Sponges as Emerging By-Product of Integrated Multitrophic Aquaculture (IMTA)
Abstract
1. Introduction
2. Past and Present Concerns
3. Bioremediation, a Way of Life
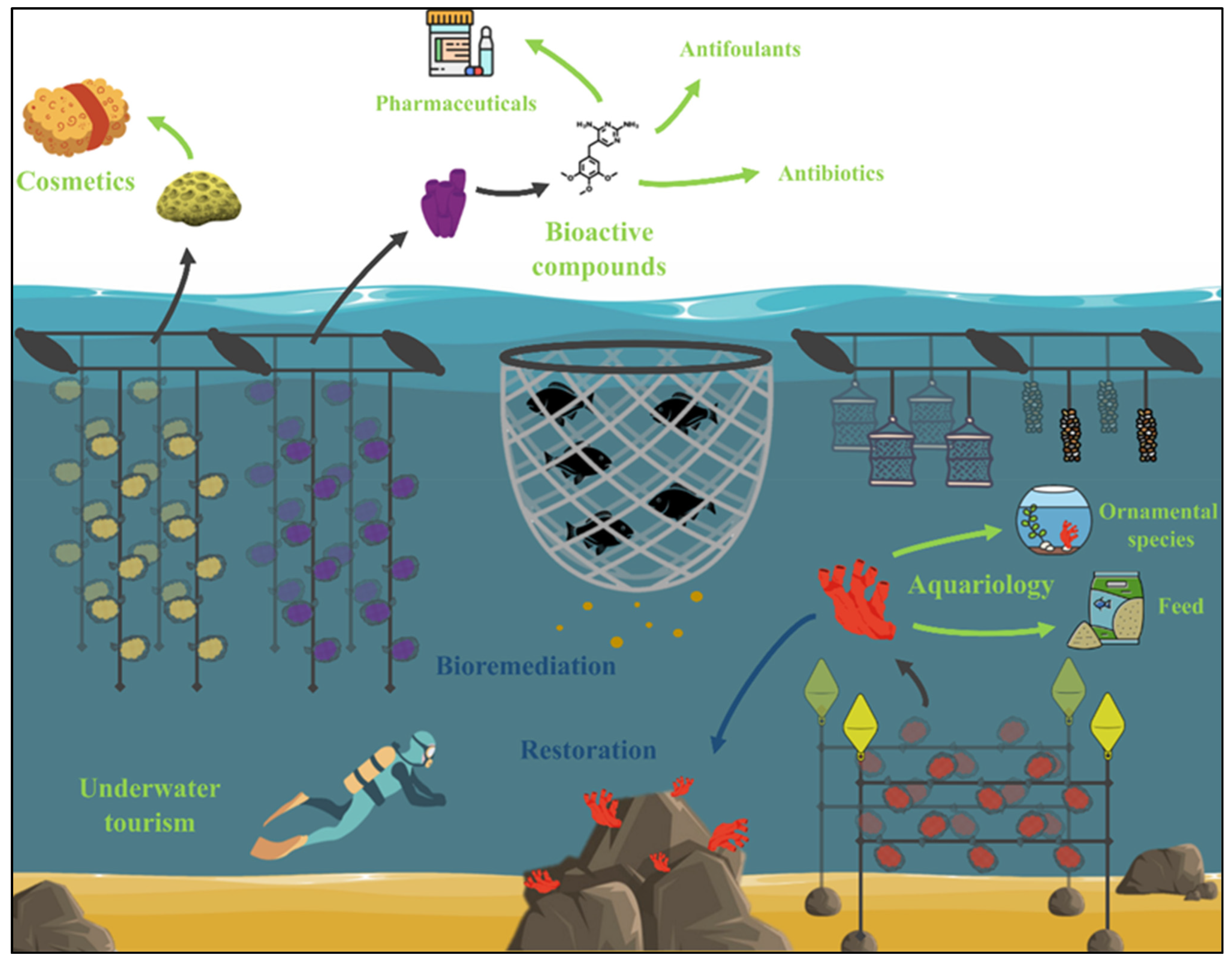
4. A Marine Bioactive Compound Factory
5. From the Sea to the Tank
6. The Challenge in Marine Sponges’ Restoration
7. Deciding the Methodological Approach
8. Fitting Strategic and Sustainable Development Goals
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaviarà, D. Le spugne e i loro pescatori dai tempi antichi ad ora. Mem. Reg. Com. Talassogr. Ital. 1920, 74, 1–49. [Google Scholar]
- Pronzato, R.; Manconi, R. Mediterranean commercial sponges: Over 5000 years of natural history and cultural heritage. Mar. Ecol. 2008, 29, 146–166. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Zahn, R.K.; Gasić, M.J.; Dogović, N.; Maidhof, A.; Becker, C.; Diehl-Seifert, B.; Eich, E. Avarol, a cytostatically active compound from the marine sponge Dysidea avara. Comp. Biochem. Physiol. Part C Comp. Pharmacol. 1985, 80, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Ferrándiz, M.L.; Sanz, M.J.; Bustos, G.; Payá, M.; Alcaraz, M.J.; De Rosa, S. Avarol and avarone, two new anti-inflammatory agents of marine origin. Eur. J. Pharmacol. 1994, 253, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Amigó, M.; Payá, M.; Braza-Boïls, A.; De Rosa, S.; Terencio, M.C. Avarol inhibits TNF-α generation and NF-κB activation in human cells and in animal models. Life Sci. 2008, 82, 256–264. [Google Scholar] [CrossRef]
- United Nations Department of Economic and Social Affairs, Population Division. World Population Prospects 2022: Summary of Results; UN DESA/POP/2022/TR/NO. 3; United Nations Publications: New York, NY, USA, 2022. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture 2022. In Towards Blue Transformation; FAO: Rome, Italy, 2022. [Google Scholar]
- Gowen, R.J.; Bradbury, N.B. The ecological impact of salmonid farming in coastal waters: A review. Oceanogr. Mar. Biol. 1987, 25, 563–575. [Google Scholar] [CrossRef]
- Holdt, S.L.; Edwards, M.D. Cost-effective IMTA: A comparison of the production efficiencies of mussels and seaweed. J. App. Phycol. 2014, 26, 933–945. [Google Scholar] [CrossRef]
- Irisarri, J.; Fernández-Reiriz, M.J.; Labarta, U.; Cranford, P.J.; Robinson, S.M. Availability and utilization of waste fish feed by mussels Mytilus edulis in a commercial integrated multi-trophic aquaculture (IMTA) system: A multi-indicator assessment approach. Ecol. Indic. 2015, 48, 673–686. [Google Scholar] [CrossRef]
- Müller, W.E.; Wimmer, W.; Schatton, W.; Böhm, M.; Batel, R.; Filic, Z. Initiation of an aquaculture of sponges for the sustainable production of bioactive metabolites in open systems: Example, Geodia cydonium. Mar. Biotechnol. 1999, 1, 569–579. [Google Scholar] [CrossRef]
- Pronzato, R.; Cerrano, C.; Cubeddu, T.; Lanza, S.; Magnino, G.; Manconi, R.; Pantelis, J.; Sarà, A.; Sidri, M. Sustainable development in coastal areas: Role of sponge farming in integrated aquaculture. In Proceedings of the Aquaculture Europe, Bordeaux, France, 7–10 October 1998. [Google Scholar] [CrossRef]
- Pronzato, R. Sponge Farming in the Mediterranean Sea: New Perspectives. Mem. Qld. Mus. 1999, 44, 485–491. [Google Scholar]
- Osinga, R.; Sidri, M.; Cerig, E.; Gokalp, S.Z.; Gokalp, M. Sponge Aquaculture Trials in the East-Mediterranean Sea: New Approaches to Earlier Ideas. Open. Mar. Biol. J. 2010, 4, 74–81. [Google Scholar] [CrossRef]
- Longo, C.; Cardone, F.; Corriero, G.; Licciano, M.; Pierri, C.; Stabili, L. The co-occurrence of the demosponge Hymeniacidon perlevis and the edible mussel Mytilus galloprovincialis as a new tool for bacterial load mitigation in aquaculture. Environ. Sci. Pollut. Res. 2016, 23, 3736–3746. [Google Scholar] [CrossRef] [PubMed]
- Gökalp, M.; Wijgerde, T.; Sarà, A.; De Goeij, J.M.; Osinga, R. Development of an integrated mariculture for the collagen-rich sponge Chondrosia reniformis. Mar. Drugs 2019, 17, 29. [Google Scholar] [CrossRef]
- Longo, C.; Scrascia, M.; Trani, R.; Pierri, C.; Cariglia, A.; Cariglia, F.; Cariglia, M. Assesment of sponge mariculture potential in polycolture system in Manfredonia Gulf toward the IMTA implementation. In Proceedings of the Aquafarm Novelfarm, Pordenone, Italy, 19–20 February 2020. [Google Scholar]
- Giangrande, A.; Pierri, C.; Arduini, D.; Borghese, J.; Licciano, M.; Trani, R.; Corriero, G.; Basile, G.; Cecere, E.; Petrocelli, A. An Innovative IMTA System: Polychaetes, Sponges and Macroalgae Co-Cultured in a Southern Italian in-Shore Mariculture Plant (Ionian Sea). J. Mar. Sci. Eng. 2020, 8, 733. [Google Scholar] [CrossRef]
- Page, M.J.; Northcote, P.T.; Webb, V.L.; Mackey, S.; Handley, S.J. Aquaculture trials for the production of biologically active metabolites in the New Zealand sponge Mycale hentscheli (Demospongiae: Poecilosclerida). Aquaculture 2005, 250, 256–269. [Google Scholar] [CrossRef]
- FAO. Available online: https://www.fao.org/3/ac286e/AC286E00.htm (accessed on 26 November 2022).
- Cavolini, F. Memorie Per Servire Alla Storia de’ Polipi Marini; Wentworth Press: Napoli, Italy, 1785; pp. 262–265. [Google Scholar]
- Crawshay, L.R. Studies in the market sponges I. Growth from the planted cutting. J. Mar. Biol. Assoc. UK 1939, 23, 553–574. [Google Scholar] [CrossRef]
- Gökalp, N. Türkiye’de Ilk Sünger Yetistirme Tecrübeleri. Balık ve Balıkçılık 1974, 12, 1–10. [Google Scholar]
- Bierwirth, J.; Mantas, T.P.; Villechanoux, J.; Cerrano, C. Restoration of marine sponges—What can we learn from over a century of experimental cultivation? Water 2022, 14, 1055. [Google Scholar] [CrossRef]
- Duckworth, A. Farming Sponges to Supply Bioactive Metabolites and Bath Sponges: A Review. Mar. Biotechnol. 2009, 11, 669–679. [Google Scholar] [CrossRef]
- Verdenal, B.; Vacelet, J. Sponge culture on vertical ropes in the Northwestern Mediterranean Sea. In New Perspectives in Sponge Biology; Rützler, K., Ed.; Smithsonian Institution Press: Washington, DC, USA, 1990; pp. 416–424. [Google Scholar]
- Corriero, G.; Longo, C.; Mercurio, M.; Marzano, C.N.; Lembo, G.; Spedicato, M.T. Rearing performance of Spongia officinalis on suspended ropes off the Southern Italian coast (Central Mediterranean Sea). Aquaculture 2004, 238, 195–205. [Google Scholar] [CrossRef]
- Duckworth, A.R.; Wolff, C. Sath sponge aquaculture in Torres Strait, Australia: Effect of explant size, farming method and the environment on culture success. Aquaculture 2007, 271, 188–195. [Google Scholar] [CrossRef]
- Duckworth, A.R.; Wolff, C.; Evans-Illidge, E. Developing methods for commercially farming bath sponges in tropical Australia. Porifera Res.-Biodivers. Innov. Sustain. Rio de Jan. Mus. Nac. Ser. Livros 2007, 28, 297–302. [Google Scholar]
- Oronti, A.; Danylchuk, A.J.; Elmore, C.E.; Auriemma, R.; Pesle, G. Assessing the Feasibility of Sponge Aquaculture as a Sustainable Industry in The Bahamas. Aquac. Int. 2012, 20, 295–303. [Google Scholar] [CrossRef]
- Tobey, J.A.; Haws, M.C.; Ellis, S.S. Aquaculture Profile for Pohnpei Federated States of Micronesia; Technical Report; Pohnpei State Division of Marine Development, Office of Economic Affairs and the Conservation Society of Pohnpei. 2004. Available online: https://repository.library.noaa.gov/view/noaa/39936 (accessed on 26 November 2022).
- Marinecultures. Available online: https://www.marinecultures.org/en/projects/spongefarming/spongefarming/ (accessed on 26 November 2022).
- Simpson, T.L. The Cell Biology of Sponges, 1st ed.; Springer: New York, NY, USA, 1984. [Google Scholar]
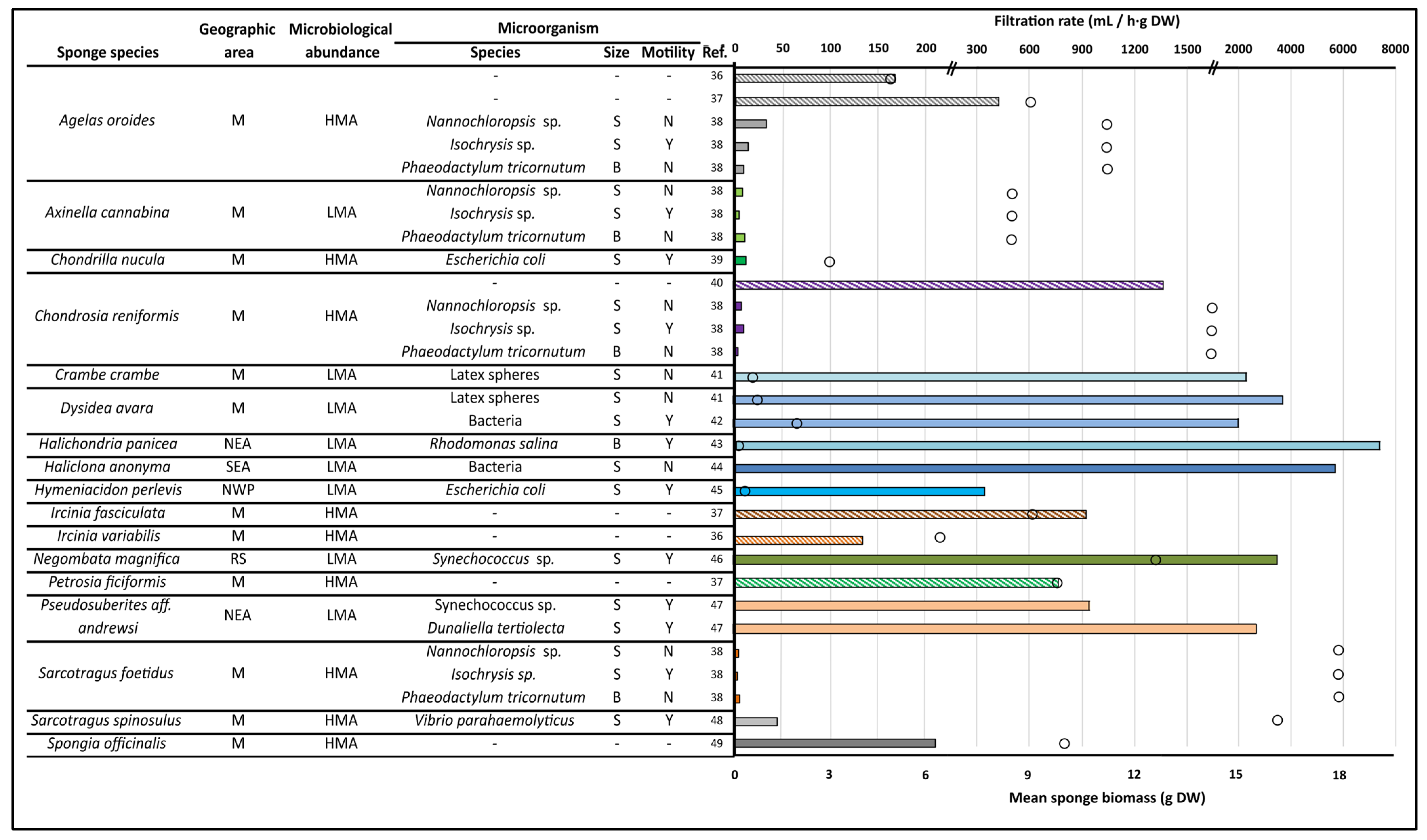
- Riisgård, H.U.; Larsen, P.S. Filter-feeding in marine macro-invertebrates: Pump characteristics, modelling and energy cost. Biol. Rev. 1995, 70, 67–106. [Google Scholar] [CrossRef]
- Hadas, E.; Marie, D.; Shpigel, M.; Ilan, M. Virus predation by sponges is a new nutrient-flow pathway in coral reef food webs. Limnol. Oceanogr. 2006, 51, 1548–1550. [Google Scholar] [CrossRef]
- Ledda, F.D.; Pronzato, R.; Manconi, R. Mariculture for bacterial and organic waste removal: A field study of sponge filtering activity in experimental farming. Aquac. Res. 2014, 45, 1389–1401. [Google Scholar] [CrossRef]
- Gili, J.M.; Bibiloni, M.A.; Montserrat, A. Tasas de filtración y retención de bacterias “in situ” de tres especies de esponjas litorales. Estudio preliminar. Misc. Zool. 1984, 8, 13–21. [Google Scholar]
- Varamogianni-Mamatsi, D.; Anastasiou, T.I.; Vernadou, E.; Papandroulakis, N.; Kalogerakis, N.; Dailianis, T.; Mandalakis, M.A. Multi-species investigation of sponges’ filtering activity towards marine microalgae. Mar. Drugs 2021, 20, 24. [Google Scholar] [CrossRef]
- Milanese, M.; Chelossi, E.; Manconi, R.; Sarà, A.; Sidri, M.; Pronzato, R. The marine sponge Chondrilla nucula Schmidt, 1862 as an elective candidate for bioremediation in integrated aquaculture. Biomol. Eng. 2003, 20, 363–368. [Google Scholar] [CrossRef]
- Gökalp, M.; Kooistra, T.; Rocha, M.S.; Silva, T.H.; Osinga, R.; Murk, A.J.; Wijgerde, T. The effect of depth on the morphology, bacterial clearance, and respiration of the mediterranean sponge Chondrosia reniformis (Nardo, 1847). Mar. Drugs 2020, 18, 358. [Google Scholar] [CrossRef]
- Turon, X.; Galera, J.; Uriz, M.J. Clearance rates and aquiferous systems in two sponges with contrasting life-history strategies. J. Exp. Zool. 1997, 278, 22–36. [Google Scholar] [CrossRef]
- Ribes, M.; Coma, R.; Gili, J.-M. Natural diet and grazing rate of the temperate sponge Dysidea avara (Demospongiae, Dendroceratida) throughout an annual cycle. Mar. Ecol. Prog. Ser. 1999, 176, 179–190. [Google Scholar] [CrossRef]
- Kumala, L.; Riisgård, H.; Canfield, D. Osculum dynamics and filtration activity in small single-osculum explants of the Demosponge Halichondria panicea. Mar. Ecol. Prog. Ser. 2017, 572, 117–128. [Google Scholar] [CrossRef]
- Stuart, V.; Klumpp, D. Evidence for food-resource partitioning by kelp-bed filter feeders. Mar. Ecol. Prog. Ser. 1984, 16, 27–37. [Google Scholar] [CrossRef]
- Fu, W.; Sun, L.; Zhang, X.; Zhang, W. Potential of the marine sponge Hymeniacidon perleve as a bioremediator of pathogenic bacteria in integrated aquaculture ecosystems. Biotechnol. Bioeng. 2006, 93, 1112–1122. [Google Scholar] [CrossRef]
- Hadas, E.; Shpigel, M.; Ilan, M. Particulate organic matter as a food source for a coral reef sponge. J. Exp. Biol. 2009, 212, 3643–3650. [Google Scholar] [CrossRef]
- Osinga, R.; Kleijn, R.; Groenendijk, E.; Niesink, P.; Tramper, J.; Wijffels, R.H. Development of in vivo sponge cultures: Particle feeding by the tropical sponge Pseudosuberites aff. andrewsi. Mar. Biotechnol. 2001, 3, 544–554. [Google Scholar] [CrossRef]
- Trani, R.; Corriero, G.; de Pinto, M.C.; Mercurio, M.; Pazzani, C.; Pierri, C.; Scrascia, M.; Longo, C. Filtering activity and nutrient release by the keratose sponge Sarcotragus spinosulus Schmidt, 1862 (Porifera, Demospongiae) at the laboratory scale. J. Mar. Sci. Eng. 2021, 9, 178. [Google Scholar] [CrossRef]
- Stabili, L.; Licciano, M.; Giangrande, A.; Longo, C.; Mercurio, M.; Marzano, C.N.; Corriero, G. Filtering activity of Spongia officinalis var. adriatica (Schmidt) (Porifera, Demospongiae) on bacterioplankton: Implications for bioremediation of polluted seawater. Water Res. 2006, 40, 3083–3090. [Google Scholar] [CrossRef]
- Riisgård, H.; Thomassen, S.; Jakobsen, H.; Weeks, J.; Larsen, P. Suspension feeding in marine sponges Halichondria panicea and Haliclona urceolus: Effects of temperature on filtration rate and energy cost of pumping. Mar. Ecol. Prog. Ser. 1993, 96, 177–188. [Google Scholar] [CrossRef]
- Ricciardi, A.; Bourget, E. Weight-to-Weight conversion factors for marine benthic macroinvertebrates. Mar. Ecol. Prog. Ser. 1998, 163, 245–251. [Google Scholar] [CrossRef]
- Moitinho-Silva, L.; Steinert, G.; Nielsen, S.; Hardoim, C.C.; Wu, Y.-C.; McCormack, G.P.; López-Legentil, S.; Marchant, R.; Webster, N.; Thomas, T. Predicting the HMA-LMA status in marine sponges by machine learning. Front. Microbiol. 2017, 8, 752. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, C.R.; Vacelet, J. Transplantation of marine sponges to different conditions of light and current. J. Exp. Mar. Biol. Ecol. 1979, 37, 91–104. [Google Scholar] [CrossRef]
- Duckworth, A.R.; Battershill, C.N.; Bergquist, P.R. Influence of explant procedures and environmental factors on culture success of three sponges. Aquaculture 1997, 156, 251–267. [Google Scholar] [CrossRef]
- Duckworth, A.R.; Battershill, C.N.; Schiel, D.R. Effects of depth and water flow on growth, survival and bioactivity of two temperate sponges cultured in different seasons. Aquaculture 2004, 242, 237–250. [Google Scholar] [CrossRef]
- Mendola, D.; De Caralt, S.; Uriz, M.; Van den End, F.; Van Leeuwen, J.L.; Wijffels, R.H. Environmental Flow Regimes for Dysidea avara Sponges. Mar. Biotechnol. 2008, 10, 622–630. [Google Scholar] [CrossRef]
- Gökalp, M.; Mes, D.; Nederlof, M.; Zhao, H.; Merijn de Goeij, J.; Osinga, R. The potential roles of sponges in integrated mariculture. Rev. Aquac. 2021, 13, 1159–1171. [Google Scholar] [CrossRef]
- Fu, W.; Wu, Y.; Sun, L.; Zhang, W. Efficient bioremediation of total organic carbon (TOC) in integrated aquaculture system by marine sponge Hymeniacidon perleve. Biotechnol. Bioeng. 2007, 97, 1387–1397. [Google Scholar] [CrossRef]
- Longo, C.; Corriero, G.; Licciano, M.; Stabili, L. Bacterial accumulation by the Demospongiae Hymeniacidon perlevis: A tool for the bioremediation of polluted seawater. Mar. Pollut. Bull. 2010, 60, 1182–1187. [Google Scholar] [CrossRef]
- Riisgård, H.U.; Larsen, P.S. Comparative ecophysiology of active zoobenthic filter feeding, essence of current knowledge. J. Sea Res. 2000, 44, 169–193. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Leatherland, T.M.; Burton, J.D. The occurrence of some trace metals in coastal organisms with particular reference to the Solent Region. J. Mar. Biol. Assoc. UK 1974, 54, 457–468. [Google Scholar] [CrossRef]
- Patel, B.; Balani, M.C.; Patel, S. Sponge ‘sentinel’ of heavy metals. Sci. Total Environ. 1985, 41, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Olesen, T.M.E.; Weeks, J.M. Accumulation of Cd by the marine sponge Halichondria panicea Pallas: Effects upon filtration rate and its relevance for biomonitoring. Bull. Environ. Contam. Toxicol. 1994, 52, 722–728. [Google Scholar] [CrossRef]
- Hansen, I.V.; Weeks, J.M.; Depledge, M.H. Accumulation of Copper, Zinc, Cadmium and Chromium by the marine sponge Halichondria panicea Pallas and the implications for biomonitoring. Mar. Pollut. Bull. 1995, 31, 133–138. [Google Scholar] [CrossRef]
- Philp, R.B. Cadmium content of the marine sponge Microciona prolifera, other sponges, water and sediment from the eastern Florida panhandle: Possible effects on Microciona cell aggregation and potential roles of low pH and low salinity. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1999, 124, 41–49. [Google Scholar] [CrossRef]
- Cebrian, E.; Martı, R.; Uriz, J.M.; Turon, X. Sublethal effects of contamination on the mediterranean sponge Crambe crambe: Metal accumulation and biological responses. Mar. Pollut. Bull. 2003, 46, 1273–1284. [Google Scholar] [CrossRef]
- Rao, J.V.; Kavitha, P.; Reddy, N.C.; Rao, T.G. Petrosia testudinaria as a biomarker for metal contamination at Gulf of Mannar, Southeast Coast of India. Chemosphere 2006, 65, 634–638. [Google Scholar] [CrossRef]
- Rao, J.V.; Kavitha, P.; Srikanth, K.; Usman, P.K.; Rao, T.G. Environmental contamination using accumulation of metals in marine sponge, Sigmadocia fibulata inhabiting the coastal waters of Gulf of Mannar, India. Toxicol. Environ. Chem. 2007, 89, 487–498. [Google Scholar] [CrossRef]
- Venkateswara Rao, J.; Srikanth, K.; Pallela, R.; Gnaneshwar Rao, T. The use of marine sponge, Haliclona tenuiramosa as bioindicator to monitor heavy metal pollution in the coasts of Gulf of Mannar, India. Environ. Monit. Assess. 2009, 156, 451. [Google Scholar] [CrossRef]
- Annibaldi, A.; Truzzi, C.; Illuminati, S.; Bassotti, E.; Finale, C.; Scarponi, G. First systematic voltammetric measurements of Cd, Pb, and Cu in hydrofluoric acid-dissolved siliceous spicules of marine sponges: Application to Antarctic specimens. Anal. Lett. 2011, 44, 2792–2807. [Google Scholar] [CrossRef]
- Mahaut, M.-L.; Basuyaux, O.; Baudinière, E.; Chataignier, C.; Pain, J.; Caplat, C. The Porifera Hymeniacidon perlevis (Montagu, 1818) as a bioindicator for water quality monitoring. Environ. Sci. Pollut. Res. 2013, 20, 2984–2992. [Google Scholar] [CrossRef] [PubMed]
- Batista, D.; Muricy, G.; Rocha, R.C.; Miekeley, N.F. Marine sponges with contrasting life histories can be complementary biomonitors of heavy metal pollution in coastal ecosystems. Environ. Sci. Pollut. Res. 2014, 21, 5785–5794. [Google Scholar] [CrossRef] [PubMed]
- Maloubier, M.; Michel, H.; Solari, P.L.; Moisy, P.; Tribalat, M.-A.; Oberhaensli, F.R.; Bottein, M.Y.D.; Thomas, O.P.; Monfort, M.; Moulin, C. Speciation of Americium in seawater and accumulation in the marine sponge Aplysina cavernicola. Dalton Trans. 2015, 44, 20584–20596. [Google Scholar] [CrossRef] [PubMed]
- Celik, F.; Camas, M.; Camas, A.S.; Ozalp, H.B. Uranium (VI) biosorption on marine sponge, Sarcotragus foetidus (Schmidt, 1862) and its statistical investigation using central composite design. Turkish J. Fish. Aquat. Sci. 2016, 16, 899–911. [Google Scholar] [CrossRef]
- Gentric, C.; Rehel, K.; Dufour, A.; Sauleau, P. Bioaccumulation of metallic trace elements and organic pollutants in marine sponges from the South Brittany Coast, France. J. Environ. Sci. Health A 2016, 51, 213–219. [Google Scholar] [CrossRef]
- Ferrante, M.; Vassallo, M.; Mazzola, A.; Brundo, M.V.; Pecoraro, R.; Grasso, A.; Copat, C. In Vivo Exposure of the marine sponge Chondrilla nucula Schmidt, 1862 to Cadmium (Cd), Copper (Cu) and Lead (Pb) and its potential use for bioremediation purposes. Chemosphere 2018, 193, 1049–1057. [Google Scholar] [CrossRef]
- Orani, A.M.; Barats, A.; Vassileva, E.; Thomas, O.P. Marine sponges as a powerful tool for trace elements biomonitoring studies in coastal environment. Mar. Pollut. Bull. 2018, 131, 633–645. [Google Scholar] [CrossRef]
- Rodríguez, G.R.; Morales, E.O. Assessment of heavy metal contamination at Tallaboa Bay (Puerto Rico) by marine sponges’ bioaccumulation and fungal community composition. Mar. Pollut. Bull. 2020, 161, 111803. [Google Scholar] [CrossRef]
- Srikanth, K.; Rao, J.V.; Rao, A.R. Trace elements in Endectyon fruticosa collected from a sewage outfall site, Therespuram, Tuticorin coast, India. Int. J. Environ. Sci. Technol. 2020, 17, 267–272. [Google Scholar] [CrossRef]
- Gravina, M.F.; Longo, C.; Puthod, P.; Rosati, M.; Colozza, N.; Scarselli, M. Heavy metal accumulation capacity of Axinella damicornis (Esper, 1794) (Porifera, Demospongiae): A tool for bioremediation of polluted seawaters. Mediterr. Mar. Sci. 2022, 23, 125–133. [Google Scholar] [CrossRef]
- Krikech, I.; Jafarabadi, A.R.; Leermakers, M.; Le Pennec, G.; Cappello, T.; Ezziyyani, M. Insights into bioaccumulation and bioconcentration of potentially toxic elements in marine sponges from the Northwestern Mediterranean coast of Morocco. Mar. Pollut. Bull. 2022, 180, 113770. [Google Scholar] [CrossRef] [PubMed]
- Orani, A.M.; Vassileva, E.; Thomas, O.P. Marine sponges as coastal bioindicators of rare earth elements bioaccumulation in the French Mediterranean Sea. Environ. Pollut. 2022, 304, 119172. [Google Scholar] [CrossRef] [PubMed]
- Genta-Jouve, G.; Cachet, N.; Oberhänsli, F.; Noyer, C.; Teyssié, J.-L.; Thomas, O.P.; Lacoue-Labarthe, T. Comparative bioaccumulation kinetics of trace elements in Mediterranean marine sponges. Chemosphere 2012, 89, 340–349. [Google Scholar] [CrossRef]
- Illuminati, S.; Annibaldi, A.; Truzzi, C.; Scarponi, G. Heavy metal distribution in organic and siliceous marine sponge tissues measured by square wave anodic stripping voltammetry. Mar. Pollut. Bull. 2016, 111, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Lacoue-Labarthe, T.; Warnau, M.; Beaugeard, L.; Pascal, P.-Y. Trophic transfer of radioisotopes in Mediterranean sponges through bacteria consumption. Chemosphere 2016, 144, 1885–1892. [Google Scholar] [CrossRef]
- Agusa, T.; Takagi, K.; Kubota, R.; Anan, Y.; Iwata, H.; Tanabe, S. Specific accumulation of arsenic compounds in green turtles (Chelonia mydas) and hawksbill turtles (Eretmochelys imbricata) from Ishigaki Island, Japan. Environ. Pollut. 2008, 153, 127–136. [Google Scholar] [CrossRef]
- Batel, R.; Bihari, N.; Rinkevich, B.; Dapper, J.; Schäcke, H.; Schröder, H.C.; Müller, W.E. Modulation of organotin-induced apoptosis by the water pollutant methyl mercury in a human lymphoblastoid tumour cell line and a marine sponge. Mar. Ecol. Prog. Ser. 1993, 93, 245–251. [Google Scholar] [CrossRef]
- Wagner, C.; Steffen, R.; Koziol, C.; Batel, R.; Lacorn, M.; Steinhart, H.; Simat, T.; Müller, W.E.G. Apoptosis in marine sponges: A biomarker for environmental stress (cadmium and bacteria). Mar. Biol. 1998, 131, 411–421. [Google Scholar] [CrossRef]
- Schröder, H.C.; Hassanein, H.M.A.; Lauenroth, S.; Koziol, C.; Mohamed, T.-A.; Lacorn, M.; Steinhart, H.; Batel, R.; Müller, W.E.G. Induction of DNA strand breaks and expression of HSP70 and GRP78 homolog by cadmium in the marine sponge Suberites domuncula. Arch. Environ. Contam. Toxicol. 1999, 36, 47–55. [Google Scholar] [CrossRef]
- Wanick, R.C.; de Sousa Barbosa, H.; Frazão, L.R.; Santelli, R.E.; Arruda, M.A.Z.; Coutinho, C.C. Evaluation of differential protein expression in Haliclona aquarius and sponge-associated microorganisms under cadmium stress. Anal. Bioanal. Chem. 2013, 405, 7661–7670. [Google Scholar] [CrossRef]
- Akpiri, R.U.; Konya, R.S.; Hodges, N.J. Development of cultures of the marine sponge Hymeniacidon perleve for genotoxicity assessment using the alkaline comet assay. Environ. Toxicol. Chem. 2017, 36, 3314–3323. [Google Scholar] [CrossRef] [PubMed]
- Zahn, R.K.; Kurelec, B.; Zahn-Daimler, G.; Müller, W.E.G.; Rijavec, M.; Batel, R.; Given, R.; Pondeljak, V.; Beyer, Ŕ. The effect of benzo[a]pyrene on sponges as model organisms in marine pollution. Chem. Biol. Interact. 1982, 39, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Perez, T.; Wafo, E.; Fourt, M.; Vacelet, J. Marine sponges as biomonitor of polychlorobiphenyl contamination: Concentration and fate of 24 congeners. Environ. Sci. Technol. 2003, 37, 2152–2158. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, K.; Kato, Y.; Ohta, C.; Koga, N.; Endo, T. Marine sponge: A potential source for methoxylated polybrominated diphenyl ethers in the Asia-Pacific food web. J. Agric. Food Chem. 2011, 59, 13102–13109. [Google Scholar] [CrossRef]
- Aresta, A.; Marzano, C.N.; Lopane, C.; Corriero, G.; Longo, C.; Zambonin, C.; Stabili, L. Analytical investigations on the lindane bioremediation capability of the Demosponge Hymeniacidon perlevis. Mar. Pollut. Bull. 2015, 90, 143–149. [Google Scholar] [CrossRef]
- Webster, L.; Russell, M.; Shepherd, N.; Packer, G.; Dalgarno, E.J.; Neat, F. Monitoring of Polycyclic Aromatic Hydrocarbons (PAHs) in Scottish Deepwater environments. Mar. Pollut. Bull. 2018, 128, 456–459. [Google Scholar] [CrossRef]
- Wu, Q.; Eisenhardt, N.; Holbert, S.S.; Pawlik, J.R.; Kucklick, J.R.; Vetter, W. Naturally occurring organobromine compounds (OBCs) including polybrominated dibenzo-p-dioxins in the marine sponge Hyrtios proteus from The Bahamas. Mar. Pollut. Bull. 2021, 172, 112872. [Google Scholar] [CrossRef]
- Batista, D.; Tellini, K.; Nudi, A.H.; Massone, T.P.; Scofield, A.D.L.; de LR Wagener, A. Marine sponges as bioindicators of oil and combustion derived PAH in coastal waters. Mar. Environ. Res. 2013, 92, 234–243. [Google Scholar] [CrossRef]
- Schröder, H.C.; Badria, F.A.; Ayyad, S.N.; Batel, R.; Wiens, M.; Hassanein, H.M.; Kurelec, B.; Müller, W.E. Inhibitory effects of extracts from the marine alga Caulerpa taxifolia and of toxin from Caulerpa racemosa on multixenobiotic resistance in the marine sponge Geodia cydonium. Environ. Toxicol. Pharmacol. 1998, 5, 119–126. [Google Scholar] [CrossRef]
- Châtel, A.; Talarmin, H.; Hamer, B.; Schröder, H.C.; Müller, W.E.G.; Dorange, G. MAP Kinase cell signaling pathway as biomarker of environmental pollution in the sponge Suberites domuncula. Ecotoxicology 2011, 20, 1727–1740. [Google Scholar] [CrossRef] [PubMed]
- Modica, L.; Lanuza, P.; García-Castrillo, G. Surrounded by microplastic, since when? Testing the feasibility of exploring past levels of plastic microfibre pollution using natural history museum collections. Mar. Pollut. Bull. 2020, 151, 110846. [Google Scholar] [CrossRef] [PubMed]
- Soares, G.M.; Barros, F.; Lanna, E.; da Silva, M.V.S.; Cavalcanti, F.F. Sponges as libraries: Increase in microplastics in Cinachyrella alloclada after 36 years. Mar. Pollut. Bull. 2022, 185, 114339. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, T.M.; Vethaak, A.D.; Almroth, B.C.; Ariese, F.; van Velzen, M.; Hassellöv, M.; Leslie, H.A. Screening for microplastics in sediment, water, marine invertebrates and fish: Method development and microplastic accumulation. Mar. Pollut. Bull. 2017, 122, 403–408. [Google Scholar] [CrossRef]
- Celis-Hernández, O.; Ávila, E.; Ward, R.D.; Rodríguez-Santiago, M.A.; Aguirre-Téllez, J.A. Microplastic distribution in urban vs pristine mangroves: Using marine sponges as bioindicators of environmental pollution. Environ. Pollut. 2021, 284, 117391. [Google Scholar] [CrossRef] [PubMed]
- Fallon, B.R.; Freeman, C.J. Plastics in Porifera: The occurrence of potential microplastics in marine sponges and seawater from Bocas Del Toro, Panamá. PeerJ 2021, 9, e11638. [Google Scholar] [CrossRef] [PubMed]
- Giametti, S.D.; Finelli, C.M. Detection of plastic-associated compounds in marine sponges. Mar. Pollut. Bull. 2022, 175, 113141. [Google Scholar] [CrossRef]
- Saliu, F.; Biale, G.; Raguso, C.; La Nasa, J.; Degano, I.; Seveso, D.; Galli, P.; Lasagni, M.; Modugno, F. Detection of plastic particles in marine sponges by a combined infrared micro-spectroscopy and pyrolysis-gas chromatography-mass spectrometry approach. Sci. Total Environ. 2022, 819, 152965. [Google Scholar] [CrossRef]
- Baird, C.A. Measuring the Effects of Microplastics on Sponges. Ph.D. Thesis, Te Herenga Waka-Victoria University of Wellington, Wellington, New Zealand, 2016. [Google Scholar]
- De Marchi, L.; Renzi, M.; Anselmi, S.; Pretti, C.; Guazzelli, E.; Martinelli, E.; Cuccaro, A.; Oliva, M.; Magri, M.; Bulleri, F. Polyethylene microplastics reduce filtration and respiration rates in the Mediterranean sponge Petrosia ficiformis. Environ. Res. 2022, 211, 113094. [Google Scholar] [CrossRef]
- Selvin, J.; Priya, S.S.; Kiran, G.S.; Thangavelu, T.; Bai, N.S. Sponge-associated marine bacteria as indicators of heavy metal pollution. Microbiol. Res. 2009, 164, 352–363. [Google Scholar] [CrossRef]
- Mori, T.; Iwamoto, K.; Wakaoji, S.; Araie, H.; Kohara, Y.; Okamura, Y.; Shiraiwa, Y.; Takeyama, H. Characterization of a novel gene involved in cadmium accumulation screened from sponge-associated bacterial metagenome. Gene 2016, 576, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Karimi, E.; Gonçalves, J.M.; Reis, M.; Costa, R. Draft genome Sequence of Microbacterium Sp. strain Alg239_V18, an Actinobacterium retrieved from the marine sponge Spongia Sp. Genome Announc. 2017, 5, e01457-16. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Liu, C.; Zhang, F.; Zhao, M.; Li, Z. Comparative genomics provides insights into the marine adaptation in sponge-derived Kocuria flava S43. Front. Microbiol. 2018, 9, 1257. [Google Scholar] [CrossRef]
- Freitas-Silva, J.; de Oliveira, B.F.R.; Vigoder, F.D.M.; Muricy, G.; Dobson, A.D.; Laport, M.S. Peeling the layers away: The genomic characterization of Bacillus pumilus 64-1, an isolate with antimicrobial activity from the marine sponge Plakina cyanorosea (Porifera, Homoscleromorpha). Front. Microbiol. 2021, 11, 592735. [Google Scholar] [CrossRef]
- Marzuki, I.; Kamaruddin, M.; Ahmad, R.; Asaf, R.; Armus, R.; Siswanty, I. Performance of cultured marine sponges-symbiotic bacteria as a heavy metal bio-adsorption. Biodiversitas 2021, 22, 5536–5543. [Google Scholar] [CrossRef]
- Chen, Y.; Pan, T.; Chai, G.; Li, Z. Complete Genome of Mycetocola spongiae MSC19T isolated from deep-sea sponge Cacospongia mycofijiensis indicates the adaptation to deep-sea environment and sponge-microbe symbioses. Mar. Genom. 2022, 63, 100955. [Google Scholar] [CrossRef] [PubMed]
- Bauvais, C.; Zirah, S.; Piette, L.; Chaspoul, F.; Domart-Coulon, I.; Chapon, V.; Gallice, P.; Rebuffat, S.; Pérez, T.; Bourguet-Kondracki, M.-L. Sponging up metals: Bacteria associated with the marine sponge Spongia officinalis. Mar. Environ. Res. 2015, 104, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Gantt, S.E.; Lopez-Legentil, S.; Erwin, P.M. Stable microbial communities in the sponge Crambe crambe from inside and outside a polluted Mediterranean harbor. FEMS Microbiol. Lett. 2017, 364, 11. [Google Scholar] [CrossRef] [PubMed]
- Webster, N.S.; Webb, R.I.; Ridd, M.J.; Hill, R.T.; Negri, A.P. The effects of copper on the microbial community of a coral reef sponge. Environ. Microbiol. 2001, 3, 19–31. [Google Scholar] [CrossRef]
- Santos-Gandelman, J.F.; Cruz, K.; Crane, S.; Muricy, G.; Giambiagi-deMarval, M.; Barkay, T.; Laport, M.S. Potential application in mercury bioremediation of a marine sponge-isolated Bacillus cereus strain Pj1. Curr. Microbiol. 2014, 69, 374–380. [Google Scholar] [CrossRef]
- Sajayan, A.; Kiran, G.S.; Priyadharshini, S.; Poulose, N.; Selvin, J. Revealing the ability of a novel polysaccharide bioflocculant in bioremediation of heavy metals sensed in a Vibrio bioluminescence reporter assay. Environ. Pollut. 2017, 228, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Orani, A.M.; Barats, A.; Zitte, W.; Morrow, C.; Thomas, O.P. Comparative study on the bioaccumulation and biotransformation of arsenic by some northeastern Atlantic and northwestern Mediterranean sponges. Chemosphere 2018, 201, 826–839. [Google Scholar] [CrossRef] [PubMed]
- Orani, A.M.; Vassileva, E.; Azemard, S.; Thomas, O.P. Comparative study on Hg bioaccumulation and biotransformation in Mediterranean and Atlantic sponge species. Chemosphere 2020, 260, 127515. [Google Scholar] [CrossRef]
- Ravindran, A.; Sajayan, A.; Priyadharshini, G.B.; Selvin, J.; Kiran, G.S. Revealing the efficacy of thermostable biosurfactant in heavy metal bioremediation and surface treatment in vegetables. Front. Microbiol. 2020, 11, 222. [Google Scholar] [CrossRef]
- Freitas-Silva, J.; de Oliveira, B.F.R.; Dias, G.R.; de Carvalho, M.M.; Laport, M.S. Unravelling the sponge microbiome as a promising source of biosurfactants. Crit. Rev. Microbiol. 2022, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Almeida, E.L.; Rincón, A.F.C.; Jackson, S.A.; Dobson, A.D. In silico screening and heterologous expression of a polyethylene terephthalate hydrolase (PETase)-like enzyme (SM14est) with polycaprolactone (PCL)-degrading activity, from the marine sponge-derived strain Streptomyces sp. SM14. Front. Microbiol. 2019, 10, 2187. [Google Scholar] [CrossRef] [PubMed]
- Perez, T.; Sarrazin, L.; Rebouillon, P.; Vacelet, J. First evidences of surfactant biodegradation by marine sponges (porifera): An experimental study with a linear alkylbenzenesulfonate. Hydrobiologia 2002, 489, 225–233. [Google Scholar] [CrossRef]
- Bonugli-Santos, R.C.; Durrant, L.R.; Sette, L.D. The production of ligninolytic enzymes by marine-derived Basidiomycetes and their biotechnological potential in the biodegradation of recalcitrant pollutants and the treatment of textile effluents. Water Air Soil Pollut. 2012, 223, 2333–2345. [Google Scholar] [CrossRef]
- Dhasayan, A.; Kiran, G.S.; Selvin, J. Production and characterisation of glycolipid biosurfactant by Halomonas Sp. MB-30 for potential application in enhanced oil recovery. Appl. Biochem. Biotechnol. 2014, 174, 2571–2584. [Google Scholar] [CrossRef]
- Loredana, S.; Graziano, P.; Antonio, M.; Carlotta, N.M.; Caterina, L.; Maria, A.A.; Carlo, Z.; Giuseppe, C.; Pietro, A. Lindane bioremediation capability of bacteria associated with the Demosponge Hymeniacidon perlevis. Mar. Drugs 2017, 15, 108. [Google Scholar] [CrossRef]
- Holert, J.; Cardenas, E.; Bergstrand, L.H.; Zaikova, E.; Hahn, A.S.; Hallam, S.J.; Mohn, W.W. Metagenomes reveal global distribution of bacterial steroid catabolism in natural, engineered, and host environments. mBio 2018, 9, e02345-17. [Google Scholar] [CrossRef] [PubMed]
- Bojko, B.; Onat, B.; Boyaci, E.; Psillakis, E.; Dailianis, T.; Pawliszyn, J. Application of in situ solid-phase microextraction on Mediterranean sponges for untargeted exometabolome screening and environmental monitoring. Front. Mar. Sci. 2019, 6, 632. [Google Scholar] [CrossRef]
- Vasconcelos, M.R.; Vieira, G.A.; Otero, I.V.; Bonugli-Santos, R.C.; Rodrigues, M.V.; Rehder, V.L.; Ferro, M.; Boaventura, S.; Bacci, M.; Sette, L.D. Pyrene degradation by marine-derived ascomycete: Process optimization, toxicity, and metabolic analyses. Environ. Sci. Pollut. Res. 2019, 26, 12412–12424. [Google Scholar] [CrossRef] [PubMed]
- Horna-Gray, I.; Lopez, N.A.; Nijenhuis, I.; Ahn, Y.; Richnow, H.H.; Häggblom, M.M. Reductive debromination by sponge-associated anaerobic bacteria coupled to carbon isotope fractionation. Int. Biodeterior. Biodegrad. 2020, 155, 105093. [Google Scholar] [CrossRef]
- Marzuki, I.; Nisaa, K.; Asaf, R.; Athirah, A.; Paena, M.; Susianingsih, E.; Nurhidayah, N.; Kadriah, I.A.K.; Kamaruddin, K.; Sahabuddin, S. Comparison of pyrene biodegradation using two types of marine bacterial isolates. Sustainability 2022, 14, 9890. [Google Scholar] [CrossRef]
- Schleuter, D.; Günther, A.; Paasch, S.; Ehrlich, H.; Kljajić, Z.; Hanke, T.; Bernhard, G.; Brunner, E. Chitin-based renewable materials from marine sponges for uranium adsorption. Carbohydr. Polym. 2013, 92, 712–718. [Google Scholar] [CrossRef]
- Wang, D.; Song, J.; Lin, S.; Wen, J.; Ma, C.; Yuan, Y.; Lei, M.; Wang, X.; Wang, N.; Wu, H. A marine-inspired hybrid sponge for highly efficient uranium extraction from seawater. Adv. Funct. Mater. 2019, 29, 1901009. [Google Scholar] [CrossRef]
- Machałowski, T.; Jankowska, K.; Bachosz, K.; Smulek, W.; Ehrlich, H.; Kaczorek, E.; Zdarta, J.; Jesionowski, T. Biocatalytic system made of 3D chitin, silica nanopowder and horseradish peroxidase for the removal of 17α-ethinylestradiol: Determination of process efficiency and degradation mechanism. Molecules 2022, 27, 1354. [Google Scholar] [CrossRef]
- Bergmann, W.; Feeney, R.J. Contributions to the Study of Marine Products. XXXII. The Nucleosides of Sponges. I. J. Org. Chem. 1951, 16, 981–987. [Google Scholar] [CrossRef]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2019, 36, 122–173. [Google Scholar] [CrossRef]
- Proksch, P.; Edrada, R.; Ebel, R. Drugs from the seas–current status and microbiological implications. Appl. Microbiol. Biotechnol. 2002, 59, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Maslin, M.; Gaertner-Mazouni, N.; Debitus, C.; Joy, N.; Ho, R. Marine sponge aquaculture towards drug development: An ongoing history of technical, ecological, chemical considerations and challenges. Aquac. Rep. 2021, 21, 100813. [Google Scholar] [CrossRef]
- Sipkema, D.; Franssen, M.C.; Osinga, R.; Tramper, J.; Wijffels, R.H. Marine sponges as pharmacy. Mar. Biotechnol. 2005, 7, 142–162. [Google Scholar] [CrossRef] [PubMed]
- Rotter, A.; Barbier, M.; Bertoni, F.; Bones, A.M.; Cancela, M.L.; Carlsson, J.; Carvalho, M.F.; Cegłowska, M.; Chirivella-Martorell, J.; Conk Dalay, M.; et al. The essentials of marine biotechnology. Front. Mar. Sci. 2021, 8, 629629. [Google Scholar] [CrossRef]
- Varijakzhan, D.; Loh, J.-Y.; Yap, W.-S.; Yusoff, K.; Seboussi, R.; Lim, S.-H.E.; Lai, K.-S.; Chong, C.M. Bioactive compounds from marine sponges: Fundamentals and applications. Mar. Drugs 2021, 19, 246. [Google Scholar] [CrossRef]
- Lipton, A.P.; Sunith, S. Mariculture of marine sponges for drug development: Bioactivity potentials of cultured sponges, Callyspongia subarmigera (Ridley) and Echinodictyum gorgonoides (Dendy). Mar. Fish. Infor. Serv. T&E Ser. 2009, 202, 7–10. Available online: http://eprints.cmfri.org.in/id/eprint/6469 (accessed on 26 November 2022).
- Page, M.J.; Handley, S.J.; Northcote, P.T.; Cairney, D.; Willan, R.C. Successes and pitfalls of the aquaculture of the sponge Mycale hentscheli. Aquaculture 2011, 312, 52–61. [Google Scholar] [CrossRef]
- Santiago, V.S.; Manzano, G.G.; Clairecynth, C.Y.; Aliño, P.M.; Salvador-Reyes, L.A. Mariculture potential of renieramycin-producing philippine blue sponge Xestospongia sp. (Porifera: Haplosclerida). Aquaculture 2019, 502, 356–364. [Google Scholar] [CrossRef]
- Mishra, S.; Das, R.; Swain, P. Status of fish diseases in aquaculture and assessment of economic loss due to disease. In Contemporary Trends in Fisheries and Aquaculture; Rao, P., Pandey, B., Pandey, P., Joshi, B.D., Eds.; Today & Tomorrow’s Printers and Publishers: New Delhi, India, 2018. [Google Scholar]
- Randall, J.E.; Hartman, W.D. Sponge-feeding fishes of the West Indies. Mar. Biol. 1968, 1, 216–225. [Google Scholar] [CrossRef]
- Wulff, J.L. Sponge Feeding by Caribbean Angelfishes, Trunkfishes, and Filefishes. In Sponges in Time and Space; van Soests, R.W.M., van Kempen, T.M.G., Braekman, J.C., Eds.; Balkema: Rotterdam, The Netherlands, 1994; pp. 265–271. [Google Scholar]
- Ruzicka, R.; Gleason, D.F. Latitudinal variation in spongivorous fishes and the effectiveness of sponge chemical defenses. Oecologia 2008, 154, 785–794. [Google Scholar] [CrossRef]
- Kaandorp, J.A.; De Kluijver, M.J. Verification of fractal growth models of the sponge Haliclona oculata (Porifera) with transplantation experiments. Mar. Biol. 1992, 113, 133–143. [Google Scholar] [CrossRef]
- van Treeck, P.; Eisinger, M.; Müller, J.; Paster, M.; Schuhmacher, H. Mariculture trials with Mediterranean sponge species: The exploitation of an old natural resource with sustainable and novel methods. Aquaculture 2003, 218, 439–455. [Google Scholar] [CrossRef]
- Kelly, M.; Handley, S.; Page, M.; Butterfield, P.; Hartill, B.; Kelly, S. Aquaculture trials of the New Zealand bath-sponge Spongia (Heterofibria) manipulatus using lanterns. N. Z. J. Mar. Freshw. Res. 2004, 38, 231–241. [Google Scholar] [CrossRef]
- De Voogd, N.J. An assessment of sponge mariculture potential in the Spermonde Archipelago, Indonesia. J. Mar. Biolog. Assoc. UK 2007, 87, 1777–1784. [Google Scholar] [CrossRef]
- de Voogd, N.J. The mariculture potential of the Indonesian reef-dwelling sponge Callyspongia (Euplacella) biru: Growth, survival and bioactive compounds. Aquaculture 2007, 262, 54–64. [Google Scholar] [CrossRef]
- Carballo, J.L.; Yañez, B.; Zubía, E.; Ortega, M.J.; Vega, C. Culture of explants from the sponge Mycale cecilia to obtain bioactive mycalazal-type metabolites. Mar. Biotechnol. 2010, 12, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Gökalp, M.; Wijgerde, T.; Murk, A.; Osinga, R. Design for large-scale maricultures of the mediterranean demosponge Chondrosia reniformis Nardo, 1847 for collagen production. Aquaculture 2022, 548, 737702. [Google Scholar] [CrossRef]
- Giangrande, A.; Gravina, M.F.; Rossi, S.; Longo, C.; Pierri, C. Aquaculture and restoration: Perspectives from mediterranean sea experiences. Water 2021, 13, 991. [Google Scholar] [CrossRef]
- Baldacconi, R.; Cardone, F.; Longo, C.; Mercurio, M.; Marzano, C.N.; Gaino, E.; Corriero, G. Transplantation of Spongia officinalis L. (Porifera, Demospongiae): A technical approach for restocking this endangered species. Mar. Ecol. 2010, 31, 309–317. [Google Scholar] [CrossRef]
- Biggs, B.C. Harnessing natural recovery processes to improve restoration outcomes: An experimental assessment of sponge-mediated coral reef restoration. PLoS ONE 2013, 8, e64945. [Google Scholar] [CrossRef]
- De Caralt, S.; Sánchez-Fontenla, J.; Uriz, M.J.; Wijffels, R.H. In situ aquaculture methods for Dysidea avara (Demospongiae, Porifera) in the Northwestern Mediterranean. Mar. Drugs 2010, 8, 1731–1742. [Google Scholar] [CrossRef] [PubMed]
- Webster, N.S.; Cobb, R.E.; Negri, A.P. Temperature thresholds for bacterial symbiosis with a sponge. ISME J. 2008, 2, 830–842. [Google Scholar] [CrossRef] [PubMed]
- United Nations Department of Economic and Social Affairs. The Sustainable Development Goals Report; United Nations: New York, NY, USA, 2022; p. 68. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilo-Arce, J.; Ferriol, P.; Trani, R.; Puthod, P.; Pierri, C.; Longo, C. Sponges as Emerging By-Product of Integrated Multitrophic Aquaculture (IMTA). J. Mar. Sci. Eng. 2023, 11, 80. https://doi.org/10.3390/jmse11010080
Aguilo-Arce J, Ferriol P, Trani R, Puthod P, Pierri C, Longo C. Sponges as Emerging By-Product of Integrated Multitrophic Aquaculture (IMTA). Journal of Marine Science and Engineering. 2023; 11(1):80. https://doi.org/10.3390/jmse11010080
Chicago/Turabian StyleAguilo-Arce, Joseba, Pere Ferriol, Roberta Trani, Patrizia Puthod, Cataldo Pierri, and Caterina Longo. 2023. "Sponges as Emerging By-Product of Integrated Multitrophic Aquaculture (IMTA)" Journal of Marine Science and Engineering 11, no. 1: 80. https://doi.org/10.3390/jmse11010080
APA StyleAguilo-Arce, J., Ferriol, P., Trani, R., Puthod, P., Pierri, C., & Longo, C. (2023). Sponges as Emerging By-Product of Integrated Multitrophic Aquaculture (IMTA). Journal of Marine Science and Engineering, 11(1), 80. https://doi.org/10.3390/jmse11010080








