A Critical Review on Selected External Physical Cues and Modulation of Cell Behavior: Magnetic Nanoparticles, Non-thermal Plasma and Lasers
Abstract
1. Introduction
- Using high-gradient magnetic fields in nanoparticle-mediated cell responses;
- Non-thermal plasma as a tool for non-specific bacterial killing;
- Highlights in understanding of cellular mechanisms of laser irradiation.
2. Using High-Gradient Magnetic Fields in Nanoparticle-Mediated Cell Responses
3. Non-Thermal Plasma as a Tool for Non-Specific Bacterial Killing
4. Highlights in Understanding of Cellular Mechanisms of Laser Irradiation
- (a)
- Power output of lasers being 0.001–0.1 Watts.
- (b)
- Wave length in the range of 300–10,600 nm.
- (c)
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Keevil, S.F. Physics and medicine: A historical perspective. Lancet 2012, 379, 1517–1524. [Google Scholar] [CrossRef]
- Melzer, A.; Cochran, S.; Prentice, P.; MacDonald, M.P.; Wang, Z.; Cuschieri, A. The importance of physics to progress in medical treatment. Lancet 2012, 379, 1534–1543. [Google Scholar] [CrossRef]
- Masic, I.; Miokovic, M.; Muhamedagic, B. Evidence based medicine—New approaches and challenges. Acta Inform. Med. 2008, 16, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Sackett, D.L.; Rosenberg, W.M.C.; Gray, J.A.M.; Haynes, R.B.; Richardson, W.S. Evidence based medicine: What it is and what it isn’t—It’s about integrating individual clinical expertise and the best external evidence. BMJ 1996, 312, 71–72. [Google Scholar] [CrossRef] [PubMed]
- Hore, P.J. Are biochemical reactions affected by weak magnetic fields? Proc. Natl. Acad. Sci. USA 2012, 109, 1357–1358. [Google Scholar] [CrossRef] [PubMed]
- Portelli, L.A.; Falldorf, K.; Thuroczy, G.; Cuppen, J. Retrospective estimation of the electric and magnetic field exposure conditions in in vitro experimental reports reveal considerable potential for uncertainty. Bioelectromagnetics 2018, 39, 231–243. [Google Scholar] [CrossRef]
- Isbary, G.; Zimmermann, J.L.; Shimizu, T.; Li, Y.F.; Morfill, G.E.; Thomas, H.M.; Steffes, B.; Heinlin, J.; Karrer, S.; Stolz, W. Non-thermal plasma—More than five years of clinical experience. Clin. Plasma Med. 2013, 1, 19–23. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Xia, Y.N. Nanomaterials at work in biomedical research. Nat. Mater. 2008, 7, 758–760. [Google Scholar] [CrossRef]
- Xie, J.; Huang, J.; Li, X.; Sun, S.; Chen, X. Iron oxide nanoparticle platform for biomedical applications. Curr. Med. Chem. 2009, 16, 1278–1294. [Google Scholar] [CrossRef]
- Pankhurst, Q.; Jones, S.; Dobson, J. Applications of magnetic nanoparticles in biomedicine: The story so far. J. Phys. D Appl. Phys. 2016, 49, 501002. [Google Scholar] [CrossRef]
- Ahrens, E.T.; Bulte, J.W.M. Tracking immune cells in vivo using magnetic resonance imaging. Nat. Rev. Immunol. 2013, 13, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Tukmachev, D.; Lunov, O.; Zablotskii, V.; Dejneka, A.; Babic, M.; Sykova, E.; Kubinova, S. An effective strategy of magnetic stem cell delivery for spinal cord injury therapy. Nanoscale 2015, 7, 3954–3958. [Google Scholar] [CrossRef] [PubMed]
- Kamau, S.W.; Hassa, P.O.; Steitz, B.; Petri-Fink, A.; Hofmann, H.; Hofmann-Amtenbrink, M.; von Rechenberg, B.; Hottiger, M.O. Enhancement of the efficiency of non-viral gene delivery by application of pulsed magnetic field. Nucleic Acids Res. 2006, 34, e40. [Google Scholar] [CrossRef] [PubMed]
- Mykhaylyk, O.; Antequera, Y.S.; Vlaskou, D.; Plank, C. Generation of magnetic nonviral gene transfer agents and magnetofection in vitro. Nat. Protoc. 2007, 2, 2391–2411. [Google Scholar] [CrossRef] [PubMed]
- Zablotskii, V.; Lunov, O.; Kubinova, S.; Polyakova, T.; Sykova, E.; Dejneka, A. Effects of high-gradient magnetic fields on living cell machinery. J. Phys. D Appl. Phys. 2016, 49, 493003. [Google Scholar] [CrossRef]
- Zablotskii, V.; Polyakova, T.; Lunov, O.; Dejneka, A. How a high-gradient magnetic field could affect cell life. Sci. Rep. 2016, 6, 37407. [Google Scholar] [CrossRef] [PubMed]
- Finegold, L.; Flamm, B.L. Magnet therapy. BMJ 2006, 332, 4. [Google Scholar] [CrossRef]
- Flamm, B.L. Magnet therapy: Healing or hogwash? Anesth. Analg. 2007, 104, 249–250. [Google Scholar] [CrossRef]
- Schenck, J.F. Physical interactions of static magnetic fields with living tissues. Prog. Biophys. Mol. Biol. 2005, 87, 185–204. [Google Scholar] [CrossRef]
- Pittler, M.H.; Brown, E.M.; Ernst, E. Static magnets for reducing pain: Systematic review and meta-analysis of randomized trials. CMAJ 2007, 177, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Lacy-Hulbert, A.; Metcalfe, J.C.; Hesketh, R. Biological responses to electromagnetic fields. FASEB J. 1998, 12, 395–420. [Google Scholar] [CrossRef] [PubMed]
- Schenck, J.F. Safety of strong, static magnetic fields. J. Magn. Reson. Imaging 2000, 12, 2–19. [Google Scholar] [CrossRef]
- Grosberg, A.Y. A few remarks evoked by Binhi and Savin’s review on magnetobiology. Phys. Usp. 2003, 46, 1113–1116. [Google Scholar] [CrossRef]
- Cepeda, M.S.; Carr, D.B.; Sarquis, T.; Miranda, N.; Garcia, R.J.; Zarate, C. Static magnetic therapy does not decrease pain or opioid requirements: A randomized double-blind trial. Anesth. Analg. 2007, 104, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Meister, M. Physical limits to magnetogenetics. Elife 2016, 5, e17210. [Google Scholar] [CrossRef]
- Plank, C.; Zelphati, O.; Mykhaylyk, O. Magnetically enhanced nucleic acid delivery. Ten years of magnetofection-progress and prospects. Adv. Drug Deliv. Rev. 2011, 63, 1300–1331. [Google Scholar] [CrossRef] [PubMed]
- Sapet, C.; Pellegrino, C.; Laurent, N.; Sicard, F.; Zelphati, O. Magnetic nanoparticles enhance adenovirus transduction in vitro and in vivo. Pharm. Res. 2012, 29, 1203–1218. [Google Scholar] [CrossRef]
- Choi, J.W.; Park, J.W.; Na, Y.; Jung, S.J.; Hwang, J.K.; Choi, D.; Lee, K.G.; Yun, C.O. Using a magnetic field to redirect an oncolytic adenovirus complexed with iron oxide augments gene therapy efficacy. Biomaterials 2015, 65, 163–174. [Google Scholar] [CrossRef]
- Grzeskowiak, B.F.; Sanchez-Antequera, Y.; Hammerschmid, E.; Doblinger, M.; Eberbeck, D.; Wozniak, A.; Slomski, R.; Plank, C.; Mykhaylyk, O. Nanomagnetic activation as a way to control the efficacy of nucleic acid delivery. Pharm. Res. 2015, 32, 103–121. [Google Scholar] [CrossRef]
- Sanchez-Antequera, Y.; Mykhaylyk, O.; van Til, N.P.; Cengizeroglu, A.; de Jong, J.H.; Huston, M.W.; Anton, M.; Johnston, I.C.; Pojda, Z.; Wagemaker, G.; et al. Magselectofection: An integrated method of nanomagnetic separation and genetic modification of target cells. Blood 2011, 117, e171–e181. [Google Scholar] [CrossRef] [PubMed]
- Kami, D.; Takeda, S.; Itakura, Y.; Gojo, S.; Watanabe, M.; Toyoda, M. Application of magnetic nanoparticles to gene delivery. Int. J. Mol. Sci. 2011, 12, 3705–3722. [Google Scholar] [CrossRef] [PubMed]
- Kami, D.; Takeda, S.; Makino, H.; Toyoda, M.; Itakura, Y.; Gojo, S.; Kyo, S.; Umezawa, A.; Watanabe, M. Efficient transfection method using deacylated polyethylenimine-coated magnetic nanoparticles. J. Artif. Organs 2011, 14, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Hisano, Y. Directional gene-transfer into the brain by an adenoviral vector tagged with magnetic nanoparticles. J. Neurosci. Meth. 2011, 194, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Shan, Z.; Andrea, K.; MacDonald, B.; Beale, S.; Curry, D.E.; Wang, L.; Wang, S.; Oakes, K.D.; Bennett, C.; et al. Chemisorption mechanism of DNA on Mg/Fe layered double hydroxide nanoparticles: Insights into engineering effective siRNA delivery systems. Langmuir 2016, 32, 2659–2667. [Google Scholar] [CrossRef] [PubMed]
- Namgung, R.; Singha, K.; Yu, M.K.; Jon, S.; Kim, Y.S.; Ahn, Y.; Park, I.K.; Kim, W.J. Hybrid superparamagnetic iron oxide nanoparticle-branched polyethylenimine magnetoplexes for gene transfection of vascular endothelial cells. Biomaterials 2010, 31, 4204–4213. [Google Scholar] [CrossRef]
- Chen, C.B.; Chen, J.Y.; Lee, W.C. Fast transfection of mammalian cells using superparamagnetic nanoparticles under strong magnetic field. J. Nanosci. Nanotechnol. 2009, 9, 2651–2659. [Google Scholar] [CrossRef]
- Zhang, S.; Gao, H.; Bao, G. Physical principles of nanoparticle cellular endocytosis. ACS Nano 2015, 9, 8655–8671. [Google Scholar] [CrossRef]
- Zhao, J.C.; Stenzel, M.H. Entry of nanoparticles into cells: The importance of nanoparticle properties. Polym. Chem. 2018, 9, 259–272. [Google Scholar] [CrossRef]
- Canton, I.; Battaglia, G. Endocytosis at the nanoscale. Chem. Soc. Rev. 2012, 41, 2718–2739. [Google Scholar] [CrossRef]
- Latham, A.H.; Williams, M.E. Controlling transport and chemical functionality of magnetic nanoparticles. Acc. Chem. Res. 2008, 41, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Estelrich, J.; Escribano, E.; Queralt, J.; Busquets, M.A. Iron oxide nanoparticles for magnetically-guided and magnetically-responsive drug delivery. Int. J. Mol. Sci. 2015, 16, 8070–8101. [Google Scholar] [CrossRef] [PubMed]
- Grief, A.D.; Richardson, G. Mathematical modelling of magnetically targeted drug delivery. J. Magn. Magn. Mater. 2005, 293, 455–463. [Google Scholar] [CrossRef]
- Ashouri, M.; Shafii, M.B. Numerical simulation of magnetic convection ferrofluid flow in a permanent magnet-inserted cavity. J. Magn. Magn. Mater. 2017, 442, 270–278. [Google Scholar] [CrossRef]
- Pedram, M.Z.; Shamloo, A.; Alasty, A.; Ghafar-Zadeh, E. Optimal magnetic field for crossing super-para-magnetic nanoparticles through the brain blood barrier: A computational approach. Biosensors 2016, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Min, K.A.; Shin, M.C.; Yu, F.; Yang, M.; David, A.E.; Yang, V.C.; Rosania, G.R. Pulsed magnetic field improves the transport of iron oxide nanoparticles through cell barriers. ACS Nano 2013, 7, 2161–2171. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Jeon, M.; Hsiao, M.H.; Patton, V.K.; Wang, K.; Press, O.W.; Zhang, M. Stable and efficient Paclitaxel nanoparticles for targeted glioblastoma therapy. Adv. Healthc. Mater. 2015, 4, 1236–1245. [Google Scholar] [CrossRef]
- Almstatter, I.; Mykhaylyk, O.; Settles, M.; Altomonte, J.; Aichler, M.; Walch, A.; Rummeny, E.J.; Ebert, O.; Plank, C.; Braren, R. Characterization of magnetic viral complexes for targeted delivery in oncology. Theranostics 2015, 5, 667–685. [Google Scholar] [CrossRef]
- Prosen, L.; Hudoklin, S.; Cemazar, M.; Stimac, M.; Lampreht Tratar, U.; Ota, M.; Scancar, J.; Romih, R.; Sersa, G. Magnetic field contributes to the cellular uptake for effective therapy with magnetofection using plasmid DNA encoding against Mcam in B16F10 melanoma in vivo. Nanomedicine 2016, 11, 627–641. [Google Scholar] [CrossRef]
- Prijic, S.; Prosen, L.; Cemazar, M.; Scancar, J.; Romih, R.; Lavrencak, J.; Bregar, V.B.; Coer, A.; Krzan, M.; Znidarsic, A.; et al. Surface modified magnetic nanoparticles for immuno-gene therapy of murine mammary adenocarcinoma. Biomaterials 2012, 33, 4379–4391. [Google Scholar] [CrossRef]
- Yanai, A.; Hafeli, U.O.; Metcalfe, A.L.; Soema, P.; Addo, L.; Gregory-Evans, C.Y.; Po, K.; Shan, X.H.; Moritz, O.L.; Gregory-Evans, K. Focused magnetic stem cell targeting to the retina using superparamagnetic iron oxide nanoparticles. Cell Transplant. 2012, 21, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.V.; Bachman, L.A.; Hann, C.R.; Bahler, C.K.; Fautsch, M.P. Human corneal endothelial cell transplantation in a human ex vivo model. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2123–2131. [Google Scholar] [CrossRef] [PubMed]
- Mimura, T.; Shimomura, N.; Usui, T.; Noda, Y.; Kaji, Y.; Yamgami, S.; Amano, S.; Miyata, K.; Araie, M. Magnetic attraction of iron-endocytosed corneal endothelial cells to Descemet’s membrane. Exp. Eye Res. 2003, 76, 745–751. [Google Scholar] [CrossRef]
- Kemp, S.J.; Ferguson, R.M.; Khandhar, A.P.; Krishnan, K.M. Monodisperse magnetite nanoparticles with nearly ideal saturation magnetization. RSC Adv. 2016, 6, 77452–77464. [Google Scholar] [CrossRef]
- Kuhn, S.J.; Hallahan, D.E.; Giorgio, T.D. Characterization of superparamagnetic nanoparticle interactions with extracellular matrix in an in vitro system. Ann. Biomed. Eng. 2006, 34, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Monzel, C.; Vicario, C.; Piehler, J.; Coppey, M.; Dahan, M. Magnetic control of cellular processes using biofunctional nanoparticles. Chem. Sci. 2017, 8, 7330–7338. [Google Scholar] [CrossRef] [PubMed]
- Meloty-Kapella, L.; Shergill, B.; Kuon, J.; Botvinick, E.; Weinmaster, G. Notch ligand endocytosis generates mechanical pulling force dependent on dynamin, epsins, and actin. Dev. Cell 2012, 22, 1299–1312. [Google Scholar] [CrossRef]
- Gordon, W.R.; Zimmerman, B.; He, L.; Miles, L.J.; Huang, J.; Tiyanont, K.; McArthur, D.G.; Aster, J.C.; Perrimon, N.; Loparo, J.J.; et al. Mechanical allostery: Evidence for a force requirement in the proteolytic activation of Notch. Dev. Cell 2015, 33, 729–736. [Google Scholar] [CrossRef]
- Ke, P.C.; Lin, S.; Parak, W.J.; Davis, T.P.; Caruso, F. A decade of the protein corona. ACS Nano 2017, 11, 11773–11776. [Google Scholar] [CrossRef]
- Vilanova, O.; Mittag, J.J.; Kelly, P.M.; Milani, S.; Dawson, K.A.; Radler, J.O.; Franzese, G. Understanding the kinetics of protein-nanoparticle corona formation. ACS Nano 2016, 10, 10842–10850. [Google Scholar] [CrossRef]
- Lunova, M.; Prokhorov, A.; Jirsa, M.; Hof, M.; Olzynska, A.; Jurkiewicz, P.; Kubinova, S.; Lunov, O.; Dejneka, A. Nanoparticle core stability and surface functionalization drive the mTOR signaling pathway in hepatocellular cell lines. Sci. Rep. 2017, 7, 16049. [Google Scholar] [CrossRef]
- Moore, T.L.; Rodriguez-Lorenzo, L.; Hirsch, V.; Balog, S.; Urban, D.; Jud, C.; Rothen-Rutishauser, B.; Lattuada, M.; Petri-Fink, A. Nanoparticle colloidal stability in cell culture media and impact on cellular interactions. Chem. Soc. Rev. 2015, 44, 6287–6305. [Google Scholar] [CrossRef] [PubMed]
- Zablotskii, V.; Lunov, O.; Dejneka, A.; Jastrabik, L.; Polyakova, T.; Syrovets, T.; Simmet, T. Nanomechanics of magnetically driven cellular endocytosis. Appl. Phys. Lett. 2011, 99, 183701. [Google Scholar] [CrossRef]
- Kuznetsov, A.A. Force acting on a cluster of magnetic nanoparticles in a gradient field: A Langevin dynamics study. J. Magn. Magn. Mater. 2019, 475, 415–420. [Google Scholar] [CrossRef]
- Kolosnjaj-Tabi, J.; Wilhelm, C.; Clement, O.; Gazeau, F. Cell labeling with magnetic nanoparticles: Opportunity for magnetic cell imaging and cell manipulation. J. Nanobiotechnol. 2013, 11 (Suppl. 1), S7. [Google Scholar] [CrossRef]
- Uzhytchak, M.; Lynnyk, A.; Zablotskii, V.; Dempsey, N.M.; Dias, A.L.; Bonfim, M.; Lunova, M.; Jirsa, M.; Kubinova, S.; Lunov, O.; et al. The use of pulsed magnetic fields to increase the uptake of iron oxide nanoparticles by living cells. Appl. Phys. Lett. 2017, 111, 243703. [Google Scholar] [CrossRef]
- Seo, D.; Southard, K.M.; Kim, J.W.; Lee, H.J.; Farlow, J.; Lee, J.U.; Litt, D.B.; Haas, T.; Alivisatos, A.P.; Cheon, J.; et al. A mechanogenetic toolkit for interrogating cell signaling in space and time. Cell 2016, 165, 1507–1518. [Google Scholar] [CrossRef]
- Tajik, A.; Zhang, Y.; Wei, F.; Sun, J.; Jia, Q.; Zhou, W.; Singh, R.; Khanna, N.; Belmont, A.S.; Wang, N. Transcription upregulation via force-induced direct stretching of chromatin. Nat. Mater. 2016, 15, 1287–1296. [Google Scholar] [CrossRef]
- Rotherham, M.; El Haj, A.J. Remote activation of the Wnt/beta-catenin signalling pathway using functionalised magnetic particles. PLoS ONE 2015, 10, e0121761. [Google Scholar] [CrossRef]
- Tseng, P.; Judy, J.W.; Di Carlo, D. Magnetic nanoparticle-mediated massively parallel mechanical modulation of single-cell behavior. Nat. Methods 2012, 9, 1113–1119. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, J.W.; Levy, M.; Kao, A.; Noh, S.H.; Bozovic, D.; Cheon, J. Magnetic nanoparticles for ultrafast mechanical control of inner ear hair cells. ACS Nano 2014, 8, 6590–6598. [Google Scholar] [CrossRef] [PubMed]
- Desprat, N.; Supatto, W.; Pouille, P.A.; Beaurepaire, E.; Farge, E. Tissue deformation modulates twist expression to determine anterior midgut differentiation in Drosophila embryos. Dev. Cell 2008, 15, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Etoc, F.; Lisse, D.; Bellaiche, Y.; Piehler, J.; Coppey, M.; Dahan, M. Subcellular control of Rac-GTPase signalling by magnetogenetic manipulation inside living cells. Nat. Nanotechnol. 2013, 8, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Rozhkova, E.A.; Ulasov, I.V.; Bader, S.D.; Rajh, T.; Lesniak, M.S.; Novosad, V. Biofunctionalized magnetic-vortex microdiscs for targeted cancer-cell destruction. Nat. Mater. 2010, 9, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Arias, L.S.; Pessan, J.P.; Vieira, A.P.M.; Lima, T.M.T.; Delbem, A.C.B.; Monteiro, D.R. Iron oxide nanoparticles for biomedical applications: A perspective on synthesis, drugs, antimicrobial activity, and toxicity. Antibiotics 2018, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Gu, H.; Xu, B. Multifunctional magnetic nanoparticles: Design, synthesis, and biomedical applications. Acc. Chem. Res. 2009, 42, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Pickard, M.R.; Adams, C.F.; Barraud, P.; Chari, D.M. Using magnetic nanoparticles for gene transfer to neural stem cells: Stem cell propagation method influences outcomes. J. Funct. Biomater. 2015, 6, 259–276. [Google Scholar] [CrossRef]
- Stanley, S.A.; Sauer, J.; Kane, R.S.; Dordick, J.S.; Friedman, J.M. Remote regulation of glucose homeostasis in mice using genetically encoded nanoparticles. Nat. Med. 2015, 21, 92–98. [Google Scholar] [CrossRef]
- Wheeler, M.A.; Smith, C.J.; Ottolini, M.; Barker, B.S.; Purohit, A.M.; Grippo, R.M.; Gaykema, R.P.; Spano, A.J.; Beenhakker, M.P.; Kucenas, S.; et al. Genetically targeted magnetic control of the nervous system. Nat. Neurosci. 2016, 19, 756–761. [Google Scholar] [CrossRef]
- Piddock, L.J.V. The crisis of no new antibiotics-what is the way forward? Lancet Infect. Dis. 2012, 12, 249–253. [Google Scholar] [CrossRef]
- Brown, E.D.; Wright, G.D. Antibacterial drug discovery in the resistance era. Nature 2016, 529, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Brown, D. Antibiotic resistance breakers: Can repurposed drugs fill the antibiotic discovery void? Nat. Rev. Drug Discov. 2015, 14, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Minden-Birkenmaier, B.A.; Bowlin, G.L. Honey-based templates in wound healing and tissue engineering. Bioengineering 2018, 5, 46. [Google Scholar] [CrossRef] [PubMed]
- Martinotti, S.; Ranzato, E. Honey, wound repair and regenerative medicine. J. Funct. Biomater. 2018, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Rau, J.V.; Fosca, M.; Graziani, V.; Egorov, A.A.; Zobkov, Y.V.; Fedotov, A.Y.; Ortenzi, M.; Caminiti, R.; Baranchikov, A.E.; Komlev, V.S. Silver-doped calcium phosphate bone cements with antibacterial properties. J. Funct. Biomater. 2016, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Casey, A.L.; Adams, D.; Karpanen, T.J.; Lambert, P.A.; Cookson, B.D.; Nightingale, P.; Miruszenko, L.; Shillam, R.; Christian, P.; Elliott, T.S. Role of copper in reducing hospital environment contamination. J. Hosp. Infect. 2010, 74, 72–77. [Google Scholar] [CrossRef]
- Hartemann, P.; Hoet, P.; Proykova, A.; Fernandes, T.; Baun, A.; De Jong, W.; Filser, J.; Hensten, A.; Kneuer, C.; Maillard, J.Y.; et al. Nanosilver: Safety, health and environmental effects and role in antimicrobial resistance. Mater. Today 2015, 18, 122–123. [Google Scholar] [CrossRef]
- Zhang, S.H.; Ye, C.S.; Lin, H.R.; Lv, L.; Yu, X. UV disinfection induces a Vbnc state in Escherichia coli and Pseudomonas aeruginosa. Environ. Sci. Technol. 2015, 49, 1721–1728. [Google Scholar] [CrossRef]
- Pokhrel, P.R.; Bermudez-Aguirre, D.; Martinez-Flores, H.E.; Garnica-Romo, M.G.; Sablani, S.; Tang, J.; Barbosa-Canovas, G.V. Combined effect of ultrasound and mild temperatures on the inactivation of E. coli in fresh carrot juice and changes on its physicochemical characteristics. J. Food Sci. 2017, 82, 2343–2350. [Google Scholar] [CrossRef]
- Evelyn; Silva, F.V.M. Resistance of Byssochlamys nivea and Neosartorya fischeri mould spores of different age to high pressure thermal processing and thermosonication. J. Food Eng. 2017, 201, 9–16. [Google Scholar] [CrossRef]
- Gupta, T.T.; Karki, S.B.; Matson, J.S.; Gehling, D.J.; Ayan, H. Sterilization of biofilm on a titanium surface using a combination of nonthermal plasma and chlorhexidine digluconate. BioMed Res. Int. 2017, 2017, 6085741. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Kylian, O.; Rauscher, H.; Hasiwa, M.; Gilliland, D. Low pressure plasma discharges for the sterilization and decontamination of surfaces. New J. Phys. 2009, 11, 115017. [Google Scholar] [CrossRef]
- Gilmore, B.F.; Flynn, P.B.; O’Brien, S.; Hickok, N.; Freeman, T.; Bourke, P. Cold plasmas for biofilm control: Opportunities and challenges. Trends Biotechnol. 2018, 36, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Scholtz, V.; Pazlarova, J.; Souskova, H.; Khun, J.; Julak, J. Nonthermal plasma—A tool for decontamination and disinfection. Biotechnol. Adv. 2015, 33, 1108–1119. [Google Scholar] [CrossRef] [PubMed]
- De Geyter, N.; Morent, R. Nonthermal plasma sterilization of living and nonliving surfaces. Annu. Rev. Biomed. Eng. 2012, 14, 255–274. [Google Scholar] [CrossRef] [PubMed]
- Sarangapani, C.; Patange, A.; Bourke, P.; Keener, K.; Cullen, P.J. Recent advances in the application of cold plasma technology in foods. Annu. Rev. Food Sci. Technol. 2018, 9, 609–629. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Naidis, G.V.; Laroussi, M.; Reuter, S.; Graves, D.B.; Ostrikov, K. Reactive species in non-equilibrium atmospheric-pressure plasmas: Generation, transport, and biological effects. Phys. Rep. Rev. Sec. Phys. Lett. 2016, 630, 1–84. [Google Scholar] [CrossRef]
- Laroussi, M. Sterilization of contaminated matter with an atmospheric pressure plasma. IEEE Trans. Plasma Sci. 1996, 24, 1188–1191. [Google Scholar] [CrossRef]
- Sung, S.J.; Huh, J.B.; Yun, M.J.; Chang, B.M.; Jeong, C.M.; Jeon, Y.C. Sterilization effect of atmospheric pressure non-thermal air plasma on dental instruments. J. Adv. Prosthodont. 2013, 5, 2–8. [Google Scholar] [CrossRef]
- Kubinova, S.; Zaviskova, K.; Uherkova, L.; Zablotskii, V.; Churpita, O.; Lunov, O.; Dejneka, A. Non-thermal air plasma promotes the healing of acute skin wounds in rats. Sci. Rep. 2017, 7, 45183. [Google Scholar] [CrossRef]
- Chatraie, M.; Torkaman, G.; Khani, M.; Salehi, H.; Shokri, B. In vivo study of non-invasive effects of non-thermal plasma in pressure ulcer treatment. Sci. Rep. 2018, 8, 5621. [Google Scholar] [CrossRef] [PubMed]
- Rupf, S.; Lehmann, A.; Hannig, M.; Schafer, B.; Schubert, A.; Feldmann, U.; Schindler, A. Killing of adherent oral microbes by a non-thermal atmospheric plasma jet. J. Med. Microbiol. 2010, 59, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Kalghatgi, S.U.; Fridman, G.; Cooper, M.; Nagaraj, G.; Peddinghaus, M.; Balasubramanian, M.; Vasilets, V.N.; Gutsol, A.F.; Fridman, A.; Friedman, G. Mechanism of blood coagulation by nonthermal atmospheric pressure dielectric barrier discharge plasma. IEEE Trans. Plasma Sci. 2007, 35, 1559–1566. [Google Scholar] [CrossRef]
- Keidar, M.; Yan, D.; Beilis, I.I.; Trink, B.; Sherman, J.H. Plasmas for treating cancer: Opportunities for adaptive and self-adaptive approaches. Trends Biotechnol. 2018, 36, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.G.; Kroesen, G.; Morfill, G.; Nosenko, T.; Shimizu, T.; van Dijk, J.; Zimmermann, J.L. Plasma medicine: An introductory review. New J. Phys. 2009, 11, 115012. [Google Scholar] [CrossRef]
- Fridman, G.; Peddinghaus, M.; Ayan, H.; Fridman, A.; Balasubramanian, M.; Gutsol, A.; Brooks, A.; Friedman, G. Blood coagulation and living tissue sterilization by floating-electrode dielectric barrier discharge in air. Plasma Chem. Plasma Process. 2006, 26, 425–442. [Google Scholar] [CrossRef]
- Dobrynin, D.; Fridman, G.; Friedman, G.; Fridman, A. Physical and biological mechanisms of direct plasma interaction with living tissue. New J. Phys. 2009, 11, 115020. [Google Scholar] [CrossRef]
- Brun, P.; Bernabe, G.; Marchiori, C.; Scarpa, M.; Zuin, M.; Cavazzana, R.; Zaniol, B.; Martines, E. Antibacterial efficacy and mechanisms of action of low power atmospheric pressure cold plasma: Membrane permeability, biofilm penetration and antimicrobial sensitization. J. Appl. Microbiol. 2018, 125, 398–408. [Google Scholar] [CrossRef]
- Joshi, S.G.; Paff, M.; Friedman, G.; Fridman, G.; Fridman, A.; Brooks, A.D. Control of methicillin-resistant Staphylococcus aureus in planktonic form and biofilms: A biocidal efficacy study of nonthermal dielectric-barrier discharge plasma. Am. J. Infect. Control 2010, 38, 293–301. [Google Scholar] [CrossRef]
- Maisch, T.; Shimizu, T.; Li, Y.F.; Heinlin, J.; Karrer, S.; Morfill, G.; Zimmermann, J.L. Decolonisation of MRSA, S-aureus and E-coli by cold-atmospheric plasma using a porcine skin model in vitro. PLoS ONE 2012, 7, e34610. [Google Scholar] [CrossRef]
- Klampfl, T.G.; Isbary, G.; Shimizu, T.; Li, Y.F.; Zimmermann, J.L.; Stolz, W.; Schlegel, J.; Morfill, G.E.; Schmidt, H.U. Cold atmospheric air plasma sterilization against spores and other microorganisms of clinical interest. Appl. Environ. Microb. 2012, 78, 5077–5082. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Li, Z.; Huang, K.; Li, Y.; Wang, J. Morphology analysis of Escherichia coli treated with nonthermal plasma. J. Appl. Microbiol. 2017, 122, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Lis, K.A.; Boulaaba, A.; Binder, S.; Li, Y.F.; Kehrenberg, C.; Zimmermann, J.L.; Klein, G.; Ahlfeld, B. Inactivation of Salmonella Typhimurium and Listeria monocytogenes on ham with nonthermal atmospheric pressure plasma. PLoS ONE 2018, 13, e0197773. [Google Scholar] [CrossRef] [PubMed]
- Lunov, O.; Churpita, O.; Zablotskii, V.; Deyneka, I.G.; Meshkovskii, I.K.; Jager, A.; Sykova, E.; Kubinova, S.; Dejneka, A. Non-thermal plasma mills bacteria: Scanning electron microscopy observations. Appl. Phys. Lett. 2015, 106, 053703. [Google Scholar] [CrossRef]
- Ben Belgacem, Z.; Carre, G.; Charpentier, E.; Le-Bras, F.; Maho, T.; Robert, E.; Pouvesle, J.M.; Polidor, F.; Gangloff, S.C.; Boudifa, M.; et al. Innovative non-thermal plasma disinfection process inside sealed bags: Assessment of bactericidal and sporicidal effectiveness in regard to current sterilization norms. PLoS ONE 2017, 12, e0180183. [Google Scholar] [CrossRef] [PubMed]
- Lunov, O.; Zablotskii, V.; Churpita, O.; Jager, A.; Polivka, L.; Sykova, E.; Dejneka, A.; Kubinova, S. The interplay between biological and physical scenarios of bacterial death induced by non-thermal plasma. Biomaterials 2016, 82, 71–83. [Google Scholar] [CrossRef]
- Han, L.; Patil, S.; Boehm, D.; Milosavljevic, V.; Cullen, P.J.; Bourke, P. Mechanisms of inactivation by high-voltage atmospheric cold plasma differ for Escherichia coli and Staphylococcus aureus. Appl. Environ. Microb. 2016, 82, 450–458. [Google Scholar] [CrossRef]
- Kvam, E.; Davis, B.; Mondello, F.; Garner, A.L. Nonthermal atmospheric plasma rapidly disinfects multidrug-resistant microbes by inducing cell surface damage. Antimicrob. Agents Chemother. 2012, 56, 2028–2036. [Google Scholar] [CrossRef]
- Shaw, P.; Kumar, N.; Kwak, H.S.; Park, J.H.; Uhm, H.S.; Bogaerts, A.; Choi, E.H.; Attri, P. Bacterial inactivation by plasma treated water enhanced by reactive nitrogen species. Sci. Rep. 2018, 8, 11268. [Google Scholar] [CrossRef]
- Panngom, K.; Lee, S.H.; Park, D.H.; Sim, G.B.; Kim, Y.H.; Uhm, H.S.; Park, G.; Choi, E.H. Non-thermal plasma treatment diminishes fungal viability and up-regulates resistance genes in a plant host. PLoS ONE 2014, 9, e99300. [Google Scholar] [CrossRef]
- Ziuzina, D.; Boehm, D.; Patil, S.; Cullen, P.J.; Bourke, P. Cold plasma inactivation of bacterial biofilms and reduction of quorum sensing regulated virulence factors. PLoS ONE 2015, 10, e0138209. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Patil, S.; Keener, K.M.; Cullen, P.J.; Bourke, P. Bacterial inactivation by high-voltage atmospheric cold plasma: Influence of process parameters and effects on cell leakage and DNA. J. Appl. Microbiol. 2014, 116, 784–794. [Google Scholar] [PubMed]
- Sinha, R.P.; Hader, D.P. UV-induced DNA damage and repair: A review. Photochem. Photobiol. Sci. 2002, 1, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Moreau, S.; Moisan, M.; Tabrizian, M.; Barbeau, J.; Pelletier, J.; Ricard, A.; Yahia, L.H. Using the flowing afterglow of a plasma to inactivate Bacillus subtilis spores: Influence of the operating conditions. J. Appl. Phys. 2000, 88, 1166–1174. [Google Scholar] [CrossRef]
- Kostov, K.G.; Rocha, V.; Koga-Ito, C.Y.; Matos, B.M.; Algatti, M.A.; Honda, R.Y.; Kayama, M.E.; Mota, R.P. Bacterial sterilization by a dielectric barrier discharge (DBD) in air. Surf. Coat. Technol. 2010, 204, 2954–2959. [Google Scholar] [CrossRef]
- Birmingham, J.G. Mechanisms of bacterial spore deactivation using ambient pressure nonthermal discharges. IEEE Trans. Plasma Sci. 2004, 32, 1526–1531. [Google Scholar] [CrossRef]
- Park, B.J.; Lee, D.H.; Park, J.C.; Lee, I.S.; Lee, K.Y.; Hyun, S.O.; Chun, M.S.; Chung, K.H. Sterilization using a microwave-induced argon plasma system at atmospheric pressure. Phys. Plasmas 2003, 10, 4539–4544. [Google Scholar] [CrossRef]
- Lee, K.Y.; Park, B.J.; Lee, D.H.; Lee, I.S.; Hyun, S.O.; Chung, K.H.; Park, J.C. Sterilization of Escherichia coli and MRSA using microwave-induced argon plasma at atmospheric pressure. Surf. Coat. Technol. 2005, 193, 35–38. [Google Scholar] [CrossRef]
- Lunov, O.; Zablotskii, V.; Churpita, O.; Lunova, M.; Jirsa, M.; Dejneka, A.; Kubinova, S. Chemically different non-thermal plasmas target distinct cell death pathways. Sci. Rep. 2017, 7, 600. [Google Scholar] [CrossRef]
- Lunov, O.; Zablotskii, V.; Churpita, O.; Jaeger, A.; Polivka, L.; Sykova, E.; Terebova, N.; Kulikov, A.; Kubinova, S.; Dejneka, A. Towards the understanding of non-thermal air plasma action: Effects on bacteria and fibroblasts. RSC Adv. 2016, 6, 25286–25292. [Google Scholar] [CrossRef]
- Rumbach, P.; Witzke, M.; Sankaran, R.M.; Go, D.B. Decoupling interfacial reactions between plasmas and liquids: Charge transfer vs plasma neutral reactions. J. Am. Chem. Soc. 2013, 135, 16264–16267. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Park, S.; Choe, W.; Yong, H.I.; Jo, C.; Kim, K. Plasma-functionalized solution: A potent antimicrobial agent for biomedical applications from antibacterial therapeutics to biomaterial surface engineering. ACS Appl. Mater. Interfaces 2017, 9, 43470–43477. [Google Scholar] [CrossRef] [PubMed]
- Laroussi, M.; Leipold, F. Evaluation of the roles of reactive species, heat, and UV radiation in the inactivation of bacterial cells by air plasmas at atmospheric pressure. Int. J. Mass Spectrom. 2004, 233, 81–86. [Google Scholar] [CrossRef]
- Laroussi, M. Nonthermal decontamination of biological media by atmospheric-pressure plasmas: Review, analysis, and prospects. IEEE Trans. Plasma Sci. 2002, 30, 1409–1415. [Google Scholar] [CrossRef]
- Liao, X.Y.; Li, J.; Suo, Y.J.; Ahn, J.; Liu, D.H.; Chen, S.G.; Hu, Y.Q.; Ye, X.Q.; Ding, T. Effect of preliminary stresses on the resistance of Escherichia coli and Staphylococcus aureus toward non-thermal plasma (NTP) challenge. Food Res. Int. 2018, 105, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.Y.; Ma, C.X.; Lin, L. Synergetic antibacterial efficacy of cold nitrogen plasma and clove oil against Escherichia coli O157:H7 biofilms on lettuce. Food Control 2016, 66, 8–16. [Google Scholar] [CrossRef]
- Fridman, G.; Brooks, A.D.; Balasubramanian, M.; Fridman, A.; Gutsol, A.; Vasilets, V.N.; Ayan, H.; Friedman, G. Comparison of direct and indirect effects of non-thermal atmospheric-pressure plasma on bacteria. Plasma Process. Polym. 2007, 4, 370–375. [Google Scholar] [CrossRef]
- Stoffels, E.; Sakiyama, Y.; Graves, D.B. Cold atmospheric plasma: Charged species and their interactions with cells and tissues. IEEE Trans. Plasma Sci. 2008, 36, 1441–1457. [Google Scholar] [CrossRef]
- Roy, S.; Khanna, S.; Nallu, K.; Hunt, T.K.; Sen, C.K. Dermal wound healing is subject to redox control. Mol. Ther. 2006, 13, 211–220. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Suzuki, N.; Miller, G.; Tognetti, V.B.; Vandepoele, K.; Gollery, M.; Shulaev, V.; Van Breusegem, F. ROS signaling: The new wave? Trends Plant Sci. 2011, 16, 300–309. [Google Scholar] [CrossRef]
- Alkawareek, M.Y.; Gorman, S.P.; Graham, W.G.; Gilmore, B.F. Potential cellular targets and antibacterial efficacy of atmospheric pressure non-thermal plasma. Int. J. Antimicrob. Agents 2014, 43, 154–160. [Google Scholar] [CrossRef]
- Yusupov, M.; Bogaerts, A.; Huygh, S.; Snoeckx, R.; van Duin, A.C.T.; Neyts, E.C. Plasma-induced destruction of bacterial cell wall components: A reactive molecular dynamics simulation. J. Phys. Chem. C 2013, 117, 5993–5998. [Google Scholar] [CrossRef]
- Beveridge, T.J. Structures of gram-negative cell walls and their derived membrane vesicles. J. Bacteriol. 1999, 181, 4725–4733. [Google Scholar]
- Malanovic, N.; Lohner, K. Gram-positive bacterial cell envelopes: The impact on the activity of antimicrobial peptides. Biochim. Biophys. Acta 2016, 1858, 936–946. [Google Scholar] [CrossRef]
- Auer, G.K.; Weibel, D.B. Bacterial cell mechanics. Biochemistry 2017, 56, 3710–3724. [Google Scholar] [CrossRef] [PubMed]
- Panieri, E.; Gogvadze, V.; Norberg, E.; Venkatesh, R.; Orrenius, S.; Zhivotovsky, B. Reactive oxygen species generated in different compartments induce cell death, survival, or senescence. Free Radic. Biol. Med. 2013, 57, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Bayles, K.W. Bacterial programmed cell death: Making sense of a paradox. Nat. Rev. Microbiol. 2014, 12, 63–69. [Google Scholar] [CrossRef]
- Dwyer, D.J.; Camacho, D.M.; Kohanski, M.A.; Callura, J.M.; Collins, J.J. Antibiotic-induced bacterial cell death exhibits physiological and biochemical hallmarks of apoptosis. Mol. Cell 2012, 46, 561–572. [Google Scholar] [CrossRef]
- Li, L.M.; Zhang, H.; Huang, Q. New insight into the residual inactivation of Microcystis aeruginosa by dielectric barrier discharge. Sci. Rep. 2015, 5, 13683. [Google Scholar] [CrossRef]
- Won, H.R.; Kang, S.U.; Kim, H.J.; Jang, J.Y.; Shin, Y.S.; Kim, C.H. Non-thermal plasma treated solution with potential as a novel therapeutic agent for nasal mucosa regeneration. Sci. Rep. 2018, 8, 13754. [Google Scholar] [CrossRef]
- Babaeva, N.Y.; Naidis, G.V. Modeling of plasmas for biomedicine. Trends Biotechnol. 2018, 36, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Kim, J.; Hong, Y.J.; Bae, W.Y.; Choi, E.H.; Jeong, J.W.; Park, H.K. Evaluation of non-thermal plasma-induced anticancer effects on human colon cancer cells. Biomed. Opt. Express 2017, 8, 2649–2659. [Google Scholar] [CrossRef] [PubMed]
- Pai, K.; Timmons, C.; Roehm, K.D.; Ngo, A.; Narayanan, S.S.; Ramachandran, A.; Jacob, J.D.; Ma, L.M.; Madihally, S.V. Investigation of the roles of plasma species generated by surface dielectric barrier discharge. Sci. Rep. 2018, 8, 16674. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.U.; Cho, J.H.; Chang, J.W.; Shin, Y.S.; Kim, K.I.; Park, J.K.; Yang, S.S.; Lee, J.S.; Moon, E.; Lee, K.; et al. Nonthermal plasma induces head and neck cancer cell death: The potential involvement of mitogen-activated protein kinase-dependent mitochondrial reactive oxygen species. Cell Death Dis. 2014, 5, e1056. [Google Scholar] [CrossRef] [PubMed]
- Daeschlein, G.; Napp, M.; Lutze, S.; Arnold, A.; von Podewils, S.; Guembel, D.; Junger, M. Skin and wound decontamination of multidrug-resistant bacteria by cold atmospheric plasma coagulation. J. Dtsch. Dermatol. Ges. 2015, 13, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, C.; Kluschke, F.; Patzelt, A.; Vandersee, S.; Czaika, V.A.; Richter, H.; Bob, A.; von Hutten, J.; Painsi, C.; Hugel, R.; et al. Clinical use of cold atmospheric pressure argon plasma in chronic leg ulcers: A pilot study. J. Wound Care 2015, 24, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Pan, J.; Ye, G.P.; Zhang, Q.; Wang, J.; Zhang, J.; Fang, J. In vitro studies of the antimicrobial effect of non-thermal plasma-activated water as a novel mouthwash. Eur. J. Oral Sci. 2017, 125, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Van Boxem, W.; Van der Paal, J.; Gorbanev, Y.; Vanuytsel, S.; Smits, E.; Dewilde, S.; Bogaerts, A. Anti-cancer capacity of plasma-treated PBS: Effect of chemical composition on cancer cell cytotoxicity. Sci. Rep. 2017, 7, 16478. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.H.; Kwok, S.J.J. Light in diagnosis, therapy and surgery. Nat. Biomed. Eng. 2017, 1, 0008. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.R. Lasers for dermatology and skin biology. J. Investig. Dermatol. 2013, 133, E21–E23. [Google Scholar] [CrossRef] [PubMed]
- Shirasu, N.; Nam, S.O.; Kuroki, M. Tumor-targeted photodynamic therapy. Anticancer Res. 2013, 33, 2823–2831. [Google Scholar] [PubMed]
- Chung, H.; Dai, T.; Sharma, S.K.; Huang, Y.Y.; Carroll, J.D.; Hamblin, M.R. The nuts and bolts of low-level laser (light) therapy. Ann. Biomed. Eng. 2012, 40, 516–533. [Google Scholar] [CrossRef] [PubMed]
- Stern, R.S. Psoralen and ultraviolet a light therapy for psoriasis. N. Engl. J. Med. 2007, 357, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Farjo, A.A.; Sugar, A.; Schallhorn, S.C.; Majmudar, P.A.; Tanzer, D.J.; Trattler, W.B.; Cason, J.B.; Donaldson, K.E.; Kymionis, G.D. Femtosecond lasers for LASIK flap creation: A report by the American Academy of Ophthalmology. Ophthalmology 2013, 120, e5–e20. [Google Scholar] [CrossRef] [PubMed]
- Chow, R.T.; Johnson, M.I.; Lopes-Martins, R.A.; Bjordal, J.M. Efficacy of low-level laser therapy in the management of neck pain: A systematic review and meta-analysis of randomised placebo or active-treatment controlled trials. Lancet 2009, 374, 1897–1908. [Google Scholar] [CrossRef]
- Yang, D.; Yi, W.; Wang, E.; Wang, M. Effects of light-emitting diode irradiation on the osteogenesis of human umbilical cord mesenchymal stem cells in vitro. Sci. Rep. 2016, 6, 37370. [Google Scholar] [CrossRef]
- Ong, W.K.; Chen, H.F.; Tsai, C.T.; Fu, Y.J.; Wong, Y.S.; Yen, D.J.; Chang, T.H.; Huang, H.D.; Lee, O.K.; Chien, S.; et al. The activation of directional stem cell motility by green light-emitting diode irradiation. Biomaterials 2013, 34, 1911–1920. [Google Scholar] [CrossRef]
- Wu, S.; Xing, D.; Gao, X.; Chen, W.R. High fluence low-power laser irradiation induces mitochondrial permeability transition mediated by reactive oxygen species. J. Cell. Physiol. 2009, 218, 603–611. [Google Scholar] [CrossRef]
- Khan, I.; Tang, E.; Arany, P. Molecular pathway of near-infrared laser phototoxicity involves ATF-4 orchestrated ER stress. Sci. Rep. 2015, 5, 10581. [Google Scholar] [CrossRef]
- Lynnyk, A.; Lunova, M.; Jirsa, M.; Egorova, D.; Kulikov, A.; Kubinova, S.; Lunov, O.; Dejneka, A. Manipulating the mitochondria activity in human hepatic cell line Huh7 by low-power laser irradiation. Biomed. Opt. Express 2018, 9, 1283–1300. [Google Scholar] [CrossRef]
- Whelan, H.T.; Buchmann, E.V.; Dhokalia, A.; Kane, M.P.; Whelan, N.T.; Wong-Riley, M.T.; Eells, J.T.; Gould, L.J.; Hammamieh, R.; Das, R.; et al. Effect of NASA light-emitting diode irradiation on molecular changes for wound healing in diabetic mice. J. Clin. Laser Med. Surg. 2003, 21, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Arany, P.R.; Cho, A.; Hunt, T.D.; Sidhu, G.; Shin, K.; Hahm, E.; Huang, G.X.; Weaver, J.; Chen, A.C.; Padwa, B.L.; et al. Photoactivation of endogenous latent transforming growth factor-beta1 directs dental stem cell differentiation for regeneration. Sci. Transl. Med. 2014, 6, 238ra69. [Google Scholar] [CrossRef] [PubMed]
- Eells, J.T.; Henry, M.M.; Summerfelt, P.; Wong-Riley, M.T.; Buchmann, E.V.; Kane, M.; Whelan, N.T.; Whelan, H.T. Therapeutic photobiomodulation for methanol-induced retinal toxicity. Proc. Natl. Acad. Sci. USA 2003, 100, 3439–3444. [Google Scholar] [CrossRef] [PubMed]
- Brosseau, L.; Robinson, V.; Wells, G.; Debie, R.; Gam, A.; Harman, K.; Morin, M.; Shea, B.; Tugwell, P. Low level laser therapy (Classes I, II and III) for treating rheumatoid arthritis. Cochrane Database Syst. Rev. 2005, 4, CD002049. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Ma, J.; Chen, J.; Shen, B.; Pei, F.; Kraus, V.B. The effectiveness of low-level laser therapy for nonspecific chronic low back pain: A systematic review and meta-analysis. Arthritis Res. Ther. 2015, 17, 360. [Google Scholar] [CrossRef] [PubMed]
- Yousefi-Nooraie, R.; Schonstein, E.; Heidari, K.; Rashidian, A.; Pennick, V.; Akbari-Kamrani, M.; Irani, S.; Shakiba, B.; Mortaz Hejri, S.A.; Mortaz Hejri, S.O.; et al. Low level laser therapy for nonspecific low-back pain. Cochrane Database Syst. Rev. 2008, 2, CD005107. [Google Scholar] [CrossRef] [PubMed]
- Tchanque-Fossuo, C.N.; Ho, D.; Dahle, S.E.; Koo, E.; Li, C.S.; Isseroff, R.R.; Jagdeo, J. A systematic review of low-level light therapy for treatment of diabetic foot ulcer. Wound Repair Regen. 2016, 24, 418–426. [Google Scholar] [CrossRef]
- Li, S.; Wang, C.; Wang, B.; Liu, L.; Tang, L.; Liu, D.; Yang, G.; Zhang, L. Efficacy of low-level light therapy for treatment of diabetic foot ulcer: A systematic review and meta-analysis of randomized controlled trials. Diabetes Res. Clin. Pract. 2018, 143, 215–224. [Google Scholar] [CrossRef]
- Karu, T. Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J. Photochem. Photobiol. B 1999, 49, 1–17. [Google Scholar] [CrossRef]
- Chen, A.C.; Arany, P.R.; Huang, Y.Y.; Tomkinson, E.M.; Sharma, S.K.; Kharkwal, G.B.; Saleem, T.; Mooney, D.; Yull, F.E.; Blackwell, T.S.; et al. Low-level laser therapy activates NF-kB via generation of reactive oxygen species in mouse embryonic fibroblasts. PLoS ONE 2011, 6, e22453. [Google Scholar] [CrossRef]
- El Sayed, S.O.; Dyson, M. Effect of laser pulse repetition rate and pulse duration on mast cell number and degranulation. Lasers Surg. Med. 1996, 19, 433–437. [Google Scholar] [CrossRef]
- Walsh, L.J.; Trinchieri, G.; Waldorf, H.A.; Whitaker, D.; Murphy, G.F. Human dermal mast cells contain and release tumor necrosis factor alpha, which induces endothelial leukocyte adhesion molecule 1. Proc. Natl. Acad. Sci. USA 1991, 88, 4220–4224. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, D.; Abrahamse, H. Biological effects of helium-neon laser irradiation on normal and wounded human skin fibroblasts. Photomed. Laser Surg. 2005, 23, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Medrado, A.R.A.P.; Pugliese, L.S.; Reis, S.R.A.; Andrade, Z.A. Influence of low level laser therapy on wound healing and its biological action upon myofibroblasts. Laser Surg. Med. 2003, 32, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Passarella, S.; Casamassima, E.; Molinari, S.; Pastore, D.; Quagliariello, E.; Catalano, I.M.; Cingolani, A. Increase of proton electrochemical potential and ATP synthesis in rat liver mitochondria irradiated in vitro by helium-neon laser. FEBS Lett. 1984, 175, 95–99. [Google Scholar] [CrossRef]
- Sazanov, L.A. A giant molecular proton pump: Structure and mechanism of respiratory complex I. Nat. Rev. Mol. Cell Biol. 2015, 16, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Karu, T.; Pyatibrat, L.; Kalendo, G. Irradiation with He-Ne laser increases ATP level in cells cultivated in vitro. J. Photochem. Photobiol. B 1995, 27, 219–223. [Google Scholar] [CrossRef]
- Pastore, D.; Greco, M.; Petragallo, V.A.; Passarella, S. Increase in <--H+/e- ratio of the cytochrome c oxidase reaction in mitochondria irradiated with helium-neon laser. Biochem. Mol. Biol. Int. 1994, 34, 817–826. [Google Scholar]
- Posten, W.; Wrone, D.A.; Dover, J.S.; Arndt, K.A.; Silapunt, S.; Alam, M. Low-level laser therapy for wound healing: Mechanism and efficacy. Dermatol. Surg. 2005, 31, 334–340. [Google Scholar] [CrossRef]
- Wang, F.; Chen, T.S.; Xing, D.; Wang, J.J.; Wu, Y.X. Measuring dynamics of caspase-3 activity in living cells using FRET technique during apoptosis induced by high fluence low-power laser irradiation. Laser Surg. Med. 2005, 36, 2–7. [Google Scholar] [CrossRef]
- Waldchen, S.; Lehmann, J.; Klein, T.; van de Linde, S.; Sauer, M. Light-induced cell damage in live-cell super-resolution microscopy. Sci. Rep. 2015, 5, 15348. [Google Scholar] [CrossRef] [PubMed]
- Carlton, P.M.; Boulanger, J.; Kervrann, C.; Sibarita, J.B.; Salamero, J.; Gordon-Messer, S.; Bressan, D.; Haber, J.E.; Haase, S.; Shao, L.; et al. Fast live simultaneous multiwavelength four-dimensional optical microscopy. Proc. Natl. Acad. Sci. USA 2010, 107, 16016–16022. [Google Scholar] [CrossRef] [PubMed]
- Naeser, M.A.; Zafonte, R.; Krengel, M.H.; Martin, P.I.; Frazier, J.; Hamblin, M.R.; Knight, J.A.; Meehan, W.P., 3rd; Baker, E.H. Significant improvements in cognitive performance post-transcranial, red/near-infrared light-emitting diode treatments in chronic, mild traumatic brain injury: Open-protocol study. J. Neurotrauma 2014, 31, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Y.; Chen, A.C.; Carroll, J.D.; Hamblin, M.R. Biphasic dose response in low level light therapy. Dose-Response 2009, 7, 358–383. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Y.; Sharma, S.K.; Carroll, J.; Hamblin, M.R. Biphasic dose response in low level light therapy—An update. Dose-Response 2011, 9, 602–618. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.L.; Cole, K.D.; Plant, A.L. Standards for cell line authentication and beyond. PLoS Biol. 2016, 14, e1002476. [Google Scholar] [CrossRef]
- Ehrenstein, M.R.; Mauri, C. If the treatment works, do we need to know why?: The promise of immunotherapy for experimental medicine. J. Exp. Med. 2007, 204, 2249–2252. [Google Scholar] [CrossRef]
- Pries, V.; Nocker, C.; Khan, D.; Johnen, P.; Hong, Z.; Tripathi, A.; Keller, A.L.; Fitz, M.; Perruccio, F.; Filipuzzi, I.; et al. Target identification and mechanism of action of picolinamide and benzamide chemotypes with antifungal properties. Cell Chem. Biol. 2018, 25, 279–290.e7. [Google Scholar] [CrossRef]
- Schenone, M.; Dancik, V.; Wagner, B.K.; Clemons, P.A. Target identification and mechanism of action in chemical biology and drug discovery. Nat. Chem. Biol. 2013, 9, 232–240. [Google Scholar] [CrossRef]
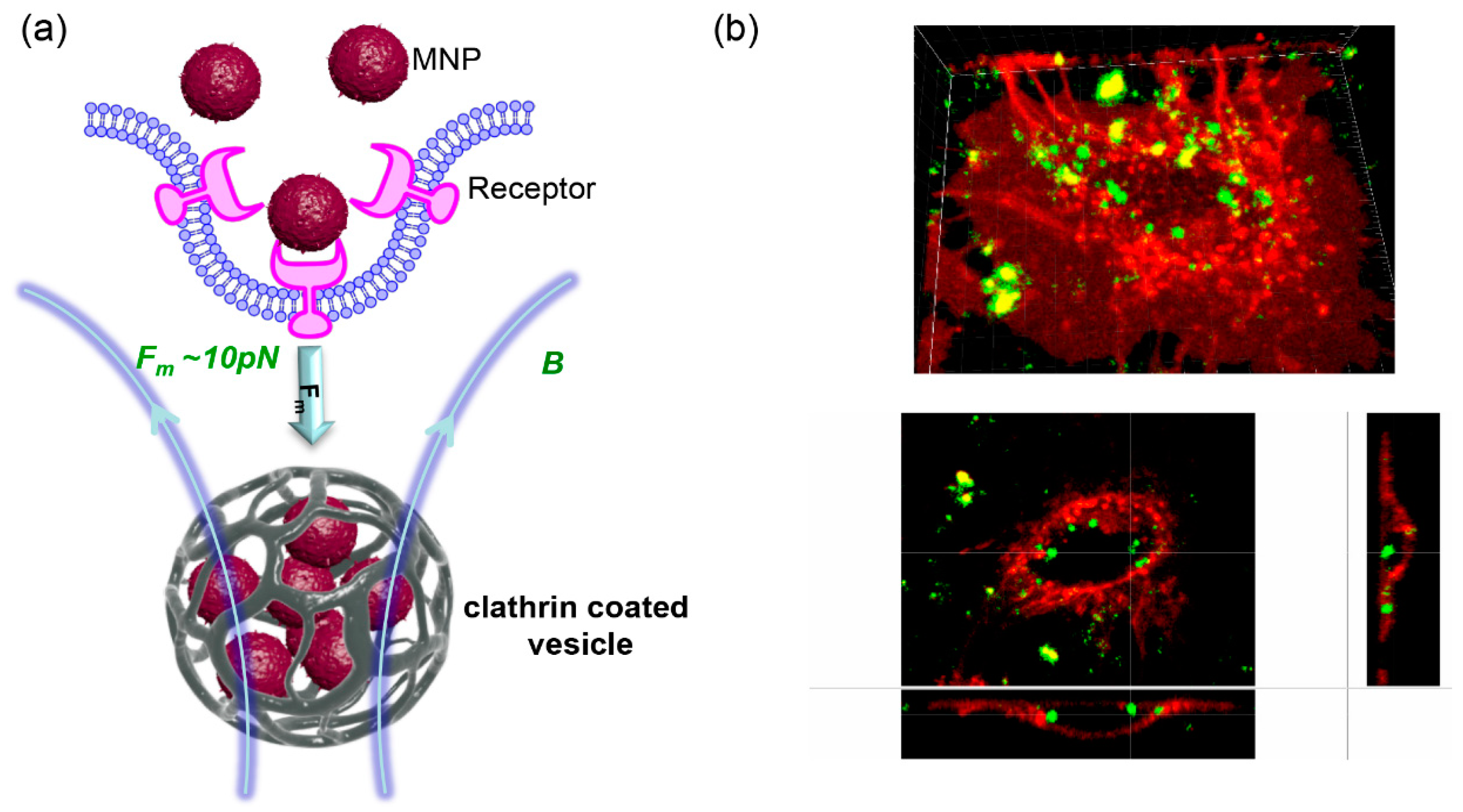
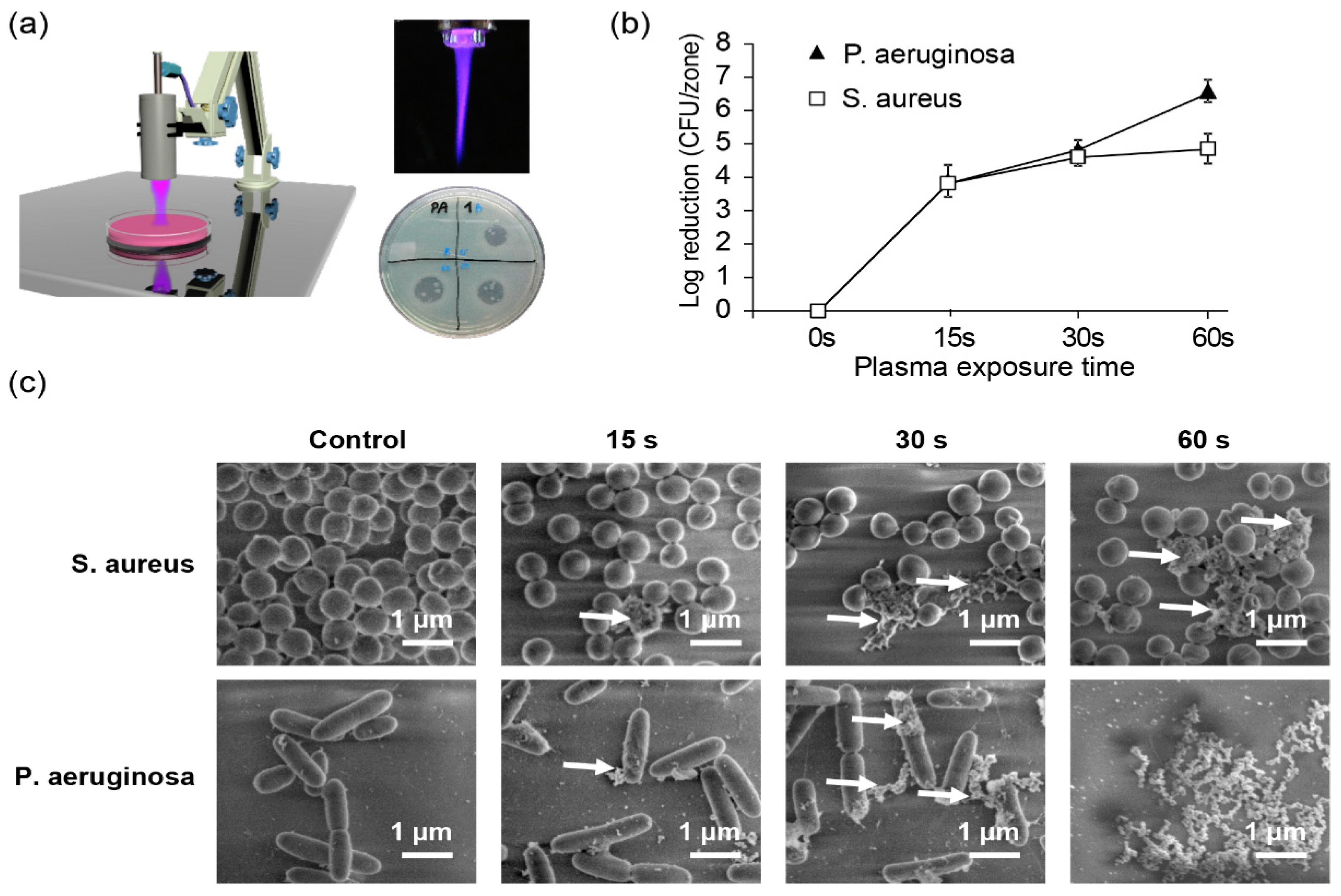
| MNP Core | Surface | Ms 1 of the Core (emu/g Iron) | Hydrodynamic Diameter (nm) | ζ-Potential (mV) | Magnetic Device | Ref. |
|---|---|---|---|---|---|---|
| magnetite | PEI 2 | 62 | 63 ± 36 | +55.4 ± 1.6 | 96-Magnets magnetic plate (OZ Biosciences, cat. no. MF10096) | [15,30] |
| magnetite | PEI + auroyl sarcosinate | N.E. 3 | 106 ± 38 | +27.7 ± 1.8 | 96-Magnets magnetic plate (OZ Biosciences, cat. no. MF10096) | [15] |
| iron oxide (γ-Fe2O3) | N.E. | N.E. | 96 ± 1 | +57.2 ± 1.7 | MidiMACS Separator magnet | [31] |
| CoFe2O4 | N.E. | 89.4 | 12.5 ± 2.0 | N.E. | No data | [32] |
| iron oxide (γ-Fe2O3) | PEI | N.E. | 121 ± 27 | N.E. | Magnetic sheet and neodymium magnet (Magna Co. Ltd.) | [33] |
| magnetite | PEI, streptavidin | N.E. | N.E. | N.E. | NdFeB magnet 5 mm in diameter | [34] |
| Mg/Fe layered double hydroxide | citrate | N.E. | ~80 | −23.2 ± 1.6 | No data | [35] |
| magnetite | BPEI 4 | ~20 | 50–100 | ~+2.7 | NdFeB magnetic plates (Chemicell, Berlin, Germany) | [36] |
| magnetite | PLL 5 | N.E. | N.E. | N.E. | Capacitor discharge impulse magnetizer (Model SUH-1220, Taiwan Ferrite Co., Taiwan) | [37] |
| magnetite | PEI | N.E. | ~50 | N.E. | Magnetic field generator ‘Dynamic Marker’ (Stetter-Elektronik, Seeheim-Jugenheim, Germany) | [14] |
| magnetite | carboxymethyl-dextran | N.E. | ~200 | N.E. | Magnetic field generator ‘Dynamic Marker’ (Stetter-Elektronik, Seeheim-Jugenheim, Germany) | [14] |
| Application | Force (pN) | Magnetic Flux Density (mT) | Field Gradient (T/m) | NP Diameter (nm) | Ref. |
|---|---|---|---|---|---|
| Notch and E-cadherin receptor activity manipulation | 1-47 | N.E. 1 | N.E. | 10-30 | [67] |
| Stretching of chromatin for gene transcription upregulation | N.E. | ~250 | N.E. | 4000 | [68] |
| Activation of the Wnt/β-Catenin signaling | N.E. | 25–120 | N.E. | ~300 | [69] |
| Stimulation of filopodia formation and oriented cell division | 100,000 | 25–100 | 2500–70,000 | ~150–500 | [70] |
| Mechanical control of inner ear hair cells | 0.1 | N.E. | 1000 | 20–120 | [71] |
| Control deformations in wild-type Drosophila embryonic tissues | 60 | ~200 | 120 | ~7.5 | [72] |
| Modulation of cell endocytosis | 1–100 | 1210 | ~10,000 | 60 | [63] |
| Enhancement of nanoparticles internalization | ~10 | ~7000 | ~6000 | 200 | [66] |
| Control of Rac-GTPase signaling | ~10–30 | ~200 | 1000–10,000 | ~500 | [73] |
| Induction of apoptosis | 20–40 | 9 | N.E. | ~1000 | [74] |
| Device | Gas | Voltage | Microorganisms | Main Result | Ref. |
|---|---|---|---|---|---|
| Dielectric barrier discharge | He | RF 1 voltage at about 45 MHz | P. aeruginosa S. aureus Bacterial biofilms | ROS/RNS accumulation Membrane depolarization | [108] |
| Floating-electrode dielectric-barrier discharge (FE-DBD) | Air | low-frequency alternating current (120 V) | E coli S. aureus methicillin-resistant Staphylococcus aureus (MRSA) | Decreasing in viability in both bacterial form (planktonic and biofilm) | [109] |
| miniFlatPlaSter | Air | 7 kV | S. aureus MRSA E. coli | Decolonization of S. aureus MRSA, E. coli in time-dependent manner | [110] |
| FlatPlaSter | Air | 9 kV | S. aureus MRSA E. coli | Decolonization of S. aureus MRSA and E. coli in time-dependent manner | [110] |
| Surface Micro-Discharge (SMD) plasma | Air | 10 kV | E. coli K12 E. coli B. cepacia P. aeruginosa Gram-positive bacteria S. aureus MRSA S. epidermidis E. faecalis E. mundtii B. cereus B. pumilus C. difficile S. pyogenes C. jeikeium C. albicans | The antimicrobial effects of SMD on various vegetative MO and endospores in time-dependent manner | [111] |
| Plasma jet | Air or Ar | 5.5 kV for air 4–5 kV for Ar | E.coli | Air plasma: completely rupture of cell walls, inactivation of bacteria by the influence of oxidative stress on peptidoglycans and lipids in the cell wall and membrane | [112] |
| Terraplasma GmbH, Garching, Germany | Air | 6.4 kV and 10 kV | S. enterica S. Typhimurium L. monocytogenes | Analysis of 4 plasma modes several properties of ham colonized by bacteria post treated with plasma as well as after storage | [113] |
| Discharge plasma device | He | 10 kV | S. aureus B. subtilis E.coli P. aeruginosa | Dose-dependent decrease in viability of all the bacterial strains, morphological changes after 60 sec of NTP treatment | [114] |
| Plasma discharge manufactured by Sominex | O2, N2 and Ar | - | P. aeruginosa S. aureus B. subtilis | Dose- dependent reduction of viability in all three studied vegetative form of bacteria, together with morphologic and structural changes of spores | [115] |
| Discharge plasma device | He | 0.5 kV10 kV | S. aureus B. subtilis E.coli P. aeruginosa | Dose-dependent effects of NTP In low-voltage mode activation of programmed cell death after 15 sec In higher-voltage mode more profound damage of bacteria and physical destruction | [116] |
| DBD system | Air | 80 kV | E. coli S.aureus | Time-dependent inactivation of both bacterial strains increased level of ROS accumulation, Possible mechanism of bacterial inactivation different between gram positive and gram negative bacteria, leakage of membrane in E. coli after plasma treatment | [117] |
| DBD system | Air | 18.6 kV | S. aureus P. aeruginosa C. albicans | Rapid inactivation of antibiotic- resistant MO, surface alternation and permeabilization of membrane, depletion of intracellular ATP production, minor changes on DNA and proteins level | [118] |
| APPJ | N2 gas in combination with different flow rates of H2O/HNO3 solution | 2.2 kV | E. coli | Effects of plasma treated water (PTW) on E. coli viability, effect of chemical composition of PTW importance of NO2- and H2O2 for antimicrobial activity, analysis of oxidative related gene expression, DNA damage and cell morphology upon PTW treatment | [119] |
| DBD system | Air or argon (Ar) | 0.75 kV | F. oxysporum f.sp. lycopersici spores | Time-dependent cell death (both apoptosis and necrosis) in Fusarium oxysporum f.sp. lycopersici spores post plasma treatment. Reduction of germination ratesWithout any effect of host plant health or growth | [120] |
| DBD system | Air | 80 kV | P. aeruginosa quorum sensing (QS)-regulated virulence factors, E. coli, L. monocytogenes and S. aureus for biofilm formation | Significant time-dependent reduction of bacterial biofilm in all three studied strains after both direct and indirect NTP treatment Disintegrated cell walls Decreasing in concentration of (QS)-regulated virulence factors (pyocyanin and elastase) | [121] |
| DBD system | Air in combination with 90% N2 + 10% O2 | 56 kV and 70 kV | L. monocytogenes E.coli | Time- dependent MO inactivation, increased with higher voltage level Dose-dependent accumulation of DNA damage and impaired membrane integrity Higher resistance of gram-negative E. coli in comparison with L. monocytogenes | [122] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smolková, B.; Uzhytchak, M.; Lynnyk, A.; Kubinová, Š.; Dejneka, A.; Lunov, O. A Critical Review on Selected External Physical Cues and Modulation of Cell Behavior: Magnetic Nanoparticles, Non-thermal Plasma and Lasers. J. Funct. Biomater. 2019, 10, 2. https://doi.org/10.3390/jfb10010002
Smolková B, Uzhytchak M, Lynnyk A, Kubinová Š, Dejneka A, Lunov O. A Critical Review on Selected External Physical Cues and Modulation of Cell Behavior: Magnetic Nanoparticles, Non-thermal Plasma and Lasers. Journal of Functional Biomaterials. 2019; 10(1):2. https://doi.org/10.3390/jfb10010002
Chicago/Turabian StyleSmolková, Barbora, Mariia Uzhytchak, Anna Lynnyk, Šárka Kubinová, Alexandr Dejneka, and Oleg Lunov. 2019. "A Critical Review on Selected External Physical Cues and Modulation of Cell Behavior: Magnetic Nanoparticles, Non-thermal Plasma and Lasers" Journal of Functional Biomaterials 10, no. 1: 2. https://doi.org/10.3390/jfb10010002
APA StyleSmolková, B., Uzhytchak, M., Lynnyk, A., Kubinová, Š., Dejneka, A., & Lunov, O. (2019). A Critical Review on Selected External Physical Cues and Modulation of Cell Behavior: Magnetic Nanoparticles, Non-thermal Plasma and Lasers. Journal of Functional Biomaterials, 10(1), 2. https://doi.org/10.3390/jfb10010002







