Elemental Composition and Morphometry of Rhyssoplax olivacea (Polyplacophora): Part I—Radula and Valves
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Study Sites
2.2. Morphometric Measurements
2.3. Energy-Dispersive Spectroscopy (EDS)
2.4. Statistical Analysis
3. Results
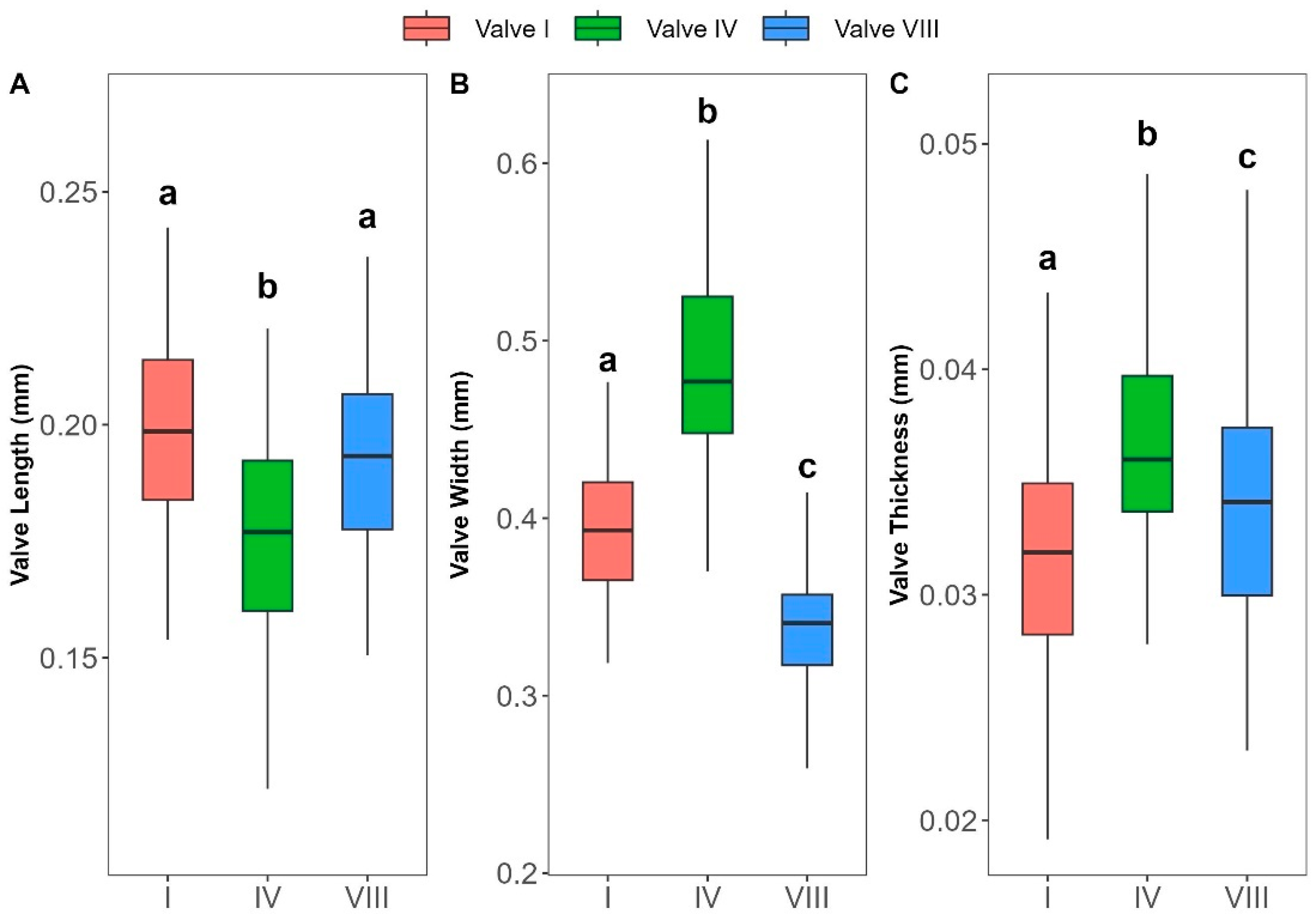
3.1. Morphometric Analysis
3.2. Elemental Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sigwart, J.D. Morphological Cladistic Analysis as a Model for Character Evaluation in Primitive Living Chitons (Polyplacophora, Lepidopleurina). Am. Malacol. Bull. 2009, 27, 95–104. [Google Scholar] [CrossRef]
- Kaas, P.; Belle, R.A.V.; Strack, H.L. Monograph of Living Chitons (Mollusca: Polyplacophora), Volume 6 Family Schizochitonidae; BRILL: Leiden, The Netherlands, 2006; ISBN 978-90-474-1837-5. [Google Scholar]
- Dethier, M.N.; Duggins, D.O. An “Indirect Commensalism” between Marine Herbivores and the Importance of Competitive Hierarchies. Am. Nat. 1984, 124, 205–219. [Google Scholar] [CrossRef]
- Elahi, R.; Sebens, K.P. Experimental Removal and Recovery of Subtidal Grazers Highlights the Importance of Functional Redundancy and Temporal Context. PLoS ONE 2013, 8, e78969. [Google Scholar] [CrossRef] [PubMed]
- Strack, H.L. The Distribution of Chitons (Polyplacophora) in Greece. Apex 1988, 3, 67–80. [Google Scholar]
- Koukouras, A.; Karachle, P. The Polyplacophoran (Eumollusca, Mollusca) Fauna of the Aegean Sea with the Description of a New Species, and Comparison with Those of the Neighbouring Seas. J. Biol. Res. 2004, 3, 23–38. [Google Scholar]
- Dell’Angelo, B.; Smriglio, C. Living Chitons of the Mediterranean; Evolver: Reston, VA, USA, 2001; ISBN 978-88-8299-007-7. [Google Scholar]
- Varkoulis, A.; Voulgaris, K.; Zachos, D.S.; Vafidis, D. Population Dynamics of Three Polyplacophora Species from the Aegean Sea (Eastern Mediterranean). Diversity 2023, 15, 867. [Google Scholar] [CrossRef]
- Sigwart, J.D.; Schwabe, E. Anatomy of the Many Feeding Types in Polyplacophoran Molluscs. Invertebr. Zool. 2017, 14, 205–216. [Google Scholar] [CrossRef]
- Lowenstam, H.A. Lepidocrocite, an Apatite Mineral, and Magnetite in Teeth of Chitons (Polyplacophora). Science 1967, 156, 1373–1375. [Google Scholar] [CrossRef]
- Wang, T.; Huang, W.; Pham, C.H.; Murata, S.; Herrera, S.; Kirchhofer, N.D.; Arkook, B.; Stekovic, D.; Itkis, M.E.; Goldman, N.; et al. Mesocrystalline Ordering and Phase Transformation of Iron Oxide Biominerals in the Ultrahard Teeth of Cryptochiton stelleri. Small Struct. 2022, 3, 2100202. [Google Scholar] [CrossRef]
- Krings, W.; Brütt, J.-O.; Gorb, S.N. Ontogeny of the Elemental Composition and the Biomechanics of Radular Teeth in the Chiton Lepidochitona cinerea. Front. Zool. 2022, 19, 19. [Google Scholar] [CrossRef]
- Sigwart, J.D.; Green, P.A.; Crofts, S.B. Functional Morphology in Chitons (Mollusca, Polyplacophora): Influences of Environment and Ocean Acidification. Mar. Biol. 2015, 162, 2257–2264. [Google Scholar] [CrossRef]
- Connors, M.J.; Ehrlich, H.; Hog, M.; Godeffroy, C.; Araya, S.; Kallai, I.; Gazit, D.; Boyce, M.; Ortiz, C. Three-Dimensional Structure of the Shell Plate Assembly of the Chiton Tonicella marmorea and Its Biomechanical Consequences. J. Struct. Biol. 2012, 177, 314–328. [Google Scholar] [CrossRef] [PubMed]
- Peebles, B.A.; Smith, A.M.; Spencer, H.G. Valve Microstructure and Phylomineralogy of New Zealand Chitons. J. Struct. Biol. 2017, 197, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Coll, M.; Piroddi, C.; Steenbeek, J.; Kaschner, K.; Lasram, F.B.R.; Aguzzi, J.; Ballesteros, E.; Bianchi, C.N.; Corbera, J.; Dailianis, T.; et al. The Biodiversity of the Mediterranean Sea: Estimates, Patterns, and Threats. PLoS ONE 2010, 5, e11842. [Google Scholar] [CrossRef] [PubMed]
- Poulos, S.; Lesioti, S.; Karditsa, A.; Angelopoulos, C. Factors Controlling the Formation and Evolution of a Beach Zone in Front of a Coastal Cliff: The Case of the East Coast of Evia Island in the Aegean Sea, Eastern Mediterranean. Water 2024, 16, 1622. [Google Scholar] [CrossRef]
- Petihakis, G.; Triantafyllou, G.; Pollani, A.; Koliou, A.; Theodorou, A. Field Data Analysis and Application of a Complex Water Column Biogeochemical Model in Different Areas of a Semi-Enclosed Basin: Towards the Development of an Ecosystem Management Tool. Mar. Environ. Res. 2005, 59, 493–518. [Google Scholar] [CrossRef]
- Zodiatis, G. Advection of the Black Sea Water in the North Aegean Sea. Glob. Atmos. Ocean. Syst. 1994, 2, 41–60. [Google Scholar]
- Zervakis, V.; Georgopoulos, D. Hydrology and Circulation in the North Aegean (Eastern Mediterranean) throughout 1997 and 1998. Mediterr. Mar. Sci. 2002, 3, 5–19. [Google Scholar] [CrossRef]
- Avila-Poveda, O.H.; Abadia-Chanona, Q.Y. Emergence, Development, and Maturity of the Gonad of Two Species of Chitons “Sea Cockroach” (Mollusca: Polyplacophora) through the Early Life Stages. PLoS ONE 2013, 8, e69785. [Google Scholar] [CrossRef]
- Warton, D.I.; Duursma, R.A.; Falster, D.S.; Taskinen, S. Smatr 3– an R Package for Estimation and Inference about Allometric Lines. Methods Ecol. Evol. 2012, 3, 257–259. [Google Scholar] [CrossRef]
- Samuel, B. Bioenergetic and Growth; Reinhold: New York, NY, USA, 1945. [Google Scholar]
- Sokal, R.R.; Rohlf, F.J. Biometry: The Principles and Practice of Statistics in Biological Research, 3rd ed.; W.H. Freeman and Co.: New York, NY, USA, 2013. [Google Scholar]
- Pitacco, V.; Orlando-Bonaca, M.; Mavrič, B.; Lipej, L. Macrofauna Associated with a Bank of Cladocora caespitosa (Anthozoa, Scleractinia) in the Gulf of Trieste (Northern Adriatic). Ann. Ser. Hist. Nat. 2014, 24, 1–14. [Google Scholar]
- Yee, A.K.; Padilla, D.K. Allometric Scaling of the Radula in the Atlantic Slippersnail Crepidula fornicata. J. Shellfish. Res. 2015, 34, 903–907. [Google Scholar] [CrossRef]
- Baxter, J.M. Allometric and Morphological Variations of Whole Animal and Valve Dimensions in the Chiton Lepidochitona cinereus (L.) (Mollusca: Polyplacophora). J. Molluscan Stud. 1982, 48, 275–282. [Google Scholar] [CrossRef]
- BAXTER, J.M.; JONES, A.M. Allometric and Morphological Characteristics of Tonicella marmorea (Fabricius, 1780) Populations (Mollusca: Polyplacophora: Ischnochitonidae). Zool. J. Linn. Soc. 1986, 88, 167–177. [Google Scholar] [CrossRef]
- Salloum, P.M.; Lavery, S.D.; de Villemereuil, P.; Santure, A.W. Local Adaptation in Shell Shape Traits of a Brooding Chiton with Strong Population Genomic Differentiation. Evolution 2023, 77, 210–220. [Google Scholar] [CrossRef]
- Ibáñez, C.M.; Sepúlveda, R.D.; Sigwart, J.D. Comparative Allometric Variation in Intertidal Chitons (Polyplacophora: Chitonidae). Zoomorphology 2018, 137, 249–256. [Google Scholar] [CrossRef]
- Saad, A.E.A. Morphometric Studies on the Rock Chiton Acanthopleura spiniger (Mollusca: Polyplacophora) from the Northwestern Region of the Red Sea. Indian J. Mar. Sci. 1997, 26, 49–52. [Google Scholar]
- Krings, W.; Brütt, J.-O.; Gorb, S.N. Elemental Analyses Reveal Distinct Mineralization Patterns in Radular Teeth of Various Molluscan Taxa. Sci. Rep. 2022, 12, 7499. [Google Scholar] [CrossRef]
- Weaver, J.C.; Wang, Q.; Miserez, A.; Tantuccio, A.; Stromberg, R.; Bozhilov, K.N.; Maxwell, P.; Nay, R.; Heier, S.T.; DiMasi, E.; et al. Analysis of an Ultra Hard Magnetic Biomineral in Chiton Radular Teeth. Mater. Today 2010, 13, 42–52. [Google Scholar] [CrossRef]
- Shaw, J.A.; Macey, D.J.; Brooker, L.R. Radula Synthesis by Three Species of Iron Mineralizing Molluscs: Production Rate and Elemental Demand. J. Mar. Biol. Assoc. U. K. 2008, 88, 597–601. [Google Scholar] [CrossRef]
- Towe, K.M.; Lowenstam, H.A. Ultrastructure and Development of Iron Mineralization in the Radular Teeth of Cryptochiton stelleri (Mollusca). J. Ultrastruct. Res. 1967, 17, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.P.; Brooker, L.R.; Macey, D.J.; van Bronswijk, W.; Webb, J. Apatite Mineralization in Teeth of the Chiton Acanthopleura echinata. Calcif. Tissue Int. 2000, 67, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Brooker, L.; Lee, A.; Macey, D.; van Bronswijk, W.; Webb, J. Multiple-Front Iron-Mineralisation in Chiton Teeth (Acanthopleura echinata: Mollusca: Polyplacophora). Mar. Biol. 2003, 142, 447–454. [Google Scholar] [CrossRef]
- Brooker, L.R.; Macey, D.J. Biomineralization in Chiton Teeth and Its Usefulness as a Taxonomic Character in the Genus Acanthopleura Guilding, 1829 (MoUusca: Polyplacophora). Am. Malacol. Bull. 2001, 16, 203–215. [Google Scholar]
- Brooker, L.R.; Lee, A.P.; Macey, D.J.; Webb, J.; van Bronswijk, W. In Situ Studies of Biomineral Deposition in the Radula Teeth of Chitons of the Suborder Chitonina. Venus 2006, 65, 71–80. [Google Scholar]
- Kim, K.-S.; Webb, J.; Macey, D.J.; Cohen, D.D. Compositional Changes during Biomineralization of the Radula of the Chiton Clavarizona hirtosa. J. Inorg. Biochem. 1986, 28, 337–345. [Google Scholar] [CrossRef]
- Macey, D.; Brooker, L. The Junction Zone: Initial Site of Mineralization in Radula Teeth of the Chiton Cryptoplax striata (Mollusca: Polyplacophora). J. Morphol. 1996, 230, 33–42. [Google Scholar] [CrossRef]
- Krings, W.; Matsumura, Y.; Brütt, J.-O.; Gorb, S.N. Material Gradients in Gastropod Radulae and Their Biomechanical Significance: A Combined Approach on the Paludomid Lavigeria Grandis. Die Naturwissenschaften 2022, 109, 52. [Google Scholar] [CrossRef]
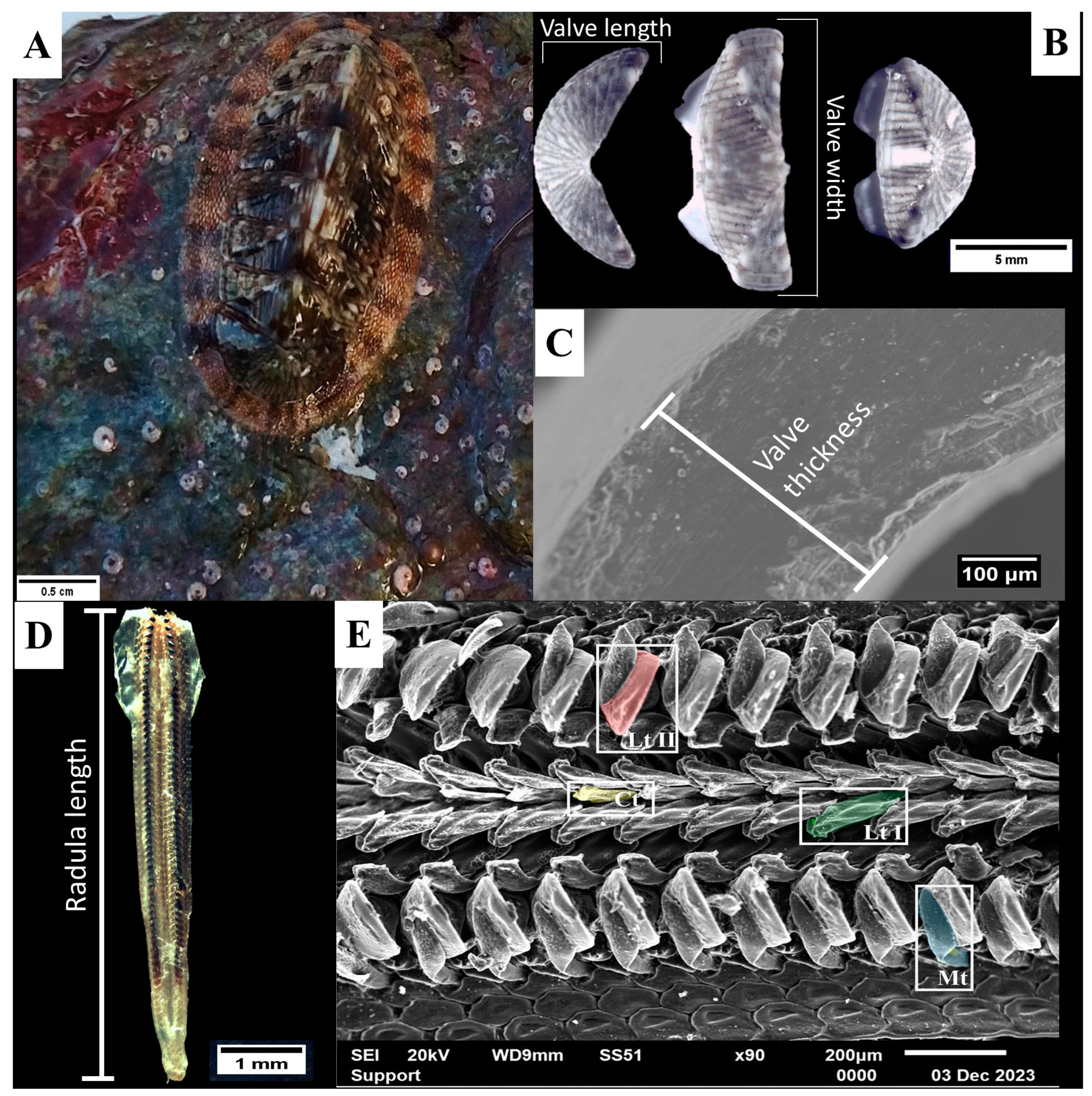

| Area | Coordinates | N | Depth (m) | Mean Length (mm) |
|---|---|---|---|---|
| Chalkidiki Peninsula | 40°23′2.73″ N, 23°55′21.19″ E | 31 | 2–2.5 | 14.4 |
| Limnos Island | 39°51′28.6″ N, 25°03′47.0″ E | 9 | 0.5–1 | 15.01 |
| Pagasitikos Gulf | 39°20′50.09″ N, 22°58′22.84″ E | 34 | 0.5–1 | 16.69 |
| Evoia Island | 38°37′31.35″ N, 24°7′16.09″ E | 15 | 0.5–1 | 20.03 |
| Paxos Island | 39°11′51.47″ N, 20°11′10.74″ E | 9 | 0.5 | 17.41 |
| Relationship | Skeletal Element | Equation | R2 | Allometry |
|---|---|---|---|---|
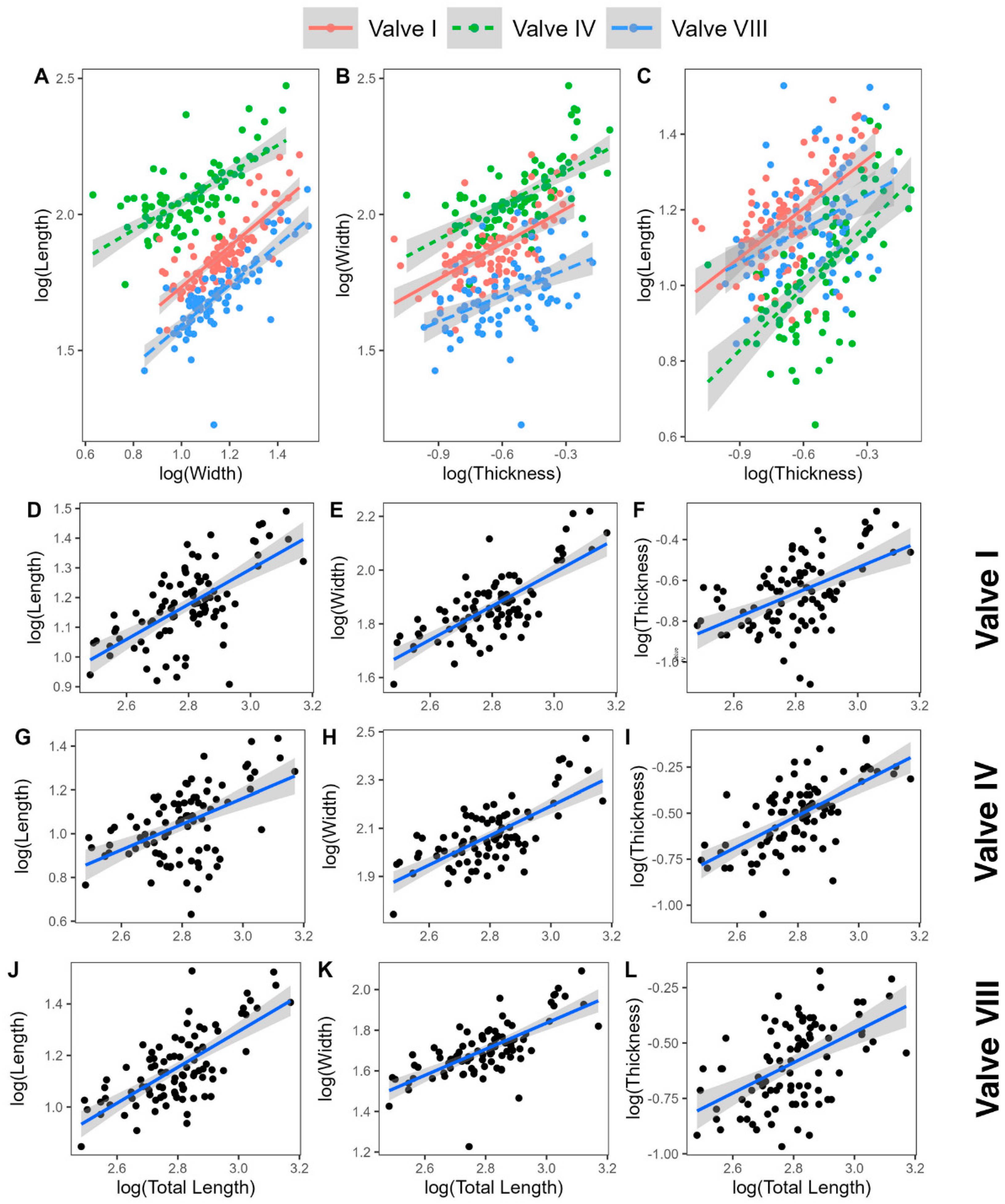
| Length–Width | I | log(L) = 1.082log(W) − 0.365 | 0.65 | Isometry |
| IV | log(L) = 1.309log(W) − 0.723 | 0.46 | Positive | |
| VIII | log(L) = 1.062log(W) − 0.286 | 0.61 | Isometry | |
| Width–Thickness | I | log(W) = 0.699log(T) + 1.011 | 0.38 | Negative |
| IV | log(W) = 0.66log(T) + 1.046 | 0.39 | Negative | |
| VIII | log(W) = 0.705log(T) + 0.921 | 0.21 | Negative | |
| Length–Thickness | I | log(L) = 0.756log(T) + 0.729 | 0.33 | Negative |
| IV | log(L) = 0.864log(T) + 0.646 | 0.41 | Isometry | |
| VIII | log(L) = 0.748log(T) + 0.692 | 0.16 | Negative | |
| Total Length–Valve Length | I | log(TL) = 1.094log(IL) + 0.646 | 0.41 | Isometry |
| IV | log(TL) = 0.874log(IVL) + 0.819 | 0.27 | Isometry | |
| VIII | log(TL) = 1.009log(VIIIL) + 0.709 | 0.49 | Isometry | |
| Total Length–Valve Width | I | log(TL) = 1.183log(IW) + 0.0.257 | 0.55 | Positive |
| IV | log(TL) = 1.145log(IVW) + 0.186 | 0.49 | Isometry | |
| VIII | log(TL) = 1.072log(VIIIW) + 0.421 | 0.47 | Isometry | |
| Total Length–Valve Thickness | I | log(TL) = 0.827log(IT) + 1.453 | 0.27 | Negative |
| IV | log(TL) = 0.756log(IVT) + 1.384 | 0.41 | Negative | |
| VIII | log(TL) = 0.755log(VIIIT) + 1.41 | 0.27 | Negative | |
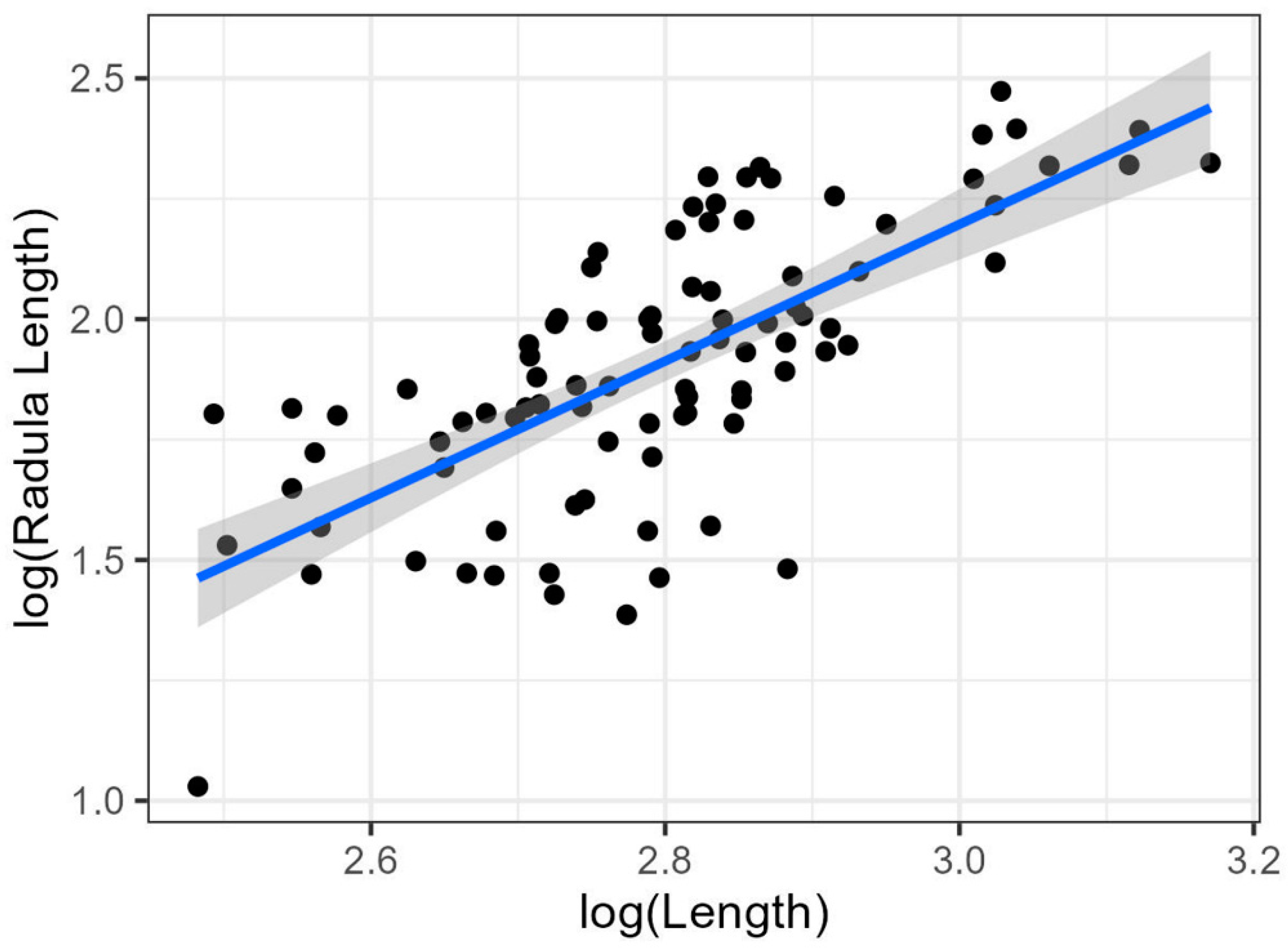
| Radula Length–Total Length | Radula | log(RL) = 2log(TL) − 1.6 | 0.5 | Positive |
| Radula | Valves | |||
|---|---|---|---|---|
| F | p | F | p | |
| Ca | 33.51 | <0.001 | 0.266 | 0.766 |
| Fe | 137 | <0.001 | 0.532 | 0.588 |
| Mg | 35.94 | <0.001 | 0.886 | 0.413 |
| K | 61.34 | <0.001 | 0.344 | 0.709 |
| P | 15.16 | <0.001 | 3.058 | 0.482 |
| Si | 10.7 | <0.001 | 0.169 | 0.844 |
| Family | Species | Region | Method | Tooth | Chemical Elements (%) | Reference |
|---|---|---|---|---|---|---|
| Acanthochitonidae | Acanthochitona fascicularis | North Sea | EDX | C | (A) Ca (<1%), Mg (<1%), P (1%), | [32] |
| Acanthochitona fascicularis | North Sea | EDX | L1 | A Fe (<1%), Ca (1%), Mg (2%),P (2%), K (1%) | [32] | |
| Acanthochitona fascicularis | North Sea | EDX | L2 | (A) Fe (29%), Ca (6%), Mg (4%), P (9%), K (1%) | [32] | |
| Acanthochitona fascicularis | North Sea | EDX | M | (A) Ca (6%), Mg (<1%) | [32] | |
| Cryptochiton stelleri | EMPA | L2 | Fe (51.8%), Ca (3.8%), P (13.3%) | [35] | ||
| Cryptochiton stelleri | Pacific Ocean | Raman; EDS | L2 | (W) Fe (69%) | [33] | |
| Chitonidae | Acanthopleura hirtosa | Indian Ocean | ICP-AES | Whole | (μg/g) Fe (59%),Ca (19%), Mg (3%), P (13%), K (<1%), Si (<1%) | [34] |
| Acanthopleura echinata | Pacific Ocean | Raman; EDS | L2 | (A) Fe (<3%), Ca (32%), P (16%) | [36] | |
| Acanthopleura echinata | Pacific Ocean | EDS | L2 | (A) Fe (66%), Ca (<1%), P (<1%) | [37] | |
| Acanthopleura echinata | Pacific Ocean | Raman; EDS | L2 | (A) Fe (1.3%), Ca (27.3%), Mg (0.4%), P (14.2%) | [37] | |
| Acanthopleura miles | Indian Ocean | Raman; EDS | L2 | (A) Fe (7.1%), Ca (28.4%), Mg (0.6%), P (13.6%) | [37] | |
| Acanthopleura spinosa | Pacific Ocean | EDS | L2 | (A) Fe (~62%), Ca (<1%), Mg (<1%), P (<1%) | [38] | |
| Chiton mamoratus | Atlantic | Raman; EDS | L2 | (A) Fe (0.8%), Ca (33.2%), Mg (0.5%), P (16.7%) | [39] | |
| Clavarizona hirtosa | Indian Ocean | PIXE and PIGME | L2 | Fe, Ca, K, P | [40] | |
| Onithochiton quercinus | Indian Ocean | EDS | L2 | (A) Fe (66%), Ca (<1%), Mg (<1%), P (<1%) | [38] | |
| Onithochiton quercinus | Indian Ocean | Raman; EDS | L2 | (A) Fe (0.2%), Ca (31.3%), Mg (0.4%), P (15.3%) | [39] | |
| Rhyssoplax olivacea | Mediterranean | EDS | C | (W) Fe (54%), Ca (15%), Mg (3%), P (4%), K (4%), Si (<1%) | Present study | |
| Rhyssoplax olivacea | Mediterranean | EDS | L1 | (W) Fe (47%), Ca (15%), Mg (4%), P (6%), K (4%), Si (<1%) | Present study | |
| Rhyssoplax olivacea | Mediterranean | EDS | L2 | (W) Fe (83%), Ca (7%), Mg (2%), P (4%), K (<1%), Si (<1%) | Present study | |
| Rhyssoplax olivacea | Mediterranean | EDS | M | (W) Fe (36%), Ca (17%), Mg (5%), P (7%), K (5%), Si (<1%) | Present study | |
| Sypharochtion pelliserpentis | Pacific Ocean | Raman; EDS | L2 | (A) Fe (4.2%), Ca (30.5%), Mg (0.6%), P (16.3%) | [39] | |
| Cryptoplacidae | Cryptoplax striata | Indian Ocean | EDS | L2 | (A) Fe (~92%), Mg (5.5%), K (1%), Si (1%) | [41] |
| Ischnochitonidae | Ischnochiton australis | Indian Ocean | EDS | L2 | (A) Fe (~62%), Ca (<1%), Mg (<1%), P (<1%) | [38] |
| Ischnochiton australis | Indian Ocean | Raman; EDS | L2 | (A) Fe (2.7%), Ca (16.2%), Mg (6.0%), P (13.6%) | [39] | |
| Mopaliidae | Plaxiphora albida | Indian Ocean | ICP-AES | Whole | (μg/g) Fe (87%), Ca (2%), Mg (1%), P (5%), K (<1%), Si (<1%) | [34] |
| Plaxiphora albida | Indian Ocean | EDS | L2 | (A) Fe (~66%), Ca (<1%), Mg (<1%), P (<1%) | [38] | |
| Plaxiphora albida | Indian Ocean | Raman; EDS | L2 | (A) Fe (26.7%), Ca (6.5%), Mg (2.4%), P (11.2%) | [39] | |
| Tonicellidae | Lepidochitona cinerea | North Sea | EDX | C | (A) Fe (2%), Ca (4%), K (<1%) | [32] |
| Lepidochitona cinerea | North Sea | EDX | L1 | (A) Ca (4%), Si (<1%) | [32] | |
| Lepidochitona cinerea | North Sea | EDX | L2 | (A) Fe (32%), Ca (8%), Mg (3%), P (7%), K (1%) | [32] | |
| Lepidochitona cinerea | North Sea | EDX | M | (A) Fe (1%), Ca (5%), K (<1%), Si (<1%) | [32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mygdalias, T.; Varkoulis, A.; Voulgaris, K.; Zaoutsos, S.; Vafidis, D. Elemental Composition and Morphometry of Rhyssoplax olivacea (Polyplacophora): Part I—Radula and Valves. J. Mar. Sci. Eng. 2024, 12, 2186. https://doi.org/10.3390/jmse12122186
Mygdalias T, Varkoulis A, Voulgaris K, Zaoutsos S, Vafidis D. Elemental Composition and Morphometry of Rhyssoplax olivacea (Polyplacophora): Part I—Radula and Valves. Journal of Marine Science and Engineering. 2024; 12(12):2186. https://doi.org/10.3390/jmse12122186
Chicago/Turabian StyleMygdalias, Thomas, Anastasios Varkoulis, Konstantinos Voulgaris, Stefanos Zaoutsos, and Dimitris Vafidis. 2024. "Elemental Composition and Morphometry of Rhyssoplax olivacea (Polyplacophora): Part I—Radula and Valves" Journal of Marine Science and Engineering 12, no. 12: 2186. https://doi.org/10.3390/jmse12122186
APA StyleMygdalias, T., Varkoulis, A., Voulgaris, K., Zaoutsos, S., & Vafidis, D. (2024). Elemental Composition and Morphometry of Rhyssoplax olivacea (Polyplacophora): Part I—Radula and Valves. Journal of Marine Science and Engineering, 12(12), 2186. https://doi.org/10.3390/jmse12122186








