Sustainable Large-Scale Aquaculture of the Northern Hemisphere Sea Lettuce, Ulva fenestrata, in an Off-Shore Seafarm
Abstract
1. Introduction
2. Materials and Methods
2.1. Algal Source Material and Fertility Induction
2.2. Experimental Setup
2.3. Growth Measurements
2.4. Protein Content and Fatty Acid Content and Composition
2.5. Carbohydrate Content and Composition
2.6. Total Phenolic Content
2.7. Pigment (Chlorophyll a, b, Carotenoids) Analysis
2.8. Statistical Analysis
3. Results
3.1. Biomass Yield, Growth and Performance
3.2. Fatty Acid Content and Relative Composition
3.3. Crude Protein Content
3.4. Carbohydrate Content and Composition
3.5. Pigment Content
3.6. Phenolic Content
4. Discussion
4.1. Biomass Yield, Growth and Performance
4.2. Fatty Acids and Proteins
4.3. Carbohydrates
4.4. Pigments and Phenols
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duarte, C.M.; Holmer, M.; Olsen, Y.; Soto, D.; Marbà, N.; Guiu, J.; Black, K.; Karakassis, I. Will the Oceans Help Feed Humanity? BioScience 2009, 59, 967–976. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2018—Meeting the Sustainable Development Goals; FAO: Rome, Italy, 2018; 120p. [Google Scholar]
- Troell, M.; Naylor, R.L.; Metian, M.; Beveridge, M.; Tyedmers, P.H.; Folke, C.; Arrow, K.J.; Barrett, S.; Crépin, A.S.; Ehrlich, P.R.; et al. Does aquaculture add resilience to the global food system? Proc. Natl. Acad. Sci. USA 2014, 111, 13257–13263. [Google Scholar] [CrossRef]
- Hanzal, H.; Divisova, M.; Murawska, D.; Janiszewski, P. The effect of dietary bio-alginate supplementation of the growth rate and body weights of common pheasant (Phasianus cochicus) chicks. Pol. J. Nat. Sci. 2016, 31, 363–371. [Google Scholar]
- Morais, T.; Inácio, A.; Coutinho, T.; Ministro, M.; Cotas, J.; Pereira, L.; Bahcevandziev, K. Seaweed Potential in the Animal Feed: A Review. J. Mar. Sci. Eng. 2020, 8, 559. [Google Scholar] [CrossRef]
- Kinley, R.D.; de Nys, R.; Vucko, M.J.; Machado, L.; Tomkins, N.W. The red macroalgae Asparagopsis taxiformis is a potent natural antimethanogenic that reduces methane production during in vitro fermentation with rumen fluid. Anim. Prod. Sci. 2016, 56, 282–289. [Google Scholar] [CrossRef]
- Xixi, L.; Norman, H.C.; Kinley, R.D.; Laurence, M.; Wilmot, M.; Bender, H.; de Nys, R.; Tomkins, N. Asparagopsis taxiformis decreases enteric methane production from sheep. Anim. Prod. Sci. 2016, 58, 681–688. [Google Scholar]
- Craigie, J. Seaweed extract stimuli in plant science and agriculture. J Appl. Phycol. 2011, 23, 371–393. [Google Scholar] [CrossRef]
- Bird, M.I.; Wurster, C.M.; de Paula Silva, P.H.; Bass, A.M.; de Nys, R. Algal biochar—Production and properties. Biores Technol. 2011, 102, 1886–1891. [Google Scholar] [CrossRef] [PubMed]
- Wahlström, N.; Nylander, F.; Malmhäll-Bah, E.; Sjövold, K.; Edlund, U.; Westman, G.; Albers, E. Composition and structure of cell wall ulvans recovered from Ulva spp. along the Swedish West coast. Carbohydr. Polym. 2020, 233, 115852. [Google Scholar] [CrossRef]
- Wahlström, N.; Steinhagen, S.; Toth, G.; Pavia, H.; Edlund, U. Ulvan dialdehyde-gelatine hydrogels for removal of heavy metals and methylene blue from aqueous solution. Carbohydr. Polym. 2020, 249. [Google Scholar] [CrossRef]
- Wahlström, N.; Edlund, U.; Pavia, H.; Toth, G.; Jaworski, A.; Pell, A.J.; Choong, F.X.; Shirani, H.; Nilsson, K.P.R.; Richter-Dahlfors, A. Cellulose from the green macroalgae Ulva lactuca: Isolation, characterization, optotracing, and production of cellulose nanofibrils. Cellulose 2020, 27, 3707–3725. [Google Scholar] [CrossRef]
- Chiellini, E.; Cinelli, P.; Ilieva, V.I.; Martera, M. Biodegradable thermoplastic composites based on polyvinyl alcohol and algae. Biomacromolecules 2008, 9, 1007–1013. [Google Scholar] [CrossRef]
- Rowbotham, J.S.; Dyer, P.W.; Greenwell, H.C.; Theodorou, M.K. Thermochemical processing of macroalgae: A late bloomer in the development of third-generation biofuels? Biofuels 2012, 3, 441–461. [Google Scholar] [CrossRef]
- Bruhn, A.; Dahl, J.; Nielsen, H.B.; Nikolaisen, L.; Rasmussen, M.B.; Markager, S.; Olesen, B.; Arias, C.; Jensen, P.D. Bioenergy potential of Ulva lactuca: Biomass yield, methane production and combustion. Bioresour Technol. 2011, 102, 2595–2604. [Google Scholar] [CrossRef]
- Bolton, J.J.; Cyrus, M.D.; Brand, M.J.; Joubert, M.; Macey, B.M. Why grow Ulva? Its potential role in the future of aquaculture. Perspect. Phycol. 2016, 3, 113–120. [Google Scholar] [CrossRef]
- Buchholz, C.M.; Krause, G.; Buck, B.H. “Seaweed and Man”. In Seaweed Biology: Ecological Studies 219; Wiencke, C., Bischof, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 471–493. [Google Scholar]
- Lawton, R.J.; Mata, L.; de Nys, R.; Paul, N.A. Algal Bioremediation of Waste Waters from Land-Based Aquaculture Using Ulva: Selecting Target Species and Strains. PLoS ONE 2013, 15, e77344. [Google Scholar] [CrossRef] [PubMed]
- Favot, G.; Cunha, M.E.; Quental-Ferreira, H.; Serrão, M. Production of Ulva sp. in multitrophic aquaculture in earth ponds. Aquac. Fish. Stud. 2019, 3, 24–31. [Google Scholar]
- Hayden, H.S.; Blomster, J.; Maggs, C.A.; Silva, P.C.; Stanhope, M.J.; Waaland, J.R. Linnaeus was right all along: Ulva and Enteromorpha are not distinct genera. Eur. J. Phycol. 2003, 38, 277–294. [Google Scholar] [CrossRef]
- Steinhagen, S.; Karez, R.; Weinberger, F. Cryptic, alien and lost species: Molecular diversity of Ulva sensu lato along the German coasts of the North and Baltic Seas. Eur. J. Phycol. 2019, 54, 466–483. [Google Scholar] [CrossRef]
- Steinhagen, S.; Karez, R.; Weinberger, F. Surveying seaweeds from the Ulvales and Fucales in the world’s most frequently used artificial waterway, the Kiel Canal. Bot. Mar. 2019, 62, 51–61. [Google Scholar] [CrossRef]
- Steinhagen, S.; Weinberger, F.; Karez, R. Molecular analysis of Ulva compressa (Chlorophyta, Ulvales) reveals its morphological plasticity, distribution and potential invasiveness on German North Sea and Baltic Sea coasts. Eur. J. Phycol. 2019, 54, 102–114. [Google Scholar] [CrossRef]
- Sebök, S.; Herppich, W.B.; Hanelt, D. Outdoor cultivation of Ulva lactuca in a recently developed ring-shaped photobioreactor: Effects of elevated CO2 concentration on growth and photosynthetic performance. Bot. Mar. 2019, 62, 179–190. [Google Scholar] [CrossRef]
- Mata, L.; Schuenhoff, A.; Santos, R. A direct comparison of the performance of the seaweed biofilters, Asparagopsis armata and Ulva Rigida. J. Appl. Phycol. 2010, 22, 639–644. [Google Scholar] [CrossRef]
- Al-Hafedh, Y.S.; Alam, A.; Buschmann, A.H. Bioremediation potential, growth and biomass yield of the green seaweed, Ulva lactuca in an integrated marine aquaculture system at the Red Sea coast of Saudi Arabia at different stocking densities and effluent flow rates. Rev. Aquacult. 2015, 7, 161–171. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Shuuluka, D.; Bolton, J.J.; Anderson, R.J. Protein content, amino acid composition and nitrogen-to-protein conversion factors of Ulva rigida and Ulva capensis from natural populations and Ulva lactuca from an aquaculture system, in South Africa. J. Appl. Phycol. 2013, 25, 677–685. [Google Scholar] [CrossRef]
- Carvalho, A.F.U.; Portela, M.C.C.; Sousa, M.B.; Martins, F.S.; Rocha, F.C.; Farias, D.F.; Feitosa, J.P.A. Physiological and physico-chemical characterization of dietary fibre from the green seaweed Ulva fasciata Delile. Braz. J. Biol. 2009, 69, 969–977. [Google Scholar] [CrossRef] [PubMed]
- McDermid, K.J.; Stuercke, B. Nutritional composition of edible Hawaiian seaweeds. J. Appl. Phycol. 2003, 15, 513–524. [Google Scholar] [CrossRef]
- Colombo, M.L.; Risè, P.; Giavarini, F.; Angelis, D.E.L.; Galli, C.; Bolis, C.L. Marine macroalgae as sources of polyunsaturated fatty acids. Plant. Foods Hum. Nutr. 2006, 61, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Abreu, M.H.; Pereira, R.; Sassi, J.F. “Marine algae and the global food industry”. In Marine Algae: Biodiversity, Taxonomy, Environmental Assessment, and Biotechnology; Pereira, L., Magalhaes Neto, J., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 300–319. [Google Scholar] [CrossRef]
- Santos, S.A.O.; Vilela, C.; Freire, C.S.R.; Abreu, M.H.; Rocha, S.M.; Silvestre, A.J.D. Chlorophyta and Rhodophyta macroalgae: A source of health promoting phytochemicals. Food Chem. 2015, 183, 122–128. [Google Scholar] [CrossRef]
- Hafting, J.T.; Craigie, J.S.; Stengel, D.B.; Loureiro, R.R.; Buschmann, A.H.; Yarish, C.; Edwards, M.D.; Critchley, A.T. Prospects and challenges for industrial production of seaweed bioactivities. J. Phycol. 2015, 51, 821–837. [Google Scholar] [CrossRef]
- Barzkar, N.; Tamadoni Jahromi, S.T.; Poorsaheli, H.B.; Vianello, F. Metabolites from marine microorganisms, micro, and macroalgae: Immense scope for pharmacology. Mar. Drugs 2019, 17, 464. [Google Scholar] [CrossRef]
- Olsson, J.; Raikova, S.; Mayers, J.J.; Steinhagen, S.; Chuck, C.J.; Nylund, G.M.; Albers, E. Effects of geographical location on potentially valuable components in Ulva intestinalis sampled along the Swedish coast. Appl. Phycol. 2020, 1, 80–92. [Google Scholar] [CrossRef]
- Olsson, J.; Toth, G.B.; Oerbekke, A.; Cvijetinovic, S.; Wahlström, N.; Harrysson, H.; Steinhagen, S.; Kinnby, A.; White, J.; Edlund, U.; et al. Cultivation conditions affect the monosaccharide composition in Ulva fenestrata. J. Appl. Phycol. 2020, 32, 3255–3263. [Google Scholar] [CrossRef]
- Gao, G.; Clare, A.S.; Chatzidimitriou, E.; Rose, C.; Caldwell, G. Effects of ocean warming and acidification, combined with nutrient enrichment, on chemical composition and functional properties of Ulva rigida. Food Chem. 2018, 258, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Clare, A.S.; Rose, C.; Caldwell, G.S. Ulva rigida in the future ocean: Potential for carbon capture, bioremediation and biomethane production. Gbc Bioenergy 2018, 10, 39–51. [Google Scholar]
- Toth, G.B.; Harrysson, H.; Wahlström, N.; Olsson, J.; Oerbekke, A.; Steinhagen, S.; Kinnby, A.; White, J.; Albers, A.; Edlund, U.; et al. Effects of irradiance, temperature, nutrients, and pCO2 on the growth and biochemical composition of cultivated Ulva fenestrata. J. Appl. Phycol. 2020, 32, 3243–3254. [Google Scholar] [CrossRef]
- Lubsch, A.; Timmermans, K. Uptake kinetics and storage capacity of dissolved inorganic phosphorus and corresponding N:P dynamics in Ulva lactuca (Chlorophyta). J. Phycol. 2018, 54, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Califano, G.; Kwantes, M.; Abreu, M.H.; Costa, R.; Wichard, T. Cultivating the macroalgal holobiont: Effects of Integrated Multi-Trophic-Aquaculture on the microbiome of Ulva rigida (Chlorophyta). Front. Mar. Sci. 2020, 7, 52. [Google Scholar] [CrossRef]
- Lapointe, B.E.; Williams, L.D.; Goldman, J.C.; Ryther, J.H. The mass outdoor culture of macroscopic marine algae. Aquaculture 1976, 8, 9–21. [Google Scholar] [CrossRef]
- Lüning, K.; Pang, S.J. Mass Cultivation of Seaweed: Current Aspects and Approaches. J. Appl. Phycol. 2003, 15, 115–119. [Google Scholar] [CrossRef]
- Titlyanov, E.A.; Titlyanova, T.V. Seaweed cultivation: Methods and problems. Russ. J. Mar. Biol. 2010, 36, 227–242. [Google Scholar] [CrossRef]
- Provasoli, L. Media and prospects for the cultivation of marine algae. In Cultures and Collections of Algae, Proceedings of the US-Japan Conference, Hakone, Japan, 12–15 September 1966; Society of Plant Physiology: Tokyo, Japan, 1968. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Angell, A.R.; Mata, L.; de Nys, R.; Paul, N.A. The protein content of seaweeds: A universal nitrogen-to-protein conversion factor of five. J. Appl. Phycol. 2016, 28, 511–524. [Google Scholar] [CrossRef]
- Harrysson, H.; Hayes, M.; Eimer, F.; Carlsson, N.G.; Toth, G.B.; Undeland, I. Production of protein extracts from Swedish red, green, and brown seaweeds, Porphyra umbilicalis Kützing, Ulva lactuca Linnaeus, and Saccharina latissima (Linnaeus) JV Lamouroux using three different methods. J. Appl. Phycol. 2018, 30, 3565–3580. [Google Scholar] [CrossRef]
- Jeffrey, S.; Humphrey, G. New spectrophotometric equations for determining a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem. Physiol. Pflanz. 1975, 167, 191–194. [Google Scholar] [CrossRef]
- Parsons, T.R.; Maita, Y.; Lalli, C.M. A manual for Chemical and Biological Methods for Seawater Analysis; Pergamon Press: Oxford, UK, 1984. [Google Scholar]
- Underwood, A.J. Techniques of analysis of variance in experimental marine biology and ecology. Oceanogr. Mar. Biol. Annu. Rev. 1981, 19, 513–603. [Google Scholar]
- Martins, M.; Fernandes, A.; Torres-Acosta, M.A.; Collén, N.; Abreu, M.H.; Ventura, S.P.M. Extraction of chlorophyll from wild and farmed Ulva spp. using aqueous solutions of ionic liquids. Sep. Purif. Technol. 2021, 254, 117589. [Google Scholar] [CrossRef]
- Palatnik, R.R.; Zilberman, D. Economics of natural resource utilization—The case of macroalgae. In Modeling, Dynamics, Optimization and Bioeconomics II; Pinto, A.A., Zilberman, D., Eds.; DGS 2014; Springer: Cham, Switzerland, 2017; pp. 1–21. [Google Scholar]
- Kessler, R.W.; Taghreed, A.; Wichard, T. Purification of sporulation and swarming inhibitors from Ulva. In Protocols for Macroalgae Research; Charrier, B., Wichard, T., Reddy, C.R.K., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 139–158. [Google Scholar]
- Carl, C.; de Nys, R.; Lawton, R.J.; Paul, N.A. Methods for the Induction of Reproduction in a Tropical Species of Filamentous Ulva. PLoS ONE 2014, 9, e97396. [Google Scholar] [CrossRef]
- Zhang, C.; Li, X.; Kim, S. Application of marine biomaterials for nutraceuticals and functional foods. Food Sci. Biotechnol. 2012, 21, 625–631. [Google Scholar] [CrossRef]
- Smaal, A.C. European mussel cultivation along the Atlantic coast: Production status, problems and perspectives. In Sustainable Increase of Marine Harvesting: Fundamental Mechanisms and New Concepts; Vadstein, O., Olsen, Y., Eds.; Developments in Hydrobiology; Springer: Dordrecht, The Netherlands, 2002; Volume 167. [Google Scholar] [CrossRef]
- Taranger, G.L.; Karlsen, Ø.; Bannister, R.J.; Glover, K.A.; Husa, V.; Karlsbakk, E.; Kvamme, B.O.; Kroon Boxaspen, K.; Bjørn, P.A.; Finstad, B.; et al. Risk assessment of the environmental impact of Norwegian Atlantic salmon farming. ICES J. Mar. Sci. 2015, 72, 997–1021. [Google Scholar] [CrossRef]
- Gao, G.; Clare, A.S.; Rose, C.; Caldwell, G.S. Eutrophication and warming-driven green tides (Ulva rigida) are predicted to increase under future climate change scenarios. Mar. Pollut. Bull. 2017, 114, 439–447. [Google Scholar] [CrossRef]
- Chen, B.B.; Lin, L.D.; Ma, Z.L.; Zhang, T.T.; Chen, W.Z.; Zou, D.H. Carbon and nitrogen accumulation and interspecific competition in two algae species, Pyropia haitanensis and Ulva lactuca, under ocean acidification conditions. Aquac. Int. 2019, 27, 721–733. [Google Scholar] [CrossRef]
- Mhatre, A.; Patil, S.; Agrawal, A.; Pandit, R.; Lali, A.M. Influence of nitrogen source on photochemistry and antenna size of the photosystems in marine green macroalgae, Ulva lactuca. Photosynth Res. 2019, 139, 539–551. [Google Scholar] [CrossRef]
- Shpigel, M.; Ragg, N.L.; Lupatsch, I.; Neori, A. Protein content determines the nutritional value of the seaweed Ulva lactuca L for the abalone Haliotis tuberculata L. and H. discus hannai Ino. J. Shellfish Res. 1999, 18, 227–234. [Google Scholar]
- Fleurence, J. Seaweed proteins: Biochemical, nutritional aspects and potential uses. Trends Food Sci. Technol. 1999, 10, 25–28. [Google Scholar] [CrossRef]
- Angell, A.R.; Angell, S.F.; de Nys, R.; Paul, N.A. Seaweed as a protein source for mono-gastric livestock. Trend Food Sci. Technol. 2016, 54, 74–84. [Google Scholar] [CrossRef]
- Øverland, M.; Mydland, L.T.; Skrede, A. Marine macroalgae as sources of protein and bioactive compounds in feed for monogastric animals. J. Sci. Food Agric. 2019, 99, 13–24. [Google Scholar] [CrossRef]
- Kumar, C.S.; Ganesan, P.; Suresh, P.V.; Bhaskar, N. Seaweeds as a source of nutritionally beneficial compounds-a review. J. Food Sci. Technol. 2008, 45, 1. [Google Scholar]
- Olsson, J.; Toth, G.; Albers, E. Biochemical composition of red, green and brown seaweeds on the Swedish west coast. J. Appl. Phycol. 2020, 32, 3305–3317. [Google Scholar] [CrossRef]
- He, Y.; Hu, C.; Wang, Y.; Cui, D.; Sun, X.; Li, Y.; Xu, N. The metabolic survival strategy of marine macroalga Ulva prolifera under temperature stress. J. Appl. Phycol. 2018, 30, 3611–3621. [Google Scholar] [CrossRef]
- Kumari, P.; Kumar, M.; Reddy, C.R.K.; Jha, B. Nitrate and phosphate regimes induced lipidomic and biochemical changes in the intertidal macroalga Ulva lactuca (Ulvophyceae, Chlorophyta). Plant. Cell Physiol. 2014, 55, 52–63. [Google Scholar] [CrossRef]
- Mohamed, S.; Ferro, V. Synthetic approaches to l-Iduronic acid and l-Idose: Key building blocks for the preparation of glycosaminoglycan oligosaccharides. In Advances in Carbohydrate Chemistry and Biochemistry; Baker, D.C., Horton, D., Eds.; Academic Press: New York, NY, USA, 2015; Volume 72, pp. 21–61. [Google Scholar]
- Muller, M.M.; Kugler, J.H.; Henkel, M.; Gerlitzki, M.; Hormann, B.; Pohnlein, M.; Syldatk, C.; Hausmann, R. Rhamnolipids-next generationsurfactants? J. Biotech. 2012, 162, 366–380. [Google Scholar] [CrossRef]
- Abd El-Baky, H.H.; El-Baz, F.K.; El-Baroty, G.S. Natural preservative ingredient from marine alga Ulva lactuca L. Int. J. Food Sci. Technol. 2009, 44, 1688–1695. [Google Scholar] [CrossRef]
- Lanfer-Marquez, U.M.; Barros, R.M.; Sinnecker, P. Antioxidant activity of chlorophylls and their derivatives. Food Res. Int. 2005, 38, 885–891. [Google Scholar] [CrossRef]
- Egner, P.A.; Muñoz, A.; Kensler, T.W. Chemoprevention with chlorophyllin in individuals exposed to dietary aflatoxin. Mutat. Res. Fundam. Mol. Mech. Mutagenesis 2003, 523, 209–216. [Google Scholar] [CrossRef]
- Khairy, H.M.; El-Sheikh, M.A. Antioxidant activity and mineral composition of three Mediterranean common seaweeds from Abu-Qir Bay, Egypt. Saudi J. Biol. Sci. 2015, 5, 623–630. [Google Scholar] [CrossRef]
- Bocanegra, A.; Bastida, S.; Benedí, J.; Rodenas, S.; Sanchez-Muniz, F.J. Characteristics and nutritional and cardiovascular-health properties of seaweeds. J. Med. Food 2009, 12, 236–258. [Google Scholar] [CrossRef]
- Eismann, A.I.; Reis, R.P.; da Silva, A.F.; Cavalcanti, D.N. Ulva spp. carotenoids: Responses to environmental conditions. Algal Res. 2020, 48, 101916. [Google Scholar] [CrossRef]
- Sirbu, R.; Stanciu, G.; Tomescu, A.; Ionescu, A.M.; Cadar, E. Evaluation of Antioxidant and Antimicrobial Activity in Relation to Total Phenolic Content of Green Algae from Black Sea. Rev. Chim. 2019, 70, 1197–1203. [Google Scholar] [CrossRef]
- Hashem, H.A.; Mansour, H.A.; El-Khawas, S.A.; Hassanein, R.A. The potentiality of marine macro-algae as bio-fertilizer to improve the productivity and salt stress tolerance of canola (Brassica napus L.) plants. Agronomy 2019, 9, 146. [Google Scholar]
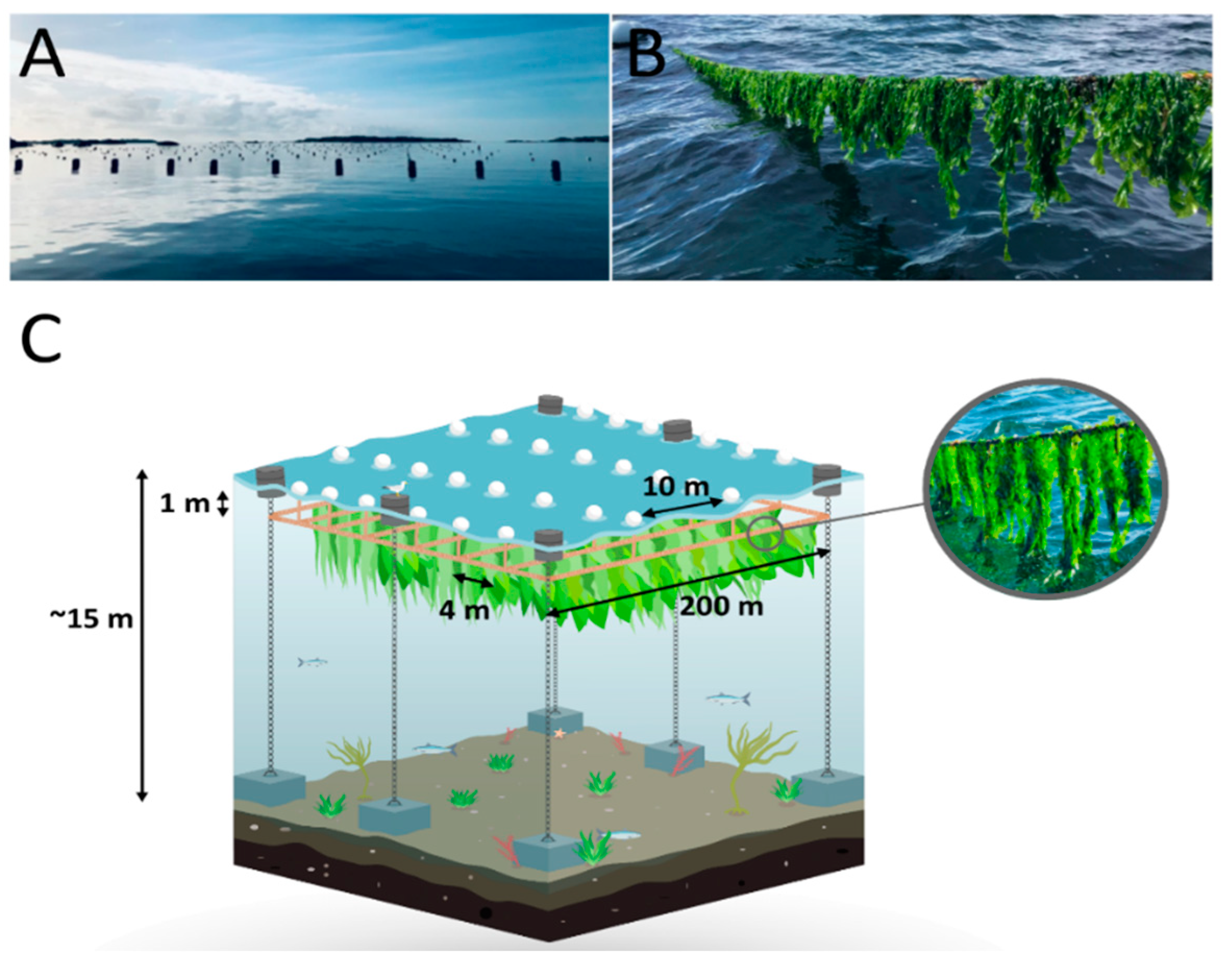
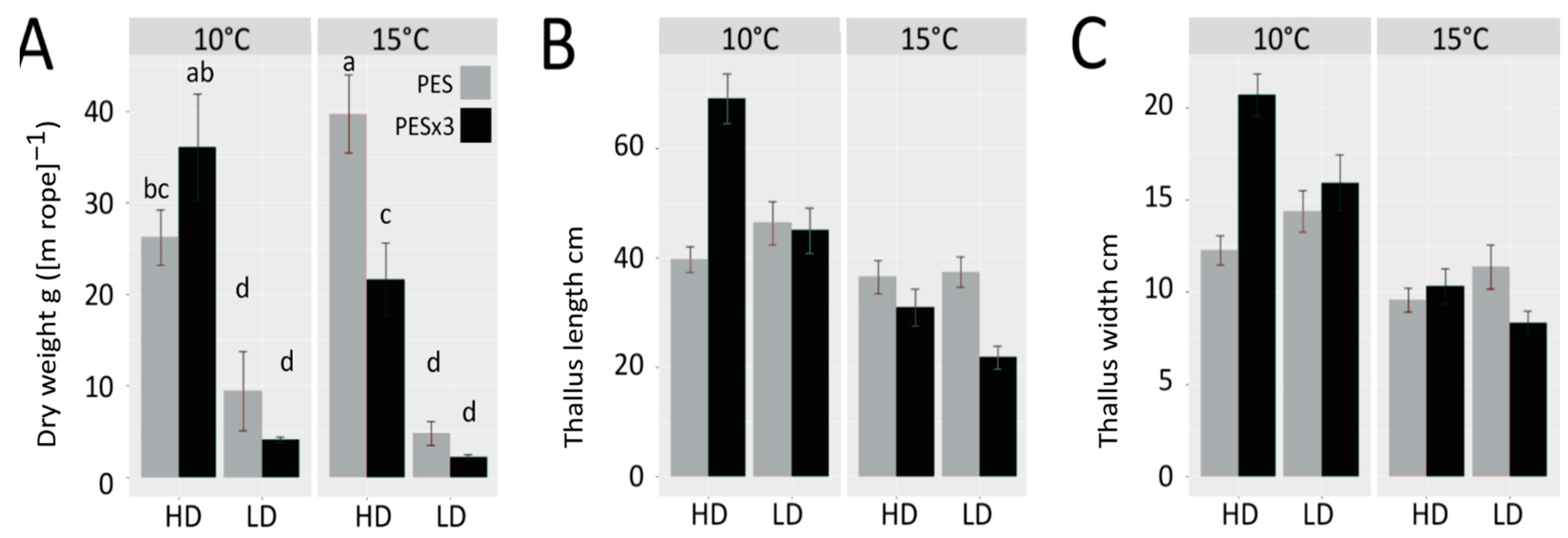
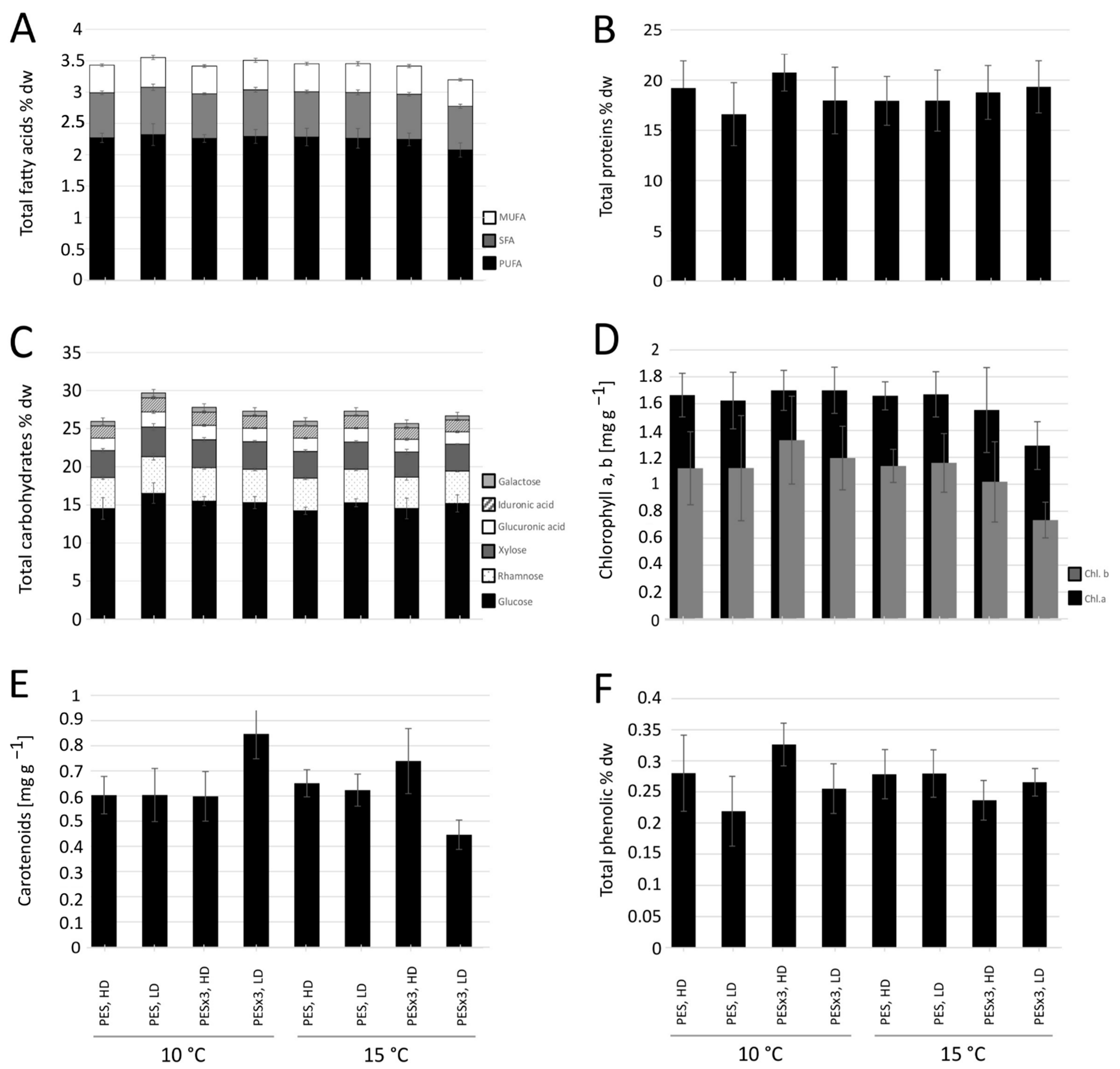
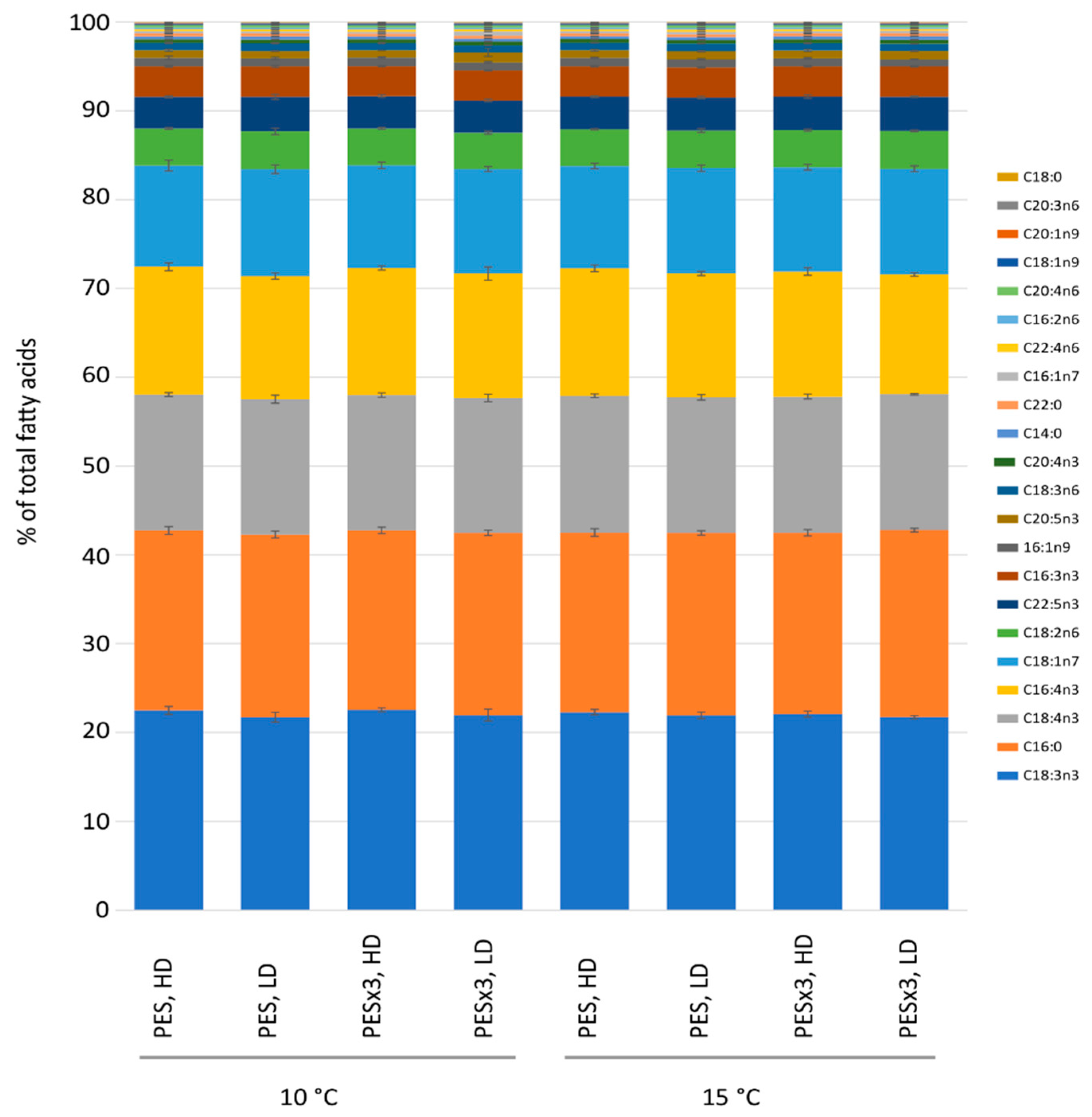
| Source of Variance | (a) Dry Weight | (b) Thallus Length | (c) Thallus Width | (d) Total Fatty Acids | (e) Crude Proteins | (f) Carbohydrates | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Df | MS | F ratio | p | MS | F ratio | p | MS | F ratio | p | MS | F ratio | p | MS | F ratio | p | MS | F ratio | p | |
| Temperature | 1 | 1.21 | 1.67 | 0.20 | 1.63 | 40.28 | <0.01 | 2.06 | 60.05 | <0.01 | 0.094 | 2.42 | 0.881 | 0.17 | 0.022 | 0.918 | 16.32 | 4.90 | 0.034 |
| Nutrients | 1 | 3.17 | 4.40 | 0.04 | <1.01 | 0.12 | 0.73 | 0.07 | 2.23 | 0.13 | 0.077 | 1.99 | 0.151 | 16.45 | 2.16 | 0.151 | 1.31 | 0.39 | 0.535 |
| Density | 1 | 114.2 | 158.4 | <0.01 | 0.15 | 3.81 | 0.052 | 0.01 | 0.51 | 0.47 | >0.001 | 0.001 | 0.179 | 14.34 | 1.88 | 0.169 | 19.15 | 5.75 | 0.022 |
| Temperature × Nutrients | 1 | 3.87 | 5.36 | 0.02 | 0.69 | 17.23 | <0.01 | 0.47 | 13.73 | <0.01 | 0.032 | 0.83 | 0.840 | 0.32 | 0.041 | 0.838 | 0.083 | 0.025 | 0.875 |
| Temperature × Density | 1 | 0.63 | 0.87 | 0.35 | <0.01 | 0.001 | 0.97 | <0.01 | 0.001 | 0.97 | 0.118 | 3.06 | 0.097 | 22.14 | 2.91 | 0.096 | 0.54 | 0.162 | 0.690 |
| Nutrients × Density | 1 | 0.19 | 0.26 | 0.60 | 0.52 | 13.01 | <0.01 | 0.39 | 11.38 | <0.01 | 0.04 | 1.05 | 0.926 | 0.06 | 0.008 | 0.907 | 13.03 | 3.914 | 0.057 |
| Temp. × Nut. × Dens. | 1 | 4.21 | 5.84 | 0.02 | 0.05 | 1.37 | 0.242 | 0.03 | 0.91 | 0.33 | 0.022 | 0.56 | 0.838 | 0.32 | 0.042 | 0.847 | 9.67 | 2.903 | 0.098 |
| Residual | 32 | 0.721 | 0.041 | 0.034 | 0.0388 | 7.59 | 3.331 | ||||||||||||
| Source of variance | (g) Chlorophyll a | (h) Chlorophyll b | (i) Carotenoids | (j) Phenolic | |||||||||||||||
| Df | MS | F ratio | p | MS | F ratio | p | MS | F ratio | p | MS | F ratio | p | |||||||
| Temperature | 1 | 0.167 | 3.648 | 0.065 | 0.318 | 3.661 | 0.065 | 0.053 | 5.391 | 0.026 | <0.01 | 0.118 | 0.733 | ||||||
| Nutrients | 1 | 0.088 | 1.925 | 0.175 | 0.042 | 0.483 | 0.492 | 0.276 | 2.797 | 0.104 | <0.01 | 0.197 | 0.660 | ||||||
| Density | 1 | 0.053 | 1.168 | 0.288 | 0.095 | 1.101 | 0.302 | 0.004 | 0.042 | 0.838 | <0.01 | 2.930 | 0.097 | ||||||
| Temperature × Nutrients | 1 | 0.224 | 4.892 | 0.034 | 0.426 | 4.896 | 0.034 | 0.645 | 6.520 | 0.015 | 0.012 | 5.411 | 0.027 | ||||||
| Temperature × Density | 1 | 0.028 | 0.620 | 0.437 | 0.010 | 0.118 | 0.734 | 0.126 | 1.277 | 0.266 | 0.016 | 7.391 | 0.011 | ||||||
| Nutrients × Density | 1 | 0.034 | 0.747 | 0.394 | 0.120 | 1.386 | 0.248 | 0.011 | 1.178 | 0.286 | <0.01 | 0.092 | 0.763 | ||||||
| Temp. × Nut. × Dens. | 1 | 0.631 | 1.376 | 0.249 | 0.187 | 0.215 | 0.646 | 0.008 | 0.886 | 0.353 | <0.01 | 0.399 | 0.531 | ||||||
| Residual | 32 | 0.045 | 0.087 | 0.009 | <0.01 | ||||||||||||||
| Interactions | (a) Thallus Length (cm) | (b) Thallus Width [cm] | (c) Chla (mg·g−1) | (d) Chlb (mg·g−1) | (e) Carotenoids (mg·g−1) | (f) Phenolic (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temp. | Nut. | Student’s Test | Mean | Student’s Test | Mean | Student’s Test | Mean | Student’s Test | Mean | Student’s Test | Mean | Student’s Test | Mean |
| 10 °C | PES | B | 43.03 | B | 13.31 | A | 1.64 | AB | 1.11 | A | 0.69 | A | 0.24 |
| 10 °C | PESx3 | A | 57.04 | A | 18.30 | A | 1.69 | A | 1.25 | A | 0.72 | A | 0.29 |
| 15 °C | PES | BC | 36.94 | C | 10.46 | A | 1.66 | A | 1.14 | A | 0.70 | A | 0.27 |
| 15 °C | PESx3 | C | 31.41 | C | 9.31 | B | 1.42 | B | 0.87 | B | 0.57 | A | 0.25 |
| Temp. | Dens. | Student’s Test | Mean | Student’s Test | Mean | ||||||||
| 10 °C | HD | - | - | - | - | - | - | - | - | - | - | A | 0.30 |
| 10 °C | LD | - | - | - | - | - | - | - | - | - | - | B | 0.23 |
| 15 °C | HD | - | - | - | - | - | - | - | - | - | - | B | 0.25 |
| 15 °C | LD | - | - | - | - | - | - | - | - | - | - | AB | 0.27 |
| Nut. | Dens. | Student’s Test | Mean | Student’s Test | Mean | ||||||||
| PES | HD | B | 38.10 | C | 15.50 | - | - | - | - | - | - | - | - |
| PES | LD | B | 41.86 | B | 12.86 | - | - | - | - | - | - | - | - |
| PESx3 | HD | A | 52.20 | A | 15.00 | - | - | - | - | - | - | - | - |
| PESx3 | LD | B | 36.25 | BC | 12.11 | - | - | - | - | - | - | - | - |
| (a) C18:3n3 | (b) C16:0 | (c) C18:4n3 | (d) C16:4n3 | (e) C18:1n7 | (f) C18:2n6 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source of variance | Df | MS | F ratio | p | MS | F ratio | p | MS | F ratio | p | MS | F ratio | p | MS | F ratio | p | MS | F ratio | p |
| Temperature | 1 | 0.323 | 1.525 | 0.226 | 0.339 | 2.210 | 0.147 | 0.067 | 0.620 | 0.437 | 0.340 | 1.602 | 0.215 | 0.047 | 0.242 | 0.626 | 0.011 | 0.299 | 0.588 |
| Nutrients | 1 | 0.006 | 0.032 | 0.859 | 0.222 | 1.451 | 0.237 | 0.010 | 0.098 | 0.757 | 0.291 | 1.368 | 0.251 | 0.016 | 0.083 | 0.775 | 0.008 | 0.216 | 0.645 |
| Density | 1 | 2.699 | 12.743 | 0.001 | 1.542 | 10.042 | 0.003 | 0.038 | 0.352 | 0.557 | 2.089 | 9.828 | 0.004 | 1.111 | 5.668 | 0.023 | 0.030 | 0.797 | 0.379 |
| T × N | 1 | 0.333 | 1.574 | 0.219 | 0.416 | 2.710 | 0.110 | 0.006 | 0.061 | 0.807 | 0.415 | 1.956 | 0.172 | 0.109 | 0.556 | 0.461 | 0.022 | 0.601 | 0.444 |
| T × D | 1 | 0.288 | 1.361 | 0.252 | 0.060 | 0.394 | 0.535 | 0.001 | 0.012 | 0.912 | 0.017 | 0.083 | 0.775 | 0.084 | 0.432 | 0.516 | 0.013 | 0.358 | 0.554 |
| N × D | 1 | 0.021 | 0.101 | 0.753 | 0.081 | 0.528 | 0.473 | 0.004 | 0.044 | 0.835 | 0.0004 | 0.002 | 0.965 | 0.213 | 1.089 | 0.305 | 0.022 | 0.602 | 0.444 |
| T × N × D | 1 | 0.023 | 0.110 | 0.743 | 0.076 | 0.498 | 0.486 | 0.005 | 0.053 | 0.819 | 0.1025 | 0.482 | 0.493 | 0.025 | 0.128 | 0.723 | 0.017 | 0.458 | 0.504 |
| Residual | 32 | 0.211 | 0.153 | 0.109 | 0.212 | 0.196 | 0.037 | ||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steinhagen, S.; Enge, S.; Larsson, K.; Olsson, J.; Nylund, G.M.; Albers, E.; Pavia, H.; Undeland, I.; Toth, G.B. Sustainable Large-Scale Aquaculture of the Northern Hemisphere Sea Lettuce, Ulva fenestrata, in an Off-Shore Seafarm. J. Mar. Sci. Eng. 2021, 9, 615. https://doi.org/10.3390/jmse9060615
Steinhagen S, Enge S, Larsson K, Olsson J, Nylund GM, Albers E, Pavia H, Undeland I, Toth GB. Sustainable Large-Scale Aquaculture of the Northern Hemisphere Sea Lettuce, Ulva fenestrata, in an Off-Shore Seafarm. Journal of Marine Science and Engineering. 2021; 9(6):615. https://doi.org/10.3390/jmse9060615
Chicago/Turabian StyleSteinhagen, Sophie, Swantje Enge, Karin Larsson, Joakim Olsson, Göran M. Nylund, Eva Albers, Henrik Pavia, Ingrid Undeland, and Gunilla B. Toth. 2021. "Sustainable Large-Scale Aquaculture of the Northern Hemisphere Sea Lettuce, Ulva fenestrata, in an Off-Shore Seafarm" Journal of Marine Science and Engineering 9, no. 6: 615. https://doi.org/10.3390/jmse9060615
APA StyleSteinhagen, S., Enge, S., Larsson, K., Olsson, J., Nylund, G. M., Albers, E., Pavia, H., Undeland, I., & Toth, G. B. (2021). Sustainable Large-Scale Aquaculture of the Northern Hemisphere Sea Lettuce, Ulva fenestrata, in an Off-Shore Seafarm. Journal of Marine Science and Engineering, 9(6), 615. https://doi.org/10.3390/jmse9060615







