1. Introduction
Cabbage (
Brassica oleracea L. var.
capitata) is one of the most extensively cultivated vegetable crops in Central Europe, with annual production in the region exceeding several million tons [
1]. Poland, Germany, and the Czech Republic are among the foremost producers in the European Union, contributing substantially to both domestic consumption and export markets [
2]. Cabbage plays a significant role in regional agricultural systems, and its economic value extends beyond fresh consumption to processing industries. Nevertheless, the cultivation of cabbage is facing mounting challenges from a variety of insect pests. Among these, the cabbage stink bug (
Eurydema ventralis Kolenati, 1846) (Hemiptera: Pentatomidae) is of particular concern, as it is becoming increasingly prevalent and significant [
1,
3,
4].
E. ventralis, a pentatomid cabbage stink bug, is distinguished by its bright coloration. It is a specialist herbivore that feeds on cruciferous plants, including economically significant crops such as cabbage, kale, and mustard. The pest’s feeding behavior involves the puncturing of leaves and stems in order to extract the juices of the plant. This results in the formation of pale, discolored spots, tissue collapse and, in certain instances, deformation of developing heads [
5]. Although historically considered a sporadic or minor pest, recent faunistic records from several Central European countries—including the Czech Republic, Slovenia, Austria, and Ukraine—indicate a broader distribution and increasing frequency of observations of
E. ventralis, pointing to its emerging importance in Brassicaceae crops [
3,
4,
6,
7]. In specific regions, infestation levels have been observed to result in visible damage to up to 30–40% of plants in untreated fields [
8]. This phenomenon has given rise to concerns among both conventional and organic growers. The increasing impact of
E. ventralis in Central Europe is likely linked to a combination of factors, including milder winters, longer growing seasons, and changes in cropping patterns that favor
Brassica monocultures [
9]. Moreover, the shift towards more environmentally sustainable pest management strategies, such as the reduced utilization of broad-spectrum insecticides in accordance with integrated pest management (IPM) guidelines and the emergence of organic farming, has given rise to management gaps for pests such as
E. ventralis, which are not consistently effectively addressed by current methodologies [
10,
11].
Despite its recent emergence as a notable pest in Brassica crops, the cabbage stink bug (Eurydema ventralis) has received relatively little scientific attention compared to other pests such as Plutella xylostella (L.) (Lepidoptera: Plutellidae) and Brevicoryne brassicae (L.) (Hemiptera: Aphididae). Knowledge of its biology, life cycle, population dynamics, and management remains limited, particularly under Central European conditions. As a result, many growers still rely on broad-spectrum plant protection products or lack region-specific guidelines—leading to inconsistent or excessive pest control measures.
This review aims to consolidate existing knowledge on the occurrence, biology, feeding behavior, and economic impact of E. ventralis in cabbage cultivation, with a special focus on Europe, including Slovenia. It evaluates current monitoring and assessment methods and reviews available control strategies—chemical, biological, cultural, and mechanical—within the context of sustainable pest management. By synthesizing fragmented literature and identifying key knowledge gaps, this article supports more effective and ecologically sound approaches to managing E. ventralis in Brassica production systems.
2. Taxonomy and Biology
2.1. Taxonomy
The cabbage stink bug is a phytophagous stink bug classified within the order Hemiptera and the suborder Heteroptera, family Pentatomidae, and subfamily Pentatominae [
5,
12]. It belongs to the tribe Strachiini, whose members are characterized by their specialization on cruciferous host plants (Brassicaceae) [
13,
14].
E. ventralis can be distinguished from closely related species, such as
Eurydema oleracea (L.) (Hemiptera: Pentatomidae) and
Eurydema ornata (L.) (Hemiptera: Pentatomidae), on the basis of specific coloration patterns and the structure of the pronotum [
15]. These characteristics are critical for field and laboratory identification.
Recent advancements in molecular taxonomy have led to a more precise delineation of species within the genus
Eurydema. The efficacy of DNA barcoding, particularly that based on the mitochondrial cytochrome oxidase I (COI) gene, has been demonstrated in the differentiation of closely related taxa and the confirmation of morphological identifications [
16,
17]. The use of integrative taxonomic approaches is crucial for the accurate identification of pests and the subsequent management of agroecosystems.
2.2. Morphology and Life Cycle
The adult cabbage stink bug (
E. ventralis) typically measures between 9–12 mm in length and exhibits sexual dimorphism, with females generally larger than males. The body is dorsoventrally flattened, the pronotum is red with six black spots, and black markings are also present on the scutellum and hemelytra (
Figure 1). The contrasting coloration has been shown to function as a warning to potential predators [
5], as these bugs can secrete foul-smelling defensive compounds [
18,
19]. The antennae are four- to five-segmented, the scutellum is triangular in shape and covers a larger part of the abdomen, and the tarsi are three-segmented [
5].
The cabbage stink bug undergoes incomplete metamorphosis, comprising egg, nymph, and adult stages (imago) [
20]. During April and May, adult cabbage stink bugs leave their overwintering locations. Moreover, with the emergence of cultivated cabbage plants, sprouts, and seedlings, the stink bugs fly over to them. After mating (
Figure 1), females deposit twelve eggs, which are arranged in two rows (six eggs in each row), predominantly on the underside of the leaves [
21]. It has been observed that females may hatch up to seven egg clutches at intervals. The eggs of the cabbage stink bug are up to 1 mm high, cylindrical in shape, with a rounded bottom, and the top is covered by a convex lid that opens when the nymph hatches. The eggs exhibit a greenish hue and are marked by a dark spot on the cover [
22]. The newly hatched nymphs are very small; they group together immediately after hatching and do not feed. Nymphs, which are yellow and black (
Figure 2), emerge approximately after 5–13 days, depending on environmental conditions.
After the first molting, individuals start to leave the group and feed. With subsequent molts, the nymphs grow and become more adult-like. The lifespan of nymphs is typically brief, lasting for a matter of weeks. The development of larvae undergoes a 25–40-day period of feeding on the plants before undergoing the metamorphosis into an imago. Furthermore, the complete life cycle from egg to imago is estimated to span of 2–2.5 months. The adults are known to overwinter in a variety of environments, including in the leaf litter at the forest edges, urban parks and gardens, slopes, and roadsides. In September, adult cabbage stink bugs that are able to fly move to a suitable overwintering site [
21]. Tanasijević and Ilić [
23] reported that in Serbia,
E. ventralis has two generations per year, and the same was reported for Croatia by Maceljski [
24]. The first generation is typically observed in the second half of June, with the second generation appearing in mid-August.
3. Distribution
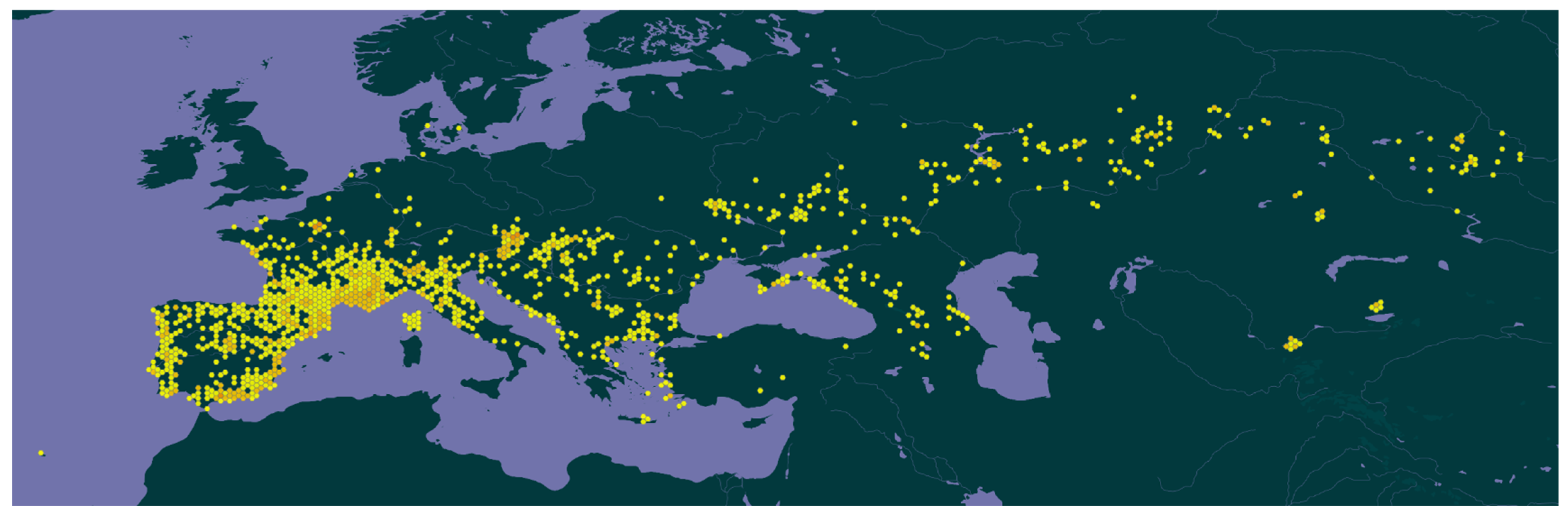
The cabbage stink bug is predominantly located within the Palearctic region and has a wide distribution across Europe and parts of Asia (
Figure 3). It is commonly found in southern and central Europe, with confirmed records in Italy, France, Austria, Slovenia, Hungary, Greece, and the Balkans, extending to the east in Ukraine, Moldova, and the Caucasus, Central Asia, and Western Siberia [
5,
25,
26,
27,
28]. These regions (i.e., Central Asia and West Siberia) represent the core distribution area, where the species is most abundant and ecologically adapted [
27]. Distribution in parts of Central Asia and West Siberia indicates a high adaptability to various temperate conditions [
5]. The species has recently been reported for the first time in Macaronesia (Madeira, Portugal), suggesting a potential expansion of its range [
25]. Moreover, this species was also recorded in the southern parts of East Siberia, in Tuva [
29] and Buriatia [
30]. While primarily associated with agricultural areas and wild habitats hosting cruciferous plants,
E. ventralis has not yet been recorded in North America, Australia, or sub-Saharan Africa, indicating its distribution is still mostly Eurasian. Moreover, the study of [
31] about the mitogenomic analysis of
Eurydema genus supports the idea of the ecological specialization of the cabbage stink bug and its wide but regionally specific distribution.
Despite its broad distribution, specific data on the frequency or population density of
E. ventralis remain limited. The majority of records are derived from sporadic field observations or museum collections, as opposed to systematic population monitoring [
27]. However, the species has been repeatedly documented in both agricultural and semi-natural habitats, suggesting that it may be locally abundant in areas where host plants are present. The cabbage stink bug is a widely distributed species. It is well established in the Mediterranean region and there are growing reports from other parts of Europe and Asia. Its adaptability and close association with cruciferous plants make it an important species for agricultural monitoring and pest management.
4. Pentatomidae Found on Brassicaceae in Slovenia
Slovenia is characterized by a diverse assemblage of stink bug species (Hemiptera: Pentatomidae), some of which are considered key agricultural pests. Among native species,
E. ventralis is considered to be one of the most significant pests of cruciferous plants, particularly cabbage. Adults overwinter and emerge in spring, with one to two generations per year. The feeding of the pest occurs on leaves and stems of the plant, resulting in chlorosis, tissue necrosis, and economic losses. As Bohinc et al. [
33] demonstrate, the damage intensity exhibited by different cabbage genotypes is subject to variation, with this variation being dependent upon characteristics such as plant color, growth duration, and cultivar type.
E. oleracea, a species closely related to E. ventralis, is another native species frequently found on Brassicaceae. Through exhibiting a slightly lesser degree of damage, it displays analogous feeding habits and seasonal biology. It is evident that both species have a substantial impact on the yield reduction of cabbage and other crucifers.
In recent years, the occurrence of invasive stink bug species has also been documented in Slovenia. The most notable of these is the brown marmorated stink bug (
Halyomorpha halys (Stål, 1855)) (Hemiptera: Pentatomidae), which is originally from East Asia. First documented in the Goriška region in 2017, it has since spread throughout Slovenia, including urban areas such as Ljubljana [
34,
35]. The brown marmorated stink bug is a highly polyphagous pest, affecting over 170 different host plants, including fruits, vegetables, and ornamentals. In the sub-Mediterranean climate of western Slovenia, the species completes two consecutive generations per year. The population abundance of the species is driven by local climatic conditions, including temperature and humidity [
36].
Another species of increasing concern is the southern green stink bug (
Nezara viridula L.) (Hemiptera: Pentatomidae). Despite its cosmopolitan character, it has been established in Slovenia since at least the 1980s, with a notable preference along the Adriatic coast. It is characterized by its highly polyphagous nature, which enables it to feed on cabbage, particularly in warm climates. Genetic analyses demonstrate that Slovenian populations of the southern green stink bug are closely related to those in other parts of Europe and the Americas [
37]. Furthermore, the species has been the focus of extensive research concerning its communication and mating behavior, with the study of substrate-borne vibrational signals providing significant insights into species recognition mechanisms [
38].
Notably, the striped shield bug (
Graphosoma lineatum L.) (Hemiptera: Pentatomidae) has also been documented in Slovenia. While it is primarily associated with the Apiaceae family of plants, its presence has been noted in agricultural ecosystems, including cabbage fields. While its impact on cabbage is negligible, its role in biodiversity and pest monitoring is worth acknowledging [
12].
The increasing diversity of both native and invasive stink bugs in Slovenia poses significant challenges for cabbage growers. The effective management of pests necessitates a combination of factors, including regular monitoring, precise species identification, and the integration of biological and chemical control measures. Recent studies also highlight the potential role of native natural enemies in suppressing pest populations, offering sustainable alternatives to chemical insecticides [
39].
5. Impact on Cabbage Production
5.1. Feeding Habits and Injuries on Plants
E. ventralis is a phytophagous insect that primarily affects cruciferous plants, with cabbage being among its preferred hosts. Among the host plants, the most frequently mentioned besides cabbage are also other plants of the family Brassicaceae, such as cauli-flower (
Brassica oleracea L. var.
botrytis L.
cauliflora), kale (
Brassica oleracea L. var.
sabauda L.), white mustard (
Sinapis alba L.), and many others [
5,
40,
41,
42].
Adult bugs and nymphs have a stylet, which they use to pierce plant tissues and extract plant sap. Their feeding activity is particularly concentrated on the softer, younger parts of the cabbage plant, such as young leaves, leaf veins, and developing parts [
43]. The feeding mechanism of
E. ventralis has been shown to cause both direct mechanical damage and indirect physiological stress to the plant. Saliva injected during feeding can lead to localized necrosis, discoloration, and tissue deformation. The symptoms of typical damage are as follows: the presence of whitish and yellowish speckling on leaves (
Figure 4 and
Figure 5) can be attributed to chlorophyll loss, with necrotic spots as a result of intense feeding, causing the deformation and curling of young leaves [
33], and, in severe infestations, stunted growth and reduced head formation have been observed [
26]. The tendency for insects to congregate and feed in groups often results in the concentration and visibility of damage, particularly on the outer leaves of the infested vegetation. Adult bugs are more mobile and can spread easily across the field, while nymphs remain localized and can cause cumulative damage over time if left uncontrolled [
44]. While specific dispersal distance data for
E. ventralis are currently unavailable, studies on related Pentatomidae species provide useful standards. For instance,
H. halys adults have been shown to fly average distances of ~2.0–2.4 km in 22 h flight mill tests, with most individuals (<89%) flying under 5 km and some reaching up to ~117 km in a single flight period [
45]. Similarly,
Euschistus servus (Say, 1832) (Hemiptera: Pentatomidae) demonstrated that ~90% of adults flew less than 1 km, while exceptional individuals flew up to ~15.9 km [
46]. These findings suggest that
E. ventralis adults may similarly achieve local field dispersal across tens to hundreds of meters, with occasional longer movements under favorable conditions.
Regarding the potential of
E. ventralis to transmit pest bacteria and viruses, current scientific literature provides limited information. Unlike some other Hemiptera species, such as aphids or whiteflies, which are well-documented vectors of plant viruses [
47,
48], there is no conclusive evidence that
E. ventralis acts as a vector for significant plant pathogens. Most research on
E. ventralis has focused on its direct feeding damage rather than pathogen transmission [
3,
4]. However, considering its piercing–sucking feeding behavior, the potential for transmission of bacterial or viral pathogens cannot be entirely ruled out and warrants further investigation. This knowledge gap represents an important direction for future research, especially in light of its increasing prevalence in cabbage crops.
The wilt is characterized by the appearance of white spots on the leaves due to the suction caused by the pest. In more severe cases, the leaf becomes translucent and perforated. The tissue within these areas has undergone necrosis; in cases where the damage is severe, the leaf may wither or the entire plant may perish. Damage is most extensive on young plants, after germination of after transplantation [
24]. Phytophagous stink bugs are renowned for their discerning approach to selecting suitable host plants. Furthermore, insect species exhibit variation in their feeding preferences, with some species specializing on specific plant parts. The selection of the host plant is contingent upon the ‘stimuli’ that are emitted by either the host or non-host plants. The insects’ ability to respond to external stimuli is the result of a complex interplay between mechanical and olfactory factors. In addition to these factors, the environment provides a context for the interaction between males and females, facilitating the process of mating. This information can be considered as a fundamental principle in the context of pest management, particularly in scenarios where the nymph and imago share the same host plant. The host plant is typically selected by the female through the deposition of eggs on a leaf or other above-ground organ. In selecting a suitable host plant, the stink bug makes a decision that is informed by a multitude of factors. The decision-making process is composed of multiple stages, which can be categorized as follows: initial orientation in space, subsequent landing on the plant, feeding, and oviposition [
49].
5.2. Economic Impact
Although
E. ventralis rarely results in complete crop failure, its impact on cabbage quality can be economically significant. The following factors have been identified as contributing to yield and revenue loss: (1) the market value of produce is reduced due to visible feeding damage, even when internal tissues remain unaffected [
26]; (2) higher culling rates are observed, especially in crops grown for fresh market sale, where visual appearance is critical [
43]; (3) increased production costs are incurred, related to pest monitoring and control measures; (4) there is a potential for secondary infections, as feeding wounds may serve as entry points for pathogens [
39].
In organic and low-input systems, where the use of synthetic insecticides is limited or should be avoided, the cabbage stink bug can present a particular challenge in terms of management, resulting in greater reliance on preventive cultural practices and biological control [
26]. To summarize, although the cabbage stink bug does not invariably result in substantial yield loss, its effect on crop quality and market value renders it an economically significant pest, especially in regions where infestations are frequent or where cabbage is cultivated on a large scale [
33].
6. Monitoring and Population Assessment Methods of Eurydema ventralis
The implementation of effective monitoring and population assessment techniques is crucial for the successful implementation of IPM strategies in cruciferous plants, such as cabbage, for the management of
E. ventralis. Monitoring facilitates the timely detection of pest outbreaks, informs decision-making processes for control interventions, and assists in the evaluation of the effectiveness of applied management strategies. The most prevalent monitoring technique entails the visual inspection and direct enumeration of adult cabbage stink bugs and nymphs on cabbage plants. It is recommended that regular scouting is carried out, typically on a weekly basis during the growing season, in order to estimate population densities and detect early signs of infestation [
38]. Visual assessments are frequently conducted by means of inspecting a predetermined number of plants or specific areas within the field, and the number of insects per plant or per square meter is recorded [
50].
Sticky traps of various colors have been tested for monitoring Pentatomidae, including species of the genus
Eurydema. Recent studies indicate that purple sticky traps are particularly effective for capturing
E. ornata, and other phytophagous stink bugs [
51,
52]. While there is currently limited evidence for the preference of
E. ventralis, color-based trapping may represent a promising tool for future monitoring efforts [
5]. These traps function as an early warning system, a particularly advantageous feature in wide-ranging fields where visual inspections are time-consuming and also more intensive. Although these methods are widely used, it is important to acknowledge that neither visual inspections nor sticky traps provide a fully comprehensive assessment. Both approaches are subject to limitations such as observer bias, environmental conditions, and variability in insect activity patterns, which can influence the accuracy and consistency of population estimates.
Furthermore, the utilization of sweep net sampling is occasionally observed, particularly within the context of mixed cropping systems. However, it is important to note that the efficacy of this technique can be influenced by different factors such as plant density and canopy structure. With regard to quantitative population assessments, mark–recapture methods have also been explored, even though less commonly, due to their comprehensive nature [
53].
The threshold levels for intervention are not yet well established for the cabbage stink bugs. However, general action thresholds for stink bugs in brassicas suggest that control measures should be considered when average densities exceed one–two individuals per plant during the head formation stage [
54]. Moreover, many authors declare that the economic threshold of
E. ventralis harmfulness represents two–three individuals per plant [
5,
55]. Further research is required in order to refine these thresholds based on local agroecological conditions.
Advancements in remote sensing and machine learning technologies are opening up new possibilities for non-invasive monitoring, such as imago-based pest detection or prediction models using climatic and crop data. Nevertheless, it should be noted that these are still in developmental stages for most stink bug species [
56]. The regular and systematic monitoring of crop damage and the implementation of pest control strategies targeting the cabbage stink bug is of crucial importance to ensure the sustainability of these strategies.
7. Integrated Pest Management as a Framework for the Control of Eurydema ventralis
The management of agricultural pests has evolved from a reliance on single-method approaches to more integrated, knowledge-based strategies. Among these, IPM is distinguished by its comprehensive nature, integrating diverse tactics to regulate pest populations in a manner that is both economically and ecologically sustainable [
57,
58,
59]. In the context of cruciferous crops such as cabbage,
E. ventralis represents a persistent pest that demands precisely such an integrated approach due to its adaptability, mobility, and potential for economic damage. IPM is not a standalone method, but rather a dynamic decision-making process that utilizes all available pest management techniques in a coordinated manner. The implementation of IPM typically follows several core principles: the prevention of pests through agronomic and cultural practices; the regular monitoring of pest and natural enemy populations; the use of economic thresholds to determine the need for intervention; and the application of control methods—biological, chemical, mechanical, and cultural—in a compatible and strategic sequence [
58,
60].
In the context of the cabbage stink bug, IPM offers a framework within which diverse suppression strategies can be efficiently integrated. Cultural techniques, including crop rotation and trap cropping, act as the primary form of defense. It has been demonstrated that early-sown trap crops, such as oilseed rape or oil radish, have been effective in attracting cabbage stink bug adults, thereby reducing their presence in the cabbage as a main crop [
43]. The efficacy of such measures is maximized when employed in conjunction with timely mechanical or chemical interventions, which are targeted exclusively at the designated trap zones.
In the context of the IPM framework, biological control is a pivotal element, especially with regard to the conservation of generalist predators and parasitoids. While specific biological control agents for
E. ventralis are still under investigation, it has been demonstrated that agroecological diversification and habitat management can enhance natural enemy activity and suppress pest outbreaks [
61]. Chemical control, whilst still necessary in some cases, is used judiciously within IPM systems. The application of insecticides is based on established thresholds and monitoring data, with an emphasis on selectivity and rotation of active ingredients to prevent resistance and preserve beneficial organisms [
62,
63].
IPM encourages the strategic integration of control tactics with a view to minimizing environmental risks and promoting long-term pest suppression. The overarching context in which the individual methods—biological, chemical, cultural, and mechanical—are most effective when applied in combination rather than isolation is provided by the aforementioned concept. In the ensuing sections, each of these approaches will be discussed in greater detail in relation to their specific application against E. ventralis.
8. Chemical Control of Eurydema ventralis
Chemical control remains a key element in managing E. ventralis, particularly in conventional cabbage production. However, the efficacy, selectivity, and environmental safety of available insecticides vary significantly depending on the chemical class, application method, and local conditions. The following section compares the main insecticide groups in terms of their performance against stink bugs and discusses their compatibility with IPM.
8.1. Synthetic Pyrethroids
Pyrethroids, such as deltamethrin and cypermethrin, are widely used against hemipteran pests due to their rapid knockdown effect and low mammalian toxicity. They act on the nervous system by modifying voltage-gated sodium channels. Field trials in Germany and Poland have demonstrated that pyrethroids can be effective against pentatomid bugs, especially when combined with organophosphates like chlorpyrifos [
64,
65].
However, pyrethroids also exhibit broad-spectrum activity and pose risks to non-target arthropods, including natural enemies and pollinators. Moreover, their efficacy is influenced by environmental factors such as temperature and humidity. For example, deltamethrin toxicity increases under low soil moisture, amplifying its ecological impact in drought-prone areas [
66].
8.2. Organophosphates and Botanical Alternatives
Organophosphates like malathion interfere with insect neurotransmission by inhibiting acetylcholinesterase. A two-year field study in Slovenia showed that malathion (0.2%) significantly reduced
E. ventralis damage and improved cabbage growth [
26]. This treatment outperformed both potassium soap and refined rapeseed oil, although the latter botanical options showed moderate efficacy under dry conditions.
Botanical formulations (e.g., rapeseed oil, potassium soap) represent low-toxicity alternatives, compatible with organic systems. However, they often require repeated applications and may be less reliable under varying environmental conditions [
26].
8.3. Neonicotinoids
Neonicotinoids, such as thiacloprid and acetamiprid, have shown strong systemic activity against sucking pests by targeting nicotinic acetylcholine receptors. Although specific data for
E. ventralis are limited, their documented success against other Hemiptera suggests potential applicability [
67,
68].
Their systemic nature makes them suitable for early-stage protection, such as applications during the yellow-bud phase of cabbage development [
67]. Nevertheless, concerns remain regarding pollinator toxicity and environmental persistence, necessitating careful use within IPM frameworks.
8.4. Inert Dusts and Physical Barriers
Inert dusts like diatomaceous earth and wood ash offer a non-chemical control option. They act by disrupting the insect cuticle and desiccating pests. A field study demonstrated that these materials reduced stink bug damage and improved yields, particularly under dry conditions [
69]. Their mode of action makes them suitable for organic farming, though their effectiveness may be weather-dependent (
Table 1).
8.5. Resistance and Sustainability Considerations
Although insecticide resistance in
E. ventralis has not yet been extensively documented, other brassica pests like the diamondback moth (
P. xylostella) have developed resistance to a wide array of insecticide classes, including pyrethroids, organophosphates, carbamates,
Bt toxins, and insect growth regulators [
70,
71,
72]. This raises concerns about the long-term sustainability of chemical control for stink bugs.
Regular use of the same chemical group increases selection pressure and resistance risk. Therefore, resistance management strategies—including insecticide rotation and combinations, use of selective agents, and reliance on economic thresholds—are essential to preserve the efficacy of available tools.
8.6. Environmental Impacts and IPM Integration
The use of chemical insecticides in the management of
E. ventralis is associated with significant environmental considerations. Broad-spectrum insecticides, particularly pyrethroids and organophosphates, are known to adversely affect non-target arthropods, including beneficial predators such as lady beetles (Coccinellidae), ground beetles (Carabidae), and pollinators like bees [
67,
73]. The disruption of natural enemy populations has the capacity to weaken biological control services and lead to secondary pest outbreaks.
Pesticide residues, resulting from repeated applications, have been shown to persist in the environment, thereby contributing to the contamination of soil and aquatic ecosystems. Studies have demonstrated that insecticides such as deltamethrin exhibit high toxicity to aquatic invertebrates and soil fauna, including earthworms and springtails [
74,
75]. The ecological risk is further amplified under conditions of low soil moisture, which enhance the bioavailability and toxicity of pyrethroids like deltamethrin [
66].
In order to mitigate the risks associated with such practices, it is essential to exercise caution and regulate the utilization of insecticides. Responsible application practices include adherence to recommended dosages, proper timing to avoid the peak activity of non-target organisms, and application methods that minimize drift and runoff [
76]. It is equally important to select insecticides that have lower environmental persistence and selectivity for target pests. The most efficacious approach to minimizing environmental harm whilst preserving efficacy is the integration of chemical control into a broader IPM strategy. IPM promotes the combined use of chemical, biological, cultural, and mechanical control measures. Cultural techniques, such as crop rotation and trap cropping, have been demonstrated to reduce pest pressure, while the conservation of natural enemies supports long-term pest suppression [
77,
78].
Botanical insecticides and inert dusts represent promising low-impact alternatives. Refined rapeseed oil and potassium soap, though somewhat variable in efficacy, have demonstrated potential under certain conditions [
26]. Diatomaceous earth and wood ash have been demonstrated to be highly effective in reducing damage caused by
E. ventralis without the associated drawbacks of chemical toxicity, making them particularly suitable for organic and low-input systems [
68,
69].
Furthermore, systemic neonicotinoids such as thiacloprid, despite their efficacy against hemipterans, necessitate judicious application due to their association with sublethal effects on pollinators and apprehensions regarding their long-term persistence in the environment [
68,
79]. Consequently, their deployment should be strategic, preferably as part of rotation schemes with other insecticide classes.
Finally, resistance management is a pivotal component of sustainable chemical control. Although resistance in
E. ventralis has not been widely reported, analogies with other crucifer pests, such as
P. xylostella, which has developed resistance to multiple insecticide groups, serve as a warning [
70,
71,
72]. It is vital to monitor susceptibility levels, to rotate insecticides with different modes of action, and to incorporate non-chemical options, in order to prevent resistance buildup. In conclusion, it is imperative that environmental considerations are integrated into the pest management of
E. ventralis. The integration of chemical control within an IPM framework enables practitioners to achieve effective pest suppression while minimizing ecological disruption and delaying resistance evolution. This integrated approach is pivotal to ensuring the efficacy of cabbage protection programs in the short and long term [
61].
9. Biological Control of Eurydema ventralis
Reliance on synthetic control methods gives rise to concerns regarding environmental contamination, the development of insecticide resistance, and negative impacts on beneficial arthropods, including pollinators and natural enemies [
80]. Consequently, there has been an increased focus on the development and implementation of sustainable pest control strategies, with biological control representing a key role.
The term ‘biological control’ is employed to denote the utilization of natural enemies, including parasitoids, predators, and entomopathogens, for the purpose of diminishing pest populations [
80,
81]. In the context of the cabbage stink bug, the primary focus of biocontrol research has been on egg parasitoids belonging to the genus
Trissolcus. These have demonstrated high levels of parasitism in field conditions [
82]. Other promising candidates include tachinid flies that parasitize nymphs and adults, generalist predators, and microbial agents such as entomopathogenic fungi [
83]. These agents may act alone or synergistically within IPM programs [
81].
This chapter provides a comprehensive overview of the known biological control agents associated with the cabbage stink bug, highlighting their mechanisms of action, field efficacy, and potential integration into sustainable pest control systems (
Table 2).
9.1. Egg Parasitoids
Egg parasitoids are among the most extensively studied natural enemies of
E. ventralis. It has been demonstrated that the species of the genus
Trissolcus (Hymenoptera: Scelionidae) exhibit high rates of parasitism on
Eurydema eggs.
Trissolcus mitsukurii (Ashmead) (Hymenoptera: Scelionidae) has been studied for its olfactory responses to plants attacked by stink bugs, highlighting its potential as a biological control agent [
84]. However, this species is not currently associated with the parasitism of
E. ventralis, and further research is needed to assess its relevance for managing this pest. These parasitoids deposit their eggs within the host’s eggs, resulting in the consumption of the developing embryo by their larvae. This process effectively prevents the hatching of the host’s eggs.
Laboratory olfactometer studies have demonstrated that
Trissolcus simoni (Mayr, 1897) (Hymenoptera: Scelionidae) exhibits behavioral responses to chemical cues from
E. ventralis, suggesting its potential as a candidate biocontrol agent [
85]. The process of oviposition by these parasitoids occurs within the eggs of cabbage stink bugs. Indihar et al. [
82] reported that in Slovenia, egg parasitoids, namely
Trissolcus scutellaris (Thomson, 1861) (Hymenoptera: Scelionidae),
Trissolcus festivae (Viktorov, 1964) (Hymenoptera: Scelionidae), and
T. viktorovi Kozlov (Hymenoptera: Scelionidae), have been documented as natural enemies of the cabbage stink bug. It is evident that none of the mentioned species are yet listed on the Positive List of the European and Mediterranean Plant Protection Organization (EPPO); it is not possible for Slovenia to include them on the list of native species of organisms for biological control. Such implementation would facilitate the use of these species in inoculative biological control in field agricultural production. The efficacy of these parasitoids in the context of biological control programs is contingent upon a number of factors, including host specificity, prevailing environmental conditions, and the compatibility of the parasitoids with alternative control methodologies. Further research is required to assess their potential for mass rearing and release in integrated pest management strategies targeting
E. ventralis.
The effectiveness of these parasitoids in biological control programs depends on several factors, including host specificity, environmental conditions, and their compatibility with other control measures. Further studies are required to evaluate their potential for mass rearing and integration into pest management strategies.
9.2. Parasitoids of Nymphs and Adults
In addition to egg parasitoids, several species of tachinid flies (Diptera: Tachinidae) have been documented as
Eurydema bug parasites, including for
E. ventralis. Of particular interest are
Clytomyia continua (Panzer, 1798) (Diptera: Tachinidae) and
Ectophasia crassipennis (Fabricius, 1794) (Diptera: Tachinidae), which are endoparasitoids capable of attacking the later developmental stages, including nymphs and adults [
5,
86,
87]. These parasitoids deposit their eggs on or in the proximity of the host insect. Upon hatching, the larvae penetrate the host’s body and feed internally, ultimately resulting in the host’s demise as they complete their development. The tachinid fly
E. crassipennis is a well-documented parasitoid of various shield bugs (Pentatomidae) across Europe and Asia. It exhibits a broad host range within this family and has been recorded parasitizing other pest species such as
Eurydema oleracea and
Nezara viridula [
88]. Though specific parasitism rates on
E. ventralis have not been extensively quantified, the presence of
E. crassipennis in habitats where
Eurydema species are abundant suggests an underutilized potential for biological control.
C. continua is another tachinid species that has been observed to be associated with stink bugs on occasion. While the scientific literature on this species is comparatively limited when viewed in relation to that on
Phasia, it is nevertheless understood to parasitize heteropteran hosts. Moreover, field observations from Eastern Europe suggest that it may play a supplementary role in regulating pentatomid populations [
5,
86].
These parasitoids are distinguished from egg parasitoids by their targeting of older life stages, a feature that is particularly advantageous in IPM programs where overlapping generations of pests are present [
89]. Their activity can complement the impact of egg parasitoids such as
Trissolcus spp., providing broader temporal coverage and reducing the likelihood of population rebounds from surviving individuals [
85]. Nevertheless, the practical application of tachinid flies in biological control programs is frequently constrained by their intricate life cycles, issues of host specificity, and difficulties in mass rearing. Furthermore, it is imperative to meticulously evaluate their ecological impact to avert any inadvertent consequences on non-target native species [
89]. Despite these limitations, further research is warranted to assess their local abundance, parasitism rates, and potential synergy with other natural enemies.
9.3. Entomopathogenic Fungi
Despite the paucity of direct studies evaluating the efficacy of entomopathogenic fungi against
E. ventralis, there is an increasing interest in their potential application based on successful use against other pentatomid bugs. It is noteworthy that certain fungi, such as
Beauveria bassiana (Bals.-Criv.) Vuill. 1912 (Hypocreales: Cordycipitaceae) and
Metarhizium anisopliae (Metschn.) Sorokin 1883 (Hypocreales: Clavicipitaceae), have demonstrated considerable pathogenicity against related species, including
N. viridula,
Piezodorus guildinii (Westwood, 1837) (Hemiptera: Pentatomidae), and
H. halys [
90,
91]. In a recent study, Ramos et al. [
90] examined the effects of two isolates of
B. bassiana on
N. viridula and
P. guildinii, reporting high mortality rates and reduced fecundity. The results suggest that these fungal isolates could be screened for efficacy against
E. ventralis, especially considering the shared ecological niches and host plants among these stink bug species. In addition,
M. anisopliae has been shown to be effective against other Hemiptera, particularly during the early life stages, which are more susceptible to infection [
91]. These findings imply that younger nymphs of
E. ventralis may also be suitable targets for fungal biocontrol, especially under favorable humidity and temperature conditions that support spore germination and infection.
However, the integration of entomopathogenic fungi with other biological control agents, such as egg parasitoids (
Trissolcus spp.), necessitates meticulous planning. A systematic review by [
92,
93] emphasized that although combinations of fungi and parasitoids can enhance pest suppression, such strategies may not always be economically viable due to increased control costs. A total of 100 studies were reviewed; however, merely two of these evaluated critical agronomic metrics, including crop damage reduction, yield improvement, and economic return. This finding serves to emphasize the necessity for more holistic assessments.
9.4. Compatibility of Biocontrol Agents
The compatibility of entomopathogens and parasitoids is crucial for the implementation of integrated pest management strategies. Studies have shown that the timing of application can impact the efficacy of these combined biocontrol agents. For instance, the application of
B. bassiana following the emergence of parasitoids has been shown to mitigate the adverse effects on parasitoid development. Similarly, the spatial separation of microbial treatments and parasitoid releases can mitigate potential conflicts between these agents [
94] (
Table 2).
10. Cultural and Mechanical Measures for Managing Eurydema ventralis on Cabbage
The effective management of the cabbage stink bug is increasingly reliant upon the integration of cultural and mechanical control strategies within an IPM framework. Among cultural techniques, the use of trap crops has emerged as one of the most promising and ecologically sustainable approaches.
The practice of trap cropping involves the deliberate cultivation of alternative host species that are more attractive to pests than the primary crop. This strategy aims to divert pest populations from economically significant crops. For the cabbage stink bug, field studies conducted in Slovenia have demonstrated that the oilseed rape (
Brassica napus L.) and oilseed radish (
Raphanus sativus L.) are particularly attractive, especially during the early growth stages, serving as effective sink habitats for feeding and oviposition [
8,
33]. Once concentrated on the trap crops, stink bugs can be efficiently targeted with localized treatments such as spot spraying, manual removal, or destruction of the crop residue, thereby reducing the need for broad-spectrum insecticide applications in the cabbage field. The success of trap cropping is contingent on several critical factors. Spatial and temporal considerations, such as the earlier planting of trap crops to ensure greater attractiveness at the time of pest migration, are key to maximizing efficacy. The efficacy of border-row and inter-row planting patterns has been demonstrated. Furthermore, the implementation of active monitoring is imperative in order to prevent such trap crops from becoming sources of secondary infestation. The application of targeted insecticide treatments may be considered if necessary [
26,
95]. Integrating trap crops with visual lures (e.g., yellow sticky traps) and repellents (e.g., neem oil, garlic extract) in a push–pull strategy has also shown potential to enhance pest diversion [
61].
Beyond the practice of trap cropping, other cultural practices have been found to be effective in disrupting the pest’s life cycle and reducing overwintering success. These include intercropping cabbage with non-cruciferous species (e.g., cereals or early potatoes), crop rotation, and the timely removal of cruciferous weeds [
5,
96]. These methods have been shown to reduce the availability of alternative hosts and to alter the field ecology of
E. ventralis in ways that are deleterious to the latter. Cover crops, though less extensively studied in this particular context, contribute indirectly by enhancing soil structure and supporting populations of natural enemies. While not primarily intended to intercept
Eurydema bugs, they can nonetheless enhance system resilience and pest suppression in polyculture systems [
26,
97].
With regard to mechanical control, the implementation of physical exclusion measures, such as the use of fine mesh netting or floating row covers, has been demonstrated to be an effective strategy for preventing cabbage stink bug adults from accessing cabbage for feeding and oviposition. This efficacy is particularly pronounced when the exclusion measures are employed during the early stages of the season. Manual removal is a viable option in small-scale or organic systems, while practices such as post-harvest deep plowing have been shown to destroy overwintering sites and reduce pest emergence the following spring [
98].
However, the implementation of trap cropping is not without challenges. In the event of inadequate management, the cultivation of trap crops has the potential to become a conductive environment for the proliferation of pest populations, thereby deviating from the intended purpose of containment. It is evident that environmental factors, including rainfall variability and drought, have the capacity to influence the attractiveness of plants to pests, and consequently, to modify the effectiveness of pest management strategies. Furthermore, the selection of the trap crop species is critical; it must be consistently more attractive than the main crop, cover a sufficient area (often cited as at least 20% of the field) and be managed to avoid unintended pest abundance [
40,
99].
Notwithstanding these limitations, when integrated and monitored correctly, trap cropping can be considered a compelling strategy for reducing pesticide usage, aligning with organic and low-input production systems. This approach is of particular importance in instances where registered insecticides are deficient or resistance is a matter of concern, thereby contributing to the overall objective of biodiversity conservation.
Role of Glucosinolates and Natural Cabbage Resistance in Defense Against E. ventralis
Glucosinolates are secondary metabolites that contain sulfur and nitrogen and are specific to the Brassicaceae family. They are known to play a crucial role in plant defense. When the plant tissue is damaged, the enzymatic hydrolysis of glucosinolates by mirosinase produces biologically active compounds, including isothiocyanates and nitriles, which are toxic or deterrent to a wide range of herbivorous insects, including stink bugs [
8,
100].
The interaction between glucosinolates and stink bugs is complex. As demonstrated by field experiments, the presence of different glucosinolates in
Brassica crops has been shown to impact feeding preferences and the levels of damage caused by
Eurydema spp. [
99]. For instance, Bohinc et al. [
100] reported that glucobrassicin exhibited a significant negative correlation with feeding damage among several Brassica crops, including white mustard (
Sinapis alba L.), oilseed rape (
Brassica napus), and white cabbage, suggesting a deterrent effect. Conversely, certain aliphatic glucosinolates, including epiprogoitrin and progoitrin, exhibited a positive correlation with the extent of damage (e.g., r = +0.69 for epiprogoitrin in white mustard, and r = +0.76 for progoitrin in specific cabbage cultivars). This suggests a potential stimulatory effect on stink bug feeding behavior [
100]. Different responses emphasize the potential of exploiting glucosinolates for the management of pests. The breeding of resistant cultivars to enhance repellent compounds such as glucobrassicin is a potential solution. In addition, the use of attractive crops with high levels of feeding-stimulant glucosinolates (e.g., oilseed rape) as trap crops to divert pests from main cabbage crops has been demonstrated to be effective [
3,
43].
In addition to chemical traits, morphological and physiological characteristics play an important role in natural resistance. Marković et al. [
4] conducted a study on 20 cabbage genotypes and found that red cabbage exhibited consistently reduced feeding damage from
Eurydema spp. compared to white cabbage. This was largely attributed to the higher antioxidant capacity in red cabbage (mean 0.68 mmol 100 g
−1 fresh weight (FW) vs. 0.48 mmol 100 g
−1 FW in white cabbage), likely due to elevated levels of anthocyanins and other phenolic compounds. This antioxidant compounds can reduce oxidative stress caused by herbivory and thereby increase plant tolerance. Furthermore, the thickness of epicuticular wax was found to contribute to resistance. Cabbage genotypes with thicker wax layers, more prevalent in red cabbage, exhibited reduced levels of feeding injury [
4]. The wax acts as a physical barrier that interferes with insect attachment and feeding, thus providing an additional defensive mechanism. These findings are consistent with behavioral studies on host selection in
Eurydema spp., where both visual and chemical cues (e.g., surface traits and glucosinolate-related volatiles) impact host preference [
101].
11. Recent Advances and Future Perspectives in the Management of E. ventralis
In recent years, considerable attention has been devoted to the development of more sustainable and innovative methods for the control of E. ventralis, especially within the framework of integrated pest management. These approaches are intended to reduce reliance on chemical pesticides and to align pest control strategies with ecological and economic sustainability. New insights from chemical ecology, biotechnology, and precision agriculture are contributing significantly to the diversification and refinement of control options.
One of the most promising areas of research has emerged from the field of semiochemical-based pest management. Recent studies have demonstrated that particular plant volatiles, notably allyl isothiocyanate, exhibit a high degree of attraction by
E. ventralis, including both male and female adults [
19,
102]. This finding offers the potential for the development of effective lure-based trapping systems that could be employed for both monitoring and mass trapping purposes. Implementation of such systems would facilitate earlier detection of infestations, thereby serving as a component of targeted, non-chemical pest management strategies. Furthermore, advancements in biotechnology, particularly within the domain of insect microbiomes, are also generating novel avenues for research. Manipulation of endosymbionts (i.e., bacteria that live within pest insects) has demonstrated potential in disrupting insect physiology and behavior [
103]. In the context of
E. ventralis, research is beginning to explore whether altering gut symbionts could reduce pest fitness or pesticide resistance. Despite their current experimental status, these approaches have the potential to offer highly selective pest suppression tools with minimal non-target effects in the future.
Another significant area of research concerns the enhancement of pest forecasting and early-warning systems. The increasing integration of digital agriculture has led to the development of increasingly accurate and accessible pest risk models that utilize climate data, crop phenology, and insect life cycles [
104]. In the case of
E. ventralis, these models have the potential to enhance the timing of interventions and reduce the necessity for preventive insecticide use, thus contributing to the realization of economic and environmental objectives.
In the domain of biological control, recent efforts have been directed towards the identification and support of natural enemies that could be applied more systematically against stink bug populations. In Slovenia and neighboring countries, the indigenous parasitoids
Cotesia glomerata (L.) (Hymenoptera: Braconidae) and
Diadegma semiclausum (Hellén, 1949) (Hymenoptera: Ichneumonidae) are being evaluated for their capacity to contribute to the biological regulation of cruciferous pests, including
E. ventralis [
33]. Although the direct parasitism of
Eurydema ventralis by these species has not yet been decisively confirmed, their role in enhancing overall agroecosystem resilience is increasingly recognized.
Generally, these developments suggest that the future of E. ventralis control relies on integrated, knowledge-based systems that combine real-time monitoring, ecological interactions, and biotechnological tools. As research continues, there is likely to be a shift in emphasis towards precision pest management, where interventions are timed and tailored with increasing specificity, supported by digital technologies and ecological principles. It is evident that sustained investment in interdisciplinary research and field validation is crucial for the practical implementation of these findings.
12. Conclusions
The cabbage stink bug (E. ventralis) is a significant pest in cruciferous plant cultivation, particularly in cabbage, due to the direct feeding damage it causes, its economic impact, and its ability to persist under varied agroecological conditions. This review article synthesizes current knowledge on the taxonomy, biology, distribution, and economic relevance of the species, as well as critically evaluating the range of available management options.
Among control strategies, chemical insecticides continue to play a crucial role in reducing cabbage stink bug populations. However, concerns over resistance development and non-target effects emphasize the need for alternative approaches. Despite the fact that biological control remains underutilized, there is considerable potential for its future development, particularly in the context of increased attention being paid to native egg parasitoids, generalist predators, and entomopathogenic fungi. Cultural and mechanical methods, including crop rotation, weed management, and the strategic use of trap crops, are recognized as important preventive tools within an IPM framework.
IPM is a holistic concept that facilitates the integration of diverse suppression tactics while ensuring the minimalization of environmental and human health risks. Recent advancements in the fields of semiochemical-based trapping, microbiome modification, and pest monitoring models have indicated a number of promising results, indicating potential avenues for future research and development. However, it is crucial to emphasize that these innovations still require rigorous validation and practical implementation before they can be fully utilized.
Despite the progress that has been made, these are several knowledge gaps that persist, including an insufficiency of information on natural enemies specific to E. ventralis, inconsistent data on the efficacy of non-chemical methods under field conditions, and a need for standardized monitoring protocols. Addressing these gaps through interdisciplinary research and field-based trials is essential to developing more sustainable and locally adapted pest management strategies.
In prospective studies, a comprehensive strategy integrating conventional knowledge with innovative technologies will be pivotal for the efficient and ecologically sustainable management of E. ventralis. The enhancement of the adoption of science-based strategies is predicated on collaboration between researchers, extension services, and growers. This collaboration will ultimately contribute to the development of more resilient crucifer cropping systems.
Author Contributions
Conceptualization, S.A.Z. and S.T.; methodology, S.A.Z. and S.T.; software, S.A.Z.; validation, S.A.Z., T.B. and S.T.; formal analysis, S.A.Z.; investigation, S.A.Z.; resources, S.T.; data curation, S.A.Z.; writing—original draft preparation, S.A.Z.; writing—review and editing, S.A.Z. and S.T.; visualization, S.A.Z., T.B. and S.T.; supervision, S.T.; project administration, S.T.; funding acquisition, S.T. All authors have read and agreed to the published version of the manuscript.
Funding
This review paper was written as a part of the L4-4554 applied research project, which received financial support from the Slovenian Research and Innovation Agency (ARIS) and the Ministry of Agriculture, Forestry, and Food of the Republic of Slovenia (MKGP). Partly, this paper was written within Horticulture No. P4-0013-0481, a program funded by the Slovenian Research and Innovation Agency.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- FAOSTAT. Crops and Livestock Products; Food and Agriculture Organization of the United Nations: Rome, Italy, 2022; Available online: https://www.fao.org/faostat (accessed on 7 May 2025).
- Eurostat. Crop Production in the EU: Vegetables Statistics; Eurostat: Luxembourg, 2021; Available online: https://ec.europa.eu/eurostat (accessed on 7 May 2025).
- Bohinc, T.; Trdan, S. Stink bugs (Pentatomidae) as crop pests and methods of their control. Acta Agric. Slov. 2011, 97, 53–61. [Google Scholar]
- Marković, D.; Bohinc, T.; Trdan, S. Association between antioxidative potential and level of injury caused by Eurydema spp. feeding on red and white cabbage genotypes. Arch. Biol. Sci. 2014, 66, 1447–1456. [Google Scholar] [CrossRef]
- Stankevych, S.; Zabrodina, I.; Yushchuk, D.; Dolya, M.; Balan, H.; Yakovlev, R.; Kosylovych, H.; Holiachuk, Y.; Zakharchuk, N.; Galagan, T.; et al. Eurydema bugs: Review of distribution, ecology, harmfulness, and control. Ukr. J. Ecol. 2021, 11, 131–149. [Google Scholar]
- Rabitsch, W. Alien true bugs of Europe (Insecta: Hemiptera: Heteroptera). Zootaxa 2008, 1827, 1–44. [Google Scholar] [CrossRef]
- Kment, P.; Zdislava, K. Catalogue of type specimens of true bugs (Hemiptera: Heteroptera) deposited in the National Museum, Prague, Czech Republic—Enicocephalomorpha, Dipsocoromorpha, Nepomorpha, Gerromorpha, and Leptopodomorpha. Acta Entomol. Musei Natl. Pragae 2013, 53, 821–890. [Google Scholar]
- Bohinc, T.; Goreta Ban, S.; Ban, D.; Trdan, S. Glucosinolates in plant protection strategies: A review. Arch. Biol. Sci. 2012, 64, 821–828. [Google Scholar] [CrossRef]
- Svobodová, E.; Trnka, M.; Dubrovský, M.; Semerádová, D.; Eitzinger, J.; Štěpánek, P.; Žalud, Z. Determination of areas with the most significant shift in persistence of pests in Europe under climate change. Pest Manag. Sci. 2014, 70, 708–715. [Google Scholar] [CrossRef]
- Baker, B.P.; Green, T.A.; Loker, A.J. Biological control and integrated pest management in organic and conventional systems. Biol. Control 2020, 140, 104095. [Google Scholar] [CrossRef]
- Pecenka, J.R.; Ingwell, L.L.; Foster, R.E.; Krupke, C.H.; Kaplan, I. IPM reduces insecticide applications by 95% while maintaining or enhancing crop yields through wild pollinator conservation. Proc. Natl. Acad. Sci. USA 2021, 118, e2108429118. [Google Scholar] [CrossRef]
- Gogala, A. Heteroptera of Slovenia, V: Pentatomorpha II and additions to the previous parts. Ann. Ser. Hist. Nat. 2008, 18, 91–126. [Google Scholar]
- Panizzi, A.R.; Grazia, J. True Bugs (Heteroptera) of the Neotropics; Springer: Dordrecht, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Schuh, R.T.; Slater, J.A. True Bugs of the World (Hemiptera: Heteroptera): Classification and Natural History; Comstock Publishing Associates: Ithaca, NY, USA, 1995; pp. 222–225. [Google Scholar]
- Panizzi, A.R.; McPherson, J.E.; James, D.G.; Javahery, M.; McPherson, R.M. Stink Bugs (Pentatomidae). In Heteroptera of Economic Importance; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Kaur, H.; Sharma, K. COI-based DNA barcoding of some species of Pentatomidae from North India (Hemiptera: Heteroptera). Mitochondrial DNA Part A DNA Mapp. Seq. Anal. 2017, 28, 756–761. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, Q.; Li, M.; Wei, J.; Zhang, X.; Zhang, H. Characterization of the complete mitochondrial genome and phylogenetic implications for Eurydema maracandica (Hemiptera: Pentatomidae). Mitochondrial DNA B Resour. 2017, 2, 550–551. [Google Scholar] [CrossRef] [PubMed]
- Aldrich, J.R.; Avery, J.W.; Lee, C.-J.; Graf, J.; Harrison, D.; Bin, F. Semiochemistry of cabbage bugs (Heteroptera: Pentatomidae): Eurydema and Murgada. J. Entomol. Sci. 1996, 31, 172–182. [Google Scholar] [CrossRef]
- Koczor, S.; Tóth, M. Field attraction of Eurydema ornata (Hemiptera: Pentatomidae) to allyl isothiocyanate. Sci. Rep. 2023, 13, 11063. [Google Scholar] [CrossRef]
- Schaefer, C.W.; Panizzi, A.R. Heteroptera of Economic Importance; CRC Press: Boca Raton, FL, USA, 2000; 828p. [Google Scholar]
- Maceljski, M.; Cvjetković, B.; Ostojić, Z.; Igrc Barčić, J.; Pagliarini, N.; Oštrec, L.; Barić, K.; Čizmić, I. Štetočinje povrća s Opsežnim Opisom Zaščite Povrća od Štetnika, Uzročnika Bolesti i Korova; Zrinski: Čakovec, Croatia, 2004; 517p. [Google Scholar]
- Janežič, F. Varstvo rastlin Pred Boleznimi in Škodljivci; Državna založba Slovenije: Ljubljana, Slovenia, 1951; 567p. [Google Scholar]
- Tanasijević, N.; Ilić, B. Posebna Entomologija; Građevinska Knjiga: Beograd, Serbia, 1969; 399p. [Google Scholar]
- Maceljski, M. Poljoprivredna Entomologija, 2nd ed.; Zrinski: Čakovec, Croatia, 2002; 519p. [Google Scholar]
- Lupoli, R.; van der Heyden, T. Review of Eurydema Laporte, 1832 species from Macaronesia and new record of Eurydema ventralis Kolenati, 1846 (Hemiptera: Pentatomidae) in Madeira (Portugal). Arq. Entomolóxicos 2024, 30, 55–57. [Google Scholar]
- Trdan, S.; Žnidarčič, D.; Valič, N. Field efficacy of three insecticides against cabbage stink bugs (Heteroptera: Pentatomidae) on two cultivars of white cabbage. Int. J. Pest Manag. 2006, 52, 79–87. [Google Scholar] [CrossRef]
- Kment, P.; Jindra, Z. New records of Eurydema fieberi from the Czech Republic with corrections to some previously published records of Palaearctic Eurydema species (Hemiptera: Heteroptera: Pentatomidae). Acta Musei Morav. Sci. Biol. 2008, 93, 11–27. [Google Scholar]
- Tsagkarakis, A.; Thanou, Z.; Chaldeou, A.; Moschou, I.; Kalaitzaki, A.; Drosopoulos, S. New records and updated checklist of the Pentatomoidea (Hemiptera: Heteroptera) of Greece. Insects 2022, 13, 749. [Google Scholar] [CrossRef]
- Kuzhuget, S.V.; Vinokurov, N.N. Species of terrestrial bugs (Heteroptera) new to the fauna of Tuva. Entmol. Rev. 2011, 91, 828–829. [Google Scholar] [CrossRef]
- Sofronova, E.V. Data on the fauna of true bugs (Heteroptera) of the Tunkinsky National Park, Buryatia, Russia. Amur. Zool. J. 2023, 15, 369–377. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, Q.; Li, M.; Wei, J.; Zhang, X.; Zhang, H. Comparative mitogenomic analysis of the Eurydema genus in the context of representative Pentatomidae (Hemiptera: Heteroptera) taxa. J. Insect Sci. 2019, 19, 20. [Google Scholar] [CrossRef]
- GBIF Secretariat. GBIF Backbone Taxonomy. Checklist Dataset. Available online: https://doi.org/10.15468/39omei (accessed on 3 June 2025).
- Bohinc, T.; Marković, D.; Trdan, S. Leaf epicuticular wax as a factor of antixenotic resistance of cabbage to cabbage flea beetles and cabbage stink bugs attack. Acta Agric. Scand. Sect. B Soil Plant Sci. 2014, 64, 493–500. [Google Scholar] [CrossRef]
- Rot, M.; Devetak, M.; Carlevaris, B.; Žežlina, J.; Žežlina, I. First record of brown marmorated stink bug (Halyomorpha halys (Stål, 1855)) (Hemiptera: Pentatomidae) in Slovenia. Acta Entomol. Slov. 2018, 26, 5–12. [Google Scholar]
- Batistič, L.; Bohinc, T.; Trdan, S. Seasonal dynamics and abundance of brown marmorated stink bug Halyomorpha halys (Stål) on four trap crops. Plant Prot. Sci. 2023, 59, 264–277. [Google Scholar] [CrossRef]
- Rot, M.; Maistrello, L.; Costi, E.; Trdan, S. Biological parameters, phenology and temperature requirements of Halyomorpha halys (Hemiptera: Pentatomidae) in the Sub-Mediterranean climate of Western Slovenia. Insects 2022, 13, 956. [Google Scholar] [CrossRef] [PubMed]
- Kavar, T.; Pavlovčič, P.; Sušnik, S.; Meglič, V.; Virant-Doberlet, M. Genetic differentiation of geographically separated populations of the southern green stink bug Nezara viridula (Hemiptera: Pentatomidae). Bull. Entomol. Res. 2006, 96, 117–128. [Google Scholar] [CrossRef]
- Žunič, A.; Virant-Doberlet, M.; Čokl, A. Species recognition during substrate-borne communication in Nezara viridula (L.) (Pentatomidae: Heteroptera). J. Insect Behav. 2011, 24, 468–487. [Google Scholar] [CrossRef]
- Trdan, S.; Laznik, Ž.; Bohinc, T. Native natural enemies of plant pests in Slovenia with an emphasis on species suitable for mass rearing. J. Insect Sci. 2023, 23, 3. [Google Scholar] [CrossRef]
- Ludwig, S.W.; Kok, L.T. Evaluation of trap crops to manage harlequin bugs, Murgantia histrionica (Hahn) (Hemiptera: Pentatomidae) on broccoli. Crop Prot. 1998, 17, 123–128. [Google Scholar] [CrossRef]
- Rather, A.H.; Azim, M.N.; Maqsood, S. Host plant selection in a pentatomid bug Eurydema pulchrum Westwood. J. Plant Prot. Res. 2010, 50, 229–232. [Google Scholar] [CrossRef]
- Ölmez-Bayhan, S.; Bayhan, E.; Ulusoy, M.R.; Hizar, F. The effect of different host plants on immature development time and mortality of Eurydema ventrale Kit. J. Agric. Fac. Ç. Ü. 2003, 18, 65–68. [Google Scholar]
- Bohinc, T.; Trdan, S. Trap crops for reducing damage caused by cabbage stink bugs (Eurydema spp.) and flea beetles (Phyllotreta spp.) on white cabbage: Fact or fantasy? J. Food Agric. Environ. 2012, 10, 1365–1370. Available online: https://www.researchgate.net/publication/263438661 (accessed on 12 June 2025).
- Venugopal, P.D.; Coffey, P.L.; Dively, G.P.; Lamp, W.O. Adjacent habitat influence on stink bug (Hemiptera: Pentatomidae) densities and the associated damage at field corn and soybean edges. PLoS ONE 2014, 9, e109917. [Google Scholar] [CrossRef]
- Lee, D.-H.; Leskey, T.C. Flight behavior of foraging and overwintering brown marmorated stink bug, Halyomorpha halys (Hemiptera: Pentatomidae). Bull. Entom. Res. 2015, 105, 566–573. [Google Scholar] [CrossRef]
- Babu, A.; Del Pozo-Valdivia, A.; Reisig, D.D. Baseline flight potential of Euschistus servus (Hemiptera: Pentatomidae) and its implications on local dispersal. Environ. Entomol. 2020, 49, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.C.K.; Perry, K.L. Transmission of plant viruses by aphid vectors. Mol. Plant Pathol. 2004, 5, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Brault, V.; Uzest, M.; Monsion, B.; Jacquot, E.; Blanc, S. Aphids as transport devices for plant viruses. Comptes Rendus Biol. 2010, 333, 524–538. [Google Scholar] [CrossRef] [PubMed]
- Visser, J.H. Host odor perception in phytophagous insects. Annu. Rev. Entomol. 1986, 31, 121–144. [Google Scholar] [CrossRef]
- Lövei, G.L.; Andow, D.A.; Arpaia, S. Transgenic insecticidal crops and natural enemies: A detailed review of laboratory studies. Environ. Entomol. 2009, 38, 293–306. [Google Scholar] [CrossRef]
- Stoianova, D.; Karakicheva, T.; Lozanova, L. True bugs (Hemiptera: Heteroptera) collected in an oil-bearing rose agrocenosis in the Kazanlak Valley, Bulgaria. Acta Zool. Bulg. 2025, 77, 231–241. [Google Scholar] [CrossRef]
- Pezzini, D.T.; Haag, M.; Walker, J.; Koch, R.L. Pentatomidae (Hemiptera: Heteroptera: Pentatomidae) captured on purple prism traps deployed for detection of emerald ash borer (Agrilus planipennis) (Coleoptera: Buprestidae) in Minnesota. Great Lakes Entomol. 2018, 51, 10–17. [Google Scholar] [CrossRef]
- Han, P.; Rodriguez-Saona, C.; Zalucki, M.P.; Liu, S.; Desneux, N. A theoretical framework to improve the adoption of green Integrated Pest Management tactics. Commun. Biol. 2024, 7, 337. [Google Scholar] [CrossRef]
- Eger, J.E. Stink bugs of economic importance in America north of Mexico. Fla. Entomol. 2002, 85, 297. [Google Scholar] [CrossRef]
- Stankevych, S.V.; Vasylieva, Y.V.; Golovan, L.V.; Zabrodina, I.V.; Lutytska, N.V.; Nakonechna, Y.O.; Molchanova, O.A.; Chupryna, Y.Y.; Zhukova, L.V. Chronicle of insect pests massive reproduction. Ukr. J. Ecol. 2019, 9, 262–274. [Google Scholar]
- Domingues, T.; Brandão, T.; Ferreira, J.C. Machine learning for detection and prediction of crop diseases and pests: A comprehensive survey. Agriculture 2022, 12, 1350. [Google Scholar] [CrossRef]
- Bajwa, W.I.; Kogan, M. Compendium of IPM Definitions (CID): What is IPM and How Is It Defined in the Worldwide Literature? Integrated Plant Protection Center, Oregon State University: Corvallis, OR, USA, 2002. [Google Scholar]
- Ehler, L.E. Integrated pest management (IPM): Definition, historical development and implementation, and the other IPM. Pest Manag. Sci. 2006, 62, 787–789. [Google Scholar] [CrossRef] [PubMed]
- Deguine, J.-P.; Aubertot, J.-N.; Flor, R.J.; Lescouret, F.; Wyckhuys, K.A.G. Integrated pest management: Good intentions, hard realities. A review. Agron. Sustain. Dev. 2021, 41, 38. [Google Scholar] [CrossRef]
- Dent, D. Insect Pest Management, 2nd ed.; CABI Publishing: Wallingford, UK, 2000. [Google Scholar]
- Cook, S.M.; Khan, Z.R.; Pickett, J.A. The use of push–pull strategies in integrated pest management. Annu. Rev. Entomol. 2007, 52, 375–400. [Google Scholar] [CrossRef]
- Breitenmoser, S.; Steinger, T.; Baux, A.; Hiltpold, I. Intercropping winter oilseed rape (Brassica napus L.) has the potential to lessen the impact of the insect pest complex. Agronomy 2022, 12, 723. [Google Scholar] [CrossRef]
- Magnin, L.; Hiltpold, I.; Jullien, A.; Baux, A. Intercropping mitigates incidence of the oilseed rape insect pest complex. Pest Manag. Sci. 2025; early view. [Google Scholar] [CrossRef]
- Jansen, J.P. Side-effects of pesticides on adult females of the predatory mites Phytoseiulus persimilis, Amblyseius cucumeris and Amblyseius degenerans (Acari: Phytoseiidae) in the laboratory. IOBC/WPRS Bull. 2000, 23, 171–180. [Google Scholar]
- Heimbach, U.; Müller, A. Pyrethroid resistance in populations of the cabbage stem flea beetle Psylliodes chrysocephala (Coleoptera: Chrysomelidae) in Germany: Monitoring and implications for control strategies. Gesunde Pflanzen 2013, 116, 17–22. [Google Scholar]
- Shi, T.; Zhang, O.; Chen, X.; Mao, G.; Feng, W.; Yang, L.; Zhao, T.; Wu, X.; Chen, Y. Overview of deltamethrin residues and toxic effects in the global environment. Environ. Geochem. Health 2024, 46, 271. [Google Scholar] [CrossRef]
- Goulson, D. An overview of the environmental risks posed by neonicotinoid insecticides. J. Appl. Ecol. 2013, 50, 977–987. [Google Scholar] [CrossRef]
- Tomizawa, M.; Casida, J.E. Neonicotinoid insecticide toxicology: Mechanisms of selective action. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 247–268. [Google Scholar] [CrossRef] [PubMed]
- Batistič, L.; Bohinc, T.; Novljan, M.; Indihar, E.; Košir, I.J.; Šilc, U.; Horvat, A.; Trdan, S. Cabbage pest control with local inert dusts: Do diatomaceous earth and wood ash have the highest potential? Acta Agric. Scand. Sect. B Soil Plant Sci. 2025, 75, 1. [Google Scholar] [CrossRef]
- Zalucki, M.P.; Shabbir, A.; Silva, R.; Adamson, D.; Shu-Sheng, L.; Furlong, M.J. Estimating the economic cost of one of the world’s major insect pests, Plutella xylostella (Lepidoptera: Plutellidae): Just how long is a piece of string? J. Econ. Entomol. 2012, 105, 1115–1129. [Google Scholar] [CrossRef]
- Furlong, M.J.; Wright, D.J.; Dosdall, L.M. Diamondback moth ecology and management: Problems, progress, and prospects. Annu. Rev. Entomol. 2013, 58, 517–541. [Google Scholar] [CrossRef]
- Sayyed, A.H.; Omar, D.; Wright, D.J.; Crickmore, N. Genetics of spinosad resistance in a multi-resistant field-selected population of Plutella xylostella. Pest Manag. Sci. 2008, 60, 827–832. [Google Scholar] [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef]
- Aktar, M.W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef]
- Yadav, R.K.; Shinde, N.D.; Patil, K.T.; Kote, A.; Kadam, P. Deltamethrin toxicity: Impacts on non-target organisms and the environment. Environ. Ecol. 2023, 41, 2039–2043. [Google Scholar] [CrossRef]
- Dubey, A.; Lewis, M.T.; Dively, G.P.; Hamby, K.A. Ecological impacts of pesticide seed treatments on arthropod communities in a grain crop rotation. J. Appl. Ecol. 2020, 57, 936–951. [Google Scholar] [CrossRef]
- Kromp, B. Carabid beetles in sustainable agriculture: A review on pest control efficacy, cultivation impacts and enhancement. Agric. Ecosyst. Environ. 1999, 74, 187–228. [Google Scholar] [CrossRef]
- Gurr, G.M.; Wratten, S.D.; Luna, J.M. Multi-function agricultural biodiversity: Pest management and other benefits. Basic Appl. Ecol. 2003, 4, 107–116. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Neonicotinoids: Risks to bees confirmed. EFSA J. 2018, 16, e05177. [Google Scholar] [CrossRef]
- Van Lenteren, J.C.; Bolckmans, K.; Köhl, J.; Ravensberg, W.J.; Urbaneja, A. Biological control using invertebrates and microorganisms: Plenty of new opportunities. BioControl 2018, 63, 39–59. [Google Scholar] [CrossRef]
- Trdan, S.; Laznik, Ž.; Bohinc, T. Thirty years of research and professional work in the field of biological control (predators, parasitoids, entomopathogenic and parasitic nematodes) in Slovenia: A review. Appl. Sci. 2020, 10, 7468. [Google Scholar] [CrossRef]
- Indihar, E.; Bohinc, T.; Neerup, P.; Trdan, S. Prve najdbe štirih vrst jajčnih parazitoidov ščitastih stenic (Pentatomidae) v Sloveniji: Trissolcus scutellaris (Thomson, 1861), Trissolcus viktorovi Kozlov, 1968, Trissolcus festivae (Viktorov, 1964) in Telenomus chloropus (Thomson, 1861) (Insecta, Hymenoptera, Scelionidae). Acta Agric. Slov. 2024, 120, 1–5. [Google Scholar] [CrossRef]
- Shah, P.A.; Pell, J.K. Entomopathogenic fungi as biological control agents. Appl. Microbiol. Biotechnol. 2003, 61, 413–423. [Google Scholar] [CrossRef]
- Rondoni, G.; Chierici, E.; Giovanni, L.; Peverieri, G.S.; Roversi, P.F.; Conti, E. Olfactory responses of Trissolcus mitsukurii to plants attacked by target and non-target stink bugs suggest low risk for biological control. Sci. Rep. 2022, 12, 1880. [Google Scholar] [CrossRef]
- Conti, E.; Salerno, G.; Bin, F.; Vinson, S.B. The role of host semiochemicals in parasitoid specificity: A case study with Trissolcus brochymenae and Trissolcus simoni on pentatomid bugs. Biol. Control 2004, 29, 435–444. [Google Scholar] [CrossRef]
- Stireman, J.O.; O’Hara, J.E.; Wood, D.M. Tachinidae: Evolution, behavior, and ecology. Annu. Rev. Entomol. 2006, 51, 525–555. [Google Scholar] [CrossRef]
- Grenier, S. Applied biological control with Tachinid flies (Diptera, Tachinidae): A review. Anz. Schadlingskd. Pflanz. 1988, 61, 49–56. [Google Scholar] [CrossRef]
- Liljesthröm, G.G.; Rabinovich, J.E. Biological control of the stink bug Nezara viridula by two parasitoids and their interaction in non-crop habitats: A simulation model. Bull. Entomol. Res. 2023, 113, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Fernández, C.A.; Punschke, E.L.; Cingolani, M.F.; Carrizo, A.P.; Barakat, M.C.; de Vilhena Perez Dios, R.; Blengino, F.; Huarte, F.; Montero, G.A. Tachinids in conservation biological control of phytophagous Pentatomidae. BioControl 2024, 69, 539–550. [Google Scholar] [CrossRef]
- Ramos, R.S.; Souza, J.D.A.; Zotti, M.J. Biological control potential of two Beauveria bassiana isolates against the stink bugs Nezara viridula L. and Piezodorus guildinii Westwood (Hemiptera: Pentatomidae) in common bean. Egypt. J. Biol. Pest Control 2024, 34, 23. [Google Scholar] [CrossRef]
- Paradza, V.M.; Khamis, F.M.; Yusuf, A.A.; Subramanian, S.; Akutse, K.S. Virulence and horizontal transmission of Metarhizium anisopliae by the adults of the greenhouse whitefly Trialeurodes vaporariorum (Hemiptera: Aleyrodidae) and the efficacy of oil formulations against its nymphs. Heliyon 2021, 7, e08277. [Google Scholar] [CrossRef]
- Lacey, L.A.; Grzywacz, D.; Shapiro-Ilan, D.I.; Frutos, R.; Brownbridge, M.; Goettel, M.S. Insect pathogens as biological control agents: Back to the future. J. Invertebr. Pathol. 2015, 132, 1–41. [Google Scholar] [CrossRef]
- Koller, J.; Sutter, L.; Gonthier, J.; Collatz, J.; Norgrove, L. Entomopathogens and parasitoids allied in biocontrol: A systematic review. Pathogens 2023, 12, 957. [Google Scholar] [CrossRef]
- Labbé, R.M.; Gillespie, D.R.; Cloutier, C.; Brodeur, J. Compatibility of an entomopathogenic fungus with a predator and a parasitoid in the biological control of greenhouse whitefly. Biocontrol Sci. Technol. 2009, 19, 429–446. [Google Scholar] [CrossRef]
- Shelton, A.M.; Nault, B.A. Dead-end trap cropping: A technique to improve management of the diamondback moth. Crop Prot. 2004, 23, 497–503. [Google Scholar] [CrossRef]
- Köneke, A.; Uesugi, R.; Herz, A.; Tabuchi, K.; Yoshimura, H.; Shimoda, T.; Nagasaka, K.; Böckmann, E. Effects of wheat undersowing and sweet alyssum intercropping on aphid and flea beetle infestation in white cabbage in Germany and Japan. J. Plant Dis. Prot. 2023, 130, 619–631. [Google Scholar] [CrossRef]
- Shelton, A.M.; Badenes-Perez, F.R. Concepts and application of trap cropping in pest management. Annu. Rev. Entomol. 2006, 51, 285–308. [Google Scholar] [CrossRef] [PubMed]
- Valantin-Morison, M.; Meynard, J.-M.; Doré, T. Effects of crop management and surrounding field environment on insect incidence in organic winter oilseed rape (Brassica napus L.). Crop Prot. 2007, 26, 1108–1120. [Google Scholar] [CrossRef]
- Gray, F.A.; Koch, D.W. Trap crops. In Encyclopedia of Pest Management; Pimental, D., Ed.; University of Wyoming: Wyoming, Lacraime, 2002; pp. 852–864. [Google Scholar]
- Bohinc, T.; Hrastar, R.; Košir, I.J.; Trdan, S. Association between glucosinolate concentration and injuries caused by cabbage stink bugs Eurydema spp. (Heteroptera: Pentatomidae) on different Brassicas. Acta Sci. Agron. 2013, 35, 1–8. [Google Scholar] [CrossRef]
- Piersanti, S.; Rebora, M.; Ederli, L.; Pasqualini, S.; Salerno, G. Role of chemical cues in cabbage stink bug host plant selection. J. Insect Physiol. 2020, 120, 103994. [Google Scholar] [CrossRef]
- Chidwala, N.; Chilumpha, G.; Makhwira, A.; Namandwa, B.; Zhou, Q.; Li, W.; Yu, T.; Nasser, R.; Mo, J. Study on the trapping effects of Brassica allelochemicals on Plutella xylostella adults. Afr. J. Agric. Res. 2024, 20, 323–336. [Google Scholar] [CrossRef]
- Nash, M.A.; Severtson, D.; Macfayden, S. New approaches to manage invertebrate pests in conservation agriculture systems—Uncoupling intensification invertebrate threats to broadacre agriculture in Australia. In Australian Agriculture in 2020: From Conservation to Automation; Australian Agronomy and Charles Sturt University: Wagga Wagga, Australia, 2019. [Google Scholar]
- Ndjomatchoua, F.T.; Guimapi, R.A.Y.; Rossini, L.; Djouda, B.S.; Pedro, S.A. A generalized risk assessment index for forecasting insect population under the effect of temperature. J. Therm. Biol. 2024, 122, 103886. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).