Stable Isotopes Analysis of Bioremediating Organisms in an Innovative Integrated Multi-Trophic Aquaculture System
Abstract
1. Introduction
2. Materials and Methods
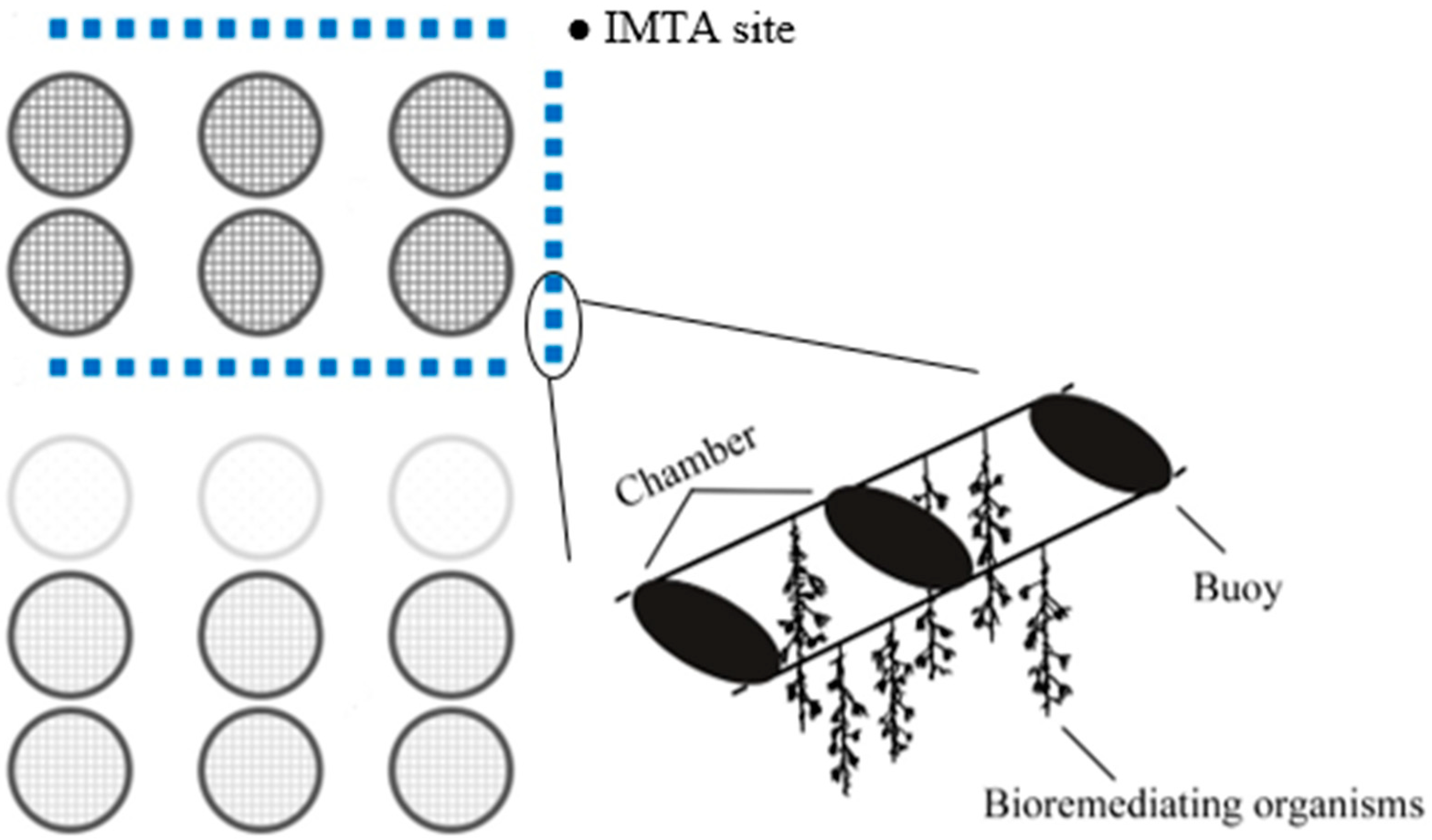
2.1. Study Area
2.2. Sample Collection
2.3. Stable Isotope Analyses
2.4. Statistical Analyses
3. Results
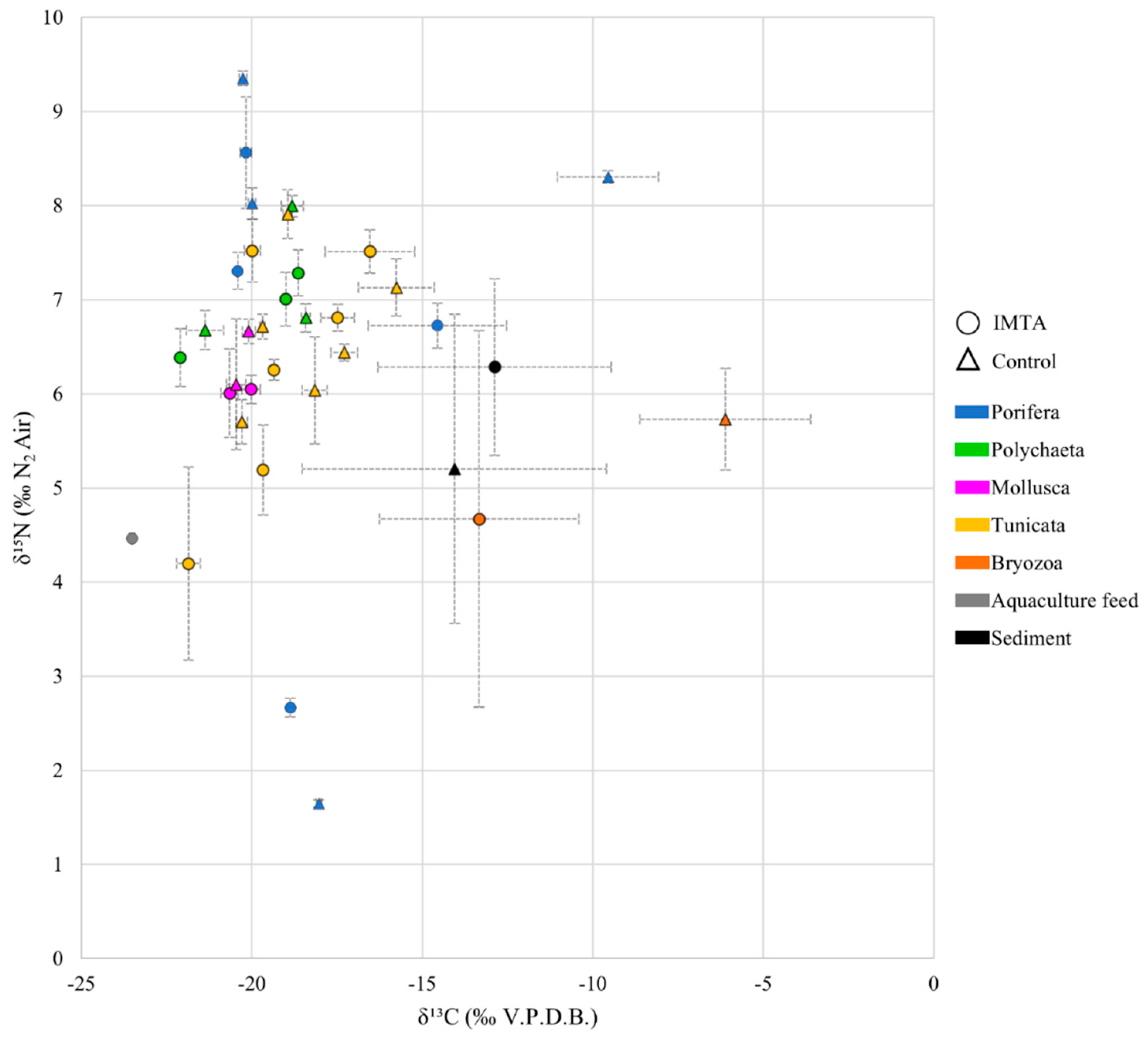
3.1. Porifera
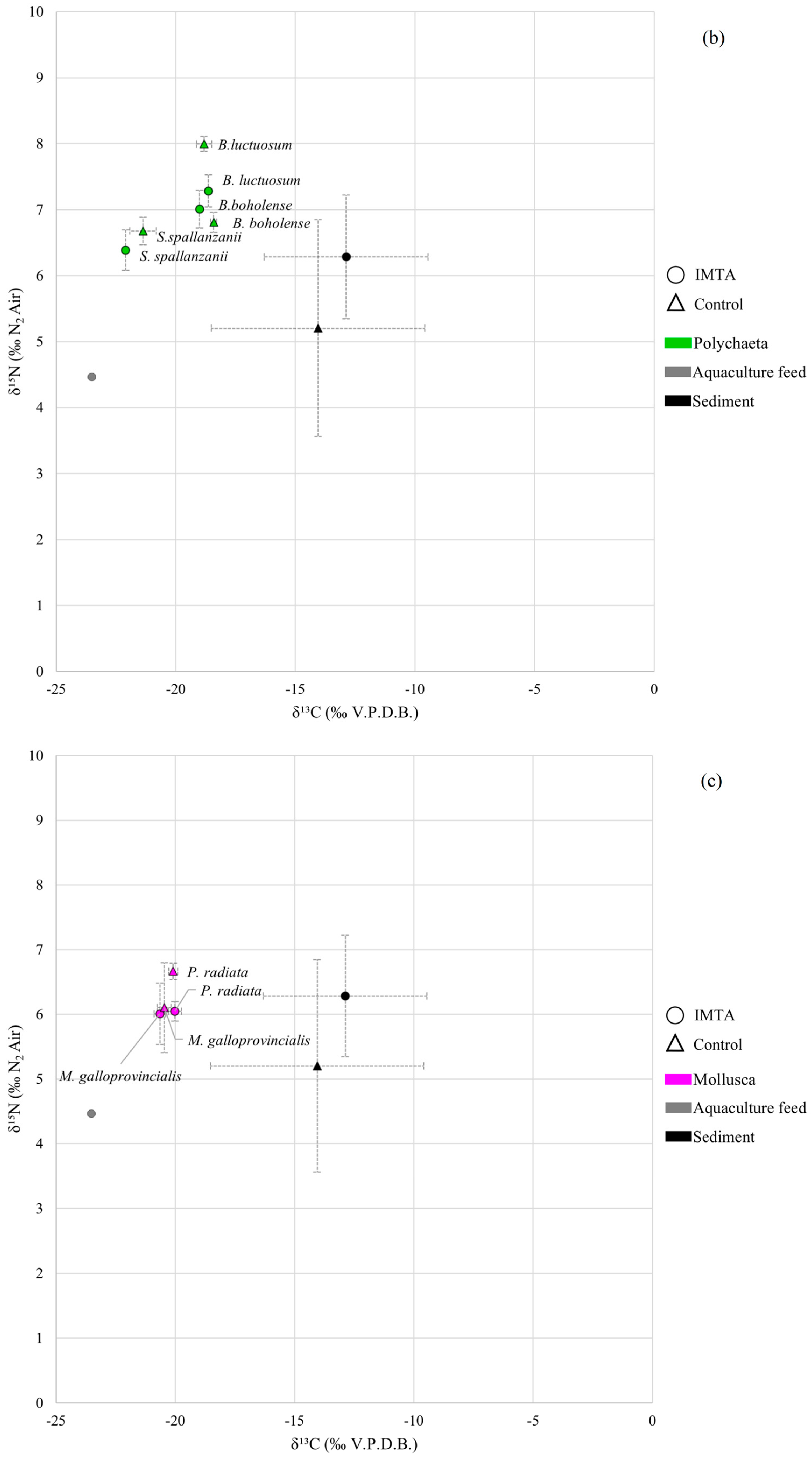
3.2. Polychaeta
3.3. Mollusca
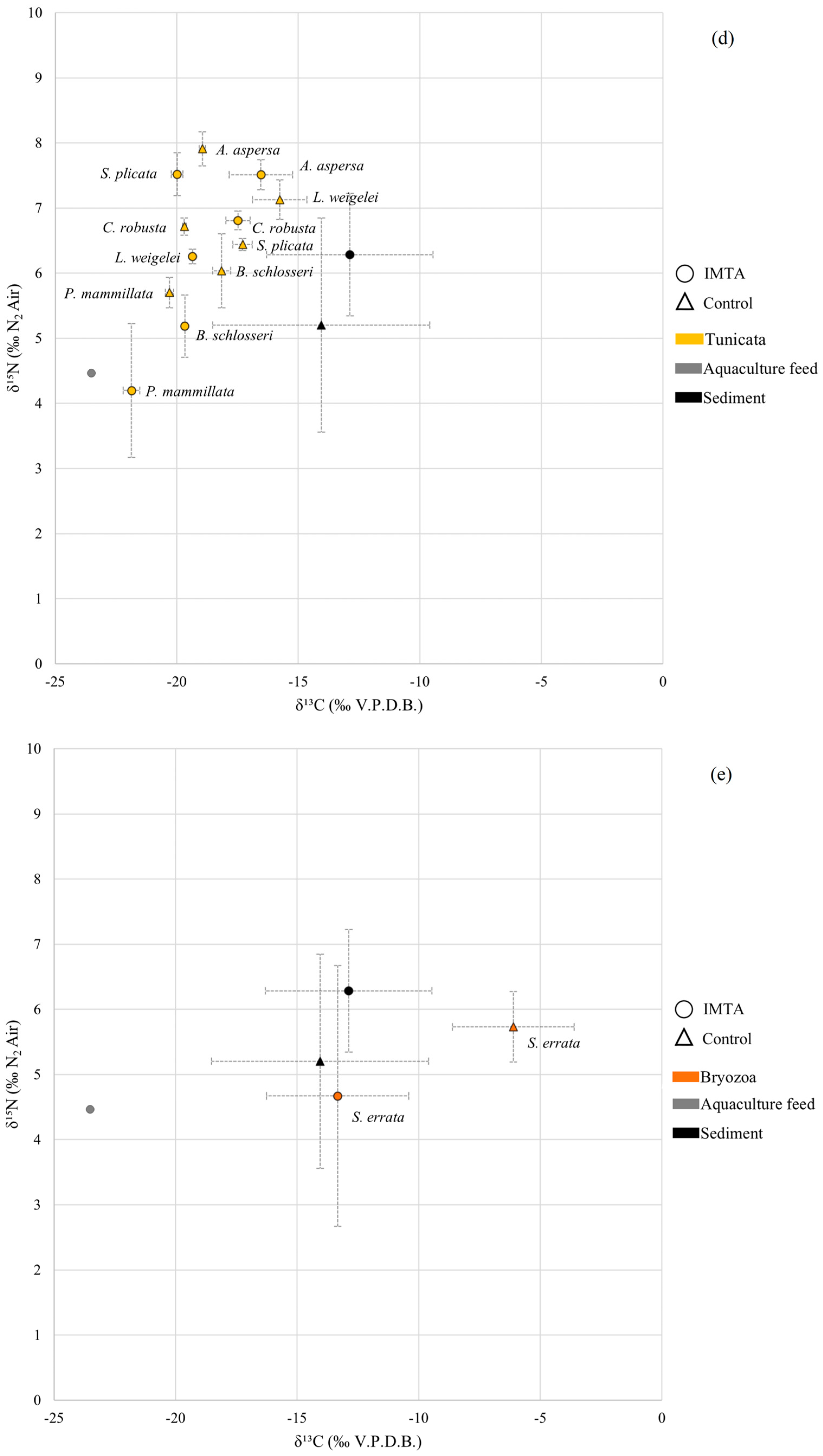
3.4. Tunicata
3.5. Bryozoa
3.6. Aquaculture Feed and Sediment Samples

4. Discussion
4.1. IMTA and Cnt Site Values
4.2. Porifera Analyses
4.3. Polychaeta Analyses
4.4. Mollusca Analyses
4.5. Tunicata Analyses
4.6. Bryozoa Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. Food and Agriculture Organization of the United Nations. The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation. Rome, FAO. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/a2090042-8cda-4f35-9881-16f6302ce757/content (accessed on 8 September 2023).
- Naylor, R.L.; Hardy, R.W.; Bureau, D.P.; Chiu, A.; Elliott, M.; Farrell, A.P.; Forster, I.; Gatlin, D.M.; Goldburg, R.J.; Hua, K.; et al. Feeding aquaculture in an era of finite resources. Proc. Natl. Acad. Sci. USA 2009, 106, 15103–15110. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Pasquet, A.; Aubin, J.; Nahon, S.; Lecocq, T. When more is more: Taking advantage of species diversity to move towards sustainable aquaculture. Biol. Rev. 2021, 96, 767–784. [Google Scholar] [CrossRef] [PubMed]
- Holmer, M.; Pérez, M.; Duarte, C.M. Benthic primary producers—A neglected environmental problem in Mediterranean maricultures? Mar. Pollut. Bull. 2003, 46, 1372–1376. [Google Scholar] [CrossRef] [PubMed]
- Tovar, A.; Moreno, C.; Mánuel-Vez, M.P.; García-Vargas, M. Environmental impacts of intensive aquaculture in marine waters. Water Res. 2000, 34, 334–342. [Google Scholar] [CrossRef]
- Soto, D. Integrated Mariculture: A Global Review, 1st ed.; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2009; 183p. [Google Scholar]
- Buck, B.H.; Troell, M.F.; Krause, G.; Angel, D.L.; Grote, B.; Chopin, T. State of the art and challenges for offshore integrated multi-trophic aquaculture (IMTA). Front. Mar. Sci. 2018, 5, 165. [Google Scholar] [CrossRef]
- Giangrande, A.; Gravina, M.F.; Rossi, S.; Longo, C.; Pierri, C. Aquaculture and restoration: Perspectives from mediterranean sea experiences. Water 2021, 13, 991. [Google Scholar] [CrossRef]
- Mahmood, T.; Fang, J.; Jiang, Z.; Zhang, J. Carbon and nitrogen flow, and trophic relationships, among the cultured species in an integrated multi-trophic aquaculture (IMTA) bay. Aquac. Environ. Interact. 2016, 8, 207–219. [Google Scholar] [CrossRef]
- Barrington, K.; Chopin, T.; Robinson, S. Integrated multi-trophic aquaculture (IMTA) in marine temperate waters. In FAO Fisheries and Aquaculture Technical Paper. No. 529; FAO: Rome, Italy, 2009; pp. 7–46. [Google Scholar]
- Chopin, T.; Buschmann, A.H.; Halling, C.; Troell, M.; Kautsky, N.; Neori, A.; Kraemer, G.P.; Zertuche-González, J.A.; Yarish, C.; Neefus, C. Minireview integrating seaweeds into marine aquaculture systems: A key toward sustainability. J. Phycol. 2001, 37, 975–986. [Google Scholar] [CrossRef]
- Martinez-Espiñeira, R.; Chopin, T.; Robinson, S.; Noce, A.; Knowler, D.; Yip, W. A contingent valuation of the biomitigation benefits of integrated multi-trophic aquaculture in Canada. Aquac. Econ. Manag. 2016, 20, 1–23. [Google Scholar] [CrossRef]
- Nahon, S.; De Brito, G.V.; Quental-Ferreira, H.; Aubin, J.; Jaeger, C.; Menniti, C.; Kerhervé, P.; Larroquet, L.; Cunha, M.E. Food web in Mediterranean coastal integrated multi-trophic aquaculture ponds: Learnings from fatty acids and stable isotope tracers. Aquaculture 2023, 567, 739292. [Google Scholar] [CrossRef]
- Fry, B.; Sherr, E.B. Δ13C Measurements as Indicators of Carbon Flow in Marine and Freshwater Ecosystems. In Stable Isotopes in Ecological Research; Rundel, P.W., Ehleringer, J.R., Nagy, K.A., Eds.; Springer: New York, NY, USA, 1989. [Google Scholar] [CrossRef]
- Post, D.M. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology 2002, 83, 703–718. [Google Scholar] [CrossRef]
- Deniro, M.J.; Epstein, S. Influence of diet on the distribution of nitrogen isotopes in animals. Geochim. Cosmochim. Acta 1981, 45, 341–351. [Google Scholar] [CrossRef]
- Smith, B.N.; Epstein, S. Two Categories of 13C/12C Ratios for Higher Plants. Plant Physiol. 1971, 47, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, S.D.; O’Donohue, M.J.; Dennison, W.C.; Loneragan, N.R.; Thomas, M. A New Approach for Detecting and Mapping Sewage Impacts. Mar. Pollut. Bull. 2001, 42, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.X.; Ritz, D.A.; Fenton, G.E.; Lewis, M.E. Tracing the influence on sediments of organic waste from a salmonid farm using stable isotope analysis. J. Exp. Mar. Biol. Ecol. 1991, 145, 161–174. [Google Scholar] [CrossRef]
- Vizzini, S.; Mazzola, A. Stable isotope evidence for the environmental impact of a land-based fish farm in the western Mediterranean. Mar. Pollut. Bull. 2004, 49, 61–70. [Google Scholar] [CrossRef]
- Yokoyama, H.; Abo, K.; Ishihi, Y. Quantifying aquaculture-derived organic matter in the sediment in and around a coastal fish farm using stable carbon and nitrogen isotope ratios. Aquaculture 2006, 254, 411–425. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, G.; Fang, J.; Mao, Y. Growth and food sources of Pacific oyster Crassostrea gigas integrated culture with Sea bass Lateolabrax japonicus in Ailian Bay, China. Aquac. Int. 2013, 21, 45–52. [Google Scholar] [CrossRef]
- Ning, Z.; Liu, S.; Zhang, G.; Ning, X.; Li, R.; Jiang, Z.; Fang, J.; Zhang, J. Impacts of an integrated multi-trophic aquaculture system on benthic nutrient fluxes: A case study in Sanggou Bay, China. Aquac. Environ. Interact. 2016, 8, 221–232. [Google Scholar] [CrossRef]
- Kerrigan, D.; Suckling, C.C. A meta-analysis of integrated multitrophic aquaculture: Extractive species growth is most successful within close proximity to open-water fish farms. Rev. Aquac. 2018, 10, 560–572. [Google Scholar] [CrossRef]
- Sarà, G.; Zenone, A.; Tomasello, A. Growth of Mytilus galloprovincialis (mollusca, bivalvia) close to fish farms: A case of integrated multi-trophic aquaculture within the Tyrrhenian Sea. Hydrobiologia 2009, 636, 129–136. [Google Scholar] [CrossRef]
- Chatzivasileiou, D.; Dimitriou, P.D.; Theodorou, J.; Kalantzi, I.; Magiopoulos, I.; Papageorgiou, N.; Pitta, P.; Tsapakis, M.; Karakassis, I. An IMTA in Greece: Co-culture of fish, bivalves, and holothurians. J. Mar. Sci. Eng. 2022, 10, 776. [Google Scholar] [CrossRef]
- Navarrete-Mier, F.; Sanz-Lázaro, C.; Marín, A. Does bivalve mollusc polyculture reduce marine fin fish farming environmental impact? Aquaculture 2010, 306, 101–107. [Google Scholar] [CrossRef]
- Sarà, G.; Scilipoti, D.; Milazzo, M.; Modica, A. Use of stable isotopes to investigate dispersal of waste from fish farms as a function of hydrodynamics. Mar. Ecol. Prog. Ser. 2006, 313, 261–270. [Google Scholar] [CrossRef]
- Giangrande, A.; Pierri, C.; Arduini, D.; Borghese, J.; Licciano, M.; Trani, R.; Corriero, G.; Basile, G.; Cecere, E.; Petrocelli, A.; et al. An innovative IMTA system: Polychaetes, sponges and macroalgae co-cultured in a Southern Italian inshore mariculture plant (Ionian Sea). J. Mar. Sci. Eng. 2020, 8, 733. [Google Scholar] [CrossRef]
- Gili, J.M.; Coma, R. Benthic suspension feeders: Their paramount role in littoral marine food webs. Trends Ecol. Evol. 1998, 13, 316–321. [Google Scholar] [CrossRef]
- Borghese, J.; Giangrande, A.; Arduini, D.; Trani, R.; Doria, L.; Anglano, M.; Rizzo, L.; Rossi, S. Influence of an innovative IMTA system (Mediterranean Sea, Italy) on environmental and biological parameters: Seasonal analysis. Aquaculture 2024, 741726. [Google Scholar] [CrossRef]
- Jacob, U.; Mintenbeck, K.; Brey, T.; Knust, R.; Beyer, K. Stable isotope food web studies: A case for standardized sample treatment. Mar. Ecol. Prog. Ser. 2005, 287, 251–253. [Google Scholar] [CrossRef]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26 1, 32–46. [Google Scholar]
- Anderson, M.J.; Braak, C.T. Permutation tests for multi-factorial analysis of variance. J. Stat. Comput. Simul. 2003, 73, 85–113. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. PRIMER v6: User Manual/Tutorial (Plymouth Routines in Multivariate Ecological Research). PRIMER-E, Plymouth. Available online: https://www.ap.smu.ca/~dclarke/home/documents/byDAC/mhd_primer.pdf (accessed on 6 November 2024).
- Anderson, M.J. Animal-sediment relationships re-visited: Characterising species’ distributions along an environmental gradient using canonical analysis and quantile regression splines. J. Exp. Mar. Biol. Ecol. 2008, 366 1, 16–27. [Google Scholar] [CrossRef]
- Dauby, P. The stable carbon isotope ratios in benthic food webs of the Gulf of Calvi, Corsica. Cont. Shelf Res. 1989, 9, 181–195. [Google Scholar] [CrossRef]
- Bongiorni, L.; Fiorentino, F.; Auriemma, R.; Aubry, F.B.; Camatti, E.; Camin, F.; Nasi, F.; Pansera, M.; Ziller, L.; Grall, J. Food web of a confined and anthropogenically affected coastal basin (the Mar Piccolo of Taranto) revealed by carbon and nitrogen stable isotopes analyses. Environ. Sci. Pollut. Res. 2016, 23, 12725–12738. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, S.; Raman, A.V.; Dauby, P.; Dehairs, F. Carbon and nitrogen stable isotope ratios of subtidal benthic invertebrates in an estuarine mangrove ecosystem (Andhra Pradesh, India). Estuar. Coast. Shelf Sci. 2002, 54, 901–913. [Google Scholar] [CrossRef]
- Rumolo, P.; Carannante, M.; Gherardi, S.; Tamburrino, S.; Vallefuoco, M.; Migliaccio, R.; D’Argenzio, C.; Barra, M. Measuring stable carbon and nitrogen isotopes in mytilus galloprovincialis to elucidate the sources of organic matter in three different nearshore marine environments. J. Coast. Res. 2017, 33, 1172–1181. [Google Scholar] [CrossRef]
- Schaal, G.; Riera, P.; Leroux, C. Trophic ecology in a Northern Brittany (Batz Island, France) kelp (Laminaria digitata) forest, as investigated through stable isotopes and chemical assays. J. Sea Res. 2010, 63, 24–35. [Google Scholar] [CrossRef]
- Cresson, P.; Ruitton, S.; Fontaine, M.F.; Harmelin-Vivien, M. Spatio-temporal variation of suspended and sedimentary organic matter quality in the Bay of Marseilles (NW Mediterranean) assessed by biochemical and isotopic analyses. Mar. Pollut. Bull. 2012, 64, 1112–1121. [Google Scholar] [CrossRef]
- Dubois, S.; Blanchet, H.; Garcia, A.; Massé, M.; Galois, R.; Grémare, A.; Charlier, K.; Guillou, G.; Richard, P.; Savoye, N. Trophic resource use by macrozoobenthic primary consumers within a semi-enclosed coastal ecosystem: Stable isotope and fatty acid assessment. J. Sea Res. 2014, 88, 87–99. [Google Scholar] [CrossRef]
- Filgueira, R.; Guyondet, T.; Reid, G.K.; Grant, J.; Cranford, P.J. Vertical particle fluxes dominate integrated multi-trophic aquaculture (IMTA) sites: Implications for shellfish-finfish synergy. Aquac. Environ. Interact. 2017, 9, 127–143. [Google Scholar] [CrossRef]
- Neofitou, N.; Klaoudatos, S. Effect of fish farming on the water column nutrient concentration in a semi-enclosed gulf of the Eastern Mediterranean. Aquac. Res. 2008, 39, 482–490. [Google Scholar] [CrossRef]
- Mohanty, B.P.; Mahanty, A.; Ganguly, S.; Mitra, T.; Karunakaran, D.; Anandan, R. Nutritional composition of food fishes and their importance in providing food and nutritional security. Food Chem. 2019, 293, 561–570. [Google Scholar] [CrossRef] [PubMed]
- De Nys, R.; Guenther, J. The impact and control of biofouling in marine finfish aquaculture. Adv. Mar. Antifouling Coat. Technol. 2009, 177–221. [Google Scholar] [CrossRef]
- Chopin, T.; Cooper, J.A.; Reid, G.; Cross, S.; Moore, C. Open-water integrated multi-trophic aquaculture: Environmental biomitigation and economic diversification of fed aquaculture by extractive aquaculture. Rev. Aquac. 2012, 4, 209–220. [Google Scholar] [CrossRef]
- Carlier, A.; Riera, P.; Amouroux, J.M.; Bodiou, J.Y.; Grémare, A. Benthic trophic network in the Bay of Banyuls-sur-Mer (northwest Mediterranean, France): An assessment based on stable carbon and nitrogen isotopes analysis. Estuar. Coast. Shelf Sci. 2007, 72, 1–15. [Google Scholar] [CrossRef]
- Woodcock, S.H.; Strohmeier, T.; Strand, Ø.; Olsen, S.A.; Bannister, R.J. Mobile epibenthic fauna consume organic waste from coastal fin-fish aquaculture. Mar. Environ. Res. 2018, 137, 16–23. [Google Scholar] [CrossRef]
- Colombo, S.M.; Parrish, C.C.; Whiticar, M.J. Fatty acid stable isotope signatures of molluscs exposed to finfish farming outputs. Aquac. Environ. Interact. 2016, 8, 611–617. [Google Scholar] [CrossRef]
- Troell, M.; Joyce, A.; Chopin, T.; Neori, A.; Buschmann, A.H.; Fang, J.G. Ecological engineering in aquaculture—Potential for integrated multi-trophic aquaculture (IMTA) in marine offshore systems. Aquaculture 2009, 297, 1–9. [Google Scholar] [CrossRef]
- Elias-Piera, F.; Rossi, S.; Gili, J.M.; Orejas, C. Trophic ecology of seven Antarctic gorgonian species. Mar. Ecol. Prog. Ser. 2013, 477, 93–106. [Google Scholar] [CrossRef]
- Vinha, B.; Rossi, S.; Gori, A.; Hanz, U.; Pennetta, A.; De Benedetto, G.E.; Mienis, F.; Huvenne, V.; Hebbeln, D.; Wienberg, C.; et al. Trophic ecology of Angolan cold-water coral reefs (SE Atlantic) based on stable isotope analyses. Sci. Rep. 2023, 13, 9933. [Google Scholar] [CrossRef]
- Weldrick, C.K.; Jelinski, D.E. Seasonal dynamics in a nearshore isotopic niche and spatial subsidies from multi-trophic aquaculture. Can. J. Fish. Aquat. Sci. 2017, 74, 1411–1421. [Google Scholar] [CrossRef]
- Aguilo-Arce, J.; Ferriol, P.; Trani, R.; Puthod, P.; Pierri, C.; Longo, C. Sponges as emerging by-product of integrated multitrophic aquaculture (IMTA). J. Mar. Sci. Eng. 2023, 11, 80. [Google Scholar] [CrossRef]
- Van Duyl, F.C.; Moodley, L.; Nieuwland, G.; Van Ljzerloo, L.; Van Soest, R.W.; Houtekamer, M.; Meesters, E.H.; Middelburg, J.J. Coral cavity sponges depend on reef-derived food resources: Stable isotope and fatty acid constraints. Mar. Biol. 2011, 158, 1653–1666. [Google Scholar] [CrossRef] [PubMed]
- Freeman, C.J.; Easson, C.G.; Baker, D.M. Metabolic diversity and niche structure in sponges from the Miskito Cays, Honduras. PeerJ 2014, 2, e695. [Google Scholar] [CrossRef] [PubMed]
- Thacker, R.W.; Freeman, C.J. Sponge–microbe symbioses: Recent advances and new directions. Adv. Mar. Biol. 2012, 62, 57–111. [Google Scholar] [CrossRef] [PubMed]
- Fiore, C.L.; Jarett, J.K.; Olson, N.D.; Lesser, M.P. Nitrogen fixation and nitrogen transformations in marine symbioses. Trends Microbiol. 2010, 18, 455–463. [Google Scholar] [CrossRef]
- Hardoim, C.C.P.; Costa, R. Temporal dynamics of prokaryotic communities in the marine sponge Sarcotragus spinosulus. Mol. Ecol. 2014, 23, 3097–3112. [Google Scholar] [CrossRef]
- Ribes, M.; Yahel, G.; Romera-Castillo, C.; Mallenco, R.; Morganti, T.M.; Coma, R. The removal of dissolved organic matter by marine sponges is a function of its composition and concentration: An in situ seasonal study of four Mediterranean species. Sci. Total Environ. 2023, 871, 161991. [Google Scholar] [CrossRef]
- Longo, C.; Pontassuglia, C.; Corriero, G.; Gaino, E. Life-cycle traits of Paraleucilla magna, a calcareous sponge invasive in a coastal Mediterranean basin. PLoS ONE 2012, 7, e42392. [Google Scholar] [CrossRef]
- Van Duyl, F.C.; Mueller, B.; Meesters, E.H. Spatio–temporal variation in stable isotope signatures (δ13C and δ15N) of sponges on the Saba Bank. PeerJ 2018, 6, e5460. [Google Scholar] [CrossRef]
- Topçu, N.E.; Pérez, T.; Grégori, G.; Harmelin-Vivien, M. In situ investigation of Spongia officinalis (Demospongiae) particle feeding coupling flow cytometry and stable isotope analysis. J. Exp. Mar. Biol. Ecol. 2010, 389, 61–69. [Google Scholar] [CrossRef]
- De Goeij, J.M.; Moodley, L.; Houtekamer, M.; Carballeira, N.M.; Van Duyl, F.C. Tracing 13C-enriched dissolved and particulate organic carbon in the bacteria-containing coral reef sponge Halisarca caerulea: Evidence for DOM-feeding. Limnol. Oceanogr. 2008, 53, 1376–1386. [Google Scholar] [CrossRef]
- De Goeij, J.M.; Van Den Berg, H.; Van Oostveen, M.M.; Epping, E.H.; Van Duyl, F.C. Major bulk dissolved organic carbon (DOC) removal by encrusting coral reef cavity sponges. Mar. Ecol. Prog. Ser. 2008, 357, 139–151. [Google Scholar] [CrossRef]
- Stabili, L.; Licciano, M.; Giangrande, A.; Caroppo, C. Filtration of the Microalga Amphidinium carterae by the Polychaetes Sabella spallanzanii and Branchiomma luctuosum: A New Tool for the Control of Harmful Algal Blooms? Microorganisms 2022, 10, 156. [Google Scholar] [CrossRef] [PubMed]
- Licciano, M.; Stabili, L.; Giangrande, A. Clearance rates of Sabella spallanzanii and Branchiomma luctuosum (Annelida: Polychaeta) on a pure culture of Vibrio alginolyticus. Water Res. 2005, 39, 4375–4384. [Google Scholar] [CrossRef] [PubMed]
- Licciano, M.; Stabili, L.; Giangrande, A.; Cavallo, R.A. Bacterial accumulation by Branchiomma luctuosum (Annelida: Polychaeta): A tool for biomonitoring marine systems and restoring polluted waters. Mar. Environ. Res. 2007, 63, 291–302. [Google Scholar] [CrossRef]
- Giangrande, A.; Cavallo, A.; Licciano, M.; Mola, E.; Pierri, C.; Trianni, L. Utilization of the filter feeder polychaete Sabella. Aquac. Int. 2005, 13, 129–136. [Google Scholar] [CrossRef]
- Stabili, L.; Licciano, M.; Giangrande, A.; Fanelli, G.; Cavallo, R.A. Sabella spallanzanii filter-feeding on bacterial community: Ecological implications and applications. Mar. Environ. Res. 2006, 61, 74–92. [Google Scholar] [CrossRef]
- Clapin, G. The Filtration Rate, Oxygen Consumption and Biomass of the Introduced Polychaete Sabella Spallanzanii Gmelin Within Cockburn Sound: Can It Control Phytoplankton Levels and Is It an Efficient Filter Feeder? Bachelor’s Thesis of Science Honours, Edith Cowan University, Perth and Bunbury, Australia, 1996. [Google Scholar]
- Borghese, J.; Giangrande, A.; Arduini, D.; Trani, R.; Doria, L.; Anglano, M.; Aguilo-Arce, J.; Toso, A.; Putignano, M.; Rizzo, L.; et al. Exploring the potential effects of IMTA on water column seston through intensive short-time cycles approach. Mar. Pollut. Bull. 2024; submitted. [Google Scholar]
- Fraissinet, S.; Arduini, D.; Martines, A.; De Benedetto, G.E.; Malitesta, C.; Giangrande, A.; Rossi, S. Seasonal occurrence and distribution of microplastics in four different benthic suspension feeders from an Integrated Multi-Trophic Aquaculture (IMTA) facility: A bioremediation perspective. Mar. Pollut. Bull. 2024, 207, 116811. [Google Scholar] [CrossRef]
- Stabili, L.; Giangrande, A.; Arduini, D.; Borghese, J.; Petrocelli, A.; Alabiso, G.; Ricci, P.; Cavallo, R.A.; Acquaviva, M.I.; Narracci, M.; et al. Environmental quality improvement of a mariculture plant after its conversion into a multi-trophic system. Sci. Total Environ. 2023, 884, 163846. [Google Scholar] [CrossRef]
- Jones, T.O.; Iwama, G.K. Polyculture of the Pacific oyster, Crassostrea gigas (Thunberg), with chinook salmon, Oncorhynchus tshawytscha. Aquaculture 1991, 92, 313–322. [Google Scholar] [CrossRef]
- Wallace, J.C. Growth rates of different populations of the edible mussel, Mytilus edulis, in north Norway. Aquaculture 1980, 19, 303–311. [Google Scholar] [CrossRef]
- Cheshuk, B.W.; Purser, G.J.; Quintana, R. Integrated open-water mussel (Mytilus planulatus) and Atlantic salmon (Salmo salar) culture in Tasmania, Australia. Aquaculture 2003, 218, 357–378. [Google Scholar] [CrossRef]
- Peharda, M.; Župan, I.; Bavčević, L.; Frankić, A.; Klanjšček, T. Growth and condition index of mussel Mytilus galloprovincialis in experimental integrated aquaculture. Aquac. Res. 2007, 38, 1714–1720. [Google Scholar] [CrossRef]
- Arduini, D.; Portacci, G.; Giangrande, A.; Acquaviva, M.I.; Borghese, J.; Calabrese, C.; Giandomenico, S.; Quarta, E.; Stabili, L. Growth Performance of Mytilus galloprovincialis Lamarck, 1819 under an Innovative Integrated Multi-Trophic Aquaculture System (IMTA) in the Mar Grande of Taranto (Mediterranean Sea, Italy). Water 2023, 15, 1922. [Google Scholar] [CrossRef]
- Irisarri, J.; Cubillo, A.M.; Fernández-Reiriz, M.J.; Labarta, U. Growth variations within a farm of mussel (Mytilus galloprovincialis) held near fish cages: Importance for the implementation of integrated aquaculture. Aquac. Res. 2015, 46, 1988–2002. [Google Scholar] [CrossRef]
- Handå, A.; Min, H.; Wang, X.; Broch, O.J.; Reitan, K.I.; Reinertsen, H.; Olsen, Y. Incorporation of fish feed and growth of blue mussels (Mytilus edulis) in close proximity to salmon (Salmo salar) aquaculture: Implications for integrated multi-trophic aquaculture in Norwegian coastal waters. Aquaculture 2012, 356–357, 328–341. [Google Scholar] [CrossRef]
- Sanz-Lazaro, C.; Sanchez-Jerez, P. Mussels do not directly assimilate fish farm wastes: Shifting the rationale of integrated multi-trophic aquaculture to a broader scale. J. Environ. Manag. 2017, 201, 82–88. [Google Scholar] [CrossRef]
- Tatian, M.; Sahade, R.; Esnal, G.B. Diet components in the food of Antarctic ascidians living at low levels of primary production. Antarct. Sci. 2004, 16, 123–128. [Google Scholar] [CrossRef]
- Ribes, M.; Coma, R.; Gili, J.M. Seasonal variation of in situ feeding rates by the temperate ascidian Halocynthia papillosa. Mar. Ecol. Prog. Ser. 1998, 175, 201–213. [Google Scholar] [CrossRef]
- Holley, M.C. Cell shape, spatial patterns of cilia, and mucus-net construction in the ascidian endostyle. Tissue Cell 1986, 18, 667–684. [Google Scholar] [CrossRef]
- Rinkevich, B.; Shapira, M. An improved diet for inland broodstock and the establishment of an inbred line from Botryllus schlosseri, a colonial sea squirt (Ascidiacea). Aquat. Living Resour. 1998, 11, 163–171. [Google Scholar] [CrossRef]
- Kosich, D.F.; Cuffey, R.J. Recognition of multilaminar cheilostome bryozoan species in modern Bermuda reef rock. Geol. Soc. Am. Abstr. Programs 1978, 10, 259. [Google Scholar]
- Scholz, J.; Hillmer, G. Reef-bryozoans and bryozoan-microreefs: Control factor evidence from the philippines and other regions. Facies 1995, 32, 109–143. [Google Scholar] [CrossRef]
- Taylor, P.D.; Allison, P.A. Bryozoan carbonates through time and space. Geology 1998, 26, 459–462. [Google Scholar] [CrossRef]
- Pouyet, S. Schizoporella violacea (Canu et Bassler, 1930) (Bryozoa cheilostomata): Variations et croissance zoariale. Geobios 1971, 4, 185–197. [Google Scholar] [CrossRef]
- Jaschinski, S.; Hansen, T.; Sommer, U. Effects of acidification in multiple stable isotope analyses. Limnol. Oceanogr. Methods 2008, 6, 12–15. [Google Scholar] [CrossRef]
 : Mar Grande Mariculture head office; ●: IMTA site; ▲: control site.
: Mar Grande Mariculture head office; ●: IMTA site; ▲: control site.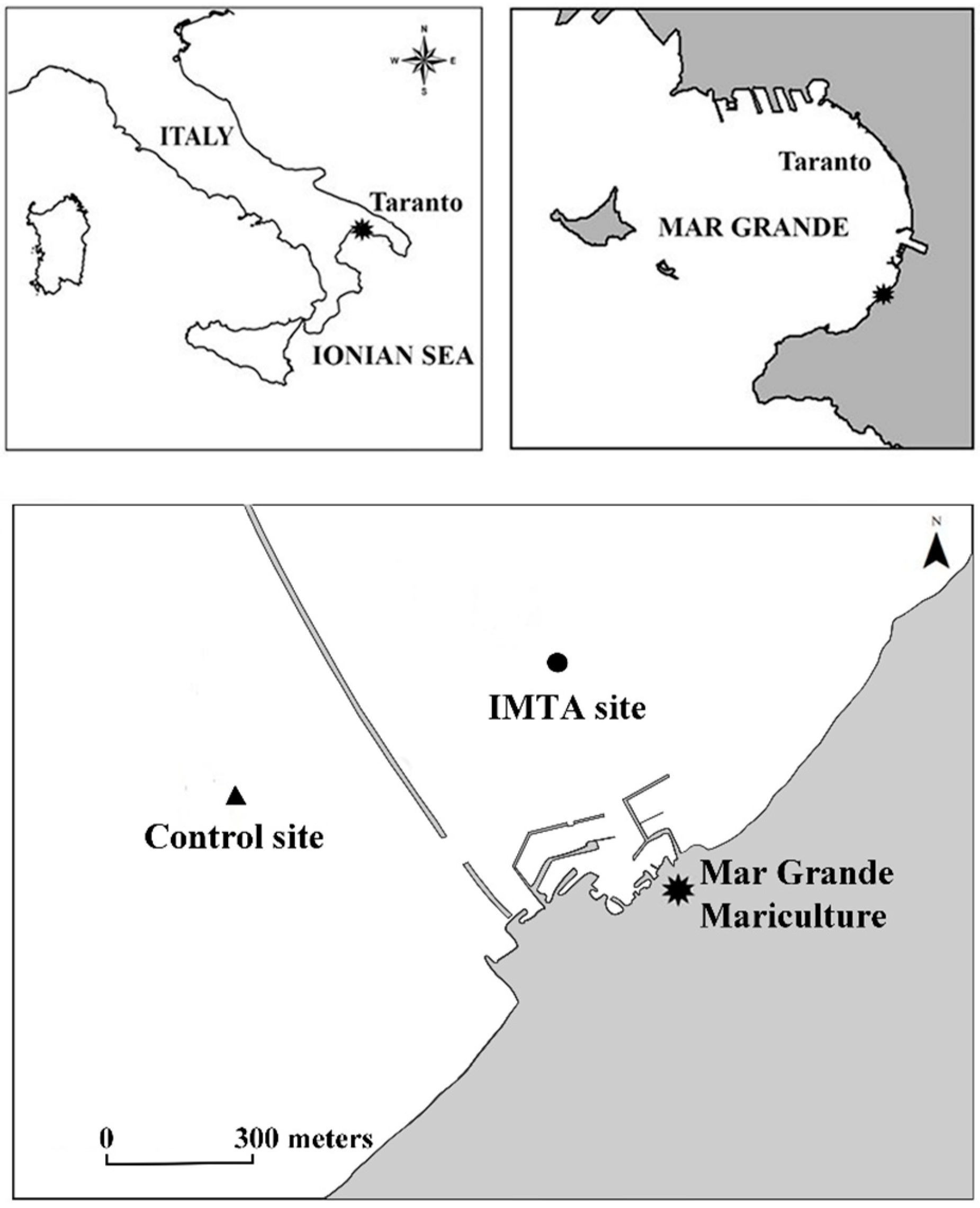
| Taxa | Sampled Species | Variables | Authors | Literature Values | IMTA | Control |
|---|---|---|---|---|---|---|
| Paraleucilla magna Klautau, Monteiro and Borojevic, 2004 | δ15N (±s.d.) (‰) | 6.72 ± 0.23 | 8.30 ± 0.10 | |||
| Porifera | δ13C (±s.d.) (‰) | −14.56 ± 2.02 | −9.55 ± 1.48 | |||
| C:N (±s.d.) | 7.45 ± 0.23 | 12.02 ± 1.63 | ||||
| Sarcotragus spinosulus Schmidt, 1862 | δ15N (±s.d.) (‰) | 2.66 ± 0.10 | 1.65 ± 0.03 | |||
| δ13C (±s.d.) (‰) | −18.87 ± 0.04 | −18.04 ± 0.07 | ||||
| C:N (±s.d.) | 3.34 ± 0.02 | 3.43 ± 0.04 | ||||
| Dysidea avara (Schmidt, 1862) | δ15N (±s.d.) (‰) | 7.30 ± 0.19 | 8.02 ± 0.16 | |||
| δ13C (±s.d.) (‰) | −20.41 ± 0.01 | −19.99 ± 0.10 | ||||
| C:N (±s.d.) | 4.64 ± 0.11 | 4.48 ± 0.07 | ||||
| Haliclona (Reniera) mediterranea Griessinger, 1971 | δ15N (±s.d.) (‰) | 8.56 ± 0.59 | 9.36 ± 0.07 | |||
| δ13C (±s.d.) (‰) | [37] | −22.50 | −20.18 ± 0.16 | −20.24 ± 0.11 | ||
| C:N (±s.d.) | 4.54 ± 0.18 | 4.48 ± 0.14 | ||||
| Branchiomma luctuosum (Grube, 1870) | δ15N (±s.d.) (‰) | 7.28 ± 0.24 | 8.00 ± 0.11 | |||
| Polychaeta | δ13C (±s.d.) (‰) | −18.64 ± 0.04 | −18.82 ± 0.32 | |||
| C:N (±s.d.) | 3.44 ± 0.05 | 3.82 ± 0.28 | ||||
| Branchiomma boholense (Grube, 1878) | δ15N (±s.d.) (‰) | 7.01 ± 0.28 | 6.81 ± 0.15 | |||
| δ13C (±s.d.) (‰) | −19.01 ± 0.06 | −18.42 ± 0.12 | ||||
| C:N (±s.d.) | 3.87 ± 0.07 | 3.75 ± 0.05 | ||||
| Sabella spallanzanii (Gmelin, 1791) | δ15N (±s.d.) (‰) | [38] | 8.85 ± 0.48 | 6.38 ± 0.30 | 6.68 ± 0.20 | |
| δ13C (±s.d.) (‰) | [38] | −21.26 ± 0.91 | −22.10 ± 0.06 | −21.37 ± 0.55 | ||
| C:N (±s.d.) | 5.72 ± 0.06 | 5.60 ± 0.40 | ||||
| Pinctada radiata (Leach, 1814) | δ15N (±s.d.) (‰) | [39] | 11.60 | 6.05 ± 0.14 | 6.66 ± 0.12 | |
| Mollusca | δ13C (±s.d.) (‰) | [39] | −17.40 | −20.02 ± 0.27 | −20.09 ± 0.19 | |
| C:N (±s.d.) | 2.80 ± 0.07 | 3.97 ± 0.34 | ||||
| Mytilus galloprovincialis Lamarck, 1819 | δ15N (±s.d.) (‰) | [38] | 6.55 ± 1.20 | 6.01 ± 0.47 | 6.10 ± 0.69 | |
| δ13C (±s.d.) (‰) | [38] | −22.61 ± 0.57 | −20.65 ± 0.24 | −20.46 ± 0.29 | ||
| C:N (±s.d.) | [40] | 4.31 to 6.01 | 5.55 ± 0.03 | 4.91 ± 0.38 | ||
| Botryllus schlosseri (Pallas, 1766) | δ15N (±s.d.) (‰) | [41] | 6.40 to 7.30 | 5.19 ± 0.47 | 6.04 ± 0.56 | |
| Tunicata | δ13C (±s.d.) (‰) | [41] | −18.30 to −18.70 | −19.67 ± 0.08 | −18.15 ± 0.36 | |
| C:N (±s.d.) | 3.53 ± 0.13 | 3.96 ± 0.08 | ||||
| Lissoclinum weigelei Lafargue, 1968 | δ15N (±s.d.) (‰) | 6.25 ± 0.11 | 7.13 ± 0.30 | |||
| δ13C (±s.d.) (‰) | −19.35 ± 0.07 | −15.76 ± 1.11 | ||||
| C:N (±s.d.) | 4.37 ± 0.08 | 3.99 ± 1.40 | ||||
| Ciona robusta Hoshino and Tokioka, 1967 | δ15N (±s.d.) (‰) | 6.81 ± 0.14 | 6.71 ± 0.13 | |||
| δ13C (±s.d.) (‰) | −17.48 ± 0.49 | −19.69 ± 0.07 | ||||
| C:N (±s.d.) | 5.54 ± 0.18 | 4.90 ± 0.04 | ||||
| Ascidiella aspersa (Müller, 1776) | δ15N (±s.d.) (‰) | [38] | 7.30 | 7.51 ± 0.23 | 7.91 ± 0.25 | |
| δ13C (±s.d.) (‰) | [38] | −20.86 | −16.54 ± 1.30 | −18.95 ± 0.13 | ||
| C:N (±s.d.) | 7.92 ± 1.22 | 6.90 ± 0.48 | ||||
| Phallusia mammillata (Cuvier, 1815) | δ15N (±s.d.) (‰) | 4.20 ± 1.02 | 5.70 ± 0.23 | |||
| δ13C (±s.d.) (‰) | [37] | −23.60 | −21.86 ± 0.34 | −20.30 ± 0.16 | ||
| C:N (±s.d.) | 7.86 ± 0.96 | 6.03 ± 0.28 | ||||
| Styela plicata (Lesueur, 1823) | δ15N (±s.d.) (‰) | [38] | 6.90 | 7.52 ± 0.33 | 6.44 ± 0.09 | |
| δ13C (±s.d.) (‰) | [38] | −22.23 | −19.99 ± 0.23 | −17.29 ± 0.39 | ||
| C:N (±s.d.) | 5.71 ± 0.75 | 6.99 ± 0.75 | ||||
| Bryozoa | Schizoporella errata (Waters, 1878) | δ15N (±s.d.) (‰) | [41] | 6.70 ± 0.23 | 4.67 ± 2.01 | 5.87 ± 0.54 |
| δ13C (±s.d.) (‰) | [41] | −16.50 | −13.33 ± 2.92 | −6.11 ± 2.50 | ||
| C:N (±s.d.) | 25.02 ± 22.96 | 53.69 ± 60.76 |
| Source | df | MS | Pseudo-F | P(perm) |
|---|---|---|---|---|
| δ15N (‰) | ||||
| Source | df | MS | Pseudo-F | P(perm) |
| Aq | 1 | 4.72 | 19.36 | 0.0002 |
| Sp | 15 | 14.26 | 58.46 | 0.0001 |
| AqxSp | 15 | 0.88 | 3.59 | 0.0003 |
| Res | 64 | 0.24 | ||
| Total | 95 | |||
| δ13C (‰) | ||||
| Aq | 1 | 35.22 | 44.07 | 0.0001 |
| Sp | 15 | 59.75 | 74.77 | 0.0001 |
| AqxSp | 15 | 9.11 | 11.40 | 0.0001 |
| Res | 64 | 0.80 | ||
| Total | 95 | |||
| C:N ratio | ||||
| Aq | 1 | 199.26 | 2.12 | 0.1628 |
| Sp | 15 | 661.53 | 7.02 | 0.0005 |
| AqxSp | 15 | 176.01 | 1.87 | 0.0265 |
| Res | 64 | 94.18 | ||
| Total | 95 | |||
| δ15N | δ13C | C:N | ||||
|---|---|---|---|---|---|---|
| t | P(MC) | t | P(MC) | t | P(MC) | |
| P. magna | ||||||
| IMTA vs. Cnt | 11.08 | 0.001 | 3.45 | 0.0256 | 4.79 | 0.0101 |
| S. spinosulus | ||||||
| IMTA vs. Cnt | 16.41 | 0.0001 | 15.70 | 0.0002 | 2.90 | 0.0442 |
| D. avara | ||||||
| IMTA vs. Cnt | 4.85 | 0.008 | 6.61 | 0.0032 | 1.95 | 0.123 |
| H. mediterranea | ||||||
| IMTA vs. Cnt | 2.30 | 0.085 | 0.81 | 0.4633 | 0.71 | 0.5066 |
| B. luctuosum | ||||||
| IMTA vs. Cnt | 4.59 | 0.0107 | 0.96 | 0.3904 | 2.28 | 0.0907 |
| B. boholense | ||||||
| IMTA vs. Cnt | 1.06 | 0.3447 | 7.15 | 0.0021 | 2.00 | 0.1194 |
| S. spallanzanii | ||||||
| IMTA vs. Cnt | 1.36 | 0.2449 | 2.26 | 0.0876 | 0.49 | 0.6373 |
| P. radiata | ||||||
| IMTA vs. Cnt | 5.41 | 0.0052 | 0.39 | 0.7164 | 5.69 | 0.0035 |
| M. galloprovincialis | ||||||
| IMTA vs. Cnt | 0.19 | 0.8562 | 0.84 | 0.4441 | 2.84 | 0.0457 |
| B. leachii | ||||||
| IMTA vs. Cnt | 1.98 | 0.1154 | 6.99 | 0.0015 | 4.79 | 0.0088 |
| L. weigelei | ||||||
| IMTA vs. Cnt | 4.66 | 0.009 | 5.58 | 0.0056 | 0.46 | 0.669 |
| C. robusta | ||||||
| IMTA vs. Cnt | 0.83 | 0.4526 | 7.69 | 0.0019 | 5.94 | 0.0039 |
| A. aspersa | ||||||
| IMTA vs. Cnt | 1.97 | 0.1188 | 3.18 | 0.0286 | 1.35 | 0.2536 |
| P. mammillata | ||||||
| IMTA vs. Cnt | 2.47 | 0.0625 | 7.11 | 0.0015 | 3.12 | 0.0374 |
| S. plicata | ||||||
| IMTA vs. Cnt | 5.44 | 0.0041 | 10.11 | 0.0005 | 2.07 | 0.1088 |
| S. errata | ||||||
| IMTA vs. Cnt | 0.90 | 0.4241 | 3.25 | 0.0324 | 1.36 | 0.245 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borghese, J.; Giangrande, A.; Arduini, D.; Doria, L.; Longo, C.; Rizzo, L.; Pennetta, A.; De Benedetto, G.E.; Rossi, S. Stable Isotopes Analysis of Bioremediating Organisms in an Innovative Integrated Multi-Trophic Aquaculture System. J. Mar. Sci. Eng. 2024, 12, 2286. https://doi.org/10.3390/jmse12122286
Borghese J, Giangrande A, Arduini D, Doria L, Longo C, Rizzo L, Pennetta A, De Benedetto GE, Rossi S. Stable Isotopes Analysis of Bioremediating Organisms in an Innovative Integrated Multi-Trophic Aquaculture System. Journal of Marine Science and Engineering. 2024; 12(12):2286. https://doi.org/10.3390/jmse12122286
Chicago/Turabian StyleBorghese, Jacopo, Adriana Giangrande, Daniele Arduini, Lorenzo Doria, Caterina Longo, Lucia Rizzo, Antonio Pennetta, Giuseppe E. De Benedetto, and Sergio Rossi. 2024. "Stable Isotopes Analysis of Bioremediating Organisms in an Innovative Integrated Multi-Trophic Aquaculture System" Journal of Marine Science and Engineering 12, no. 12: 2286. https://doi.org/10.3390/jmse12122286
APA StyleBorghese, J., Giangrande, A., Arduini, D., Doria, L., Longo, C., Rizzo, L., Pennetta, A., De Benedetto, G. E., & Rossi, S. (2024). Stable Isotopes Analysis of Bioremediating Organisms in an Innovative Integrated Multi-Trophic Aquaculture System. Journal of Marine Science and Engineering, 12(12), 2286. https://doi.org/10.3390/jmse12122286









