.; Narula, S.C.; Sharma, R.K.; Tewari, S.; Yadav, R. Clinical evaluation of guided tissue regeneration combined with autogenous bone or autogenous bone mixed with bioactive glass in intrabony defects. J. Oral Sci. 2011, 53, 481–488. [Google Scholar] [CrossRef]
- Balasubramanian, P.; Buettner, T.; Pacheco, V.M.; Boccaccini, A.R. Boron-containing bioactive glasses in bone and soft tissue engineering. J. Eur. Ceram. Soc. 2018, 38, 855–869. [Google Scholar] [CrossRef]
- Dreesmann, H.U. Knochenplombierung. Beitr. Klin. Chir. 1892, 9, 804–810. [Google Scholar]
- Shaffer, C.D.; App, G.R. The use of plaster of paris in treating infrabony periodontal defects in humans. J. Periodontol. 1971, 42, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Ramsdell, L.; Partridge, E. The crystal forms of calcium sulphate. Am. Mineral. J. Earth Planet. Mater. 1929, 14, 59–74. [Google Scholar]
- Couri, C.J.; Maze, G.I.; Hinkson, D.W.; Collins III, B.H.; Dawson, D.V. Medical grade calcium sulfate hemihydrate versus expanded polytetrafluoroethylene in the treatment of mandibular class II furcations. J. Periodontol. 2002, 73, 1352–1359. [Google Scholar] [CrossRef] [PubMed]
- Moore, W.R.; Graves, S.E.; Bain, G.I. Synthetic bone graft substitutes. ANZ J. Surg. 2001, 71, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Oh, J.H.; Han, I.; Kim, H.-S.; Chung, S.W. Grafting using injectable calcium sulfate in bone tumor surgery: Comparison with demineralized bone matrix-based grafting. Clin. Orthopedic Surg. 2011, 3, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Sukumar, S.; Drízhal, I.; Bukac, J.; Paulusová, V.; Pilathadka, S. Surgical treatment of periodontal intrabony defects with calcium sulphate in combination with beta tricalcium phosphate—A 12-month retrospective clinical evaluation. Acta Med. 2010, 53, 229–234. [Google Scholar] [CrossRef]
- Sukumar, S.; Drízhal, I.; Paulusová, V.; Bukac, J. Surgical treatment of periodontal intrabony defects with calcium sulphate in combination with beta-tricalcium phosphate: Clinical observations two years post-surgery. Acta Med. 2011, 54, 13–20. [Google Scholar] [CrossRef]
- Maragos, P.; Bissada, N.F.; Wang, R.; Cole, B.P. Comparison of Three Methods Using Calcium Sulfate as a Graft/Barrier Material for the Treatment of Class II Mandibular Molar Furcation Defects. Int. J. Periodontics Restor. Dent. 2002, 22, 493–501. [Google Scholar]
- Orsini, M.; Orsini, G.; Benlloch, D.; Aranda, J.J.; Lazaro, P.; Sanz, M.; Luca, M.D.; Piattelli, A. Comparison of calcium sulfate and autogenous bone graft to bioabsorbable membranes plus autogenous bone graft in the treatment of intrabony periodontal defects: A split-mouth study. J. Periodontol. 2001, 72, 296–302. [Google Scholar] [CrossRef]
- Gitelis, S.; Brebach, G.T. The treatment of chronic osteomyelitis with a biodegradable antibiotic-impregnated implant. J. Orthopaedic Surg. 2002, 10, 53–60. [Google Scholar] [CrossRef]
- Bell, W.H. Resorption characteristics of bone and bone substitutes. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 1964, 17, 650–657. [Google Scholar] [CrossRef]
- Horowitz, R.A.; Rohrer, M.D.; Prasad, H.S.; Tovar, N.; Mazor, Z. Enhancing Extraction Socket Therapy with a Biphasic Calcium Sulfate. Compend. Contin. Educ. Dent. (Jamesburg NJ 1995) 2012, 33, 420–426. [Google Scholar]
- Thomas, M.V.; Puleo, D.A. Calcium sulfate: Properties and clinical applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 88, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Varoni, E.M.; Iriti, M.; Rimondini, L. Plant products for innovative biomaterials in dentistry. Coatings 2012, 2, 179–194. [Google Scholar] [CrossRef]
- Dong, C.; Lv, Y. Application of collagen scaffold in tissue engineering: Recent advances and new perspectives. Polymers 2016, 8, 42. [Google Scholar] [CrossRef]
- Gelse, K.; Pöschl, E.; Aigner, T. Collagens—Structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef]
- Chevallay, B.; Herbage, D. Collagen-based biomaterials as 3D scaffold for cell cultures: Applications for tissue engineering and gene therapy. Med. Biol. Eng. Comput. 2000, 38, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Bunyaratavej, P.; Wang, H.L. Collagen membranes: A review. J. Periodontol. 2001, 72, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Black, L.; Santacana-Laffitte, G.; Patrick, C.W., Jr. Preparation and assessment of glutaraldehyde-crosslinked collagen–chitosan hydrogels for adipose tissue engineering. J. Biomed. Mater. Res. Part A 2007, 81, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Gough, J.E.; Scotchford, C.A.; Downes, S. Cytotoxicity of glutaraldehyde crosslinked collagen/poly (vinyl alcohol) films is by the mechanism of apoptosis. J. Biomed. Mater. Res. 2002, 61, 121–130. [Google Scholar] [CrossRef]
- Dutta, P.K.; Dutta, J.; Tripathi, V. Chitin and chitosan: Chemistry, properties and applications. J. Sci. Ind. Res. 2004, 63, 20–31. [Google Scholar]
- Goy, R.C.; de Britto, D.; Assis, O.B. A review of the antimicrobial activity of chitosan. Polímeros 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Chang, Y.-Z.; Lin, J.-T.; Prasannan, A.; Chen, P.-C.; Ko, C.-Y.; Tsai, H.-C. Evaluation of the bacterial anti-adhesive properties of polyacrylic acid, chitosan and heparin-modified medical grade Silicone rubber substrate. J. Polym. Res. 2015, 22, 131. [Google Scholar] [CrossRef]
- Peng, L.; Cheng, X.R.; Wang, J.W.; Xu, D.X.; Wang, G. Preparation and evaluation of porous chitosan/collagen scaffolds for periodontal tissue engineering. J. Bioact. Compat. Polym. 2006, 21, 207–220. [Google Scholar] [CrossRef]
- Xu, C.; Lei, C.; Meng, L.; Wang, C.; Song, Y. Chitosan as a barrier membrane material in periodontal tissue regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 1435–1443. [Google Scholar] [CrossRef]
- Lee, Y.M.; Park, Y.J.; Lee, S.J.; Ku, Y.; Han, S.B.; Klokkevold, P.R.; Choi, S.M.; Chung, C.P. Tissue engineered bone formation using chitosan/tricalcium phosphate sponges. J. Periodontol. 2000, 71, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, M. Synthesis and characterization of macroporous chitosan/calcium phosphate composite scaffolds for tissue engineering. J. Biomed. Mater. Res. 2001, 55, 304–312. [Google Scholar] [CrossRef]
- Morris, G.A.; Kök, S.M.; Harding, S.E.; Adams, G.G. Polysaccharide drug delivery systems based on pectin and chitosan. Biotechnol. Genet. Eng. Rev. 2010, 27, 257–284. [Google Scholar] [CrossRef]
- Archana, D.; Upadhyay, L.; Tewari, R.; Dutta, J.; Huang, Y.; Dutta, P. Chitosan-pectin-alginate as a novel scaffold for tissue engineering applications. Indian J. Biotechnol. 2013, 12, 475–482. [Google Scholar]
- Rashidova, S.S.; Milusheva, R.Y.; Semenova, L.; Mukhamedjanova, M.Y.; Voropaeva, N.; Vasilyeva, S.; Faizieva, R.; Ruban, I. Characteristics of interactions in the pectin–chitosan system. Chromatographia 2004, 59, 779–782. [Google Scholar] [CrossRef]
- Li, W.; Hao, W.; Xiaohua, Z.; Yinchen, H.; Wangwang, L.; Gongming, Y.; Aimin, J. Pectin-chitosan complex: Preparation and application in colon-specific capsule. Int. J. Agric. Biol. Eng. 2015, 8, 151–160. [Google Scholar]
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Löfgren, C.; Guillotin, S.; Hermansson, A.-M. Microstructure and kinetic rheological behavior of amidated and nonamidated LM pectin gels. Biomacromolecules 2006, 7, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Sriamornsak, P. Chemistry of pectin and its pharmaceutical uses: A review. Silpakorn Univ. Int. J. 2003, 3, 206–228. [Google Scholar]
- Mishra, R.; Banthia, A.; Majeed, A.B. Pectin based formulations for biomedical applications: A review. Asian J. Pharm. Clin. Res. 2012, 5, 1–7. [Google Scholar]
- Kastner, H.; Einhorn-Stoll, U.; Senge, B. Structure formation in sugar containing pectin gels–Influence of Ca2+ on the gelation of low-methoxylated pectin at acidic pH. Food Hydrocoll. 2012, 27, 42–49. [Google Scholar] [CrossRef]
- De Vries, J.; Rombouts, F.; Voragen, A.; Pilnik, W. Distribution of methoxyl groups in apple pectic substances. Carbohydr. Polym. 1983, 3, 245–258. [Google Scholar] [CrossRef]
- Monfregola, L.; Bugatti, V.; Amodeo, P.; De Luca, S.; Vittoria, V. Physical and water sorption properties of chemically modified pectin with an environmentally friendly process. Biomacromolecules 2011, 12, 2311–2318. [Google Scholar] [CrossRef]
- Bigucci, F.; Luppi, B.; Cerchiara, T.; Sorrenti, M.; Bettinetti, G.; Rodriguez, L.; Zecchi, V. Chitosan/pectin polyelectrolyte complexes: Selection of suitable preparative conditions for colon-specific delivery of vancomycin. Eur. J. Pharm. Sci. 2008, 35, 435–441. [Google Scholar] [CrossRef]
- Bigucci, F.; Luppi, B.; Monaco, L.; Cerchiara, T.; Zecchi, V. Pectin-based microspheres for colon-specific delivery of vancomycin. J. Pharm. Pharmacol. 2009, 61, 41–46. [Google Scholar] [CrossRef]
- Chen, P.-H.; Kuo, T.-Y.; Kuo, J.-Y.; Tseng, Y.-P.; Wang, D.-M.; Lai, J.-Y.; Hsieh, H.-J. Novel chitosan–pectin composite membranes with enhanced strength, hydrophilicity and controllable disintegration. Carbohydr. Polym. 2010, 82, 1236–1242. [Google Scholar] [CrossRef]
- Munarin, F.; Guerreiro, S.; Grellier, M.; Tanzi, M.C.; Barbosa, M.; Petrini, P.; Granja, P. Pectin-based injectable biomaterials for bone tissue engineering. Biomacromolecules 2011, 12, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Kokkonen, H.; Cassinelli, C.; Verhoef, R.; Morra, M.; Schols, H.; Tuukkanen, J. Differentiation of osteoblasts on pectin-coated titanium. Biomacromolecules 2008, 9, 2369–2376. [Google Scholar] [CrossRef]
- Liu, L.; Won, Y.J.; Cooke, P.H.; Coffin, D.R.; Fishman, M.L.; Hicks, K.B.; Ma, P.X. Pectin/poly (lactide-co-glycolide) composite matrices for biomedical applications. Biomaterials 2004, 25, 3201–3210. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Jain, D.; Verma, K.; Verma, S. Pectin-based colon-specific drug delivery. Chron. Young Sci. 2011, 2, 83. [Google Scholar] [CrossRef]
- Munarin, F.; Petrini, P.; Tanzi, M.C.; Barbosa, M.A.; Granja, P.L. Biofunctional chemically modified pectin for cell delivery. Soft Matter 2012, 8, 4731–4739. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.H.; Ross-Murphy, S.B. Structural and mechanical properties of biopolymer gels. In Biopolymers; Springer: Berlin/Heidelberg, Germany, 1987; pp. 57–192. [Google Scholar]
- Dobie, K.; Smith, G.; Sloan, A.; Smith, A. Effects of alginate hydrogels and TGF-β1 on human dental pulp repair in vitro. Connect. Tissue Res. 2002, 43, 387–390. [Google Scholar] [CrossRef]
- Srinivasan, S.; Jayasree, R.; Chennazhi, K.; Nair, S.; Jayakumar, R. Biocompatible alginate/nano bioactive glass ceramic composite scaffolds for periodontal tissue regeneration. Carbohydr. Polym. 2012, 87, 274–283. [Google Scholar] [CrossRef]
- Necas, J.; Bartosikova, L.; Brauner, P.; Kolar, J. Hyaluronic acid (hyaluronan): A review. Vet. Med. 2008, 53, 397–411. [Google Scholar] [CrossRef]
- Inuyama, Y.; Kitamura, C.; Nishihara, T.; Morotomi, T.; Nagayoshi, M.; Tabata, Y.; Matsuo, K.; Chen, K.K.; Terashita, M. Effects of hyaluronic acid sponge as a scaffold on odontoblastic cell line and amputated dental pulp. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 92, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Lapčík, L.; Lapčík, L.; De Smedt, S.; Demeester, J.; Chabreček, P. Hyaluronan: Preparation, Structure, Properties, and Applications. Chem. Rev. 1998, 98, 2663–2684. [Google Scholar] [CrossRef]
- Collins, M.N.; Birkinshaw, C. Hyaluronic acid based scaffolds for tissue engineering—A review. Carbohydr. Polym. 2013, 92, 1262–1279. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, D.A.; Merrill, E.; Smith, K.A.; Balazs, E. Rheology of hyaluronic acid. Biopolym. Orig. Res. Biomol. 1968, 6, 777–791. [Google Scholar] [CrossRef] [PubMed]
- Bansal, J.; Kedige, S.D.; Anand, S. Hyaluronic acid: A promising mediator for periodontal regeneration. Indian J. Dent. Res. 2010, 21, 575. [Google Scholar] [PubMed]
- Drago, L.; Cappelletti, L.; De Vecchi, E.; Pignataro, L.; Torretta, S.; Mattina, R. Antiadhesive and antibiofilm activity of hyaluronic acid against bacteria responsible for respiratory tract infections. Apmis 2014, 122, 1013–1019. [Google Scholar] [CrossRef]
- Gudiña, E.J.; Rocha, V.; Teixeira, J.; Rodrigues, L. Antimicrobial and antiadhesive properties of a biosurfactant isolated from Lactobacillus paracasei ssp. paracasei A20. Lett. Appl. Microbiol. 2010, 50, 419–424. [Google Scholar] [CrossRef]
- Shi, Z.; Neoh, K.; Kang, E.; Poh, C.; Wang, W. Bacterial adhesion and osteoblast function on titanium with surface-grafted chitosan and immobilized RGD peptide. J. Biomed. Mater. Res. Part A 2008, 86, 865–872. [Google Scholar] [CrossRef]
- Woodard, J.R.; Hilldore, A.J.; Lan, S.K.; Park, C.; Morgan, A.W.; Eurell, J.A.C.; Clark, S.G.; Wheeler, M.B.; Jamison, R.D.; Johnson, A.J.W. The mechanical properties and osteoconductivity of hydroxyapatite bone scaffolds with multi-scale porosity. Biomaterials 2007, 28, 45–54. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Hutmacher, D.W.; Schantz, J.T.; Lam, C.X.F.; Tan, K.C.; Lim, T.C. State of the art and future directions of scaffold-based bone engineering from a biomaterials perspective. J. Tissue Eng. Regen. Med. 2007, 1, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Groeneveld, E.; Van Den Bergh, J.; Holzmann, P.; Ten Bruggenkate, C.; Tuinzing, D.; Burger, E. Mineralization processes in demineralized bone matrix grafts in human maxillary sinus floor elevations. J. Biomed. Mater. Res. 1999, 48, 393–402. [Google Scholar] [CrossRef]
- Reznikov, N.; Shahar, R.; Weiner, S. Bone hierarchical structure in three dimensions. Acta Biomater. 2014, 10, 3815–3826. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.; Matyas, J.; Katzenberg, M.; Hallgrimsson, B. Comparison of microcomputed tomographic and microradiographic measurements of cortical bone porosity. Calcif. Tissue Int. 2004, 74, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Keaveny, T.M.; Morgan, E.F.; Niebur, G.L.; Yeh, O.C. Biomechanics of trabecular bone. Ann. Rev. Biomed. Eng. 2001, 3, 307–333. [Google Scholar] [CrossRef] [PubMed]
- Currey, J.D. Tensile yield in compact bone is determined by strain, post-yield behaviour by mineral content. J. Biomech. 2004, 37, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Kuboki, Y.; Takita, H.; Kobayashi, D.; Tsuruga, E.; Inoue, M.; Murata, M.; Nagai, N.; Dohi, Y.; Ohgushi, H. BMP-induced osteogenesis on the surface of hydroxyapatite with geometrically feasible and nonfeasible structures: Topology of osteogenesis. J. Biomed. Mater. Res. 1998, 39, 190–199. [Google Scholar] [CrossRef]
- Hing, K.A. Bioceramic bone graft substitutes: Influence of porosity and chemistry. Int. J. Appl. Ceram. Technol. 2005, 2, 184–199. [Google Scholar] [CrossRef]
- Xynos, I.; Hukkanen, M.; Batten, J.; Buttery, L.; Hench, L.; Polak, J. Bioglass® 45S5 stimulates osteoblast turnover and enhances bone formation in vitro: Implications and applications for bone tissue engineering. Calcif. Tissue Int. 2000, 67, 321–329. [Google Scholar] [CrossRef]
- Kim, H.D.; Valentini, R.F. Retention and activity of BMP-2 in hyaluronic acid-based scaffolds in vitro. J. Biomed. Mater. Res. 2002, 59, 573–584. [Google Scholar] [CrossRef]
- El-Ghannam, A.R. Advanced bioceramic composite for bone tissue engineering: Design principles and structure–bioactivity relationship. J. Biomed. Mater. Res. Part A 2004, 69, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Yin, Y.; Lu, W.W.; Leong, J.C.; Zhang, W.; Zhang, J.; Zhang, M.; Yao, K. Preparation and histological evaluation of biomimetic three-dimensional hydroxyapatite/chitosan-gelatin network composite scaffolds. Biomaterials 2002, 23, 3227–3234. [Google Scholar] [CrossRef]
- Iviglia, G.; Cassinelli, C.; Bollati, D.; Baino, F.; Torre, E.; Morra, M.; Vitale-Brovarone, C. Engineered porous scaffolds for periprosthetic infection prevention. Mater. Sci. Eng. C 2016, 68, 701–715. [Google Scholar] [CrossRef] [PubMed]
- Gmeiner, R.; Deisinger, U.; Schönherr, J.; Lechner, B.; Detsch, R.; Boccaccini, A.; Stampfl, J. Additive manufacturing of bioactive glasses and silicate bioceramics. J. Ceram. Sci. Technol 2015, 6, 75–86. [Google Scholar]
- Hulbert, S.; Young, F.; Mathews, R.; Klawitter, J.; Talbert, C.; Stelling, F. Potential of ceramic materials as permanently implantable skeletal prostheses. J. Biomed. Mater. Res. 1970, 4, 433–456. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.P.; Vehof, J.W.; Dean, D.; van der Waerden, J.P.C.; Holland, T.A.; Mikos, A.G.; Jansen, J.A. Soft and hard tissue response to photocrosslinked poly (propylene fumarate) scaffolds in a rabbit model. J. Biomed. Mater. Res. 2002, 59, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Ayers, R.; Simske, S.; Bateman, T.; Petkus, A.; Sachdeva, R.; Gyunter, V. Effect of nitinol implant porosity on cranial bone ingrowth and apposition after 6 weeks. J. Biomed. Mater. Res. 1999, 45, 42–47. [Google Scholar] [CrossRef]
- El-Rashidy, A.A.; Roether, J.A.; Harhaus, L.; Kneser, U.; Boccaccini, A.R. Regenerating bone with bioactive glass scaffolds: A review of in vivo studies in bone defect models. Acta Biomater. 2017, 62, 1–28. [Google Scholar] [CrossRef]
- Akin, F.A.; Zreiqat, H.; Jordan, S.; Wijesundara, M.B.; Hanley, L. Preparation and analysis of macroporous TiO2 films on Ti surfaces for bone–tissue implants. J. Biomed. Mater. Res. 2001, 57, 588–596. [Google Scholar] [CrossRef]
- Akay, G.; Birch, M.; Bokhari, M. Microcellular polyHIPE polymer supports osteoblast growth and bone formation in vitro. Biomaterials 2004, 25, 3991–4000. [Google Scholar] [CrossRef]
- Takahashi, Y.; Tabata, Y. Effect of the fiber diameter and porosity of non-woven PET fabrics on the osteogenic differentiation of mesenchymal stem cells. J. Biomater. Sci. Polym. Ed. 2004, 15, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.M.; Takita, H.; Kohgo, T.; Atsumi, K.; Itoh, H.; Kuboki, Y. Effects of geometry of hydroxyapatite as a cell substratum in BMP-induced ectopic bone formation. J. Biomed. Mater. Res. 2000, 52, 841–851. [Google Scholar] [CrossRef]
- Kuboki, Y.; Jin, Q.; Kikuchi, M.; Mamood, J.; Takita, H. Geometry of artificial ECM: Sizes of pores controlling phenotype expression in BMP-induced osteogenesis and chondrogenesis. Connect. Tissue Res. 2002, 43, 529–534. [Google Scholar] [CrossRef]
- Kuboki, Y.; Jin, Q.; Takita, H. Geometry of carriers controlling phenotypic expression in BMP-induced osteogenesis and chondrogenesis. JBJS 2001, 83, S1–S105. [Google Scholar] [CrossRef]
- Tsuruga, E.; Takita, H.; Itoh, H.; Wakisaka, Y.; Kuboki, Y. Pore size of porous hydroxyapatite as the cell-substratum controls BMP-induced osteogenesis. J. Biochem. 1997, 121, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Baino, F.; Spriano, S.; Pugno, N.M.; Vitale-Brovarone, C. Modelling of the strength–porosity relationship in glass-ceramic foam scaffolds for bone repair. J. Eur. Ceram. Soc. 2014, 34, 2663–2673. [Google Scholar] [CrossRef]
- Baino, F.; Vitale-Brovarone, C. Three-dimensional glass-derived scaffolds for bone tissue engineering: Current trends and forecasts for the future. J. Biomed. Mater. Res. Part A 2011, 97, 514–535. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, M. Three-dimensional macroporous calcium phosphate bioceramics with nested chitosan sponges for load-bearing bone implants. J. Biomed. Mater. Res. 2002, 61, 1–8. [Google Scholar] [CrossRef]
- Bollati, D.; Morra, M.; Cassinelli, C.; Cascardo, G. Implantable Devices Having Antibacterial Properties and Multifunctional Surfaces. U.S. Patent No. 9999706, 19 June 2018. [Google Scholar]
- Finkemeier, C.G. Bone-grafting and bone-graft substitutes. JBJS 2002, 84, 454–464. [Google Scholar] [CrossRef]
- Han, P.; Wu, C.; Chang, J.; Xiao, Y. The cementogenic differentiation of periodontal ligament cells via the activation of Wnt/β-catenin signalling pathway by Li+ ions released from bioactive scaffolds. Biomaterials 2012, 33, 6370–6379. [Google Scholar] [CrossRef]
- Iviglia, G.; Cassinelli, C.; Torre, E.; Baino, F.; Morra, M.; Vitale-Brovarone, C. Novel bioceramic-reinforced hydrogel for alveolar bone regeneration. Acta Biomater. 2016, 44, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Nyman, S.; Lindhe, J.; Karring, T.; Rylander, H. New attachment following surgical treatment of human periodontal disease. J. Clin. Periodontol. 1982, 9, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Bartee, B.K.; Carr, J. Evaluation of a high-density polytetrafluoroethylene (n-PTFE) membrane as a barrier material to facilitate guided bone regeneration in the rat mandible. J. Oral Implantol. 1995, 21, 88–95. [Google Scholar] [PubMed]
- Marouf, H.A.; El-Guindi, H.M. Efficacy of high-density versus semipermeable PTFE membranes in an elderly experimental model. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2000, 89, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Babo, P.S.; Pires, R.L.; Reis, R.L.; Gomes, M.E. Membranes for periodontal tissues regeneration. Ciênc. Tecnol. Mater. 2014, 26, 108–117. [Google Scholar] [CrossRef]
- Monteiro, A.; Macedo, L.; Macedo, N.-L.; Balducci, I. Polyurethane and PTFE membranes for guided bone regeneration: Histopathological and ultrastructural evaluation. Med. Oral Patol. Oral Cir. Bucal 2010, 15, e401–e406. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.G. Postoperative Healing Complications Associated with Gore-Tex Periodontal Material. Part I. Incidence and Characterization. Int. J. Periodontics Restor. Dent. 1995, 15, 363–375. [Google Scholar]
- Hardwick, R.; Hayes, B.K.; Flynn, C. Devices for dentoalveolar regeneration: An up-to-date literature review. J. Periodontol. 1995, 66, 495–505. [Google Scholar] [CrossRef]
- Sigurdsson, T.J.; Hardwick, R.; Bogle, G.C.; Wikesjö, U.M. Periodontal repair in dogs: Space provision by reinforced ePTFE membranes enhances bone and cementum regeneration in large supraalveolar defects. J. Periodontol. 1994, 65, 350–356. [Google Scholar] [CrossRef]
- Wikesjö, U.M.; Selvig, K.A. Periodontal wound healing and regeneration. Periodontology 2000 1999, 19, 21–39. [Google Scholar] [CrossRef]
- Becker, W.; Becker, B.E. Periodontal regeneration: A contemporary re-evaluation. Periodontology 2000 1999, 19, 104–114. [Google Scholar] [CrossRef]
- Cortellini, P.; Prato, G.P.; Tonetti, M.S. Interproximal free gingival grafts after membrane removal in guided tissue regeneration treatment of intrabony defects. A randomized controlled clinical trial. J. Periodontol. 1995, 66, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Minabe, M. A critical review of the biologic rationale for guided tissue regeneration. J. Periodontol. 1991, 62, 171–179. [Google Scholar] [CrossRef]
- Khor, E. Methods for the treatment of collagenous tissues for bioprostheses. Biomaterials 1997, 18, 95–105. [Google Scholar] [CrossRef]
- Pitaru, S.; Tal, H.; Soldinger, M.; Grosskopf, A.; Noff, M. Partial regeneration of periodontal tissues using collagen barriers: Initial observations in the canine. J. Periodontol. 1988, 59, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Minabe, M.; Kodama, T.; Kogou, T.; Tamura, T.; Hori, T.; Watanabe, Y.; Miyata, T. Different cross-linked types of collagen implanted in rat palatal gingiva. J. Periodontol. 1989, 60, 35–43. [Google Scholar] [CrossRef]
- Wang, H.L.; Miyauchi, M.; Takata, T. Initial attachment of osteoblasts to various guided bone regeneration membranes: An in vitro study. J. Periodontal Res. 2002, 37, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Evans, G.; Yukna, R.; Cambre, K.; Gardiner, D. Clinical regeneration with guided tissue barriers. Curr. Opin. Periodontol. 1997, 4, 75–81. [Google Scholar]
- Bergsma, J.E.; Rozema, F.; Bos, R.; Boering, G.; de Bruijn, W.; Pennings, A. In vivo degradation and biocompatibility study of in vitro pre-degraded as-polymerized polylactide particles. Biomaterials 1995, 16, 267–274. [Google Scholar] [CrossRef]
- Ignatius, A.; Claes, L.E. In vitro biocompatibility of bioresorbable polymers: Poly (L, DL-lactide) and poly (L-lactide-co-glycolide). Biomaterials 1996, 17, 831–839. [Google Scholar] [CrossRef]
- Lundgren, D.; Laurell, L.; Gottlow, J.; Rylander, H.; Mathisen, T.; Nyman, S.; Rask, M. The influence of the design of two different bioresorbable barriers on the results of guided tissue regeneration therapy. An intra-individual comparative study in the monkey. J. Periodontol. 1995, 66, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Hürzeler, M.B.; Quiñones, C.R.; Caffesse, R.G.; Schüpbach, P.; Morrison, E.C. Guided periodontal tissue regeneration in interproximal intrabony defects following treatment with a synthetic bioabsorbable barrier. J. Periodontol. 1997, 68, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Fleisher, N.; de Waal, H.; Bloom, A. Regeneration of lost attachment apparatus in the dog using Vicryl absorbable mesh (Polyglactin 910). Int. J. Periodontics Restor. Dent. 1988, 8, 44–55. [Google Scholar]
- Polson, A.M.; Southard, G.L.; Dunn, R.L.; Polson, A.P.; Yewey, G.L.; Swanbom, D.D.; Fulfs, J.C.; Rodgers, P.W. Periodontal healing after guided tissue regeneration with Atrisorb barriers in beagle dogs. Int. J. Periodontics Restor. Dent. 1995, 15, 574–589. [Google Scholar]
- Warrer, K.; Karring, T.; Nyman, S.; Gogolewski, S. Guided tissue regeneration using biodegradable membranes of polylactic acid or polyurethane. J. Clin. Periodontol. 1992, 19, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Leghissa, G.C.; Botticelli, A.R. Resistance to bacterial aggression involving exposed nonresorbable membranes in the oral cavity. Int. J. Oral Maxillofac. Implants 1996, 11, 210–215. [Google Scholar]
- Giannobile, W. Periodontal tissue engineering by growth factors. Bone 1996, 19, S23–S37. [Google Scholar] [CrossRef]
- Whang, K.; Tsai, D.; Nam, E.; Aitken, M.; Sprague, S.; Patel, P.; Healy, K. Ectopic bone formation via rhBMP-2 delivery from porous bioabsorbable polymer scaffolds. J. Biomed. Mater. Res. 1998, 42, 491–499. [Google Scholar] [CrossRef]
- Fournier, N.; Doillon, C.J. Biological molecule-impregnated polyester: An in vivo angiogenesis study. Biomaterials 1996, 17, 1659–1665. [Google Scholar] [CrossRef]
- Howard, D.; Buttery, L.D.; Shakesheff, K.M.; Roberts, S.J. Tissue engineering: Strategies, stem cells and scaffolds. J. Anat. 2008, 213, 66–72. [Google Scholar] [CrossRef]
- Barron, V.; Pandit, A. Combinatorial Approaches in Tissue Engineering: Progenitor Cells, Scaffolds, and Growth Factors; Ashammakhi, N., Ferretti, P., Eds.; Topics in Tissue Engineering; University of Oulu: Oulu, Finland, 2003; pp. 1–21. [Google Scholar]
- Taba, M., Jr.; Jin, Q.; Sugai, J.; Giannobile, W. Current concepts in periodontal bioengineering. Orthodont. Craniofac. Res. 2005, 8, 292–302. [Google Scholar]
- Lyngstadaas, S.; Wohlfahrt, J.; Brookes, S.; Paine, M.; Snead, M.; Reseland, J. Enamel matrix proteins; old molecules for new applications. Orthodont. Craniofac. Res. 2009, 12, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Giannobile, W.V.; Somerman, M.J. Growth and amelogenin-like factors in periodontal wound healing. A systematic review. Ann. Periodontol. 2003, 8, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Tabata, Y. Tissue regeneration based on growth factor release. Tissue Eng. 2003, 9, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Takahashi, Y.; Tabata, Y. Controlled release by biodegradable hydrogels enhances the ectopic bone formation of bone morphogenetic protein. Biomaterials 2003, 24, 4375–4383. [Google Scholar] [CrossRef]
- Woo, B.H.; Fink, B.F.; Page, R.; Schrier, J.A.; Jo, Y.W.; Jiang, G.; DeLuca, M.; Vasconez, H.C.; DeLuca, P.P. Enhancement of bone growth by sustained delivery of recombinant human bone morphogenetic protein-2 in a polymeric matrix. Pharm. Res. 2001, 18, 1747–1753. [Google Scholar] [CrossRef] [PubMed]
- Dennison, D.K.; Vallone, D.R.; Pinero, G.J.; Rittman, B.; Caffesse, R.G. Differential effect of TGF-β1 and PDGF on proliferation of periodontal ligament cells and gingival fibroblasts. J. Periodontol. 1994, 65, 641–648. [Google Scholar] [CrossRef]
- Oates, T.W.; Rouse, C.A.; Cochran, D.L. Mitogenic effects of growth factors on human periodontal ligament cells in vitro. J. Periodontol. 1993, 64, 142–148. [Google Scholar] [CrossRef]
- Giannobile, W.; Whitson, S.; Lynch, S. Non-coordinate control of bone formation displayed by growth factor combinations with IGF-I. J. Dent. Res. 1997, 76, 1569–1578. [Google Scholar] [CrossRef]
- Lynch, S.E.; Buser, D.; Hernandez, R.A.; Weber, H.; Stich, H.; Fox, C.H.; Williams, R.C. Effects of the platelet-derived growth factor/insulin-like growth factor-I combination on bone regeneration around titanium dental implants. Results of a pilot study in beagle dogs. J. Periodontol. 1991, 62, 710–716. [Google Scholar] [CrossRef]
- Nevins, M.; Camelo, M.; Nevins, M.L.; Schenk, R.K.; Lynch, S.E. Periodontal regeneration in humans using recombinant human platelet-derived growth factor-BB (rhPDGF-BB) and allogenic bone. J. Periodontol. 2003, 74, 1282–1292. [Google Scholar] [CrossRef]
- Giannobile, W.V.; Hernandez, R.A.; Finkelman, R.D.; Ryarr, S.; Kiritsy, C.P.; D’Andrea, M.; Lynch, S.E. Comparative effects of plateletderived growth factor-BB and insulin-like growth factor-I, individually and in combination, on periodontal regeneration in Macaca fascicularis. J. Periodontal Res. 1996, 31, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Izumi, Y.; Aoki, A.; Yamada, Y.; Kobayashi, H.; Iwata, T.; Akizuki, T.; Suda, T.; Nakamura, S.; Wara-Aswapati, N.; Ueda, M. Current and future periodontal tissue engineering. Periodontology 2000 2011, 56, 166–187. [Google Scholar] [CrossRef] [PubMed]
- Sigurdsson, T.J.; Lee, M.B.; Kubota, K.; Turek, T.J.; Wozney, J.M.; Wikesjö, U.M. Periodontal repair in dogs: Recombinant human bone morphogenetic protein-2 significantly enhances periodontal regeneration. J. Periodontol. 1995, 66, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, A.; Oda, S.; Takahashi, K.; Yokota, S.; Ishikawa, I. Periodontal regeneration by application of recombinant human bone morphogenetic protein-2 to horizontal circumferential defects created by experimental periodontitis in beagle dogs. J. Periodontol. 1997, 68, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Wikesjö, U.M.; Guglielmoni, P.; Promsudthi, A.; Cho, K.S.; Trombelli, L.; Selvig, K.A.; Jin, L.; Wozney, J.M. Periodontal repair in dogs: Effect of rhBMP-2 concentration on regeneration of alveolar bone and periodontal attachment. J. Clin. Periodontol. 1999, 26, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Paralkar, V.M.; Nandedkar, A.; Pointer, R.H.; Kleinman, H.K.; Reddi, A. Interaction of osteogenin, a heparin binding bone morphogenetic protein, with type IV collagen. J. Biol. Chem. 1990, 265, 17281–17284. [Google Scholar]
- Luan, X.; Ito, Y.; Diekwisch, T.G. Evolution and development of Hertwig’s epithelial root sheath. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2006, 235, 1167–1180. [Google Scholar] [CrossRef]
- Zeichner-David, M.; Oishi, K.; Su, Z.; Zakartchenko, V.; Chen, L.S.; Arzate, H.; Bringas, P., Jr. Role of Hertwig’s epithelial root sheath cells in tooth root development. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2003, 228, 651–663. [Google Scholar] [CrossRef]
- Moradian-Oldak, J. Protein-mediated enamel mineralization. Front. Biosci. J. Virtual Libr. 2012, 17, 1996. [Google Scholar] [CrossRef]
- Heijl, L.H.G.; Svärdström, G.; Ostgren, A. Enamel matrix derivative (EMDOGAIN) in the treatment of intrabony periodontal defects. J. Clin. Periodontol. 1997, 24, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Hammarström, L. Enamel matrix, cementum development and regeneration. J. Clin. Periodontol. 1997, 24, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Cambini, A.F.T.; Ordesi, P.; Arcara, C.; Caccianiga, G. Rigenerazione tissutale guidata in difetti infraossei mediante innesto di amelogenine. Dent. Clin. N. Am. 2012, 3, 19–26. [Google Scholar]
- Zanatta, F.B.; Souza, F.G.d.; Pinto, T.M.P.; Antoniazzi, R.P.; Rösing, C.K. Do the clinical effects of enamel matrix derivatives in infrabony defects decrease overtime? A systematic review and meta-analysis. Braz. Dent. J. 2013, 24, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Zhu, Y.-Y.; Smiley, E.; Bonadio, J.; Rouleau, J.P.; Goldstein, S.A.; McCauley, L.K.; Davidson, B.L.; Roessler, B.J. Stimulation of new bone formation by direct transfer of osteogenic plasmid genes. Proc. Natl. Acad. Sci. USA 1996, 93, 5753–5758. [Google Scholar] [CrossRef] [PubMed]
- Nussenbaum, B.; Krebsbach, P.H. The role of gene therapy for craniofacial and dental tissue engineering. Adv. Drug Deliv. Rev. 2006, 58, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Schek, R.M.; Hollister, S.J.; Krebsbach, P.H. Delivery and protection of adenoviruses using biocompatible hydrogels for localized gene therapy. Mol. Ther. 2004, 9, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Gansbacher, B. Report of a second serious adverse event in a clinical trial of gene therapy for X-linked severe combined immune deficiency (X-SCID) Position of the European Society of Gene Therapy (ESGT). J. Gene Med. 2003, 5, 261–262. [Google Scholar]
- Anusaksathien, O.; Webb, S.A.; Jin, Q.-M.; Giannobile, W.V. Platelet-derived growth factor gene delivery stimulates ex vivo gingival repair. Tissue Eng. 2003, 9, 745–756. [Google Scholar] [CrossRef]
- Anusaksathien, O.; Jin, Q.; Zhao, M.; Somerman, M.J.; Giannobile, W.V. Effect of sustained gene delivery of platelet-derived growth factor or its antagonist (PDGF-1308) on tissue-engineered cementum. J. Periodontol. 2004, 75, 429–440. [Google Scholar] [CrossRef]
- Jin, Q.; Anusaksathien, O.; Webb, S.A.; Printz, M.A.; Giannobile, W.V. Engineering of tooth-supporting structures by delivery of PDGF gene therapy vectors. Mol. Ther. 2004, 9, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.M.; Anusaksathien, O.; Webb, S.; Rutherford, R.; Giannobile, W. Gene therapy of bone morphogenetic protein for periodontal tissue engineering. J. Periodontol. 2003, 74, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Neel, E.A.A.; Chrzanowski, W.; Salih, V.M.; Kim, H.-W.; Knowles, J.C. Tissue engineering in dentistry. J. Dent. 2014, 42, 915–928. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, T.A. Clinical applications of recombinant human BMPs: Early experience and future development. JBJS 2003, 85, 82–88. [Google Scholar] [CrossRef]
- Kargozar, S.; Mozafari, M. Nanotechnology and Nanomedicine: Start small, think big. Mater. Today Proc. 2018, 5, 15492–15500. [Google Scholar] [CrossRef]
- Sowmya, S.; Mony, U.; Jayachandran, P.; Reshma, S.; Kumar, R.A.; Arzate, H.; Nair, S.V.; Jayakumar, R. Tri-Layered Nanocomposite Hydrogel Scaffold for the Concurrent Regeneration of Cementum, Periodontal Ligament, and Alveolar Bone. Adv. Healthc. Mater. 2017, 6, 1601251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Miron, R.J.; Li, S.; Shi, B.; Sculean, A.; Cheng, X. Novel Meso Porous BioGlass/silk scaffold containing ad PDGF-B and ad BMP 7 for the repair of periodontal defects in beagle dogs. J. Clin. Periodontol. 2015, 42, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, Y.; Miao, L.; Wang, Y.; Ren, S.; Yang, X.; Hu, Y.; Sun, W. Controlled release of recombinant human cementum protein 1 from electrospun multiphasic scaffold for cementum regeneration. Int. J. Nanomed. 2016, 11, 3145. [Google Scholar]
- Tobón, S.I.; Arismendi, J.A.; Marín, M.L.; Mesa, A.L.; Valencia, J.A. Comparison between a conventional technique and two bone regeneration techniques in periradicular surgery. Int. Endod. J. 2002, 35, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, G.; Murashima, Y.; Wadachi, R.; Sawada, N.; Suda, H. Guided bone regeneration (GBR) using membranes and calcium sulphate after apicectomy: A comparative histomorphometrical study. Int. Endod. J. 2002, 35, 255–263. [Google Scholar] [CrossRef]
- Britain, S.K.; von Arx, T.; Schenk, R.K.; Buser, D.; Nummikoski, P.; Cochran, D.L. The Use of Guided Tissue Regeneration Principles in Endodontic Surgery for Induced Chronic Periodontic-Endodontic Lesions: A Clinical, Radiographic, and Histologic Evaluation. J. Periodontol. 2005, 76, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Pini-Prato, G.; Cortellini, P. Effect of cigarette smoking on periodontal healing following GTR in infrabony defects: A preliminary retrospective study. J. Clin. Periodontol. 1995, 22, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, P.; Prato, G.P.; Tonetti, M.S. Periodontal regeneration of human intrabony defects with bioresorbable membranes. A controlled clinical trial. J. Periodontol. 1996, 67, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Machtei, E.E.; Cho, M.I.; Dunford, R.; Norderyd, J.; Zambon, J.J.; Genco, R.J. Clinical, microbiological, and histological factors which influence the success of regenerative periodontal therapy. J. Periodontol. 1994, 65, 154–161. [Google Scholar] [CrossRef] [PubMed]


| Commercial Name | Company | Sources | Cross-Linking Agent | Main Components | Resorption Rate |
|---|---|---|---|---|---|
| BioMend | Zimmer, Frankfurt, Germany | Bovine tendon | Formaldehyde | 100% type I collagen | 6–8 weeks |
| BioMend-Extend | Zimmer, Frankfurt, Germany | Bovine tendon | Formaldehyde | 100% type I collagen | 18 weeks |
| Periogen | Collagen Inc., Palo Alto, CA | Bovine dermis | Glutaraldehyde | Type I and III collagen | 4–8 weeks |
| Paroguide | Coletica, Lyon, France | Clafskin | DPPA | 96% type I collagen and 4% chondroitin-4-sulfate | 4–8 weeks |
| Biostite | Coletica, Lyon, France | Calfskin | DPPA | 88% HA 9.5% type I collagen, and 2.5% chondroitin-4-4sulfate | 4–8 weeks |
| BioGide | Geistlich, Wolhusen, Switzerland | Porcine dermis | None | Type I and III collagen | 24 weeks |
| Tissue Guide | Koken Co., Tokyo, Japan | Bovine dermis + tendon | HMDIC | Atelocollagen (I°) + tendon collagen | 4–8 weeks |
| BioBar | Colbar Research & Dev. Ltd., Ramat-Hasharon, Israel | Bovine dermis | N/A | 100% type I collagen | 6–8 moths |
| Osteobiol | Tecnoss srl, Giaveno, Italy | Heterologous mesenchymal tissue | None | 100% equine collagen | 8 weeks |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iviglia, G.; Kargozar, S.; Baino, F. Biomaterials, Current Strategies, and Novel Nano-Technological Approaches for Periodontal Regeneration. J. Funct. Biomater. 2019, 10, 3. https://doi.org/10.3390/jfb10010003
Iviglia G, Kargozar S, Baino F. Biomaterials, Current Strategies, and Novel Nano-Technological Approaches for Periodontal Regeneration. Journal of Functional Biomaterials. 2019; 10(1):3. https://doi.org/10.3390/jfb10010003
Chicago/Turabian StyleIviglia, Giorgio, Saeid Kargozar, and Francesco Baino. 2019. "Biomaterials, Current Strategies, and Novel Nano-Technological Approaches for Periodontal Regeneration" Journal of Functional Biomaterials 10, no. 1: 3. https://doi.org/10.3390/jfb10010003
APA StyleIviglia, G., Kargozar, S., & Baino, F. (2019). Biomaterials, Current Strategies, and Novel Nano-Technological Approaches for Periodontal Regeneration. Journal of Functional Biomaterials, 10(1), 3. https://doi.org/10.3390/jfb10010003







