Walleye Pollock Gadus chalcogrammus, a Species with Continuous Range from the Norwegian Sea to Korea, Japan, and California: New Records from the Siberian Arctic
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
- -
- comparative analysis of fossil records, modern distribution and variability of mtDNA genes (see e.g., [87]);
- -
- -
- comparison of the chemical composition of otoliths (see e.g., [90]);
- -
- comparative analysis of the diet composition and feeding habits (see e.g., [91]), including evaluation of microplastic, macroplastic and other marine litter content in the stomach and its impact on the biological condition of fish (see e.g., [92,93,94]) since pollution of the Siberian Arctic in recent years become a serious ecological problem [95,96].
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shuntov, V.P.; Volkov, A.F.; Temnykh, O.S.; Dulepova, E.P. Walleye pollock in the Ecosystems of Far Eastern Seas; TINRO: Vladivostok, Russia, 1993. [Google Scholar]
- Bailey, K.M.; Powers, D.M.; Quattro, J.M.; Villa, G.; Nishimura, A.; Traynor, J.J.; Walters, G. Population ecology and structure dynamics of walleye pollock (Theragra chalcogramma). In Dynamics of the Bering Sea; Thomas, R.L., Ohtani, K., Eds.; University of Alaska Sea Grant: Fairbanks, AK, USA, 1999; pp. 581–614. [Google Scholar]
- Cohen, D.M.; Inada, T.; Iwamoto, T.; Scialabba, N. Gadiform Fishes of the World (Order Gadiformes). An Annotated and Illustrated Catalogue of Cods, Hakes, Grenadiers and Other Gadiform Fishes Known to Date; Food and Agriculture Organization (FAO): Rome, Italy, 1990; Volume 10. [Google Scholar]
- Balykin, P.A. Fecundity of walleye pollock in the western Bering Sea. Vopr. Ikhtiol. 1986, 26, 164–168. [Google Scholar]
- Orlov, A.M.; Rabazanov, N.I.; Nikiforov, A.I. Transoceanic migrations of fishlike animals and fish: Norm or exclusion? J. Ichthyol. 2020, 60, 242–262. [Google Scholar] [CrossRef]
- Mecklenburg, C.W.; Lynghammar, A.; Johannesen, E.; Byrkjedal, I.; Christiansen, J.S.; Dolgov, A.V.; Karamushko, O.V.; Mecklenburg, T.; Møller, P.R.; Steinke, D.; et al. Marine Fishes of the Arctic Region; CAFF: Akureyri, Iceland, 2018; Volume 1. [Google Scholar]
- Makhrov, A.A.; Lajus, D.L. Postglacial colonization of the North European seas by Pacific fishes and lamprey. Contemp. Prob. Ecol. 2018, 11, 247–258. [Google Scholar] [CrossRef]
- Springer, A.M. A review: Walleye pollock in the North Pacific—How much difference do they really make? Fish. Oceanogr. 1992, 1, 80–96. [Google Scholar] [CrossRef]
- Fadeev, N.S.; Vespestad, V. Overview of walleye pollock fishery. Izv. TINRO 2001, 128, 75–91. [Google Scholar]
- Bulatov, O.A. Walleye pollock: Global overview. Fish. Sci. 2014, 80, 109–116. [Google Scholar] [CrossRef]
- Shevchenko, V.V.; Datsky, A.V. Bioeconomics of the Use of Walleye Pollock Commercial Resources of the Northern Pacific; VNIRO Publishing: Moscow, Russia, 2014. [Google Scholar]
- Bulatov, O.A. On the question of the methodology of stock assessment forecasting and pollock fishery strategy. Tr. VNIRO 2015, 157, 45–70. [Google Scholar]
- Livingston, P.A. Importance of predation by groundfish, marine mammals and birds on walleye pollock Theragra chalcogramma and Pacific herring Clupea pallasi in the eastern Bering Sea. Mar. Ecol. Prog. Ser. 1993, 102, 205–215. [Google Scholar] [CrossRef]
- Pallas, P.S. Zoographia Rosso-Asiatica, Sistens Omnium Animalium In Extenso Imperio Rossico et Adjacentibus Maribus Observatorum Recensionem, Domicilia, Mores et Descriptiones Anatomen Atque Icones Plurimorum; Academia Scientiarum: Petropolis, Brazil, 1814; Volume 3. [Google Scholar]
- Cope, E.D. A contribution to the ichthyology of Alaska. Proc. Amer. Philos. Soc. 1873, 13, 24–32. [Google Scholar]
- Steindachner, F.; Döderlein, L. Beiträge zur Kenntniss der Fische Japan’s. (IV). Denkschr. Kais. Akad. Wiss. Wien Math.-Nat. Cl. 1887, 53, 257–296. [Google Scholar]
- Jordan, D.S.; Gilbert, C.H. Note on the wall-eyed pollack (Pollachius chalcogrammus fucensis) of Puget Sound. Proc. U. S. Natl. Mus. 1893, 16, 315–316. [Google Scholar] [CrossRef][Green Version]
- Jordan, D.S.; Evermann, B.W. The fishes of North and Middle America: A descriptive catalogue of the species of fish-like vertebrates found in the waters of North America north of the Isthmus of Panama. Part III. Bull. U. S. Natl. Mus. 1898, 47, 2183–3136. [Google Scholar]
- Fricke, R., Eschmeyer, W.N., van der Laan, R., Eds.; Eschmeyer’s Catalog of Fishes: Genera, Species, References. Available online: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp (accessed on 30 July 2021).
- Froese, R.; Pauly, D. (Eds.) FishBase, version (06/2021); Available online: www.fishbase.org (accessed on 24 September 2021).
- Koefoed, E. Theragra finnmarchica n. sp.: Gadus poutassou, Risso, Raia spinicauda, Jensen: Eumicrotremus spinosus subsp. nov. eggvinii. Rep. Norweg. Fish. Mar. Investig. 1956, 11, 1–24. [Google Scholar]
- Christiansen, J.S.; Fevolden, S.E.; Byrkjedal, I. The occurrence of Theragra finnmarchica Koefoed, 1956 (Teleostei, Gadidae), 1932–2004. J. Fish Biol. 2005, 66, 1193–1197. [Google Scholar] [CrossRef]
- Privalikhin, A.M.; Norvillo, G.V. On the finding of a rare species—Norwegian pollock Theragra finnmarchica Koefoed, 1956 (Gadidae)—In the Barents Sea. J. Ichthyol. 2010, 50, 143–147. [Google Scholar] [CrossRef]
- Zhukova, K.A.; Privalikhin, A.M. New data on distribution of Norwegian (Atlantic) pollock Theragra finnmarchica (Gadidae) in the Barents Sea. J. Ichthyol. 2014, 54, 217–222. [Google Scholar] [CrossRef]
- Coulson, M.W.; Marshall, H.D.; Pepin, P.; Carr, S.M. Mitochondrial genomics of gadine fishes: Implications for taxonomy and biogeographic origins from whole-genome data sets. Genome 2006, 49, 1115–1130. [Google Scholar] [CrossRef]
- Teletchea, F.; Laudet, V.; Hänni, C. Phylogeny of the Gadidae (sensu Svetovidov, 1948) based on their morphology and two mitochondrial genes. Mol. Phylogenet. Evol. 2006, 38, 189–199. [Google Scholar] [CrossRef]
- Ursvik, A.; Breines, R.; Christiansen, J.S.; Fevolden, S.-E.; Coucheron, D.H.; Johansen, S.D. A mitogenomic approach to the taxonomy of pollocks: Theragra chalcogramma and T. finnmarchica represent one single species. BMC Evol. Biol. 2007, 7, 86. [Google Scholar] [CrossRef]
- Byrkjedal, I.; Rees, D.J.; Christiansen, J.S.; Fevolden, S.-E. The taxonomic status of Theragra finnmarchica Koefoed, 1956 (Teleostei: Gadidae): Perspectives from morphological and molecular data. J. Fish Biol. 2008, 73, 1183–1200. [Google Scholar] [CrossRef]
- Carr, S.M.; Marshall, H.D. Phylogeographic analysis of complete mtDNA genomes from walleye pollock (Gadus chalcogrammus Pallas, 1811) shows an ancient origin of genetic biodiversity. DNA Seq. 2008, 19, 490–496. [Google Scholar] [CrossRef]
- Svetovidov, A.N. Gadiformes. Fauna of the USSR. Fishes; Academy of Sciences of USSR: Moscow/Leningrad, Soviet Union, 1948. [Google Scholar]
- Svetovidov, A.N. Gadidae. In Fishes of the Northeastern Atlantic and the Mediterranean; Whitehead, P.J.P., Bauchot, M.-L., Hureau, J.-C., Nielsen, J., Tortonese, E., Eds.; UNESCO: Paris, France, 1986; Volume 2, pp. 680–710. [Google Scholar]
- Laevatsu, T. Manual of Methods in Fishery Biology; FAO Manuals in Fisheries Science; Food and Agriculture Organization (FAO): Rome, Italy, 1965. [Google Scholar]
- Pravdin, I.F. Guide to the Study of Fish; Pishchevaya Promyshlennost’: Moscow, Soviet Union, 1966. [Google Scholar]
- Beamish, R.J.; Chilton, D.E. Preliminary evaluation of a method to determine the age of sablefish (Anoplopoma fimbria). Can. J. Fish. Aquat. Sci. 1982, 39, 277–287. [Google Scholar] [CrossRef]
- Beamish, R.J.; McFarlane, G.A. Current trends in age determination methodology. In Age and Growth of Fish; Summerfelt, R.C., Hall, G.E., Eds.; Iowa State University Press: Ames, IA, USA, 1987; pp. 15–42. [Google Scholar]
- D’Iglio, C.; Albano, M.; Famulari, S.; Savoca, S.; Panarello, G.; di Paola, D.; Perdichizzi, A.; Rinelli, P.; Lanteri, G.; Spanò, N.; et al. Intra- and interspecific variability among congeneric Pagellus otoliths. Sci. Rep. 2021, 11, 16315. [Google Scholar] [CrossRef]
- Sakun, O.F.; Butskaya, N.A. Determination of Gonad Maturity Degree and Analysis of Sexual Cycles of Fishes; Znanie: Moscow, Soviet Union, 1963. [Google Scholar]
- Andriashev, A.P. Fishes of the Northern Seas of the USSR; Israel Program for Scientific Translations: Washington, DC, USA, 1964. [Google Scholar]
- Lindberg, G.U.; Legeza, M.I. Fishes of the Sea of Japan and the Adjacent Areas of the Sea of Okhotsk and the Yellow Sea. Part II; Nauka: Moscow/Leningrad, Soviet Union, 1965. [Google Scholar]
- Nizovtsev, G.P. Theragra finnmachica. In Commercial Biological Resources of the Northern Atlantic and Adjacent Seas of the Arctic Ocean; PINRO: Murmansk, Soviet Union, 1977; p. 314. [Google Scholar]
- Gritsenko, O.F.; Kotlyar, A.N.; Kotenev, B.N. (Eds.) Commercial Fishes of Russia; VNIRO: Moscow, Russia, 2006. [Google Scholar]
- Ivanova, N.V.; Zemlak, T.S.; Hanner, R.H.; Hebert, P. Universal primer cocktails for fish DNA barcoding. Mol. Ecol. Notes 2007, 7, 544–548. [Google Scholar] [CrossRef]
- Ward, R.D.; Zemlak, T.S.; Innes, B.H.; Last, P.R.; Hebert, P.D. DNA barcoding Australia’s fish species. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 1847–1857. [Google Scholar] [CrossRef]
- Silva, J.W.; Costa, M.; Valente, V.; Sousa, J.D.F.; Paçó-Larson, M.; Espreafico, E.; Camargo, S.; Monteiro, E.; Holanda, A.D.J.; Zago, M.; et al. PCR template preparation for capillary DNA sequencing. Biotechniques 2001, 30, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Drummond, A.J.; Ashton, B.; Buxton, S.; Cheung, M.; Cooper, A.; Duran, C.; Field, M.; Heled, J.; Kearse, M.; Markowitz, S.; et al. Geneious v5.4. 2011. Available online: http://www.geneious.com (accessed on 24 September 2021).
- Leigh, J.W.; Bryant, D. Popart: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Villesen, P. FaBox: An online toolbox for fasta sequences. Mol. Ecol. Notes 2007, 7, 965–968. [Google Scholar] [CrossRef]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [PubMed]
- Roa-Varón, A.; Ortí, G. Phylogenetic relationships among families of Gadiformes (Teleostei, Paracanthopterygii) based on nuclear and mitochondrial data. Mol. Phylogenet. Evol. 2009, 52, 688–704. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Emelyanova, O.R.; Grigorov, I.V.; Orlov, A.M.; Orlova, S.Y. Polymorphism of mtDNA gene Cyt b of the Chukchi Sea polar cod, Boreogadus saida (Gadidae, Gadiformes). Deep Sea Res. Part II Top. Stud. Oceanogr. (Under review).
- Fadeev, N.S. Handbook on the Biology and Fishery of the North Pacific Fishes; TINRO: Vladivostok, Russia, 2005. [Google Scholar]
- Hoff, G.R.; Stevenson, D.E.; Orr, J.W. Guide to the Gadiform Fishes of the Eastern North Pacific; NOAA Technical Memorandum NMFS-AFSC 309; US Department of Commerce: Washington, DC, USA, 2015; pp. 1–68. [Google Scholar] [CrossRef]
- Orlov, A.; Benzik, A.; Vedishcheva, E.; Gafitsky, S.; Gorbatenko, K.; Goryanina, S.; Zubarevich, V.; Kodryan, K.; Nosov, M.; Orlova, S.Y.; et al. Fisheries research in the Chukchi Sea at the RV “Professor Levanidov” in August 2019: Some preliminary results. Tr. VNIRO 2019, 178, 206–220. [Google Scholar] [CrossRef]
- Orlov, A.; Savin, A.; Gorbatenko, K.; Benzik, A.; Morozov, T.; Rybakov, M.; Terentiev, D.; Vedishcheva, E.; Kurbanov, Y.; Nosov, M.A.; et al. Biological studies in the Russian Far Eastern and Arctic seas. Tr. VNIRO 2020, 181, 102–143. [Google Scholar] [CrossRef]
- Orlov, A.M.; Gorbatenko, K.M.; Benzik, A.N.; Rybakov, M.O.; Nosov, M.A.; Orlova, S.Y. Biological research in the Siberian Arctic seas in summer–autumn 2019 (cruise of the R/V professor Levanidov). Oceanology 2021, 61, 295–296. [Google Scholar] [CrossRef]
- Antonov, N.P.; Emelin, P.O.; Maznikova, O.A.; Sheibak, A.Y.; Trofimova, A.O.; Benzik, A.N.; Nosov, M.A.; Orlov, A.M. Walleye pollock Gadus chalcogrammus in the western Chukchi Sea: Promising target of Arctic fishery? Deep Sea Res. Part II Top. Stud. Oceanogr. (In preparation).
- Orlov, A.M.; Tokranov, A.M. Checklist of deep-sea fishes of the Russian northwestern Pacific Ocean found at depths below 1000 m. Prog. Oceanogr. 2019, 176, 102143. [Google Scholar] [CrossRef]
- Eschmeyer, W.N.; Herald, E.S.; Hammann, H. A Field Guide to Pacific Coast Fishes of North America; Houghton Mifflin Company: Boston, MA, USA, 1983. [Google Scholar]
- Balykin, P.A. Biology and Condition of Stocks of the Western Bering Sea Walleye Pollock. Ph.D. Thesis, KoTINRO, Petropavlovsk-Kamchatsky, Soviet Union, 1990. [Google Scholar]
- Antonov, N.P. Biology and Dynamics of Abundance of the East Kamchatka Walleye Pollock. Ph.D. Thesis, TINRO, Vladivostok, Soviet Union, 1991. [Google Scholar]
- Nuzhdin, V.A. Biology and Condition of Stocks of Walleye Pollock Theragra chalcogramma in Waters of Primorye. Ph.D. Thesis, TINRO-Centre, Vladivostok, Russia, 2008. [Google Scholar]
- Smith, G.B. The biology of walleye pollock. In The Eastern Bering Sea Shelf: Oceanography and Resources; Hood, D.W., Calder, J.A., Eds.; University of Washington Press: Seattle, WA, USA, 1981; Volume 1, pp. 527–551. [Google Scholar]
- Sergeeva, N.P. The walleye pollock of the Eastern Sea of Okhotsk. In Condition of Biological Resources of the North-West Pacific; Siniakov, S.A., Naumenko, N.I., Diakov, Y.P., Zolotov, O.G., Vronsky, B.B., Eds.; Kamchat NIRO: Petropavlovsk-Kamchatsky, Russia, 2003; pp. 18–20. [Google Scholar]
- Munk, K.M. Maximum ages of groundfishes in waters off Alaska and British Columbia and consideration of age determination. Alsk. Fish. Res. Bull. 2001, 8, 12–21. [Google Scholar]
- Gritsai, E.V. Biology and Fishery of Walleye Pollock Theragra chalcogramma in the Northern Bering Sea. Ph.D. Thesis, TINRO-Centre, Vladivostok, Russia, 2008. [Google Scholar]
- Fadeev, N.S. Biology and fishery of the East-Korean Pollock. Izv. TINRO 2005, 142, 113–133. [Google Scholar]
- Fadeev, N.S. Fisheries, population structure and biology of walleye pollock in the Sakhalin-Kuril-Hokkaido waters. Izv. TINRO 2006, 147, 3–35. [Google Scholar]
- Frost, K.J. Descriptive key to the otoliths of gadid fishes of the Bering, Chukchi, and Beaufort seas. Arctic 1981, 34, 55–59. [Google Scholar] [CrossRef]
- Vedishcheva, E.V.; Maznikova, O.; Orlov, A. New data on the age and growth of Greenland halibut, Reinhardtius hippoglossoides (Pleuronectidae), from the Laptev Sea. J. Ichthyol. 2018, 58, 845–850. [Google Scholar] [CrossRef]
- Mishin, A.V.; Evseenko, S.A.; Bol’shakov, D.V.; Bol’shakova, Y.Y. Ichthyoplankton of Russian Arctic seas: 1. Polar cod Boreogadus saida. J. Ichthyol. 2018, 58, 710–716. [Google Scholar] [CrossRef]
- Orlov, A.M.; Benzik, A.N.; Vedishcheva, E.V.; Gorbatenko, K.M.; Goryanina, S.V.; Zubarevich, V.L.; Kodryan, K.V.; Nosov, M.A.; Orlova, S.Y.; Pedchenko, A.P.; et al. Preliminary results of fisheries research in the Laptev Sea at RV “Professor Levanidov” in September 2019. Tr. VNIRO 2020, 179, 206–225. [Google Scholar] [CrossRef]
- Karamushko, L.I.; Raskhozheva, E.V.; Karamushko, O.V. Population structure and growth of polar cod Boreogadus saida in the Sea of Laptev. J. Ichthyol. 2021, 61, 564–575. [Google Scholar] [CrossRef]
- Iwata, M. Population identification of walleye pollock, Theragra chalcogramma (Pallas), in the vicinity of Japan. Mem. Fac. Fish. Hokkaido Univ. 1975, 22, 193–258. [Google Scholar]
- Haryu, T. Larval distribution of walleye pollock, Theragra chalcogramma (Pallas), in the Bering Sea, with special reference to morphological changes. Bull. Fac. Fish. Hokkaido Univ. 1980, 31, 121–136. [Google Scholar]
- Tollit, D.J.; Heaslip, S.G.; Zeppelin, T.K.; Joy, R.; Call, K.A.; Trites, A.W. A method to improve size estimates of walleye pollock (Theragra chalcogramma) and Atka mackerel (Pleurogrammus monopterygius) consumed by pinnipeds: Digestion correction factors applied to bones and otoliths recovered in scats. Fish. Bull. 2004, 102, 498–508. [Google Scholar]
- Short, J.A.; Gburski, C.M.; Kimura, D.K. Using otolith morphometrics to separate small walleye pollock Theragra chalcogramma from Arctic Cod Boreogadus saida in mixed samples. Alsk. Fish. Res. Bull. 2006, 12, 147–152. [Google Scholar]
- Byrkjedal, I.; Langhelle, A. Walleye pollock Gadus chalcogrammus Pallas, 1814 found north of Spitsbergen indicates a Pacific-Atlantic connection in the species. Fauna Norveg. 2020, 40, 137–140. [Google Scholar] [CrossRef]
- Dodd, P.A.; Rabe, B.; Hansen, E.; Falck, E.; Mackensen, A.; Rohling, E.; Stedmon, C.; Kristiansen, S. The freshwater composition of the fram strait outflow derived from a decade of tracer measurements. J. Geophys. Res. Space Phys. 2012, 117, 11005. [Google Scholar] [CrossRef]
- Maeda, T. Fishing grounds of the Alaska pollock. Bull. Jpn. Soc. Sci. Fish. 1972, 38, 362–371. [Google Scholar] [CrossRef][Green Version]
- Pushnikov, V.V. Results of the Sea of Okhotsk walleye pollock tagging. In Population Structure, Dynamics of Abundance and Ecology of Walleye Pollock; TINRO: Vladivostok, Russia, 1987; pp. 202–208. [Google Scholar]
- Glubokov, A.I.; Kotenev, B.N. Population Structure of Walleye Pollock in the Northern Bering Sea; VNIRO Publishing: Moscow, Russia, 2006. [Google Scholar]
- Proshutinsky, A.; Yang, J.; Krishfield, R.; Gerdes, R.; Karcher, M.; Kauker, F.; Koeberle, C.; Häkkinen, S.; Hibler, W.; Holland, D.; et al. Arctic ocean study: Synthesis of model results and observations. Eos 2005, 86, 368–371. [Google Scholar] [CrossRef]
- Golubeva, E.N.; Platov, G.; Iakshina, D.F. Numerical simulations of the current state of waters and sea ice in the Arctic Ocean. Ice Snow 2015, 130, 81. [Google Scholar] [CrossRef]
- Glebov, I.I.; Nadtochii, V.A.; Savin, A.B.; Slabinskii, A.M.; Borilko, O.Y.; Chul’chekov, D.N.; Sokolov, A.S. Results of complex research in the East Siberian Sea in August 2015. Izv. TINRO 2016, 186, 81–92. [Google Scholar] [CrossRef]
- Orlov, A.M.; Benzik, A.N.; Vedishcheva, E.V.; Gorbatenko, K.M.; Goryanina, S.V.; Zubarevich, V.L.; Kodryan, K.V.; Nosov, M.A.; Orlova, S.Y.; Pedchenko, A.P.; et al. Preliminary results of fisheries research in the East Siberian Sea at the RV “Professor Levanidov” in September 2019. Tr. VNIRO 2020, 179, 187–205. [Google Scholar] [CrossRef]
- Orlov, A.M.; Bannikov, A.F.; Orlova, S.Y. Hypothesis of Antimora spp. (Moridae) dispersion in the world oceans based on data on modern distribution, genetic analysis, and ancient records. J. Ichthyol. 2020, 60, 399–410. [Google Scholar] [CrossRef]
- Tuset, V.M.; Otero-Ferrer, J.L.; Gómez-Zurita, J.; Venerus, L.A.; Stransky, C.; Imondi, R.; Orlov, A.M.; Ye, Z.; Santschi, L.; Afanasiev, P.K.; et al. Otolith shape lends support to the sensory drive hypothesis in rockfishes. J. Evol. Biol. 2016, 29, 2083–2097. [Google Scholar] [CrossRef] [PubMed]
- Afanasyev, P.K.; Orlov, A.; Rolsky, A.Y. Otolith shape analysis as a tool for species identification and studying the population structure of different fish species. Biol. Bull. 2017, 44, 952–959. [Google Scholar] [CrossRef]
- Korostelev, N.B.; Orlov, A.M. Micro- and ultramicroelemental content in otoliths of blue antimora Antimora rostrata and Pacific flatnose A. microlepis (Moridae, Teleostei). Oceanology 2020, 60, 798–802. [Google Scholar] [CrossRef]
- Botterell, Z.L.; Beaumont, N.; Dorrington, T.; Steinke, M.; Thompson, R.C.; Lindeque, P.K. Bioavailability and effects of microplastics on marine zooplankton: A review. Environ. Pollut. 2019, 245, 98–110. [Google Scholar] [CrossRef]
- Mancuso, M.; Savoca, S.; Bottari, T. First record of microplastics ingestion by European hake Merluccius merluccius from the Tyrrhenian Sicilian coast (Central Mediterranean Sea). J. Fish Biol. 2019, 94, 517–519. [Google Scholar] [CrossRef] [PubMed]
- Capillo, G.; Lauriano, E.; Icardo, J.; Siriyappagouder, P.; Kuciel, M.; Karapanagiotis, S.; Zaccone, G.; Fernandes, J. Structural identification of the pacemaker cells and expression of hyperpolarization-activated cyclic nucleotide-gated (HCN) channels in the heart of the wild Atlantic cod, Gadus morhua (Linnaeus, 1758). Int. J. Mol. Sci. 2021, 22, 7539. [Google Scholar] [CrossRef] [PubMed]
- Savoca, S.; Matanović, K.; D’Angelo, G.; Vetri, V.; Anselmo, S.; Bottari, T.; Mancuso, M.; Kužir, S.; Spanò, N.; Capillo, G.; et al. Ingestion of plastic and non-plastic microfibers by farmed gilthead sea bream (Sparus aurata) and common carp (Cyprinus carpio) at different life stages. Sci. Total Environ. 2021, 782, 146851. [Google Scholar] [CrossRef]
- Benzik, A.N.; Orlov, A.M.; Novikov, M.A. Marine seabed litter in Siberian Arctic: A first attempt to assess. Mar. Pollut. Bull. 2021, 172, 112836. [Google Scholar] [CrossRef]
- Novikov, M.A.; Gorbacheva, E.A.; Prokhorova, T.A.; Kharlamova, M.N. Composition and distribution of marine anthropogenic litter in the Barents Sea. Oceanology 2021, 61, 48–57. [Google Scholar] [CrossRef]
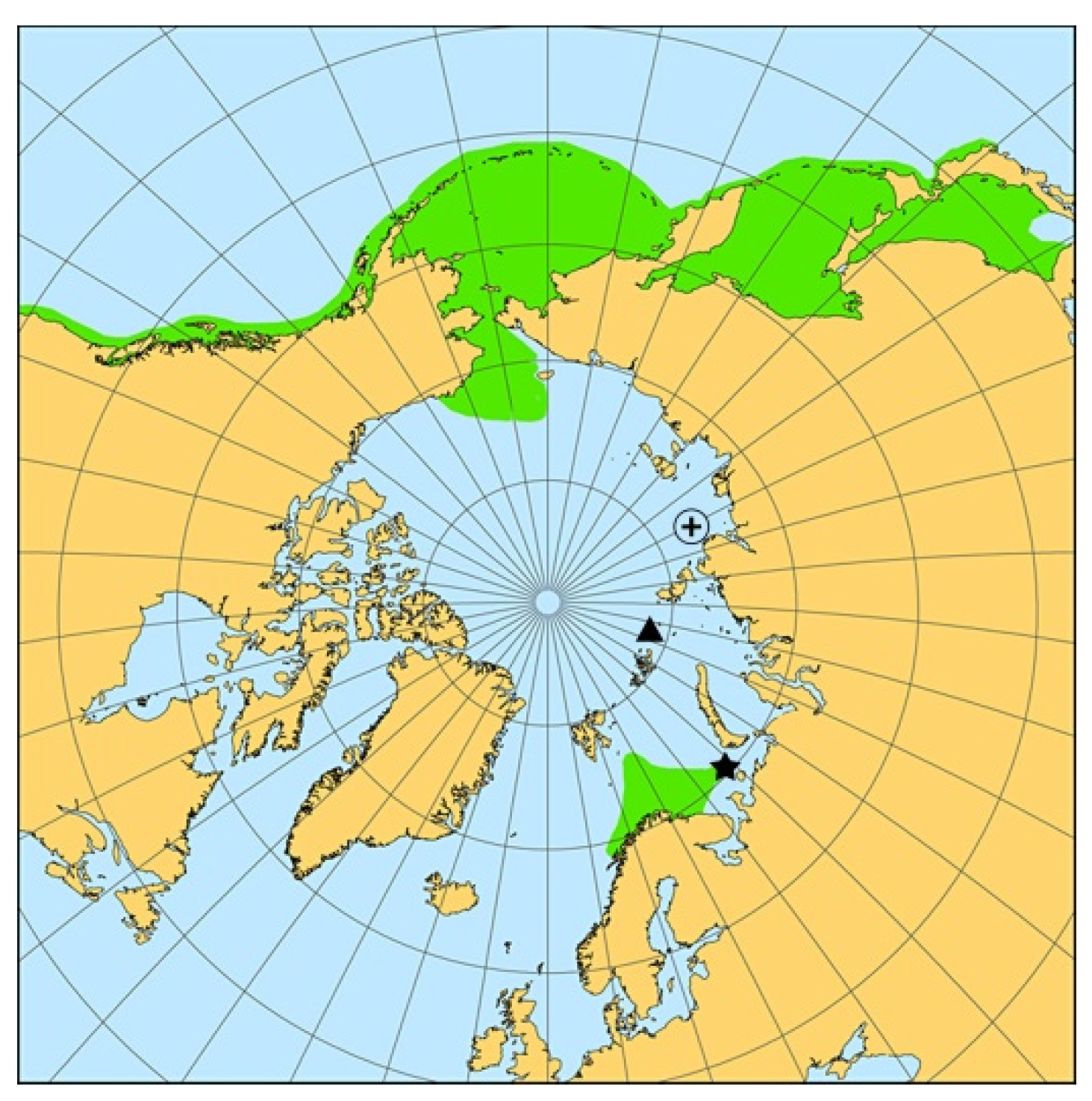

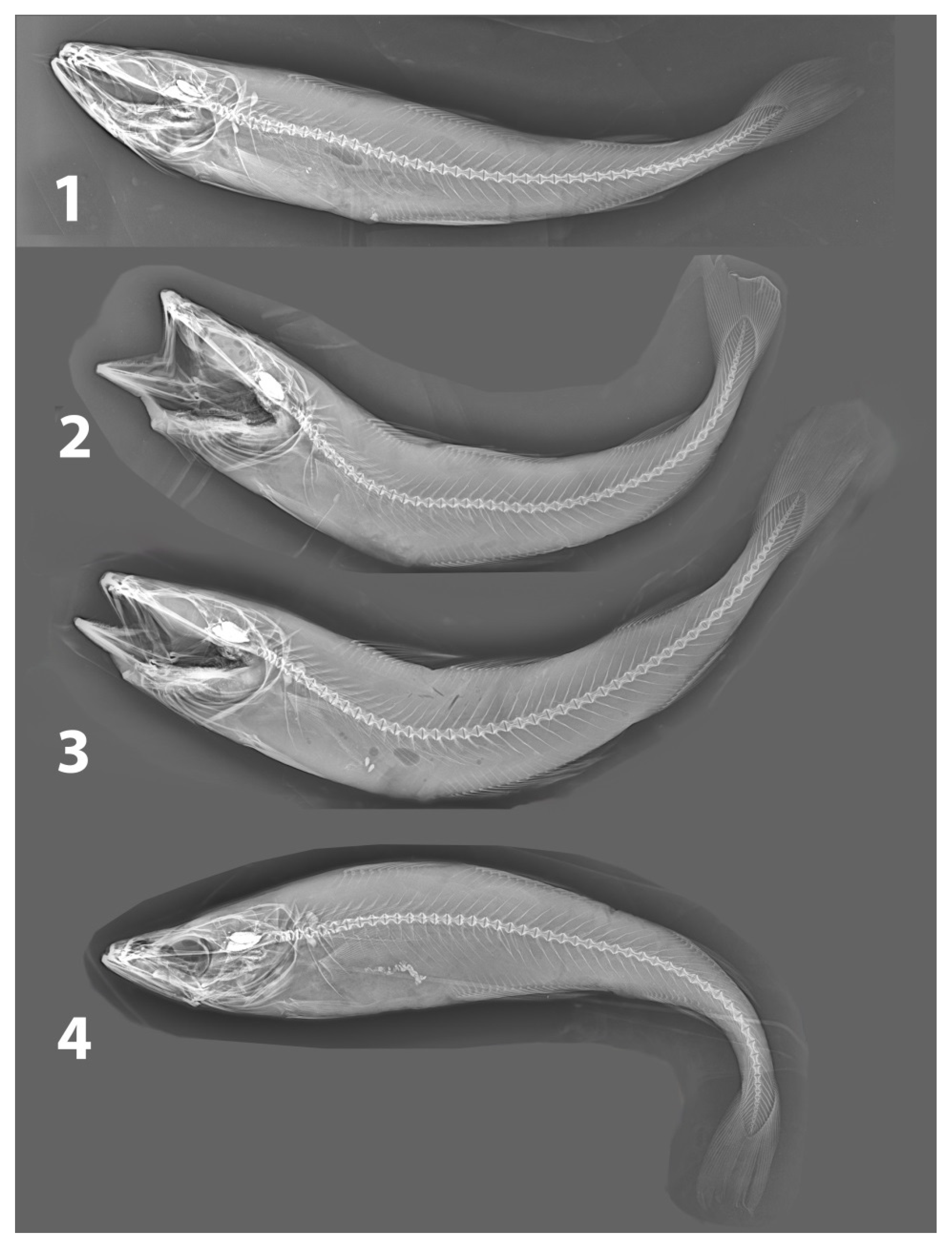
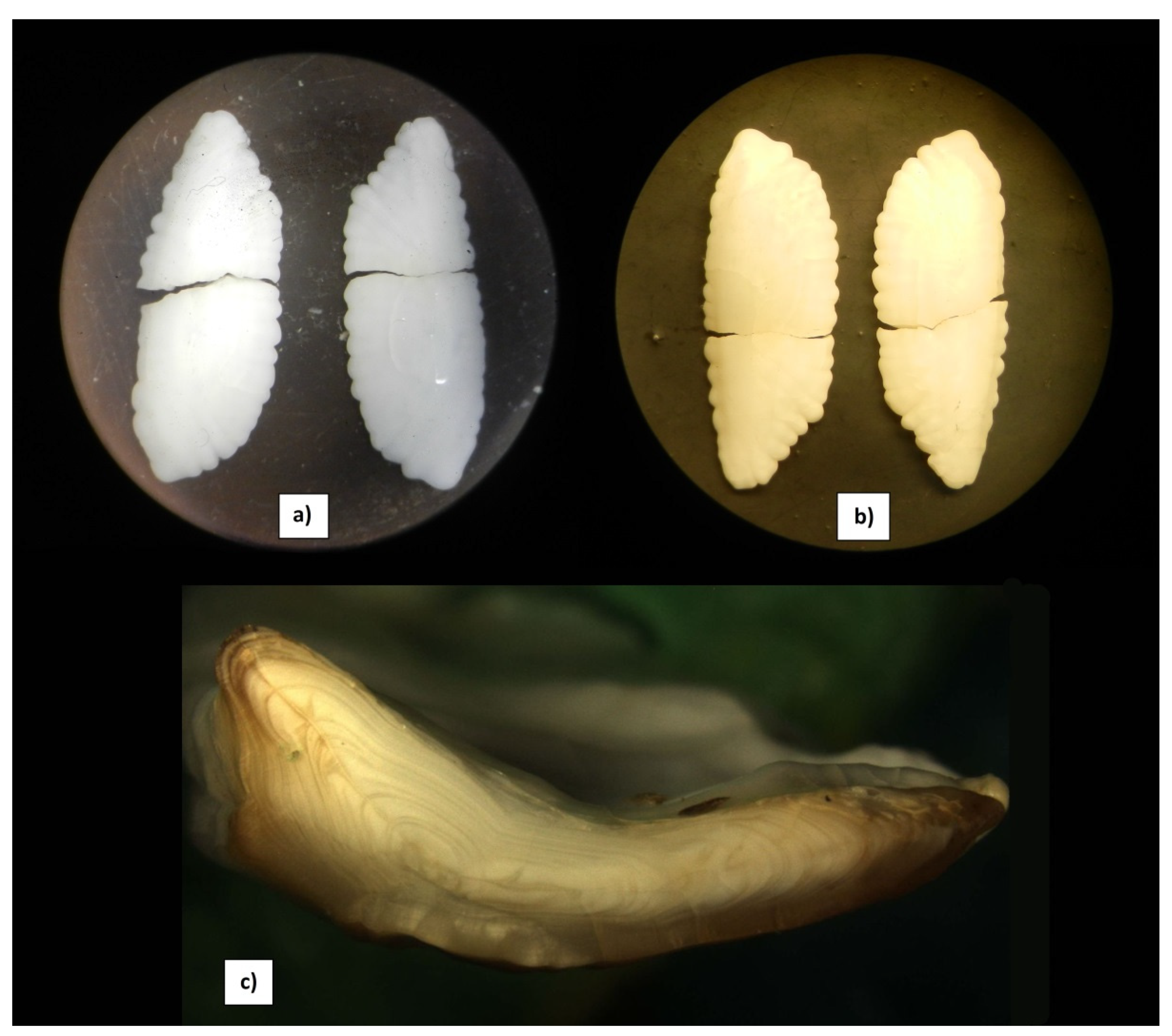

| Vessel/Gear | Date | Latitude, N | Longitude, E | Depth, m | Total Length, cm | Weight, g | Sex | Maturity Stage | Liver Weight, g | Gonad Weight, g | Food Weight, g | Age, Years |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Barents Sea | ||||||||||||
| FV “Kotoyarvi”/longline | 06.12.2018 | 69°31′50″ | 47°25′30″ | 42 | 77 | 2930 | M | 3–4 | 120 | 90 | 0 | 13+ |
| Kara Sea | ||||||||||||
| RV “Obva”/bottom trawl | 02.09.2008 | 82°00′00″ | 77°08′00″ | 383 | 35 | 310 | F | 3 | 18 | 8 | 12 | 5+ |
| 43 | 530 | F | 4 | 21 | 16 | 17 | 7+ | |||||
| 41 | 452 | F | 3 | 14 | 8 | 8 | 6+ | |||||
| 41 | 495 | F | 4 | 16 | 34 | 5 | 7+ | |||||
| 40 | 474 | F | 4 | 36 | 27 | 4 | 6+ | |||||
| 42 | 420 | F | 4 | 12 | 14 | 2 | 6+ | |||||
| 37 | 360 | M | 4 | 18 | 13 | 19 | 5+ | |||||
| 34 | 250 | F | 3 | 12 | 4 | 0 | 5+ | |||||
| 33 | 230 | F | 2 | 9 | 2 | 4 | 4+ | |||||
| Laptev Sea | ||||||||||||
| RV “Professor Levanidov”/bottom trawl | 12.09.2019 | 77°18′54″ | 122°44′12″ | 603 | 32 | 195 | na | na | na | na | na | na |
| 34 | 247 | na | na | na | na | na | na | |||||
| 77°20′06″ | 120°12′18″ | 452 | 35 | 272 | na | na | na | na | na | na | ||
| 13.09.2019 | 77°54′06″ | 115°52′36″ | 408 | 32 | 216 | na | na | na | na | na | na | |
| No. Sample | Number in VNIRO Genetic Database | Genbank Accession Number | Sampling Date | Locality | Country | Source |
|---|---|---|---|---|---|---|
| European hake Merluccius (outgroup) | ||||||
| 1 | na | KX782815 | 11.06.2013 | Germany | NCBI | |
| Greenland cod Gadus ogac (sister group 1) | ||||||
| 2 | na | LC146707 | 26.07.2016 | Greenland | NCBI | |
| Atlantic cod Gadus morhua (sister group 2) | ||||||
| 3 | na | KJ204879 | 2010–2012 | North Sea | na | NCBI |
| 4 | na | KJ204880 | 2010–2012 | North Sea | na | NCBI |
| Pacific cod Gadus macrocephalus (sister group 3) | ||||||
| 5 | na | ABCBF117-10 | na | na | Canada | BOLD Systems |
| 6 | na | ABCBF118-10 | na | na | Canada | BOLD Systems |
| Walleye pollock Gadus chalcogrammus (main group) | ||||||
| 7 | na | ABFJ129-06 | 09.10.2005 | Hachinohe | Japan | BOLD Systems |
| 8 | na | DSFAL234-07 | 12.09.2007 | N Bering Sea | USA | BOLD Systems |
| 9 | na | DSFAL479-08 | 09.08.2008 | W Beaufort Sea | USA | BOLD Systems |
| 10 | na | DSFAL480-08 | 09.08.2008 | W Beaufort Sea | USA | BOLD Systems |
| 11 | na | DSFAL481-08 | 09.08.2008 | W Beaufort Sea | USA | BOLD Systems |
| 12 | na | FHAK128-19 | 02.08.2018 | British Columbia | Canada | BOLD Systems |
| 13 | na | FHAK129-19 | 02.08.2018 | British Columbia | Canada | BOLD Systems |
| 14 | na | FMV085-08 | 29.04.2003 | Puget Sound, Washington | USA | BOLD Systems |
| 15 | na | FMV086-08 | 29.04.2003 | Puget Sound, Washington | USA | BOLD Systems |
| 16 | na | FMV441-09 | 02.02.2009 | Puget Sound, Washington | USA | BOLD Systems |
| 17 | na | GBGC4810-08 | na | Finnmark | Norway | BOLD Systems |
| 18 | na | GBGC4811-08 | na | Finnmark | Norway | BOLD Systems |
| 19 | na | GBGC4812-08 | na | Finnmark | Norway | BOLD Systems |
| 20 | na | NBMF027-15 | 14.08.2011 | Svalbard | Norway | BOLD Systems |
| 21 | na | SDP113001-13 | 03.08.2012 | Aleutian Islands | USA | BOLD Systems |
| 22 | na | SDP113002-13 | 03.08.2012 | Aleutian Islands | USA | BOLD Systems |
| 23 | na | SDP131037-14 | 03.08.2012 | Aleutian Islands | USA | BOLD Systems |
| 24 | THE5962 | MZ750950 | 11.09.2019 | E Chukchi Sea | USA | NCBI |
| 25 | THE5980 | MZ750951 | 11.09.2019 | E Chukchi Sea | USA | NCBI |
| 26 | lapLevArk88 | MZ750942 | 12.09.2019 | Laptev Sea | Russia | NCBI |
| 27 | lapTHE5897 | MZ750943 | 13.09.2019 | Laptev Sea | Russia | NCBI |
| 28 | LevArk89 | MZ750944 | 12.09.2019 | Laptev Sea | Russia | NCBI |
| 29 | THE5896 | MZ750948 | 13.09.2019 | Laptev Sea | Russia | NCBI |
| 30 | THE5898 | MZ750949 | 13.09.2019 | Laptev Sea | Russia | NCBI |
| 31 | barTHE2842 | MZ750938 | 06.12.2018 | SE Barents Sea | Russia | NCBI |
| 32 | chuk1THE5881 | MZ750939 | 23.08.2019 | W Chukchi Sea | Russia | NCBI |
| 33 | chuk1THE5884 | MZ750940 | 23.08.2019 | W Chukchi Sea | Russia | NCBI |
| 34 | chuk2THE5983 | MZ750941 | 11.09.2019 | W Chukchi Sea | Russia | NCBI |
| 35 | THE5882 | MZ750945 | 23.08.2019 | W Chukchi Sea | Russia | NCBI |
| 36 | THE5885 | MZ750946 | 23.08.2019 | W Chukchi Sea | Russia | NCBI |
| 37 | THE5889 | MZ750947 | 23.08.2019 | W Chukchi Sea | Russia | NCBI |
| Character/Source | North Atlantic | North Pacific | Laptev Sea | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |||
| Min. | Max. | Mean | |||||||||||
| Total length (TL), mm | 620 | 585 | 422 | 322 | 352 | 333.0 | |||||||
| Standard length (SL), mm | 550 | 555 | 507 | 309 | 292 | 320 | 302.5 | ||||||
| Morphometrics | |||||||||||||
| % TL | |||||||||||||
| Predorsal length | 40.0 | 42.6 | 40.7 | 40.4–44.1 | 39.6 | 41.2 | 40.8 | ||||||
| Preanal length | 27.1 | 29.9 | 29.4 | 27.3–29.2 | 25.0 | 28.6 | 26.3 | ||||||
| Pectoral fin length | 16.1 | 15.0 | 17.5–21.4 | 15.0 | 16.3 | 15.9 | |||||||
| Pelvic fin length | 14.7 | 15.6 | 9.5–11.8 | 12.6 | 15.1 | 13.6 | |||||||
| Body depth | 15.5 | 22.6 | 16.7–18.2 | 9.8–15.2 | 15.2 | 15.9 | 15.5 | ||||||
| Depth of caudal peduncle | 4.0 | 4.2 | 4.4–4.5 | 3.0–3.5 | 3.6 | 4.2 | 3.9 | ||||||
| Head length | 21.8 | 24.6 | 25.0–26.7 | 21.8–24.2 | 22.2 | 23.4 | 22.7 | ||||||
| % SL | |||||||||||||
| Predorsal length | 47.2 | 45.7 | 43.9 | 45.3 | 44.9 | ||||||||
| Preanal length | 31.2 | 32.4 | 27.5 | 31.4 | 29.0 | ||||||||
| Pectoral fin length | 20.0 | 18.4 | 17.7 | 16.6 | 18.0 | 17.5 | |||||||
| Pelvic fin length | 18.7 | 15.7 | 15.9 | 13.8 | 16.6 | 14.9 | |||||||
| Body depth | 16.7 | 17.4 | 17.0 | ||||||||||
| Depth of caudal peduncle | 4.5 | 4.2 | 4.6 | 4.0 | 4.6 | 4.3 | |||||||
| Pectoral fin length | 24.5 | 25.7 | 26.4 | 24.5 | 25.8 | 25.0 | |||||||
| Upper jaw length | 11.1 | 9.7 | 11.1 | 10.5 | |||||||||
| Interorbital length | 29.6 | 5.7 | 6.8 | 6.3 | |||||||||
| Horizontal eye diameter | 4.9 | 5.8 | 5.9 | 6.6 | 6.3 | ||||||||
| Vertical eye diameter | 5.3 | 6.0 | 5.7 | ||||||||||
| Preorbital length | 8.4 | 8.6 | 7.5 | 8.5 | 7.9 | ||||||||
| Postorbital length | 12.9 | 12.6 | 11.4 | 12.9 | 11.9 | ||||||||
| Length of D1 base | 12.1 | 12.1 | 9.0 | 11.5 | 10.4 | ||||||||
| Length of D2 base | 17.5 | 17.6 | 14.8 | 16.8 | 15.8 | ||||||||
| Length of D3 base | 16.0 | 15.9 | 13.8 | 15.6 | 15.0 | ||||||||
| Length of A1 base | 20.7 | 21.4 | 17.2 | 20.2 | 18.3 | ||||||||
| Length of A2 base | 16.8 | 16.6 | 11.4 | 17.2 | 15.1 | ||||||||
| Meristics | |||||||||||||
| Number of D1 fin rays | 13 | 12 | 13 | 12.8 | 12.5 | 12–14 | 12–14 | 12–14 | 13–14 | 12 | 14 | 12.8 | |
| Number of D2 fin rays | 16 | 15 | 16–17 | 16.1 | 16.3 | 12–18 | 12–18 | 12–18 | 15–19 | 14 | 18 | 16.3 | |
| Number of D3 fin rays | 17 | 16 | 18–20 | 18.4 | 18.1 | 17.5 | 20–21 | 17–21 | 20–21 | 18–23 | 18 | 20 | 19.0 |
| Number of A1 fin rays | 17 | 20 | 19–20 | 19.7 | 20.8 | 19–23 | 19–23 | 19–23 | 19–24 | 18 | 22 | 20.8 | |
| Number of A2 fin rays | 20 | 19 (21) | 19–20 | 18.9 | 19.4 | 21–23 | 20–23 | 21–23 | 20–23 | 19 | 21 | 20.0 | |
| Number of pectoral fin rays | 19 | 16 | 19 | 18.8 | 18.8 | 18–19 | 16 | 20 | 18.5 | ||||
| Number of pelvic fin rays | 6 | 7 | 6.1 | 6.2 | 5–6 | 6 | 7 | 6.8 | |||||
| Number of caudal fin rays | 45.5 | 42.2 | 50 * | 52 * | 50.5 | ||||||||
| Number of vertebrae | 50 | 50–52 | 50 | 50–52 | 51 | 52 | 51.5 | ||||||
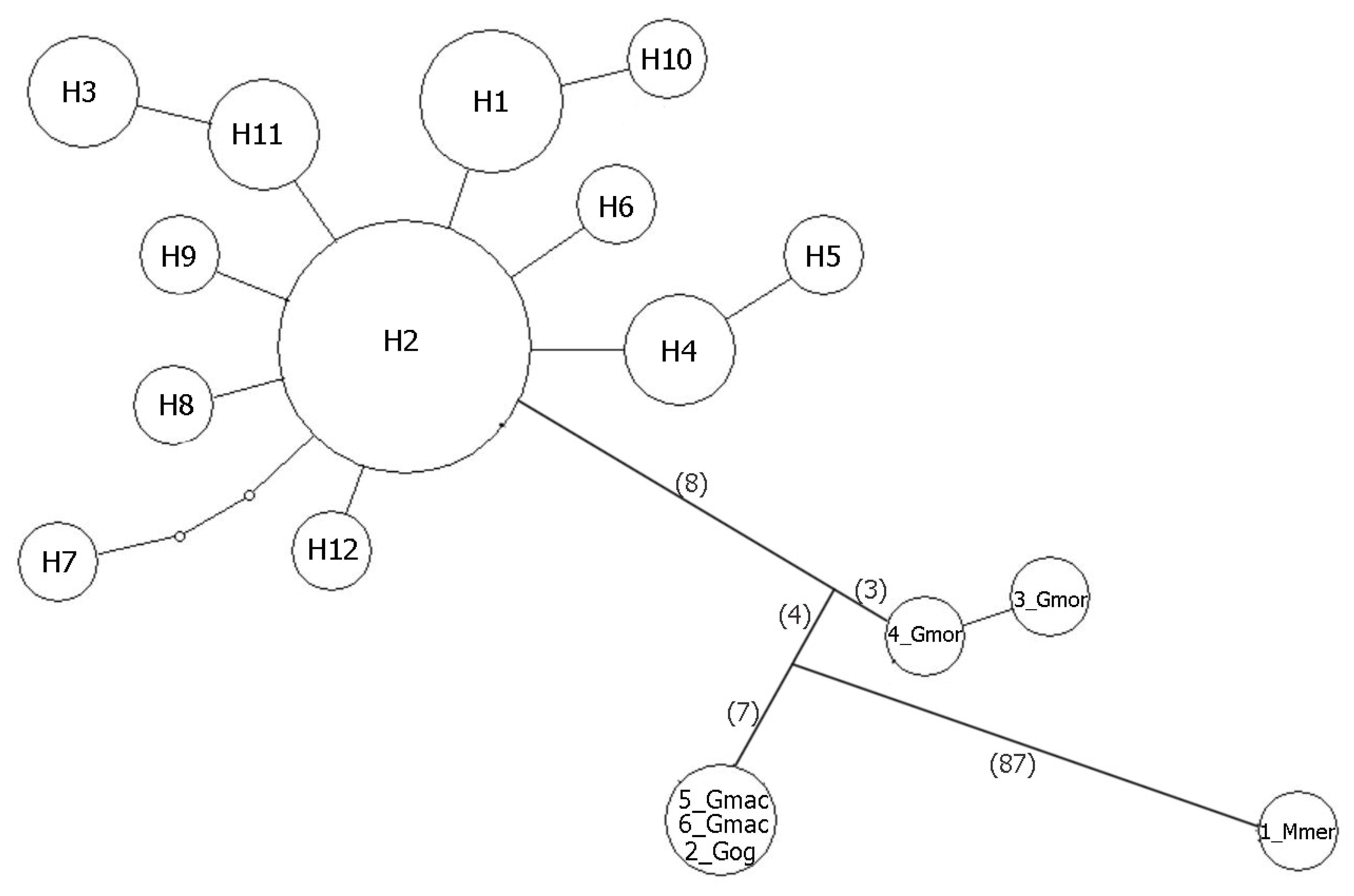
| No. Sample | No. Haplotype | Country, Locality | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
| 7 | Japan, Hachinohe | ||||||||||||
| 8 | USA, Bering Sea | ||||||||||||
| 9 | USA, Beaufort Sea | ||||||||||||
| 10 | USA, Beaufort Sea | ||||||||||||
| 11 | USA, Beaufort Sea | ||||||||||||
| 12 | Canada, British Columbia | ||||||||||||
| 13 | Canada, British Columbia | ||||||||||||
| 14 | USA, Puget Sound | ||||||||||||
| 15 | USA, Puget Sound | ||||||||||||
| 16 | USA, Puget Sound | ||||||||||||
| 17 | Norway, Finnmark | ||||||||||||
| 18 | Norway, Finnmark | ||||||||||||
| 19 | Norway, Finnmark | ||||||||||||
| 20 | Norway, Svalbard | ||||||||||||
| 21 | USA, Aleutians | ||||||||||||
| 22 | USA, Aleutians | ||||||||||||
| 23 | USA, Aleutians | ||||||||||||
| 24 | USA, Chukchi Sea | ||||||||||||
| 25 | USA, Chukchi Sea | ||||||||||||
| 26 | Russia, Laptev Sea | ||||||||||||
| 27 | Russia, Laptev Sea | ||||||||||||
| 28 | Russia, Laptev Sea | ||||||||||||
| 29 | Russia, Laptev Sea | ||||||||||||
| 30 | Russia, Laptev Sea | ||||||||||||
| 31 | Russia, Barents Sea | ||||||||||||
| 32 | Russia, Chukchi Sea | ||||||||||||
| 33 | Russia, Chukchi Sea | ||||||||||||
| 34 | Russia, Chukchi Sea | ||||||||||||
| 35 | Russia, Chukchi Sea | ||||||||||||
| 36 | Russia, Chukchi Sea | ||||||||||||
| 37 | Russia, Chukchi Sea | ||||||||||||
| Total number of particular haplotype | 3 | 15 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlov, A.M.; Rybakov, M.O.; Vedishcheva, E.V.; Volkov, A.A.; Orlova, S.Y. Walleye Pollock Gadus chalcogrammus, a Species with Continuous Range from the Norwegian Sea to Korea, Japan, and California: New Records from the Siberian Arctic. J. Mar. Sci. Eng. 2021, 9, 1141. https://doi.org/10.3390/jmse9101141
Orlov AM, Rybakov MO, Vedishcheva EV, Volkov AA, Orlova SY. Walleye Pollock Gadus chalcogrammus, a Species with Continuous Range from the Norwegian Sea to Korea, Japan, and California: New Records from the Siberian Arctic. Journal of Marine Science and Engineering. 2021; 9(10):1141. https://doi.org/10.3390/jmse9101141
Chicago/Turabian StyleOrlov, Alexei M., Maxim O. Rybakov, Elena V. Vedishcheva, Alexander A. Volkov, and Svetlana Yu. Orlova. 2021. "Walleye Pollock Gadus chalcogrammus, a Species with Continuous Range from the Norwegian Sea to Korea, Japan, and California: New Records from the Siberian Arctic" Journal of Marine Science and Engineering 9, no. 10: 1141. https://doi.org/10.3390/jmse9101141
APA StyleOrlov, A. M., Rybakov, M. O., Vedishcheva, E. V., Volkov, A. A., & Orlova, S. Y. (2021). Walleye Pollock Gadus chalcogrammus, a Species with Continuous Range from the Norwegian Sea to Korea, Japan, and California: New Records from the Siberian Arctic. Journal of Marine Science and Engineering, 9(10), 1141. https://doi.org/10.3390/jmse9101141







