Research Progress on Techniques for Quantitative Detection of Starch in Food in the Past Five Years
Abstract
1. Introduction
2. Titration Methods
3. Spectrophotometric Methods
4. Chromatography
5. Polarimetric Methods
6. Thermogravimetric Analysis
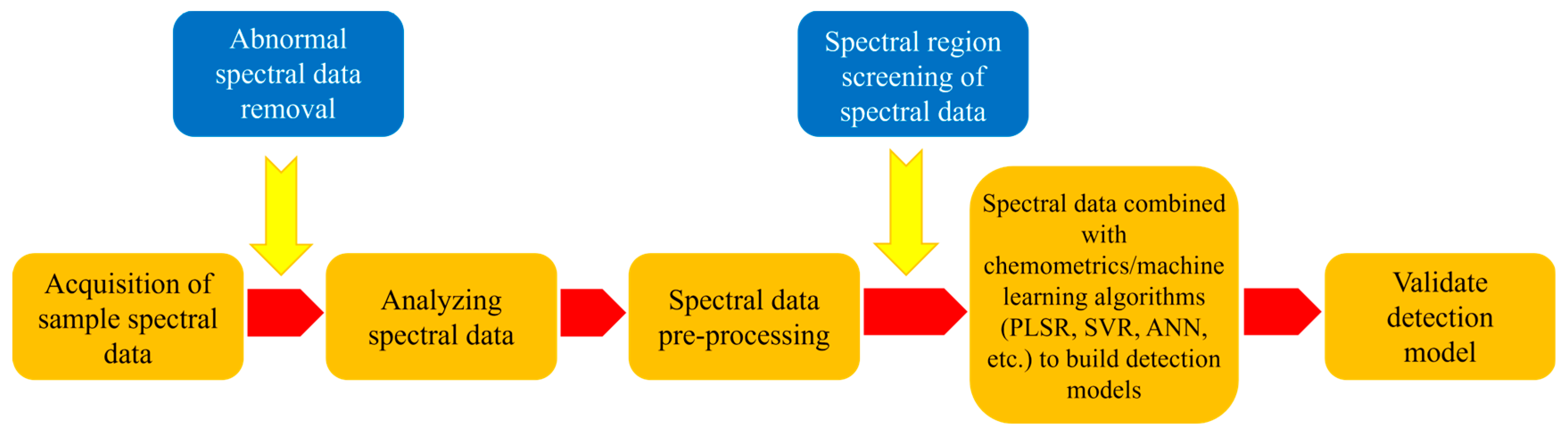
7. Near-Infrared Spectroscopy
8. Hyperspectral Imaging Technology
9. Mid-Infrared, Raman, and Terahertz Spectroscopy Technology
10. Challenges and Future Trends
11. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arefi, A.; Sturm, B.; Hoffmann, T. Explainability of deep convolutional neural networks when it comes to NIR spectral data: A case study of starch content estimation in potato tubers. Food Control 2025, 169, 110979. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Wang, X.; Wang, H.; Liu, S.; Chen, S. A transfer learning method for near infrared models of potato starch content and traceability from different origins. J. Food Compos. Anal. 2025, 137, 106909. [Google Scholar] [CrossRef]
- Xu, Y.; Luo, J.; Xue, S.; Jin, Q.; Zhu, J.; Lu, S.; Meng, Q.; Du, H.; Fu, M.; Zhong, Y. Development of comprehensive prediction models for pumpkin fruit sensory quality using physicochemical analysis, near-infrared spectroscopy, and machine learning. J. Food Compos. Anal. 2024, 134, 106530. [Google Scholar] [CrossRef]
- Gonzalez-Vidal, T.; Calvo-Malvar, M.; Fernandez-Merino, C.; Sanchez-Castro, J.; Lado-Baleato, O.; Diaz-Louzao, C.; Pazos-Couselo, M.; Alonso-Sampedro, M.; Matabuena, M.; Gude, F. Divergent hypoglycemic and hyperglycemic responses to the components of evening meals. A general adult population study in individuals without diabetes (aegis study). Clin. Nutr. 2024, 43, 379–390. [Google Scholar] [CrossRef] [PubMed]
- GB/T 13213-2017; Canned Pork Mince. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, Standardization Administration of the People’s Republic of China: Beijing, China, 2017.
- GB/T 36187-2018; Frozen Surimi. State Administration for Market Regulation, Standardization Administration of the People’s Republic of China: Beijing, China, 2018.
- Zhao, G.; Xu, Z.; Tang, L.; Li, X.; Dai, Z.; Xie, Z.; Jiang, Y.; Wu, Y.; Zhang, P.; Wang, Q. Research on rapid determination methods for main compositions and sensory quality of pumpkins based on hyperspectral imaging technology. J. Food Compos. Anal. 2025, 138, 107028. [Google Scholar] [CrossRef]
- Zhu, J.; Ji, G.; Chen, B.; Yan, B.; Ren, F.; Li, N.; Zhu, X.; He, S.; Mu, Z.; Liu, H. High-throughput near-infrared spectroscopy for detection of major components and quality grading of peas. Front. Nutr. 2024, 11, 1505407. [Google Scholar] [CrossRef]
- Namakula, B.F.; Nuwamanya, E.; Kanaabi, M.; Wembambazi, E.; Kawuki, R.S. Predicting starch content of cassava with near infrared spectroscopy in Ugandan cassava germplasm. J. Near Infrared Spectrosc. 2023, 31, 256–262. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, G. Starch content and physicochemical properties of green wheat starch. Int. J. Food Prop. 2019, 22, 1463–1474. [Google Scholar] [CrossRef]
- Vitelli, M.; Mehrtash, H.; Assatory, A.; Tabtabaei, S.; Legge, R.L.; Rajabzadeh, A.R. Rapid and non-destructive determination of protein and starch content in agricultural powders using near-infrared and fluorescence spectroscopy, and data fusion. Powder Technol. 2021, 381, 620–631. [Google Scholar] [CrossRef]
- Zeng, W.; Zhang, H.Z.; Lee, T.C. Direct determination of the starch content in gravy by near-infrared spectroscopy. J. Agric. Food Chem. 1996, 44, 1460–1463. [Google Scholar] [CrossRef]
- Su, W.; Xue, H. Imaging spectroscopy and machine learning for intelligent determination of potato and sweet potato quality. Foods 2021, 10, 2146. [Google Scholar] [CrossRef]
- GB/T 20378-2006; Native Starch—Determination of Starch Content—Ewers Polarimetric Method. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, Standardization Administration of the People’s Republic of China: Beijing, China, 2006.
- ISO 10520-1997; Native Starch-Determination of Starch Content-Ewers Polarimetric Method. International Organization for Standardization: Geneva, Switzerland, 1997.
- GB/T 25219-2010; Inspection of Grain and Oils—Determination of Starch Content in Maize—Near-Infrared Method. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, Standardization Administration of the People’s Republic of China: Beijing, China, 2010.
- 17. AOAC 940.30; Starch in Prepared Mustard. Association of Official Analytical Chemists: Gaithersburg, MD, USA, 1940.
- AOAC 925.50; Starch in Confectionery. Association of Official Analytical Chemists: Gaithersburg, MD, USA, 1925.
- 19. AOAC 920.10; Sulfur in Plants. Sodium Peroxide Method. Association of Official Analytical Chemists: Gaithersburg, MD, USA, 1920.
- AOAC 920.44; Starch in Baking Powders. Association of Official Analytical Chemists: Gaithersburg, MD, USA, 1920.
- AOAC 920.83; Starch in Cacao Products Direct Acid Hydrolysis Method. Association of Official Analytical Chemists: Gaithersburg, MD, USA, 1920.
- AOAC 979.10; Starch in Cereals. Association of Official Analytical Chemists: Gaithersburg, MD, USA, 1979.
- AOAC 996.11; Starch(Total)In Cereal Products Amyloglucosidase-a-Amylase Method. Association of Official Analytical Chemists: Gaithersburg, MD, USA, 1979.
- AOAC 958.06; Starch in Meat Titrimetric Method. Association of Official Analytical Chemists: Gaithersburg, MD, USA, 1958.
- ISO 13965-1998; Meat and Meat Products-Determination of Starch and Glucose Contents-Enzymatic Method. International Organization for Standardization: Geneva, Switzerland, 1998.
- NY/T 802-2004; Method for Determination of Starch in Raw Milk and Dairy Food Enzyme-Colorimetric method. The Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2004.
- AACC 76-11; Starch—Glucoamylase Method with Subsequent Measurement of Glucose with Glucose Oxidase. American Association of Cereal Chemists: St. Paul, MN, USA, 1976.
- 28. GB 5009.9-2016; National Food Safety Standard Determination of Starch in Food. National Health and Family Planning Commission of the People’s Republic of China, National Medical Products Administration: Beijing, China, 2016.
- Tang, Y.; Zhang, J.; Xu, Y.; Li, C.; Li, Y.; Li, G.; Hu, Y.; Li, W. Combining with acid-base titration, HPLC, ATR-FTIR and chemometrics to study the effects of sulfur fumigation on medicinal and edible starchy samples. J. Food Compos. Anal. 2025, 137, 106967. [Google Scholar] [CrossRef]
- Letoffe, A.; Hosseinpourpia, R.; Silveira, V.; Adamopoulos, S. Effect of Fenton reaction parameters on the structure and properties of oxidized wheat starch. Carbohydr. Res. 2024, 542, 109190. [Google Scholar] [CrossRef]
- Yogurtcu, H.; Gurler, N. Evaluation of effect of boric acid on thermoplastic starch: Morphological, mechanical, barrier, and optical properties. Polym. Eng. Sci. 2024, 64, 2230–2240. [Google Scholar] [CrossRef]
- Huang, M.; Li, L.; Lei, G.; Qiu, R.; Wang, Y.; Wu, J.; Zong, X. Preparation of fern root resistant starch by pullulanase and glucoamylase combined with autoclaving-enzymatic method: Physicochemical properties and structural characterization. J. Sci. Food Agric. 2025, 105, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Liu, Q. Enzymatic determination of total starch and degree of starch gelatinization in various products. Food Hydrocoll. 2020, 103, 105639. [Google Scholar] [CrossRef]
- Souto, L.R.F.; Caliari, M.; Soares Junior, M.S.; Fiorda, F.A.; Garcia, M.C. Utilization of residue from cassava starch processing for production of fermentable sugar by enzymatic hydrolysis. Food Sci. Technol. 2017, 37, 19–24. [Google Scholar] [CrossRef]
- Lehoczki, G.; Kandra, L.; Gyemant, G. The use of starch azure for measurement of alpha-amylase activity. Carbohydr. Polym. 2018, 183, 263–266. [Google Scholar] [CrossRef]
- Halim, A.; Torley, P.J.; Farahnaky, A.; Majzoobi, M. Investigating the effects of acid hydrolysis on physicochemical properties of quinoa and faba bean starches as compared to cassava starch. Foods 2024, 13, 3885. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Lu, X.; Cheng, J.; Lu, G.; Pang, L. Comparison and optimization of determination methods for starch in sweet potato. J. Chin. Cereals Oils Assoc. 2023, 38, 199–204. [Google Scholar] [CrossRef]
- Nascimento, M.V.F.; Vicente, J.; Silva, F.O.M.d.; La, O.R. Acid hydrolysis optimization of starch by lane-eynon method. Bol. Cent. Pesqui. Process. Aliment. 2016, 34, 37–44. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, L. Study on determination of starch content in rice by acid hydrolysis—Fehlings reagent titration. Sci. Technol. Food Ind. 2017, 38, 256–259+265. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, R.; Fu, G.; Wen, J.; Xing, L.; Wu, S. Determination of starch in food by high temperature and high pressure acid hydrolysis liquid phase method. Cereal Feed. Ind. 2023, 2, 62–65. [Google Scholar]
- Gaspari, P.F.; Bernardes, C.F. Experimental characterization of carbohydrates. Rev. Ensino Bioquim. 2020, 18, 49–55. [Google Scholar]
- Rautenstrauch, H.; Rebenstorff, A.; Gudenschwager, S.; Ruppersberg, K. The new molisch sample for teaching a safe carbohydrate detection. Chem. Unserer Zeit 2023, 57, 172–179. [Google Scholar] [CrossRef]
- Zhao, C.; Zhao, X.; Gu, H.; Zhang, J.; Zou, W.; Liu, J.; Yang, Q. Qualitative analysis of components of bioflocculant prepared with Bacillus fusiformis for the treatment of tannery wastewater. Clean Technol. Environ. Policy 2016, 18, 973–978. [Google Scholar] [CrossRef]
- Fan, J.; Gu, D.; Xv, W.; Zhou, T.; Chen, A.; Lu, J.; Wang, Y.; Jin, H.; Wei, F.; Ma, S. Comparison of polysaccharide profiles of different seaweeds based on ion chromatography and ultrahigh-performance liquid chromatography. Sep. Sci. Plus 2024, 7, e202400060. [Google Scholar] [CrossRef]
- Wang, Z.; Li, X.; Arif, M.; Shamshad, J.; Wu, A.; Zhan, W.; Ahmad, B.; Tan, N.; Al-Anazi, K.M.; Farah, M.A.; et al. Analyzing the impact of phosphorous and nitrogen on Castanopsis sclerophylla early growth stages. J. King Saud Univ. Sci. 2024, 36, 103517. [Google Scholar] [CrossRef]
- Wu, Y.; Peng, C.; Yu, X.; Shen, Y. Biochemical changes during fruit and seed development in Nanjing Linden (Tilia miqueiana M.). Forests 2023, 14, 969. [Google Scholar] [CrossRef]
- Kanchan, M.; Tambe, P.K.; Bharati, S.; Powar, O.S. Convolutional neural network for colorimetric glucose detection using a smartphone and novel multilayer polyvinyl film microfluidic device. Sci. Rep. 2024, 14, 28377. [Google Scholar] [CrossRef]
- Bastida, A.; Garrido, L.; Fernandez-Mayoralas, A. Use of supramolecular chemistry based on β-cyclodextrin-grafted chitosan beads to prepare green biocatalytic materials. Mater. Adv. 2025, 6, 311–318. [Google Scholar] [CrossRef]
- Zulfiqar, S.; Blando, F.; Orfila, C.; Marshall, L.J.; Boesch, C. Chromogenic assay is more efficient in identifying α-amylase inhibitory properties of anthocyanin-rich samples when compared to the 3,5-dinitrosalicylic acid (DNS) assay. Molecules 2023, 28, 6399. [Google Scholar] [CrossRef]
- Xie, S.; Chen, H.; Jiang, X.; Zhou, B.; Guo, Z.; Zeng, H.; Zhang, Y. Structural and physicochemical properties of a Chinese yam starch-tea polyphenol complex prepared using autoclave-assisted pullulanase treatment. Foods 2023, 12, 3763. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, K.; Sumi, T.S.; Golder, M.; Biswas, B.; Maknoon Kerr, P.G. Antidiabetic profiling, cytotoxicity and acute toxicity evaluation of aerial parts of Phragmites karka (Retz.). J. Ethnopharmacol. 2021, 270, 113781. [Google Scholar] [CrossRef]
- Pakaew, K.; Chonpathompikunlert, P.; Wongmanee, N.; Rojanaverawong, W.; Sitdhipol, J.; Thaveethaptaikul, P.; Charoenphon, N.; Hanchang, W. Lactobacillus reuteri TISTR 2736 alleviates type 2 diabetes in rats via the hepatic IRS1/PI3K/AKT signaling pathway by mitigating oxidative stress and inflammatory mediators. Eur. J. Nutr. 2025, 64, 27. [Google Scholar] [CrossRef]
- Ichikawa, S.; Kodama, Y. Fluorescent staining and quantification of starch granules in chloroplasts of live plant cells using fluorescein. Bio-Protocol 2024, 14, e5103. [Google Scholar] [CrossRef] [PubMed]
- Dhir, A.; Kaur, C.; Devi, V.; Singh, A.; Das, A.K.; Rakshit, S.; Chaudhary, D.P. A rapid single kernel screening method for preliminary estimation of amylose in maize. Food Anal. Methods 2022, 15, 2163–2171. [Google Scholar] [CrossRef]
- Jiang, Z.; Jin, D.; Zhang, H.; Qu, J.; Liu, S.; Guan, S.; Ma, Y. Effects of overexpression of zmapo1-9 gene on maize yield. Plant Growth Regul. 2023, 99, 493–503. [Google Scholar] [CrossRef]
- He, J.; Yan, F.; Huang, F.; Xiao, Y.; Xie, L. Determination of amylose and amylopectin contents in yam and taros by dual-wavelength spectrophotometry. Sci. Technol. Food Ind. 2022, 43, 303–309. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Gao, F.; Zeng, Z.; Li, X.; Liang, Y.; Zhang, H.; Yang, S. The effects of different conditions (dispersion temperature and time) in dual wavelength violet spectrophotographic determination of rice starch content. J. Southwest Univ. (Nat. Sci. Ed.) 2020, 42, 49–55. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, Z.; Wang, D.; Gao, H.; Ding, Y.; Cheng, J.; Han, Y. Fe(II)/Fe(III) cycle enhanced the Electro-Fenton degradation of methylene blue with Fe3O4@C as three-dimensional electrode. Appl. Surf. Sci. 2025, 683, 161764. [Google Scholar] [CrossRef]
- GB 5009.8-2016; Determination of Fructose, Glucose, Sucrose, Maltose and Lactose in Food According to National Food Safety Standards. National Health and Family Planning Commission of the People’s Republic of China, National Medical Products Administration: Beijing, China, 2016.
- Li, O.; Zhang, Q.; Shi, X.; Huang, Y.; Yin, J.; Zhang, S.; Nie, S. The quality control of extracts from Dictyophora echino-volvata zane by high performance gel permeation chromatography and ion chromatography. J. Chin. Inst. Food Sci. Technol. 2019, 19, 213–219. [Google Scholar] [CrossRef]
- Xie, W.; Gong, Y.; Yu, K. Quantitative analysis of total starch content in wheat flour by reaction headspace gas chromatography. Anal. Bioanal. Chem. 2017, 409, 5195–5200. [Google Scholar] [CrossRef] [PubMed]
- Weng, C. The Determination of Starch in Meat Floss and the Effect of Different Processing Techniques on Its Characteristics and Content Determination. Master’s Thesis, Zhejiang University of Technology, Zhejiang, China, 2018. [Google Scholar]
- Han, X.; Ando, H.; Kudo, Y.; Sasaki, Y. Development of highly sensitive method for sugar determination in herbal medicine; application of monosaccharides and oligosaccharides in Japanese Angelica root and Rehmannia root. Chem. Pharm. Bull. 2022, 70, 796–804. [Google Scholar] [CrossRef]
- Yeganeh-Zare, S.; Farhadi, K.; Amiri, S. Rapid detection of apple juice concentrate adulteration with date concentrate, fructose and glucose syrup using HPLC-RID incorporated with chemometric tools. Food Chem. 2022, 370, 131015. [Google Scholar] [CrossRef]
- Puscas, A.; Tanislav, A.E.; Marc, R.A.; Muresan, V.; Muresan, A.E.; Pall, E.; Cerbu, C. Cytotoxicity evaluation and antioxidant activity of a novel drink based on roasted avocado seed powder. Plants 2022, 11, 1083. [Google Scholar] [CrossRef]
- Zhou, X.; Lei, B.; Zhang, F.; Liu, Y.; Fan, X. Uncertainty evaluation for the determination of starch content in Manihot esculenta by polarimetric method. Food Sci. 2016, 37, 144–147. [Google Scholar]
- Na, B.; Ao, W.; Wang, X.; Bao, X. Determination of starch content in rice by the polarimetric method. J. North Pharm. 2020, 17, 6–8. [Google Scholar]
- Ma, J.; Jiang, Q.-T.; Zhang, X.-W.; Wei, L.; Chen, G.-Y.; Qi, P.-F.; Wei, Y.-M.; Lan, X.-J.; Lu, Z.-X.; Zheng, Y.-L. Effect of lipids on starch determination through various methods. Pak. J. Agric. Sci. 2014, 51, 751–757. [Google Scholar]
- Valkova, V.; Duranova, H.; Bilcikova, J.; Zofajova, A.; Havrlentova, M. The content and quality of starch in different wheat varieties growing in experimental conditions. J. Microbiol. Biotechnol. Food Sci. 2019, 9, 462–466. [Google Scholar] [CrossRef]
- Li, X.; Xue, C.; Gao, L.; Ren, G.; Cao, Y.; Zhang, D. Potato starch content determination and comparision from different areas of Yulin city. Appl. Chem. Ind. 2015, 44, 1892–1896. [Google Scholar] [CrossRef]
- Zhao, Y. On determining starch content of green bean food with polarimetry. Jiangsu Condiment Subsid. Food 2014, 2, 37–39. [Google Scholar] [CrossRef]
- Kong, D.; Zhao, R.; Wang, X.; Song, B. Comparison of starch contents of corn samples using different standard methods. J. Henan Univ. Technol. (Nat. Sci. Ed.) 2016, 37, 23–27. [Google Scholar] [CrossRef]
- Castillo-Paz, A.M.; Correa-Pina, B.L.; Pineda-Gomez, P.; Barron-Garcia, O.Y.; Londono-Restrepo, S.M.; Rodriguez-Garcia, M.E. Structural, morphological, compositional, thermal, pasting, and functional properties of isolated achira (Canna indica L.) starch: Review. Int. J. Biol. Macromol. 2024, 282, 136710. [Google Scholar] [CrossRef]
- Zhan, H.; Cui, L.; Li, J.; Bai, J. Determination of starch content in corn by differential thermal analysis. J. Henan Univ. Technol. (Nat. Sci. Ed.) 2012, 33, 31–36. [Google Scholar] [CrossRef]
- Zhan, H.; Zhang, J.; Xu, F.; Yang, D. Determination of main component content in soybean by hermogravimetric analysis. Cereal Feed Industry 2016, 11, 56–61. [Google Scholar]
- Cui, L.; Zhan, H.; Zhang, J.; Hu, Y. Determination of starch content in rice by thermogravimetric analysis. J. Chin. Cereals Oils Assoc. 2017, 32, 167–170. [Google Scholar]
- Lopez-Calabozo, R.; Liberal, A.; Fernandes, A.; Revilla, I.; Ferreira, I.C.F.R.; Barros, L.; Vivar-Quintana, A.M. Determination of carbohydrate composition in lentils using near-infrared spectroscopy. Sensors 2024, 24, 4232. [Google Scholar] [CrossRef]
- Li, X.; Xu, Z.; Tang, L.; Zhao, G.; Wu, Y.; Zhang, P.; Wang, Q. An effective moisture interference correction method for maize powder NIR spectra analysis. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2024, 312, 124033. [Google Scholar] [CrossRef]
- Hou, S.; Han, J.; Men, Y.; Yang, Y.; Long, L.; Liu, L.; Sun, Z. Analysis of genotype-by-environment effects on starch content in 281 Tartary buckwheat varieties and evaluation of the physicochemical properties of two elite varieties. LWT 2024, 197, 115866. [Google Scholar] [CrossRef]
- Bantadjan, Y.; Rittiron, R.; Malithong, K.; Narongwongwattana, S. Rapid starch evaluation in fresh cassava root using a developed portable visible and near-infrared spectrometer. ACS Omega 2020, 5, 11210–11216. [Google Scholar] [CrossRef]
- Mbanjo, E.G.N.; Hershberger, J.; Peteti, P.; Agbona, A.; Ikpan, A.; Ogunpaimo, K.; Kayondo, S.I.; Abioye, R.S.; Nafiu, K.; Alamu, E.O.; et al. Predicting starch content in cassava fresh roots using near-infrared spectroscopy. Front. Plant Sci. 2022, 13, 990250. [Google Scholar] [CrossRef]
- Maraphum, K.; Saengprachatanarug, K.; Wongpichet, S.; Phuphuphud, A.; Posom, J. Achieving robustness across different ages and cultivars for an NIRS-PLSR model of fresh cassava root starch and dry matter content. Comput. Electron. Agric. 2022, 196, 106872. [Google Scholar] [CrossRef]
- Posom, J.; Maraphum, K. Achieving prediction of starch in cassava (Manihot esculenta Crantz) by data fusion of Vis-NIR and Mid-NIR spectroscopy via machine learning. J. Food Compos. Anal. 2023, 122, 105415. [Google Scholar] [CrossRef]
- Chaiareekitwat, S.; Mahayothee, B.; Rungpichayapichet, P.; Khuwijitjaru, P.; Nagle, M.; Latif, S.; Mueller, J. The potential of near-infrared spectroscopy as a rapid method for quality evaluation of cassava leaves and roots. J. Food Compos. Anal. 2024, 126, 105913. [Google Scholar] [CrossRef]
- Ding, J.; Han, D.; Li, Y.; Peng, Y.; Wang, Q.; Han, X. Simultaneous non-destructive on-line detection of potato black-heart disease and starch content based on visible/near infrared diffuse transmission spectroscopy. Spectrosc. Spectr. Anal. 2020, 40, 1909–1915. [Google Scholar]
- Wang, F.; Li, Y.; Peng, Y.; Yang, B.; Li, L.; Liu, Y. Multi-parameter potato quality non-destructive rapid detection by visible/near-infrared spectra. Spectrosc. Spectr. Anal. 2018, 38, 3736–3742. [Google Scholar]
- Wang, F.; Li, Y.; Peng, Y.; Yang, B.; Li, L.; Yin, X. Hand-held device for non-destructive detection of potato quality parameters. Trans. Chin. Soc. Agric. Mach. 2018, 49, 348–354. [Google Scholar]
- Tang, C.; Jiang, B.; Ejaz, I.; Ameen, A.; Zhang, R.; Mo, X.; Wang, Z. High-throughput phenotyping of nutritional quality components in sweet potato roots by near-infrared spectroscopy and chemometrics methods. Food Chem. X 2023, 20, 100916. [Google Scholar] [CrossRef]
- Alamu, E.O.; Adesokan, M.; Asfaw, A.; Maziya-Dixon, B. Effect of sample preparation methods on the prediction performances of near infrared reflectance spectroscopy for quality traits of fresh yam (Dioscorea spp.). Appl. Sci. 2020, 10, 6035. [Google Scholar] [CrossRef]
- John, R.; Bartwal, A.; Jeyaseelan, C.; Sharma, P.; Ananthan, R.; Singh, A.K.; Singh, M.; Gayacharan Rana, J.C.; Bhardwaj, R. Rice bean-adzuki bean multitrait near infrared reflectance spectroscopy prediction model: A rapid mining tool for trait-specific germplasm. Front. Nutr. 2023, 10, 1224955. [Google Scholar] [CrossRef] [PubMed]
- Padhi, S.R.; John, R.; Bartwal, A.; Tripathi, K.; Gupta, K.; Wankhede, D.P.; Mishra, G.P.; Kumar, S.; Rana, J.C.; Riar, A.; et al. Development and optimization of NIRS prediction models for simultaneous multi-trait assessment in diverse cowpea germplasm. Front. Nutr. 2022, 9, 1001551. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Guo, J.; Zhang, M.; Zhang, X.; Jingjing, E. Establishment of rapid detection model of buckwheat nutritional components based on near infrared spectroscopy. J. Chin. Cereals Oils Assoc. 2020, 35, 151–158. [Google Scholar]
- Joe, A.A.F.; Gopal, A.; Pandian, R. Performance evaluation of chemometric prediction models-key components of wheat grain. J. Sci. Ind. Res. 2020, 79, 148–152. [Google Scholar]
- He, M.H.; Hu, J.Q.; Wu, Y.W.; Ouyang, J. Determination of starch and amylose contents in various cereals using common model of near-infrared reflectance spectroscopy. Int. Food Res. J. 2021, 28, 987–995. [Google Scholar] [CrossRef]
- Tomar, M.; Bhardwaj, R.; Kumar, M.; Singh, S.P.; Krishnan, V.; Kansal, R.; Verma, R.; Yadav, V.K.; Dahuja, A.; Ahlawat, S.P.; et al. Development of NIR spectroscopy based prediction models for nutritional profiling of pearl millet (Pennisetum glaucum (L.)) R.Br: A chemometrics approach. LWT 2021, 149, 111813. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Wang, F.; Zhao, F.; Li, X.; Fan, G.; Zhao, Z.; Guo, P. Rapid prediction of apparent amylose, total starch, and crude protein by near-infrared reflectance spectroscopy for foxtail millet (Setaria italica). Cereal Chem. 2020, 97, 653–660. [Google Scholar] [CrossRef]
- John, R.; Bhardwaj, R.; Jeyaseelan, C.; Bollinedi, H.; Singh, N.; Harish, G.D.; Singh, R.; Nath, D.J.; Arya, M.; Sharma, D.; et al. Germplasm variability-assisted near infrared reflectance spectroscopy chemometrics to develop multi-trait robust prediction models in rice. Front. Nutr. 2022, 9, 946255. [Google Scholar] [CrossRef]
- Vichasilp, C.; Kawano, S. Prediction of starch content in meatballs using near infrared spectroscopy (NIRS). Int. Food Res. J. 2015, 22, 1501–1506. [Google Scholar]
- Lu, Y.; Saeys, W.; Kim, M.; Peng, Y.; Lu, R. Hyperspectral imaging technology for quality and safety evaluation of horticultural products: A review and celebration of the past 20-year progress. Postharvest Biol. Technol. 2020, 170, 111318. [Google Scholar] [CrossRef]
- Polder, G.; Dieleman, J.A.; Hageraats, S.; Meinen, E. Imaging spectroscopy for monitoring the crop status of tomato plants. Comput. Electron. Agric. 2024, 216, 108504. [Google Scholar] [CrossRef]
- Wang, F.; Wang, C. Improved model for starch prediction in potato by the fusion of near-infrared spectral and textural data. Foods 2022, 11, 3133. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, C.; Song, S. A study of starch content detection and the visualization of fresh-cut potato based on hyperspectral imaging. RSC Adv. 2021, 11, 13636–13643. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, C.; Song, S.; Xie, S.; Kang, F. Study on starch content detection and visualization of potato based on hyperspectral imaging. Food Sci. Nutr. 2021, 9, 4420–4430. [Google Scholar] [CrossRef]
- He, H.; Wang, Y.; Wang, Y.; Al-Maqtari, Q.A.; Liu, H.; Zhang, M.; Ou, X. Towards rapidly quantifying and visualizing starch content of sweet potato [Ipomoea batatas (L.) Lam] based on NIR spectral and image data fusion. Int. J. Biol. Macromol. 2023, 242, 124748. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Zhou, C.; Zhou, J.; Nan, T.; Yang, J.; Huang, L. Rapid and nondestructive identification of origin and index component contents of Tiegun yam based on hyperspectral imaging and chemometric method. J. Food Qual. 2023, 2023, 6104038. [Google Scholar] [CrossRef]
- Hu, H.; Wang, T.; Wei, Y.; Xu, Z.; Cao, S.; Fu, L.; Xu, H.; Mao, X.; Huang, L. Non-destructive prediction of isoflavone and starch by hyperspectral imaging and deep learning in Puerariae Thomsonii Radix. Front. Plant Sci. 2023, 14, 1271320. [Google Scholar] [CrossRef]
- Bu, Y.; Jiang, X.; Tian, J.; Hu, X.; Fei, X.; Huang, D.; Luo, H. Rapid and accurate detection of starch content in mixed sorghum by hyperspectral imaging combined with data fusion technology. J. Food Process Eng. 2022, 45, e14129. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, Z.; Ren, Z.; Wang, S.; Yue, M.; Zhang, S.; Yin, X.; Gong, K.; Ma, C. Rapid determination of protein, starch and moisture content in wheat flour by near-infrared hyperspectral imaging. J. Food Compos. Anal. 2023, 117, 105134. [Google Scholar] [CrossRef]
- Qiao, M.; Cui, T.; Xia, G.; Xu, Y.; Li, Y.; Fan, C.; Han, S.; Dong, J. Integration of spectral and image features of hyperspectral imaging for quantitative determination of protein and starch contents in maize kernels. Comput. Electron. Agric. 2024, 218, 108718. [Google Scholar] [CrossRef]
- Liu, C.; Huang, W.; Yang, G.; Wang, Q.; Li, J.; Chen, L. Determination of starch content in single kernel using near-infrared hyperspectral images from two sides of corn seeds. Infrared Phys. Technol. 2020, 110, 103462. [Google Scholar] [CrossRef]
- Liang, Y.; Tian, J.; Hu, X.; Huang, Y.; He, K.; Xie, L.; Yang, H.; Huang, D.; Zhou, Y.; Xia, Y. Rapid determination of starch and alcohol contents in fermented grains by hyperspectral imaging combined with data fusion techniques. J. Food Sci. 2024, 89, 3540–3553. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yin, X.; Ma, C. Development of simplified models for the nondestructive testing of rice with husk starch content using hyperspectral imaging technology. Anal. Methods 2019, 11, 5910–5918. [Google Scholar] [CrossRef]
- Lu, X. Research on Nondestructive Detection of Varieties, Moisture and Starch in Rice Based on Hyperspectral Imaging Technology. Master’s Thesis, Jiangsu University, Zhenjiang, China, 2017. [Google Scholar]
- Ichinose, J.; Oba, K.; Arase, Y.; Kaneshiro, J.; Tate, S.-i.; Watanabe, T.M. Quantitative prediction of rice starch digestibility using Raman spectroscopy and multivariate calibration analysis. Food Chem. 2024, 435, 137505. [Google Scholar] [CrossRef]
- An, H.; Ma, Q.; Zhang, F.; Zhai, C.; Sun, J.; Tang, Y.; Wang, W. Insight into microstructure evolution during starch retrogradation by infrared and Raman spectroscopy combined with two-dimensional correlation spectroscopy analysis. Food Hydrocoll. 2024, 146, 109174. [Google Scholar] [CrossRef]
- An, H.; Zhai, C.; Ma, Q.-Y.; Zhang, F.; Wang, S.-Y.; Sun, J.-F.; Wang, W.-X. Quantitative characterization of wheat starch retrogradation by combining 2d-cos and spectral fusion. Spectrosc. Spectr. Anal. 2023, 43, 162–168. [Google Scholar]
- Cozzolino, D.; Roumeliotis, S.; Eglinton, J. Prediction of starch pasting properties in barley flour using ATR-MIR spectroscopy. Carbohydr. Polym. 2013, 95, 509–514. [Google Scholar] [CrossRef]
- Guo, H.; Shiraga, K.; Kondo, N.; Chen, S.; Yamashige, Y.; Ogawa, Y. Determining changes in crystallinity of rice starch after heat-moisture treatment using terahertz spectroscopy. Food Chem. 2023, 425, 136237. [Google Scholar] [CrossRef]
- Pezzotti, G.; Zhu, W.; Chikaguchi, H.; Marin, E.; Boschetto, F.; Masumura, T.; Sato, Y.-I.; Nakazaki, T. Raman molecular fingerprints of rice nutritional quality and the concept of Raman barcode. Front. Nutr. 2021, 8, 663569. [Google Scholar] [CrossRef]
- Pezzotti, G.; Zhu, W.; Hashimoto, Y.; Marin, E.; Masumura, T.; Sato, Y.-I.; Nakazaki, T. Raman fingerprints of rice nutritional quality: A comparison between Japanese Koshihikari and internationally renowned cultivars. Foods 2021, 10, 2936. [Google Scholar] [CrossRef]
- Fakhlaei, R.; Babadi, A.A.; Ariffin, N.M.; Zou, X. Development of FTIR-ATR spectra and pls regression combination model for discrimination of pure and adulterated acacia honey. Food Control 2025, 169, 110996. [Google Scholar] [CrossRef]
- Kandpal, L.M.; Mouazen, A.M.; Masithoh, R.E.; Mishra, P.; Lohumi, S.; Cho, B.-K.; Lee, H. Sequential data-fusion of near-infrared and mid-infrared spectroscopy data for improved prediction of quality traits in tuber flours. Infrared Phys. Technol. 2022, 127, 104371. [Google Scholar] [CrossRef]
- Pielorz, S.; Kita, A.; Rytel, E.; Szostak, R.; Mazurek, S. Application of vibrational and fluorescence spectroscopy to the compositional analysis of colored-flesh potatoes. J. Sci. Food Agric. 2024, 104, 1399–1407. [Google Scholar] [CrossRef]
- Karunakaran, C.; Vijayan, P.; Stobbs, J.; Bamrah, R.K.; Arganosa, G.; Warkentin, T.D. High throughput nutritional profiling of pea seeds using Fourier transform mid-infrared spectroscopy. Food Chem. 2020, 309, 125585. [Google Scholar] [CrossRef]
- Su, W.; Sun, D. Fourier transform infrared and Raman and hyperspectral imaging techniques for quality determinations of powdery foods: A review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 104–122. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Li, Y.; Yuan, Y.; Li, S.; Song, X.; Yin, J. Comparison of NIR and Raman spectra combined with chemometrics for the classification and quantification of mung beans (Vigna radiata L.) of different origins. Food Control 2023, 145, 109498. [Google Scholar] [CrossRef]
- Nakajima, S.; Kuroki, S.; Ikehata, A. Selective detection of starch in banana fruit with Raman spectroscopy. Food Chem. 2023, 401, 134166. [Google Scholar] [CrossRef]
- Wei, X.; Li, F.; Perumal, A.B.; Sanaeifar, A.; Guindo, M.L.; Shi, Y.; He, Y.; Liu, F. Confocal Raman microspectroscopy combined with spectral screening algorithms for quantitative analysis of starch in rice. Food Hydrocoll. 2023, 141, 108737. [Google Scholar] [CrossRef]
- Wei, X.; Zheng, W.; Zhu, S.; Zhou, S.; Wu, W.; Xie, Z. Application of terahertz spectrum and interval partial least squares method in the identification of genetically modified soybeans. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 238, 118453. [Google Scholar] [CrossRef]
- Wei, X.; Zhu, S.; Zhou, S.; Zheng, W.; Li, S. Identification of soybean origin by terahertz spectroscopy and chemometrics. IEEE Access 2020, 8, 184988–184996. [Google Scholar] [CrossRef]
- Wei, X.; Li, S.; Zhu, S.; Zheng, W.; Zhou, S.; Wu, W.; Xie, Z. Quantitative analysis of soybean protein content by terahertz spectroscopy and chemometrics. Chemom. Intell. Lab. Syst. 2021, 208, 104199. [Google Scholar] [CrossRef]
- Wei, X.; Li, S.; Zhu, S.; Zheng, W.; Xie, Y.; Zhou, S.; Hu, M.; Miao, Y.; Ma, L.; Wu, W.; et al. Terahertz spectroscopy combined with data dimensionality reduction algorithms for quantitative analysis of protein content in soybeans. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 253, 119571. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Kong, D.; Zhu, S.; Li, S.; Zhou, S.; Wu, W. Rapid identification of soybean varieties by terahertz frequency-domain spectroscopy and grey wolf optimizer-support vector machine. Front. Plant Sci. 2022, 13, 823865. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, S.; Shiraga, K.; Suzuki, T.; Kondo, N.; Ogawa, Y. Quantification of starch content in germinating mung bean seedlings by terahertz spectroscopy. Food Chem. 2019, 294, 203–208. [Google Scholar] [CrossRef]

| Standard Name | Object | Test Method |
|---|---|---|
| GB/T 20378-2006 [14] | Native starch | Polarimetric method |
| ISO 10520-1997 [15] | Native starch | Polarimetric method |
| GB/T 25219-2010 [16] | Corn | NIRS |
| AOAC 940.30 [17] | Prepared mustard | Titration methods |
| AOAC 925.50 [18] | Confectionery | Titration methods |
| AOAC 920.10 [19] | Coffee | Titration methods |
| AOAC 920.44 [20] | Baking powders | Titration methods |
| AOAC 920.83 [21] | Cacao products | Titration methods |
| AOAC 979.10 [22] | Cereals | Spectrophotometric methods |
| AOAC 996.11 [23] | Cereal products | Spectrophotometric methods |
| AOAC 958.06 [24] | Meat | Titration methods |
| ISO 13965-1998 [25] | Meat and meat products | Spectrophotometric methods |
| NY/T 802-2004 [26] | Milk and milk products | Spectrophotometric methods |
| AACC 76-11 [27] | Food | Spectrophotometric methods |
| GB 5009.9-2016 [28] | Food | Titration methods |
| Research Object | Spectral Technology | Model-Building Algorithm | Result (R2 or R) | Ref. |
|---|---|---|---|---|
| Cassava | NIRS | PLSR, SVR, RT, ER, GR | R2: 0.88 | Posom, J et al. [83] |
| Fresh cassava roots | NIRS | PLSR | R: 0.825 | Bantadjan, Y et al. [80] |
| Fresh cassava roots | NIRS | PLSR, RF, SVR | R2: 0.84–0.90 | Mbanjo, EGN et al. [81] |
| Fresh cassava roots | NIRS | PLSR | R2: 0.91 | Maraphum, K et al. [82] |
| Cassava | NIRS | PLSR | R2: 0.673 | Chaiareekitwat, S et al. [84] |
| Potato | NIRS | PLSR | R: 0.893 | Ding, JG et al. [85] |
| Potato | NIRS | PLSR | R: 0.9122 | Wang, F et al. [86,87] |
| Sweet potato | NIRS | PLSR | R2: 0.94 | Tang, CC et al. [88] |
| Fresh yam | NIRS | MPLS | R2:0.83 | Alamu, EO et al. [89] |
| Rice bean and adzuki bean | NIRS | MPLS | R2: 0.962 | John, R et al. [90] |
| Cowpea | NIRS | MPLS | R: 0.93 | Padhi, SR et al. [91] |
| Buckwheat | NIRS | PLSR | R2: 0.9986 | Zhang, J et al. [92] |
| Wheat Grain | NIRS | PLSR, MLR, SVR | R2: 0.998 | Joe, AAF et al. [93] |
| Wheat, glutinous rice, and other cereals | NIRS | PCR, PLSR | R2 greater than 0.9 | He, MH et al. [94] |
| Pearl millet | NIRS | MPLS | R2: 0.915 | Tomar, M et al. [95] |
| Foxtail millet | NIRS | PLSR | R2: 0.827, 0.906 | Zhang, HY et al. [96] |
| Rice | NIRS | PLSR, MPLS, PCR | R2: 0.8195 | John, R et al. [97] |
| Meatballs | NIRS | PLSR | R2: 0.98 | Vichasilp, C et al. [98] |
| Potato | Hyperspectral imaging technology | PLSR, SVR | R: 0.9467 | Wang, FX [101,102,103] |
| Sweet potato | Hyperspectral imaging technology | PLSR, MLR | R: 0.970 | He, HJ et al. [104] |
| Tiegun Yam | Hyperspectral imaging technology | PLSR, RF, SVR | R2: 0.9677 | Zhang, Y et al. [105] |
| Puerariae Thomsonii Radix | Hyperspectral imaging technology | PLSR, SVR, CatBoost, 1DCNN | R2: 0.9091 | Hu, HQ et al. [106] |
| Mixed sorghum | Hyperspectral imaging technology | SVR, BPNN | R2: 0.9948, 0.9985 | Bu, YH et al. [107] |
| Wheat flour | Hyperspectral imaging technology | PLSR, PCR, SVR, MLR | R2: 0.9243 | Zhang, J et al. [108] |
| Maize kernels | Hyperspectral imaging technology | PLSR, SVR, ELM | R: 0.8847 | Qiao, MM et al. [109] |
| Corn seeds | Hyperspectral imaging technology | PLSR, ANN | R: 0.96 | Liu, C et al. [110] |
| Fermented grains | Hyperspectral imaging technology | SVR | R2: 0.9976 | Liang, Y et al. [111] |
| Rice (with husk) | Hyperspectral imaging technology | PLSR, SVR, PCR | R2: 0.8029 | Zhang, ZH [112] |
| Rice | Hyperspectral imaging technology | SVR | R2: 0.991 | Lu, XZ [113] |
| Tuber flours | NIRS, MIRS | PLSR, SOPLS | R2: 0.95 | Kandpal, LM et al. [122] |
| Colored-flesh potatoes | NIRS, MIRS, Raman, Fluorescence | PLSR | R: 0.949 | Pielorz, S et al. [123] |
| Pea seeds | MIRS | PLSR | R: 0.749 | Karunakaran, C et al. [124] |
| Mung beans | NIRS, Raman | PLSR | R: 0.469 | Wu, ML et al. [126] |
| Banana fruit | Raman | linear regression, PLSR | R2: 0.88 | Nakajima, S et al. [127] |
| Rice | Raman | PLSR, SVR, BPNN | R: 0.8915 | Wei, X et al. [128,129,130,131,132,133] |
| Germinating mung bean seedlings | THz | PLSR | R: 0.98 | Nakajima, S et al. [134] |
| Detection Technology | Advantages | Disadvantages |
|---|---|---|
| Enzymatic hydrolysis–titration | Highly specific and accurate. | Cumbersome, time-consuming, and costly to operate. |
| Acid hydrolysis–titration | Faster and simpler to operate, with better accuracy and detection efficiency, and easier to popularize (compared with enzymatic hydrolysis). | Not as selective as enzyme hydrolysis, with many factors interfering with the test results, requiring a higher level of operator skills. |
| Spectrophotometric methods | Relatively simple operation and a large number of samples can be tested. | Easily affected by the color components in the sample to be measured, weak anti-interference. |
| Chromatography | Glucose content can be accurately detected (compared with titration and spectrophotometric methods), easy to batch experimental samples, with good precision. | Complicated and time-consuming operation process. |
| Polarimetric methods | Higher precision and better repeatability. | The acidic calcium chloride solution has strict standard requirements for pH and temperature, etc., and the test results are generally on the high side. |
| Thermogravimetric analysis | Simultaneous quantitative detection of multiple components. | Highly interfering with experimental conditions, not suitable for testing samples with low starch content. |
| Near-infrared spectroscopy | No complicated pre-treatment, saving time and effort, no use of chemical reagents, and non-destructive to the experimental samples. | Low sensitivity, and other components in the experimental samples have a great influence on the results of starch content detection. |
| Hyperspectral imaging technology | Simple pre-processing, fast and easy to operate, simultaneous detection of multiple components, acquisition of two-dimensional sample image, strong visualization. | Detection model accuracy is limited, it is difficult to apply to the detection needs of samples with complex compositions, and the generalization ability of the detection model among different brands and models of equipment is poor. |
| Mid-infrared, Raman, and Terahertz spectroscopy technology | No complicated pre-treatment, no additional waste and solvent generation, more in line with the green testing requirements. The mid-infrared region has many characteristic absorption peaks of starch functional groups and molecular bonds, which is convenient for quantitative detection of starch content. Raman spectroscopy is not easily interfered by sample moisture. THz spectroscopy generally does not cause radiation damage to the sample, and the weak interactions of molecules and low-frequency vibrational absorption, etc., are in the THz band. | The research of these methods is in its infancy, the mechanism analysis is not thorough enough, the accuracy of the detection model is generally not high, and the stability is still to be verified. Corresponding spectroscopic equipment is required, and the cost is high. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, X.; Li, F.; Liu, Y.; Li, S.; Liu, Y.; Dong, D. Research Progress on Techniques for Quantitative Detection of Starch in Food in the Past Five Years. Agriculture 2025, 15, 1250. https://doi.org/10.3390/agriculture15121250
Wei X, Li F, Liu Y, Li S, Liu Y, Dong D. Research Progress on Techniques for Quantitative Detection of Starch in Food in the Past Five Years. Agriculture. 2025; 15(12):1250. https://doi.org/10.3390/agriculture15121250
Chicago/Turabian StyleWei, Xiao, Fang Li, Yinfeng Liu, Song Li, Yachao Liu, and Daming Dong. 2025. "Research Progress on Techniques for Quantitative Detection of Starch in Food in the Past Five Years" Agriculture 15, no. 12: 1250. https://doi.org/10.3390/agriculture15121250
APA StyleWei, X., Li, F., Liu, Y., Li, S., Liu, Y., & Dong, D. (2025). Research Progress on Techniques for Quantitative Detection of Starch in Food in the Past Five Years. Agriculture, 15(12), 1250. https://doi.org/10.3390/agriculture15121250






