Bacterial Diversity Associated with Millepora alcicornis, Phyllogorgia dilatata and Mussismilia harttii Collected from Two Distinct Corals Reefs on the Brazilian Coast
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Sampling
2.2. DNA Isolation, PCR Amplification and 16S Sequencing
2.3. Illumina 16S Sequencing
2.4. Bioinformatics Analysis and Statistics
3. Results
3.1. Bacterial Sequencing and Sample Coverage
3.2. Bacterial Composition Analysis
3.3. Diversity Analysis of the Microbiota
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garcia, T.M.; Matthews-Cascon, H.; Franklin-Junior, W. Millepora Alcicornis (Cnidaria: Hydrozoa) as Substrate for Benthic Fauna. Braz. J. Oceanogr. 2009, 57, 153–155. [Google Scholar] [CrossRef]
- Gates, R.D.; Ainsworth, T.D. The Nature and Taxonomic Composition of Coral Symbiomes as Drivers of Performance Limits in Scleractinian Corals. J. Exp. Mar. Biol. Ecol. 2011, 408, 94–101. [Google Scholar] [CrossRef]
- Burt, J.A.; Camp, E.F.; Enochs, I.C.; Johansen, J.L.; Morgan, K.M.; Riegl, B.; Hoey, A.S. Insights from Extreme Coral Reefs in a Changing World. Coral Reefs 2020, 39, 495–507. [Google Scholar] [CrossRef]
- Blackall, L.L.; Wilson, B.; Van Oppen, M.J.H. Coral—The World’s Most Diverse Symbiotic Ecosystem. Mol. Ecol. 2015, 24, 5330–5347. [Google Scholar] [CrossRef]
- Davy, S.K.; Allemand, D.; Weis, V.M. Cell Biology of Cnidarian-Dinoflagellate Symbiosis. Microbiol. Mol. Biol. Rev. 2012, 76, 229–261. [Google Scholar] [CrossRef]
- Muller-Parker, G.; D’Elia, C.F.; Cook, C.B. Interactions Between Corals and Their Symbiotic Algae. In Coral Reefs in the Anthropocene; Springer: Dordrecht, The Netherlands, 2015; pp. 99–116. [Google Scholar] [CrossRef]
- Goulet, T.L.; Erill, I.; Ascunce, M.S.; Finley, S.J.; Javan, G.T. Conceptualization of the Holobiont Paradigm as It Pertains to Corals. Front. Physiol. 2020, 11, 566968. [Google Scholar] [CrossRef]
- Boilard, A.; Dubé, C.E.; Gruet, C.; Mercière, A.; Hernandez-Agreda, A.; Derome, N. Defining Coral Bleaching as a Microbial Dysbiosis within the Coral Holobiont. Microorganisms 2020, 8, 1682. [Google Scholar] [CrossRef]
- Stabili, L.; Parisi, M.G.; Parrinello, D.; Cammarata, M. Cnidarian Interaction with Microbial Communities: From Aid to Animal’s Health to Rejection Responses. Mar. Drugs 2018, 16, 296. [Google Scholar] [CrossRef]
- Rosenberg, E.; Koren, O.; Reshef, L.; Efrony, R.; Zilber-Rosenberg, I. The Role of Microorganisms in Coral Health, Disease and Evolution. Nat. Rev. Microbiol. 2007, 5, 355–362. [Google Scholar] [CrossRef]
- Hernandez-Agreda, A.; Leggat, W.; Bongaerts, P.; Ainsworth, T.D. The Microbial Signature Provides Insight into the Mechanistic Basis of Coral Success across Reef Habitats. mBio 2016, 7, e00560-16. [Google Scholar] [CrossRef]
- Littman, R.A.; Bourne, D.G.; Willis, B.L. Responses of Coral-Associated Bacterial Communities to Heat Stress Differ with Symbiodinium Type on the Same Coral Host. Mol. Ecol. 2010, 19, 1978–1990. [Google Scholar] [CrossRef] [PubMed]
- Meron, D.; Atias, E.; Iasur Kruh, L.; Elifantz, H.; Minz, D.; Fine, M.; Banin, E. The Impact of Reduced PH on the Microbial Community of the Coral Acropora Eurystoma. ISME J. 2011, 5, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.L.; Castellanos-Gell, J.; Aeby, G.S.; Häse, C.C.; Ushijima, B.; Paul, V.J. Microbial Community Shifts Associated With the Ongoing Stony Coral Tissue Loss Disease Outbreak on the Florida Reef Tract. Front. Microbiol. 2019, 10, 2244. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Yu, K.; Liang, J.; Yao, Q.; Chen, B. Significant Changes in Microbial Communities Associated With Reef Corals in the Southern South China Sea During the 2015/2016 Global-Scale Coral Bleaching Event. J. Geophys. Res. Oceans 2020, 125, e2019JC015579. [Google Scholar] [CrossRef]
- Morrow, K.M.; Moss, A.G.; Chadwick, N.E.; Liles, M.R. Bacterial Associates of Two Caribbean Coral Species Reveal Species-Specific Distribution and Geographic Variability. Appl. Environ. Microbiol. 2012, 78, 6438–6449. [Google Scholar] [CrossRef]
- Leão, Z.M.A.N.; Kikuchi, R.K.P.; Ferreira, B.P.; Neves, E.G.; Sovierzoski, H.H.; Oliveira, M.D.M.; Maida, M.; Correia, M.D.; Johnsson, R. Brazilian Coral Reefs in a Period of Global Change: A Synthesis. Braz. J. Oceanogr. 2016, 64, 97–116. [Google Scholar] [CrossRef]
- Leão, Z.M.A.N.; Kikuchi, R.K.P.; Testa, V. Corals and Coral Reefs of Brazil. In Latin American Coral Reefs; Elsevier: Amsterdam, The Netherlands, 2003; pp. 9–52. [Google Scholar] [CrossRef]
- Leão, Z.M.A.N.; Kikuchi, R.K.P. The Abrolhos Reefs of Brazil. In Coastal Marine Ecosystems of Latin America; Springer: Berlin/Heidelberg, Germany, 2001; pp. 83–96. [Google Scholar] [CrossRef]
- Teixeira, C.D.; Leitão, R.L.L.; Ribeiro, F.V.; Moraes, F.C.; Neves, L.M.; Bastos, A.C.; Pereira-Filho, G.H.; Kampel, M.; Salomon, P.S.; Sá, J.A.; et al. Sustained Mass Coral Bleaching (2016–2017) in Brazilian Turbid-Zone Reefs: Taxonomic, Cross-Shelf and Habitat-Related Trends. Coral Reefs 2019, 38, 801–813. [Google Scholar] [CrossRef]
- Duarte, G.A.S.; Villela, H.D.M.; Deocleciano, M.; Silva, D.; Barno, A.; Cardoso, P.M.; Vilela, C.L.S.; Rosado, P.; Messias, C.S.M.A.; Chacon, M.A.; et al. Heat Waves Are a Major Threat to Turbid Coral Reefs in Brazil. Front. Mar. Sci. 2020, 7, 515393. [Google Scholar] [CrossRef]
- Corazza, B.M.; Lacerda, C.H.F.; Güth, A.Z.; Marcançoli, R.K.M.; Bianchini, A.; Calderon, E.N.; Capel, K.C.C.; Conceição, E.; Faria, S.C.; Francini-Filho, R.B.; et al. No Coral Recovery Three Years after a Major Bleaching Event in Reefs in the Southwestern Atlantic Refugium. Mar. Biol. 2024, 171, 114. [Google Scholar] [CrossRef]
- Li, J.; Yang, Q.; Dong, J.; Sweet, M.; Zhang, Y.; Liu, C.; Zhang, Y.; Tang, X.; Zhang, W.; Zhang, S. Microbiome Engineering: A Promising Approach to Improve Coral Health. Engineering 2023, 28, 105–116. [Google Scholar] [CrossRef]
- Thompson, J.R.; Rivera, H.E.; Closek, C.J.; Medina, M. Microbes in the Coral Holobiont: Partners through Evolution, Development, and Ecological Interactions. Front. Cell Infect. Microbiol. 2015, 4, 176. [Google Scholar] [CrossRef] [PubMed]
- Vilela, C.L.S.; Villela, H.D.M.; da Costa Rachid, C.T.C.; Do Carmo, F.L.; Vermelho, A.B.; Peixoto, R.S. Exploring the Diversity and Biotechnological Potential of Cultured and Uncultured Coral-Associated Bacteria. Microorganisms 2021, 9, 2235. [Google Scholar] [CrossRef] [PubMed]
- De Castro, A.P.; Araújo, S.D.; Reis, A.M.M.; Pompeu, M.; Hatay, M.; de Moura, R.L.; Francini-Filho, R.B.; Thompson, F.L.; Krüger, R.H. Bacterial Communities Associated with Three Brazilian Endemic Reef Corals (Mussismilia spp.) in a Coastal Reef of the Abrolhos Shelf. Cont. Shelf Res. 2013, 70, 135–139. [Google Scholar] [CrossRef]
- Rosser, N.L.; Thomas, L.; Stankowski, S.; Richards, Z.T.; Kennington, W.J.; Johnson, M.S. Phylogenomics Provides New Insight into Evolutionary Relationships and Genealogical Discordance in the Reef-Building Coral Genus Acropora. Proc. R. Soc. B Biol. Sci. 2017, 284, 20162182. [Google Scholar] [CrossRef]
- Leite, D.C.A.; Leão, P.; Garrido, A.G.; Lins, U.; Santos, H.F.; Pires, D.O.; Castro, C.B.; van Elsas, J.D.; Zilberberg, C.; Rosado, A.S.; et al. Broadcast Spawning Coral Mussismilia Hispida Can Vertically Transfer Its Associated Bacterial Core. Front. Microbiol. 2017, 8, 239169. [Google Scholar] [CrossRef]
- Andrade Galvão de Medeiros, T.; Seoane, J.C.S.; Macedo de Mello Baptista, G.; Leal, P.R.; Dekker, A. Effect of Temperature and PH on the Millepora Alcicornis and Mussismilia Harttii Corals in Light of a Spectral Reflectance Response. Int. J. Remote Sens. 2022, 43, 2475–2502. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing Taxonomic Classification of Marker-Gene Amplicon Sequences with QIIME 2’s Q2-Feature-Classifier Plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- Doering, T.; Tandon, K.; Topa, S.H.; Pidot, S.J.; Blackall, L.L.; van Oppen, M.J.H. Genomic Exploration of Coral-Associated Bacteria: Identifying Probiotic Candidates to Increase Coral Bleaching Resilience in Galaxea Fascicularis. Microbiome 2023, 11, 185. [Google Scholar] [CrossRef]
- Ziegler, M.; Seneca, F.O.; Yum, L.K.; Palumbi, S.R.; Voolstra, C.R. Bacterial Community Dynamics Are Linked to Patterns of Coral Heat Tolerance. Nat. Commun. 2017, 8, 14213. [Google Scholar] [CrossRef] [PubMed]
- McCauley, M.; Goulet, T.L.; Jackson, C.R.; Loesgen, S. Systematic Review of Cnidarian Microbiomes Reveals Insights into the Structure, Specificity, and Fidelity of Marine Associations. Nat. Commun. 2023, 14, 4899. [Google Scholar] [CrossRef]
- Raina, J.B.; Tapiolas, D.; Willis, B.L.; Bourne, D.G. Coral-Associated Bacteria and Their Role in the Biogeochemical Cycling of Sulfur. Appl. Environ. Microbiol. 2009, 75, 3492–3501. [Google Scholar] [CrossRef] [PubMed]
- Macedo, M.W.F.S.; Cunha, N.B.; Carneiro, J.A.; da Costa, R.A.; de Alencar, S.A.; Cardoso, M.H.; Franco, O.L.; Dias, S.C. Marine Organisms as a Rich Source of Biologically Active Peptides. Front. Mar. Sci. 2021, 8, 667764. [Google Scholar] [CrossRef]
- Macedo, M.W.F.S.; Costa, R.A.; de Lima, R.G.; Gomes, J.E.M.; Rocha, G.I.Y.; dos Santos, H.F.; Dias, S.C.; da Cunha, N.B. Bacterial Diversity Associated with Millepora Alcicornis and Phyllogorgia Dilatata Corals and Prospection for Genes Encoding Bioactive Molecules. Reg. Stud. Mar. Sci. 2023, 59, 102811. [Google Scholar] [CrossRef]
- Ritchie, K.B. Bacterial Symbionts of Corals and Symbiodinium. In Beneficial Microorganisms in Multicellular Life Forms; Springer: Berlin/Heidelberg, Germany, 2012; pp. 139–150. [Google Scholar] [CrossRef]
- Glasl, B.; Webster, N.S.; Bourne, D.G. Microbial Indicators as a Diagnostic Tool for Assessing Water Quality and Climate Stress in Coral Reef Ecosystems. Mar. Biol. 2017, 164, 91. [Google Scholar] [CrossRef]
- Tignat-Perrier, R.; van de Water, J.A.J.M.; Allemand, D.; Ferrier-Pagès, C. Holobiont Responses of Mesophotic Precious Red Coral Corallium Rubrum to Thermal Anomalies. Environ. Microbiome 2023, 18, 70. [Google Scholar] [CrossRef]
- Almeidaa, M.T.R.; Moritz, M.I.G.; Capel, K.C.C.; Pérez, C.D.; Schenkel, E.P. Chemical and Biological Aspects of Octocorals from the Brazilian Coast. Rev. Bras. Farmacogn. 2014, 24, 446–467. [Google Scholar] [CrossRef]
- Maia, L.F.; Fernandes, R.F.; Almeida, M.R.; de Oliveira, L.F.C. Rapid Assessment of Chemical Compounds from Phyllogorgia Dilatata Using Raman Spectroscopy. Rev. Bras. Farmacogn. 2015, 25, 619–626. [Google Scholar] [CrossRef]
- Van De Water, J.A.J.M.; Allemand, D.; Ferrier-Pagès, C. Host-Microbe Interactions in Octocoral Holobionts—Recent Advances and Perspectives. Microbiome 2018, 6, 64. [Google Scholar] [CrossRef]
- Xu, M.; Cheng, K.; Xiao, B.; Tong, M.; Cai, Z.; Jong, M.-C.; Chen, G.; Zhou, J. Bacterial Communities Vary from Different Scleractinian Coral Species and between Bleached and Non-Bleached Corals. Microbiol. Spectr. 2023, 11, e0491022. [Google Scholar] [CrossRef] [PubMed]
- Vartoukian, S.R.; Palmer, R.M.; Wade, W.G. Strategies for Culture of “unculturable” Bacteria. FEMS Microbiol. Lett. 2010, 309, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pantos, O.; Cooney, R.P.; Le Tissier, M.D.A.; Barer, M.R.; O’Donnell, A.G.; Bythell, J.C. The Bacterial Ecology of a Plague-like Disease Affecting the Caribbean Coral Montastrea Annularis. Environ. Microbiol. 2003, 5, 370–382. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Koenen, M.; Bale, N.J.; Reitsma, W.; Engelmann, J.C.; Stefanova, K.; Damsté, J.S.S.; Villanueva, L. Organic Matter Degradation in the Deep, Sulfidic Waters of the Black Sea: Insights into the Ecophysiology of Novel Anaerobic Bacteria. Microbiome 2024, 12, 98. [Google Scholar] [CrossRef]
- Flieder, M.; Buongiorno, J.; Herbold, C.W.; Hausmann, B.; Rattei, T.; Lloyd, K.G.; Loy, A.; Wasmund, K. Novel Taxa of Acidobacteriota Implicated in Seafloor Sulfur Cycling. ISME J. 2021, 15, 3159–3180. [Google Scholar] [CrossRef]
- de Castro, A.P.; Araújo, S.D.; Reis, A.M.M.; Moura, R.L.; Francini-Filho, R.B.; Pappas, G.; Rodrigues, T.B.; Thompson, F.L.; Krüger, R.H. Bacterial Community Associated with Healthy and Diseased Reef Coral Mussismilia Hispida from Eastern Brazil. Microb. Ecol. 2010, 59, 658–667. [Google Scholar] [CrossRef]
- Reis, A.M.M.; Araújo, S.D.; Moura, R.L.; Francini-Filho, R.B.; Pappas, G.; Coelho, A.M.A.; Krüger, R.H.; Thompson, F.L. Bacterial Diversity Associated with the Brazilian Endemic Reef Coral Mussismilia Braziliensis. J. Appl. Microbiol. 2009, 106, 1378–1387. [Google Scholar] [CrossRef]
- Vilela, C.L.S.; Villela, H.D.M.; Duarte, G.A.S.; Santoro, E.P.; Rachid, C.T.C.C.; Peixoto, R.S. Estrogen Induces Shift in Abundances of Specific Groups of the Coral Microbiome. Sci. Rep. 2021, 11, 2767. [Google Scholar] [CrossRef]
- Bayer, T.; Neave, M.J.; Alsheikh-Hussain, A.; Aranda, M.; Yum, L.K.; Mincer, T.; Hughen, K.; Apprill, A.; Voolstra, C.R. The Microbiome of the Red Sea Coral Stylophora Pistillata Is Dominated by Tissue-Associated Endozoicomonas Bacteria. Appl. Environ. Microbiol. 2013, 79, 4759–4762. [Google Scholar] [CrossRef]
- Fernando, S.C.; Wang, J.; Sparling, K.; Garcia, G.D.; Francini-Filho, R.B.; de Moura, R.L.; Paranhos, R.; Thompson, F.L.; Thompson, J.R. Microbiota of the Major South Atlantic Reef Building Coral Mussismilia. Microb. Ecol. 2015, 69, 267–280. [Google Scholar] [CrossRef]
- Dubé, C.E.; Ziegler, M.; Mercière, A.; Boissin, E.; Planes, S.; Bourmaud, C.A.F.; Voolstra, C.R. Naturally Occurring Fire Coral Clones Demonstrate a Genetic and Environmental Basis of Microbiome Composition. Nat. Commun. 2021, 12, 6402. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Matos, A.E.; Rosado, W.; Govind, N.S. Bacterial Diversity Associated with the Caribbean Tunicate Ecteinascidia Turbinata. Antonie Leeuwenhoek 2007, 92, 155–164. [Google Scholar] [CrossRef]
- Moss, C.; Green, D.H.; Pérez, B.; Velasco, A.; Henríquez, R.; McKenzie, J.D. Intracellular Bacteria Associated with the Ascidian Ecteinascidia Turbinata: Phylogenetic and in Situ Hybridisation Analysis. Mar. Biol. 2003, 143, 99–110. [Google Scholar] [CrossRef]
- Rath, C.M.; Janto, B.; Earl, J.; Ahmed, A.; Hu, F.Z.; Hiller, L.; Dahlgren, M.; Kreft, R.; Yu, F.; Wolff, J.J.; et al. Meta-Omic Characterization of the Marine Invertebrate Microbial Consortium That Produces the Chemotherapeutic Natural Product ET-743. ACS Chem. Biol. 2011, 6, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Erwin, P.M.; Pineda, M.C.; Webster, N.; Turon, X.; López-Legentil, S. Down under the Tunic: Bacterial Biodiversity Hotspots and Widespread Ammonia-Oxidizing Archaea in Coral Reef Ascidians. ISME J. 2014, 8, 575–588. [Google Scholar] [CrossRef]
- Mohamed, A.R.; Ochsenkühn, M.A.; Kazlak, A.M.; Moustafa, A.; Amin, S.A. The Coral Microbiome: Towards an Understanding of the Molecular Mechanisms of Coral-Microbiota Interactions. FEMS Microbiol. Rev. 2023, 47, fuad005. [Google Scholar] [CrossRef]
- Bourne, D.; Iida, Y.; Uthicke, S.; Smith-Keune, C. Changes in Coral-Associated Microbial Communities during a Bleaching Event. ISME J. 2008, 2, 350–363. [Google Scholar] [CrossRef]
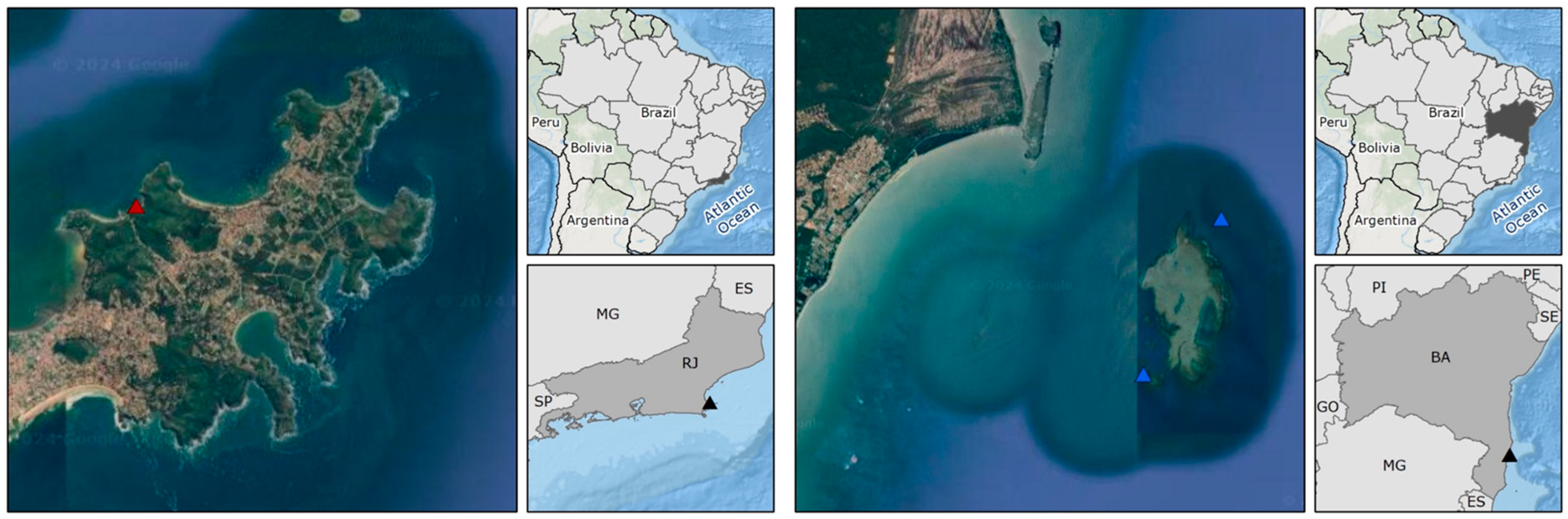

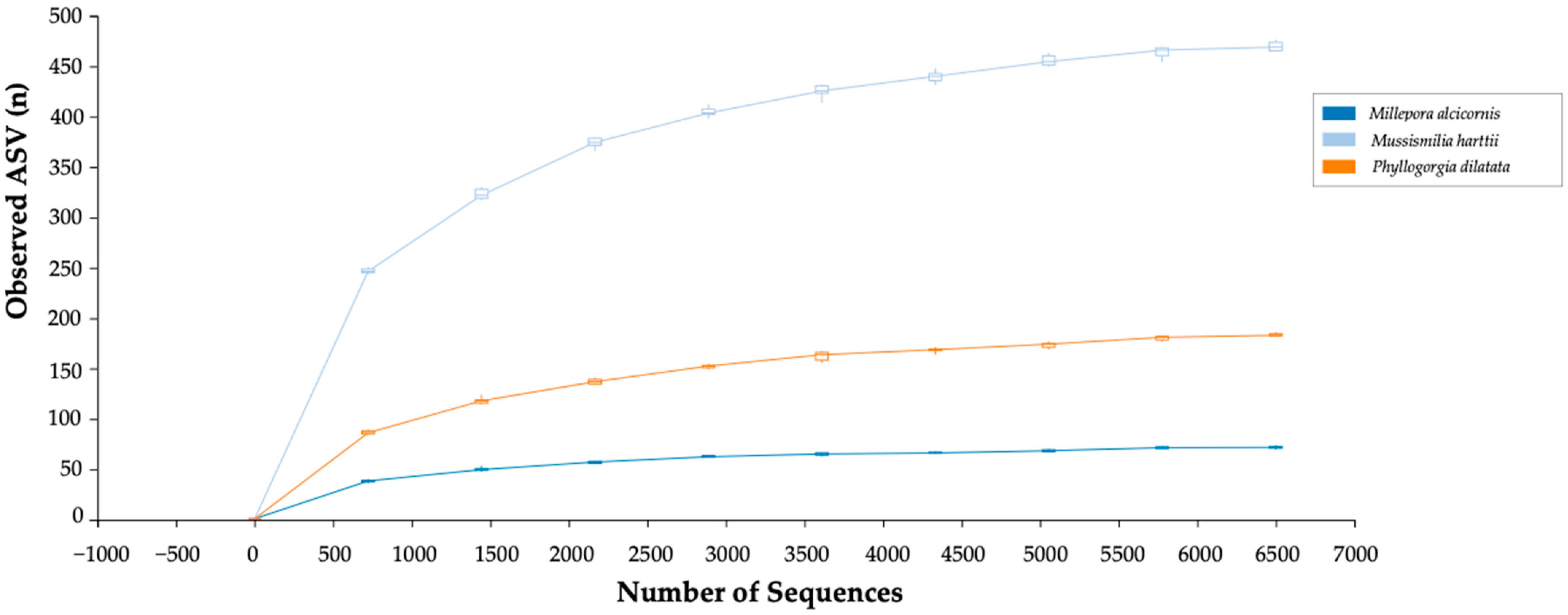
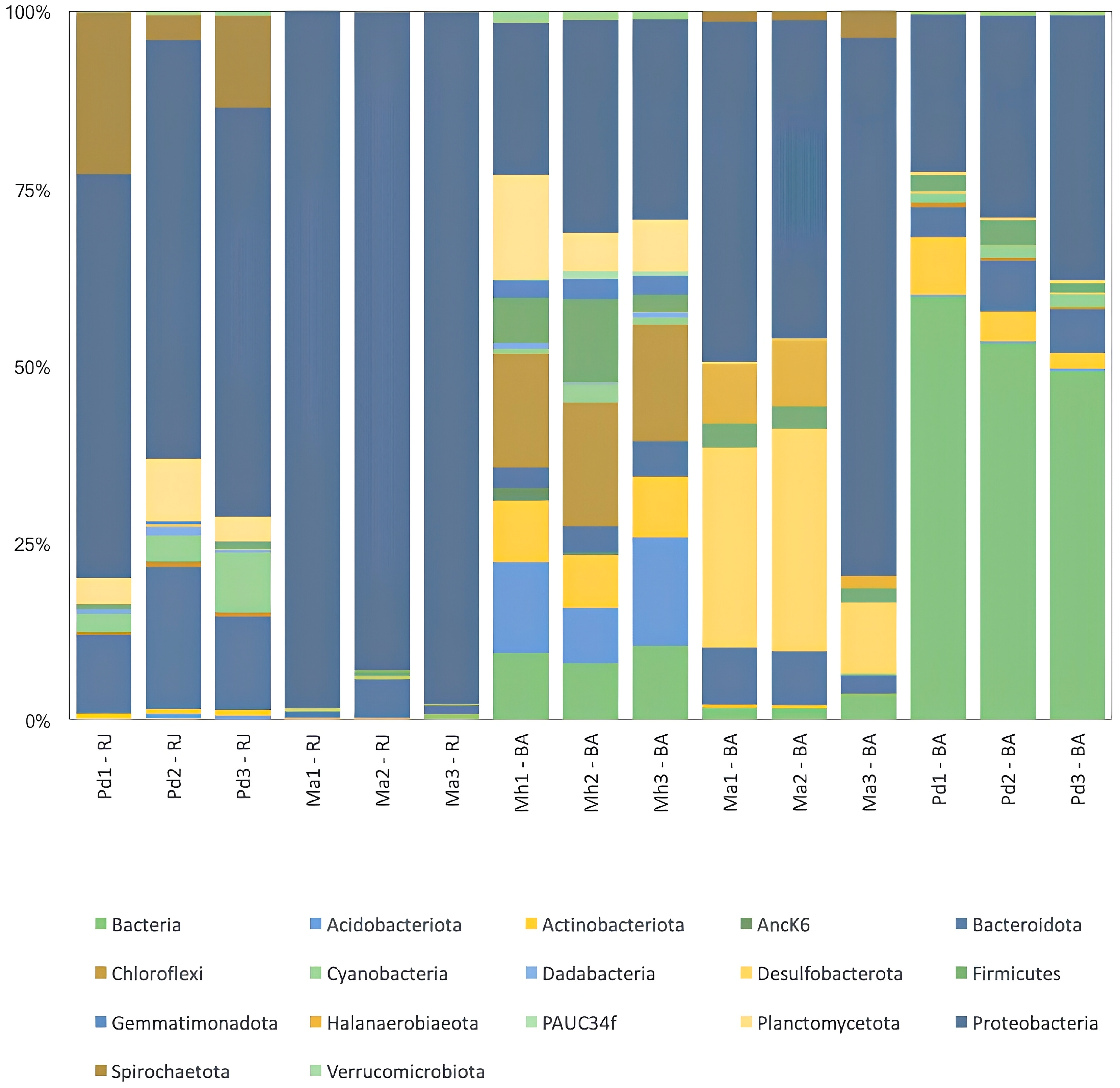

| Species | Total Num. of Reads | Total Num. Processed Reads | % Data Filtered |
|---|---|---|---|
| Phyllogorgia dilatata (Búzios) | 274,442 | 263,372 | 4.0 |
| Millepora alcicornis (Rio de Janeiro) | 24,475 | 226,541 | 7.4 |
| Phyllogorgia dilatata (Bahia) | 27,493 | 266,144 | 3.2 |
| Millepora alcicornis (Bahia) | 24,337 | 23,525 | 3.3 |
| Mussismilia harttii (Bahia) | 229,776 | 223,561 | 2.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade da Costa, R.; Wanna Figueiredo, M.; Fragoso dos Santos, H.; Bezerra Pinto, O.H.; Chaves Barreto, C.; Amorim de Alencar, S.; Campos Dias, S. Bacterial Diversity Associated with Millepora alcicornis, Phyllogorgia dilatata and Mussismilia harttii Collected from Two Distinct Corals Reefs on the Brazilian Coast. J. Mar. Sci. Eng. 2025, 13, 358. https://doi.org/10.3390/jmse13020358
Andrade da Costa R, Wanna Figueiredo M, Fragoso dos Santos H, Bezerra Pinto OH, Chaves Barreto C, Amorim de Alencar S, Campos Dias S. Bacterial Diversity Associated with Millepora alcicornis, Phyllogorgia dilatata and Mussismilia harttii Collected from Two Distinct Corals Reefs on the Brazilian Coast. Journal of Marine Science and Engineering. 2025; 13(2):358. https://doi.org/10.3390/jmse13020358
Chicago/Turabian StyleAndrade da Costa, Rosiane, Maria Wanna Figueiredo, Henrique Fragoso dos Santos, Otávio Henrique Bezerra Pinto, Cristine Chaves Barreto, Sérgio Amorim de Alencar, and Simoni Campos Dias. 2025. "Bacterial Diversity Associated with Millepora alcicornis, Phyllogorgia dilatata and Mussismilia harttii Collected from Two Distinct Corals Reefs on the Brazilian Coast" Journal of Marine Science and Engineering 13, no. 2: 358. https://doi.org/10.3390/jmse13020358
APA StyleAndrade da Costa, R., Wanna Figueiredo, M., Fragoso dos Santos, H., Bezerra Pinto, O. H., Chaves Barreto, C., Amorim de Alencar, S., & Campos Dias, S. (2025). Bacterial Diversity Associated with Millepora alcicornis, Phyllogorgia dilatata and Mussismilia harttii Collected from Two Distinct Corals Reefs on the Brazilian Coast. Journal of Marine Science and Engineering, 13(2), 358. https://doi.org/10.3390/jmse13020358










