Impact of High Temperature on Germination, Seedling Growth and Enzymatic Activity of Wheat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seed Material
2.2. Experimentation
2.3. Observations Recorded
2.4. Statistical Analysis
3. Results
3.1. Analysis of Variance
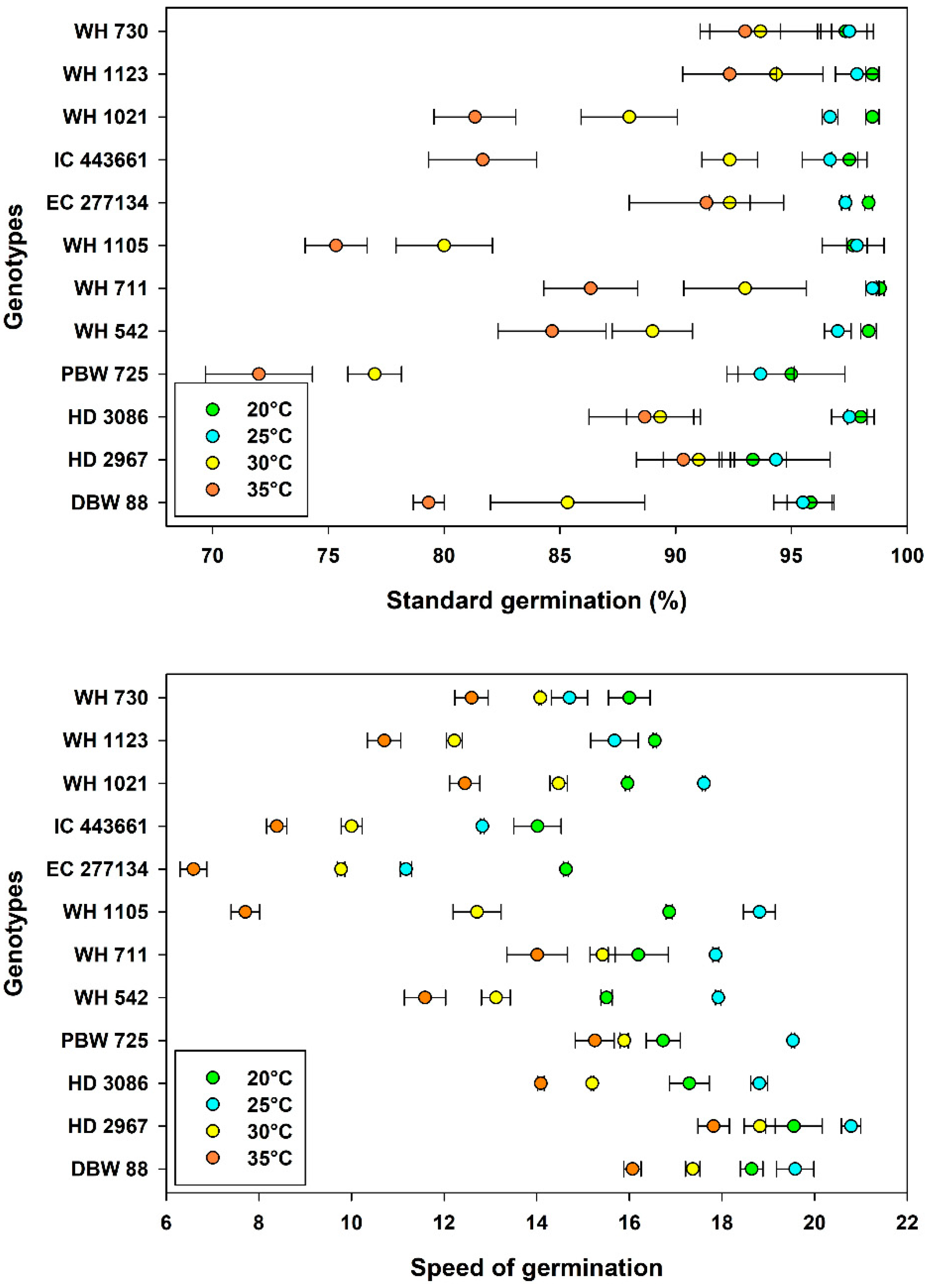
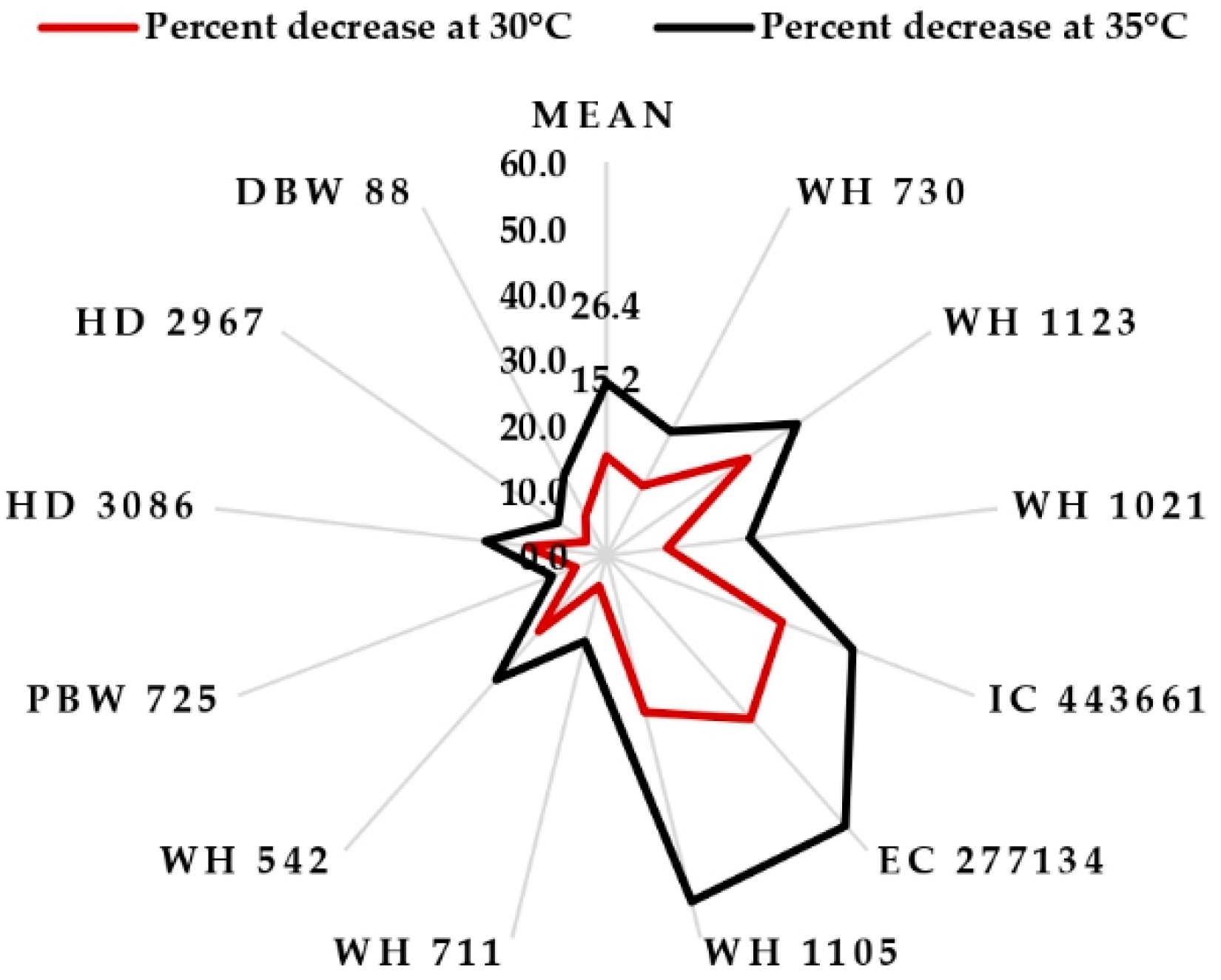
3.2. Germination Characteristics
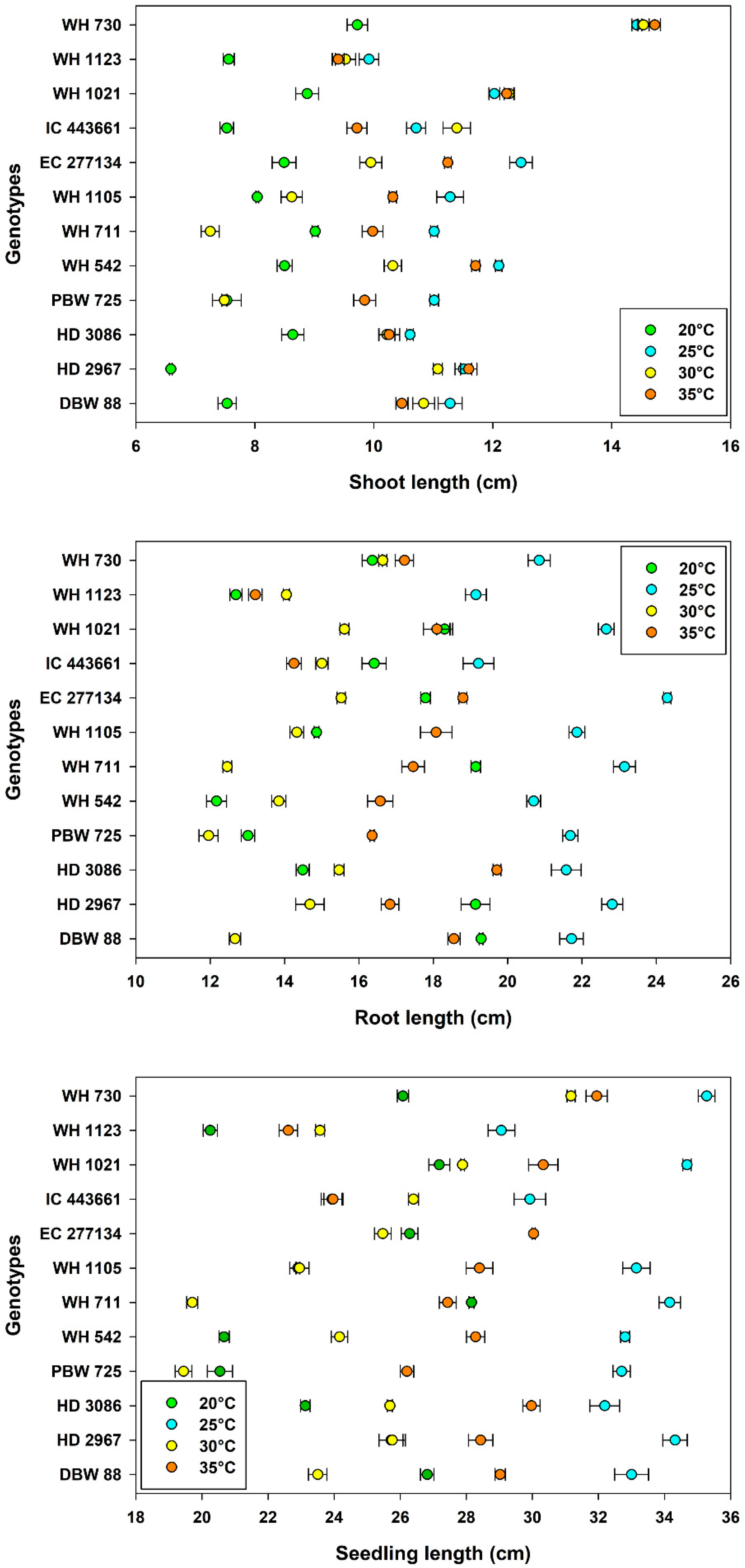
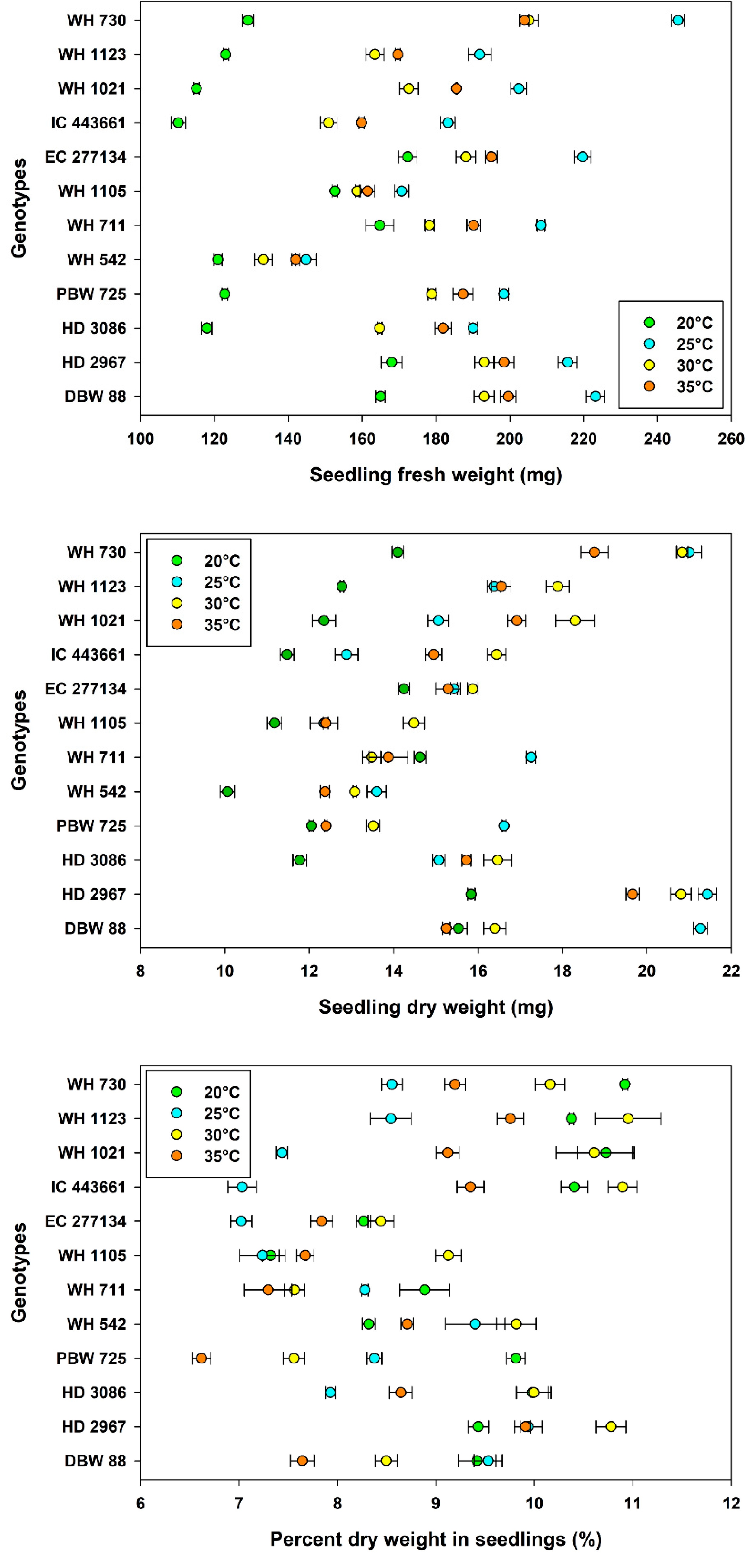
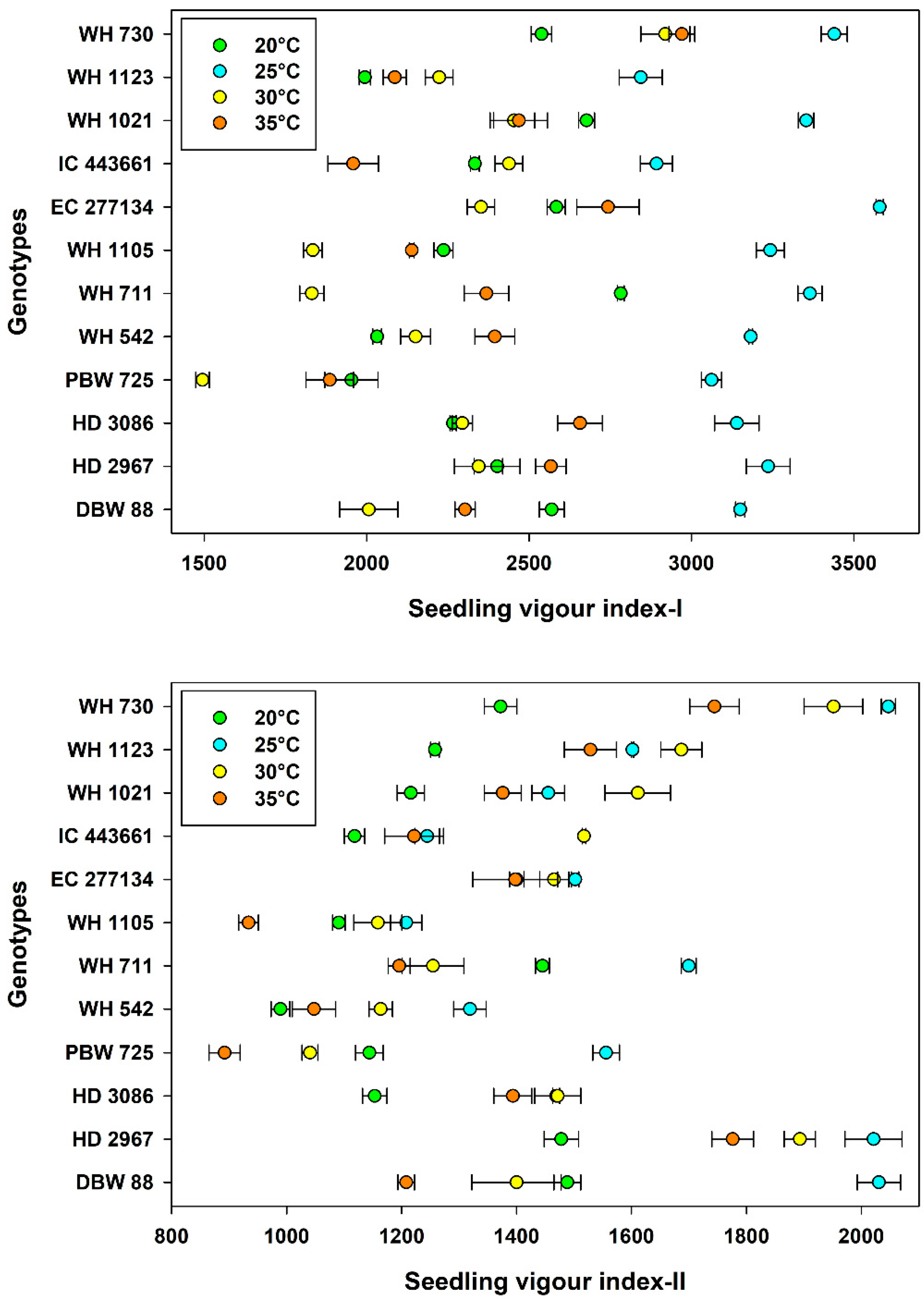
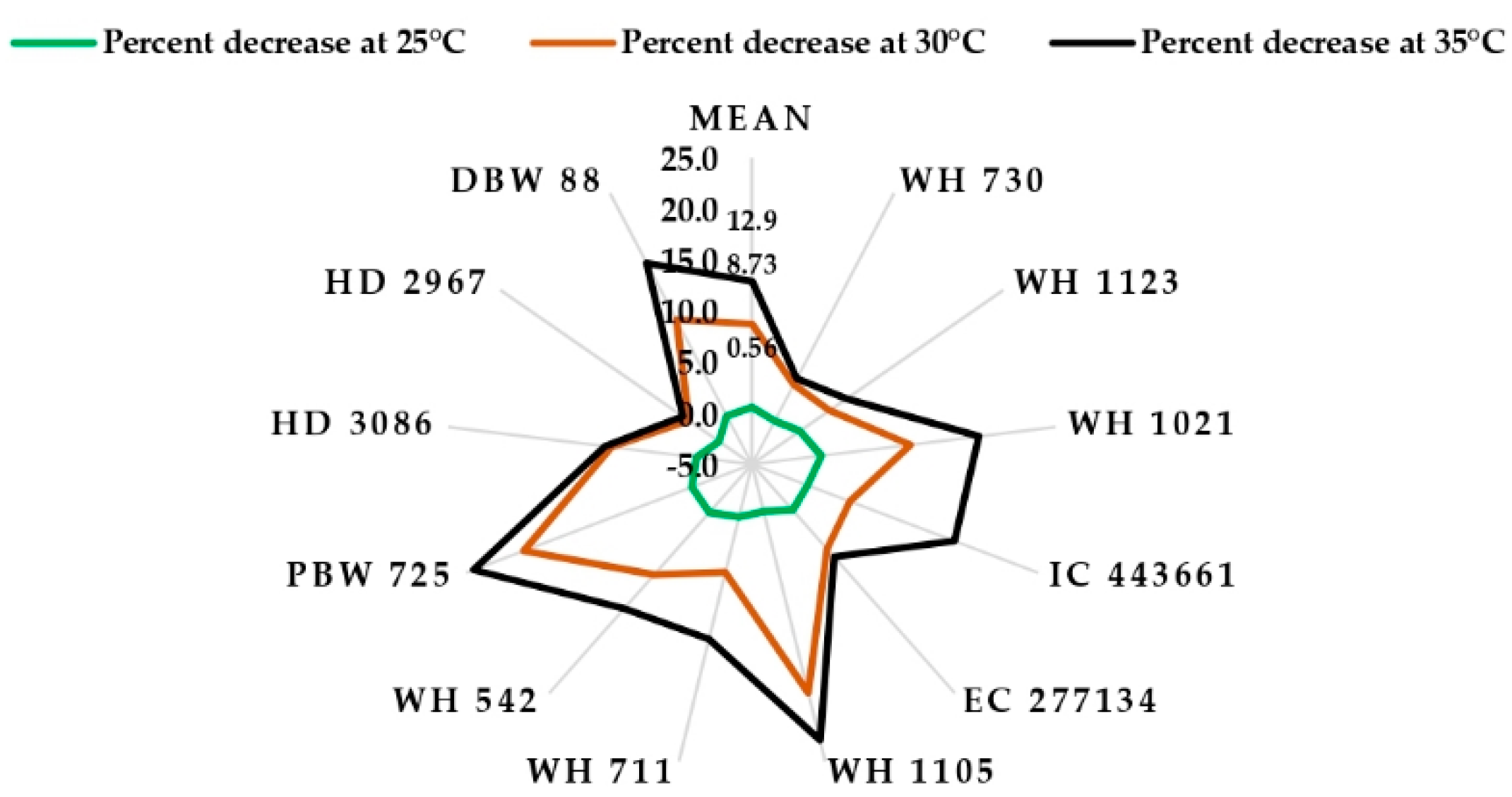
3.3. Seedling Growth and Vigor
3.4. Antioxidant Enzymes
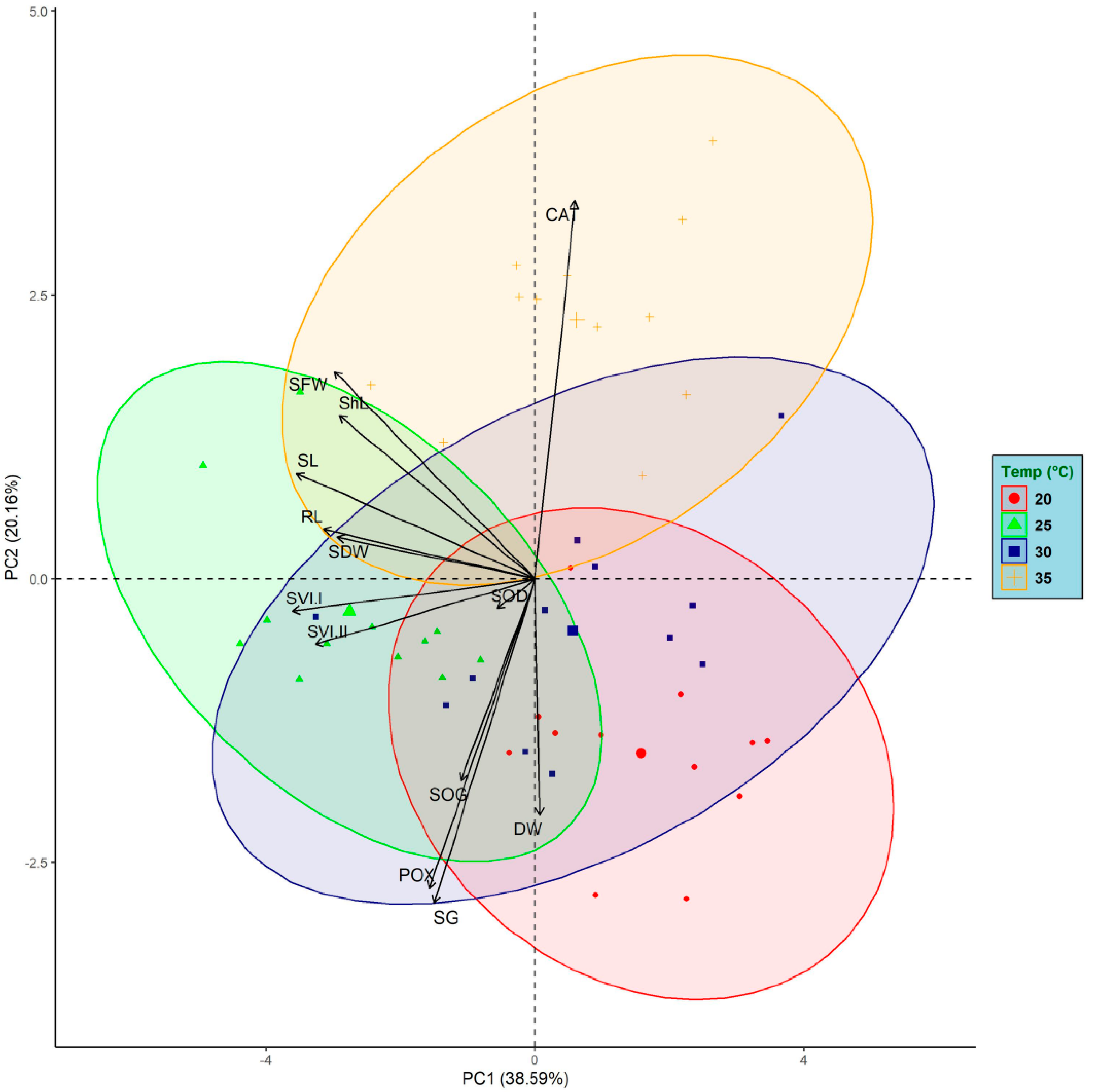
3.5. Principal Component Analysis (PCA)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seneviratne, S.I.; Nicholls, N.; Easterling, D.; Goodess, C.M.; Kanae, S. Changes in climate extremes and their impacts on the natural physical environment. In Managing the Risks of Extreme Events and Disasters to Advance Climate Change Adaptation; Field, C.B., Barros, V., Stocker, T.F., Qin, D., Dokken, D.J., Ebi, K.L., Mastrandrea, M.D., Mach, K.J., Plattner, G.-K., Allen, S.K., et al., Eds.; A Special Report of Working Groups I and II of the Intergovernmental Panel on Climate Change (IPCC); Cambridge University Press: Cambridge, UK; New York, NY, USA, 2012; pp. 109–230. [Google Scholar]
- Kerasa, S.; Baric, M.; Sarcevic, H.; Marchette, S.; Drasner, G. Callus Induction and Plant Regeneration from Immature and Mature Embryos of Winter Wheat (Triticum aestivum L.) genotypes. Plant Breeding Sustaining the Future. In Proceedings of the XVIth Eucarpia Congress, Edinburgh, UK, 10–14 September 2001; p. 41. [Google Scholar]
- Barun, H.J.; Atlin, G.; Payne, T. Multi-location testing as a tool to identify plant response to global climate change. In Climate Change and Crop Production; Reynolds, M.P., Ed.; CABI: Wallingford, UK, 2010; pp. 115–138. [Google Scholar]
- Acevedo, M.; Zurn, J.D.; Molero, G.; Singh, P.; He, X.; Aoun, M.; Juliana, P.; Bockleman, H.; Bonman, M.; El-Sohl, M.; et al. The role of wheat in global food security. In Agricultural Development and Sustainable Intensification; Nagothu, U.S., Ed.; Routledge: London, UK, 2018; pp. 81–110. [Google Scholar] [CrossRef]
- Joshi, A.K.; Mishra, B.; Chatrath, R.; Ferrara, G.O.; Singh, R.P. Wheat improvement in India: Present status, emerging challenges and future prospects. Euphytica 2007, 157, 431–446. [Google Scholar] [CrossRef]
- Howard, A. Crop production in India: A Critical Survey of Its Problems; Oxford University Press: Oxford, UK, 1924; p. 156. [Google Scholar]
- Johkan, M.; Oda, M.; Maruo, T.; Shinohara, Y. Crop production and global warming. In Global Warming Impacts-Case Studies on the Economy, Human Health, and on Urban and Natural Environments; Casalegno, S., Ed.; IntechOpen: Rijeka, Croatia; London, UK, 2011; pp. 139–152. [Google Scholar]
- Hossain, A.; Sarker, M.; Saifuzzaman, M.; da Silva, J.T.; Lozovskaya, M.; Akhter, M. Evaluation of growth, yield, relative performance and heat susceptibility of eight wheat (Triticum aestivum L.) genotypes grown under heat stress. Int. J. Plant Prod. 2013, 7, 615–636. [Google Scholar] [CrossRef]
- Buriro, M.; Oad, F.C.; Keerio, M.I.; Tunio, S.; Gandahi, A.W. Wheat Seed Germination under Influence of Temperature Regimes. Sarhad J. Agric. 2011, 27, 4. [Google Scholar]
- Hampson, C.R.; Simpson, G.M. Effect of temperature, salt and osmotic potential on early growth of wheat (Triticum aestivum) germination. Can. J. Bot. 1990, 68, 524–528. [Google Scholar] [CrossRef]
- Prasad, P.V.V.; Boote, K.J.; Allen, L.H., Jr. Adverse high temperature effects on pollen viability, seed-set, seed yield and harvest index of grain-sorghum [Sorghum bicolor (L.) Moench] are more severe at elevated carbon dioxide due to higher tissue tem-peratures. Agric. For. Meteorol. 2006, 39, 237–251. [Google Scholar] [CrossRef]
- ISTA. International Rules for Seed Testing; International Seed Testing Association: Bassersdorf, Switzerland, 2015. [Google Scholar]
- Maguire, J.D. Speed of germination-aid selection and evaluation for seedling emergence and vigor. Crop Sci. 1962, 2, 176–177. [Google Scholar] [CrossRef]
- Abdul-Baki, A.A.; Anderson, J.D. Vigour determination in soybean seed by multiple criteria. Crop Sci. 1973, 13, 630–633. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide Dismutases: Occurrence in Higher Plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Sinha, A.K. Calorimetric assay of catalase. Anal. Biochem. 1972, 47, 2. [Google Scholar] [CrossRef]
- Rao, M.B.; Tanksale, A.M.; Ghatge, M.S.; Deshpande, V.V. Molecular and Biotechnological Aspects of Microbial Proteases. Microbiol. Mol. Biol. Rev. 1998, 62, 597–635. [Google Scholar] [CrossRef]
- Sandhu, S.S.; Kaur, P.; Gill, K.K.; Vashisth, B.B. The effect of recent climate shifts on optimal sowing windows for wheat in Punjab, India. J. Water Clim. Chang. 2019, 11, 1177–1190. [Google Scholar] [CrossRef]
- Khodabandeh, N. Cereals, 7th ed.; Tehran University Press: Tehran, Iran, 2003; pp. 78–111. [Google Scholar]
- Thuzar, M.; Puteh, A.B.; Abdullah, N.A.P.; Lassim, M.B.M.; Jusoff, K. The Effects of Temperature Stress on the Quality and Yield of Soya Bean [(Glycine max L.) Merrill.]. J. Agric. Sci. 2010, 2, 172. [Google Scholar] [CrossRef]
- Toh, S.; Imamura, A.; Watanabe, A.; Nakabayashi, K.; Okamoto, M.; Jikumaru, Y.; Hanada, A.; Aso, Y.; Ishiyama, K.; Tamura, N.; et al. High Temperature-Induced Abscisic Acid Biosynthesis and Its Role in the Inhibition of Gibberellin Action in Arabidopsis Seeds. Plant Physiol. 2008, 146, 1368–1385. [Google Scholar] [CrossRef]
- Essemine, J.; Ammar, S.; Bouzid, S. Impact of Heat Stress on Germination and Growth in Higher Plants: Physiological, Biochemical and Molecular Repercussions and Mechanisms of Defence. J. Biol. Sci. 2010, 10, 565–572. [Google Scholar] [CrossRef]
- Blacklow, W.M. Influence of Temperature on Germination and Elongation of the Radicle and Shoot of Corn (Zea mays L.) 1. Crop Sci. 1972, 12, 647–650. [Google Scholar] [CrossRef]
- Kumar, S.; Kaur, R.; Kaur, N.; Bhandhari, K.; Kaushal, N.; Gupta, K.; Bains, T.S.; Nayyar, H. Heat-stress induced inhibition in growth and chlorosis in mungbean (Phaseolus aureus Roxb.) is partly mitigated by ascorbic acid application and is related to reduction in oxidative stress. Acta Physiol. Plant. 2011, 33, 2091–2101. [Google Scholar] [CrossRef]
- Piramila, B.H.M.; Prabha, A.L.; Nandagopalan, V.; Stanley, A.L. Effect of heat treatment on germination, seedling growth and some biochemical parameters of dry seeds of black gram. Int. J. Pharm. Phytopharm. Res. 2011, 1, 194–202. [Google Scholar]
- Iloh, A.C.; Omatta, G.; Ogbadu, G.H.; Onyenekwe, P.C. Effects of elevated temperature on seed germination and seedling growth on three cereal crops in Nigeria. Sci. Res. Essays 2014, 9, 806–813. [Google Scholar] [CrossRef]
- Sikder, S.; Hasan, M.; Hossain, M. Germination Characteristics and Mobilization of Seed Reserves in Maize Varieties as Influenced by Temperature Regimes. J. Agric. Rural Dev. 1970, 7, 51–58. [Google Scholar] [CrossRef]
- Roberts, E.H. Temperature and seed germination. Symp. Soc. Exp. Biol. 1988, 42, 32. [Google Scholar]
- Sikder, S.; Paul, N.K. Study of influence of temperature regimes on germination characteristics and seed reserves mobilization in wheat. Afr. J. Plant Sci. 2010, 4, 401–408. [Google Scholar]
- Jorenush, M.H.; Rajabi, M. Effect of Drought and Salinity Tensions on Germination and Seedling Growth of Artichoke (Cynara scolymus L.). Int. J. Adv. Biol. Biomed. Res. 2015, 3, 297–302. [Google Scholar]
- Özaktan, H.; Çiftçi, C.Y.; Kaya, M.D.; Uzun, S.; Akdogan, G. Chloride salts inhibit emergence and seedling growth of chickpea rather than germination. Legum. Res. Int. J. 2017, 40, 60–66. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Yin, H.; Chen, Q.; Yi, M. Effects of short-term heat stress on oxidative damage and responses of antioxidant system in Lilium longiflorum. Plant Growth Regul. 2007, 54, 45–54. [Google Scholar] [CrossRef]
- Hernández, J.A.; Almansa, M. Short-term effects of salt stress on antioxidant systems and leaf water relations of pea leaves. Physiol. Plant. 2002, 115, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Dionisio-Sese, M.L.; Tobita, S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998, 135, 1–9. [Google Scholar] [CrossRef]
- Sudhakar, C.; Lakshmi, A.; Giridarakumar, S. Changes in the antioxidant enzyme efficacy in two high yielding genotypes of mulberry (Morus alba L.) under NaCl salinity. Plant Sci. 2001, 161, 613–619. [Google Scholar] [CrossRef]
- Lin, C.C.; Kao, C.H. Osmotic stress-induced changes in cell wall peroxidase activity and hydrogen peroxide level in roots of rice seedlings. Plant Growth Regul. 2002, 37, 177–184. [Google Scholar] [CrossRef]
- Foyer, C.; Noctor, G. Oxygen processing in photosynthesis: Regulation and signaling. New Phytol. 2000, 146, 359–388. [Google Scholar] [CrossRef]
- Badawi, G.H.; Tahir, I.S.A.; Nakata, N.; Tanaka, K. Induction of some antioxidant enzymes in selected wheat genotypes. In Proceedings of the Eighth African Crop Science Conference Proceedings, El-Minia, Egypt, 27–31 October 2007; pp. 841–848. [Google Scholar]
- Wang, Z.-Y.; Li, F.-M.; Xiong, Y.-C.; Xu, B.-C. Soil-Water Threshold Range of Chemical Signals and Drought Tolerance Was Mediated by ROS Homeostasis in Winter Wheat During Progressive Soil Drying. J. Plant Growth Regul. 2008, 27, 309–319. [Google Scholar] [CrossRef]
- Ibrahim, M.; Alsahli, A.; Al-Ghamdi, A. Cumulative abiotic stresses and their effect on the antioxidant defense system in two species of wheat, Triticum durum Desf and Triticum aestivum L. Arch. Biol. Sci. 2013, 65, 1423–1433. [Google Scholar] [CrossRef]
- Gupta, N.K.; Agarwal, S.; Agarwal, V.P.; Nathawat, N.S.; Gupta, S.; Singh, G. Effect of short-term heat stress on growth, physiology and antioxidative defence system in wheat seedlings. Acta Physiol. Plant. 2013, 35, 1837–1842. [Google Scholar] [CrossRef]
- Caverzan, A.; Casassola, A.; Brammer, S.P. Antioxidant responses of wheat plants under stress. Genet. Mol. Biol. 2016, 39, 1–6. [Google Scholar] [CrossRef]
- Pang, C.-H.; Wang, B.-S. Role of Ascorbate Peroxidase and Glutathione Reductase in Ascorbate–Glutathione Cycle and Stress Tolerance in Plants. In Ascorbate-Glutathione Pathway and Stress Tolerance in Plants; Anjum, N., Chan, M.T., Umar, S., Eds.; Springer: Dordrecht, The Netherlands, 2010. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Catalase, superoxide dismutase and ascorbate-glutathione cycle enzymes confer drought tolerance of Amaranthus tricolor. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Zheng, B.; Chenu, K.; Dreccer, M.F.; Chapman, S.C. Breeding for the future: What are the potential impacts of future frost and heat events on sowing and flowering time requirements for Australian bread wheat (Triticum aestivum) varieties? Glob. Change Biol. 2012, 18, 2899–2914. [Google Scholar] [CrossRef]

| Sr. No. | Name of the Genotype | Parentage | Details |
|---|---|---|---|
| 1. | WH 730 | CPAN 2092/Improved Lok-1 | Heat tolerance advance genotype from NBPGR (National Bureau of Plant Genetic Resources) |
| 2. | WH 1123 | NI5663/RAJ3765//K9330 | Advance genotype (heat tolerant) |
| 3. | WH 1021 | NYOT95/SONAK | High-yielding variety released for late sown conditions |
| 4. | IC 443661 | NA | Indigenous line selected from core germplasm for heat tolerance (NBPGR) |
| 5. | EC 277134 | NA | Exotic line selected from core germplasm for heat tolerance (NBPGR) |
| 6. | WH 1105 | MILAN/S87230//BABAX | Popular varieties under cultivation in north-west plains (recommended for timely sown irrigated conditions) |
| 7. | WH 711 | ALD ‘S’ HUAC//HD 2285/3/HFW- 17 | |
| 8. | WH 542 | JUP/BJY”S”//URES | |
| 9. | PBW 725 | PBW621//GLUPR O/3*PBW 568/3/PBW 621 | |
| 10. | HD 3086 | DBW14/HD2733//HUW468 | |
| 11. | HD 2967 | ALD/COC//URES/HD216 0M/HD2278 | |
| 12. | DBW 88 | KAUZ//ALTAR84/AOS/3/MILAN/KAUZ/4/HUITES |
| Germination Parameters | Seedling Growth Parameters | Biochemical Parameters | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source of Variation | d.f. | Standard Germination | Speed of Germination | Shoot Length | Root Length | Seedling Length | Seedling Fresh Weight | Seedling Dry Weight | Percent Dry Weight in Seedlings | Seedling Vigor Index-I | Seedling Vigor Index-II | CAT Activity | POX Activity | SOD Activity |
| Temperature (T) | 3 | 170 ** | 610 ** | 1296 ** | 2066 ** | 2550 ** | 2022 ** | 728 ** | 211 ** | 975 ** | 255 ** | 8470 ** | 1928 ** | 7912 ** |
| Genotype (G) | 11 | 17.1 ** | 256 ** | 286 ** | 130 ** | 228 ** | 404 ** | 437 ** | 105 ** | 89.9 ** | 189 ** | 416 ** | 266 ** | 560 ** |
| Temperature × Genotype (T × G) | 33 | 4.58 ** | 17.5 ** | 43.7 ** | 45.0 ** | 50.3 ** | 40.7 ** | 49.7 ** | 30.7 ** | 17.7 ** | 22.6 ** | 221 ** | 55.6 ** | 395 ** |
| Main Effects | Levels | Standard Germination (%) (Mean ± SE) | Speed of Germination (Mean ± SE) | Shoot Length (cm) (Mean ± SE) | Root Length (cm) (Mean ± SE) | Seedling Length (cm) (Mean ± SE) |
|---|---|---|---|---|---|---|
| Temperature, Tm | 20 °C | 97.3 ± 0.37 a | 16.5 ± 0.32 b | 8.17 ± 0.14 d | 16.1 ± 0.43 c | 24.3 ± 0.46 c |
| 25 °C | 96.7 ± 0.35 a | 17.1 ± 0.59 a | 11.5 ± 0.19 a | 21.6 ± 0.25 a | 33.2 ± 0.36 a | |
| 30 °C | 88.8 ± 1.01 b | 14.1 ± 0.54 c | 10.3 ± 0.33 c | 14.4 ± 0.24 d | 24.6 ± 0.53 c | |
| 35 °C | 84.7 ± 1.22 c | 12.3 ± 0.69 d | 11.0 ± 0.24 b | 17.1 ± 0.31 b | 28.1 ± 0.44 b | |
| Genotype, Gn | WH 730 | 95.4 ± 0.94 a | 14.3 ± 0.48 gh | 13.4 ± 0.63 a | 17.8 ± 0.55 c | 31.1 ± 1.00 a |
| WH 1123 | 95.8 ± 1.00 a | 13.8 ± 0.92 h | 9.10 ± 0.28 h | 14.8 ± 0.78 g | 23.9 ± 0.98 i | |
| WH 1021 | 91.1 ± 2.16 bc | 15.1 ± 0.72 f | 11.4 ± 0.44 b | 18.7 ± 0.77 b | 30.0 ± 0.89 b | |
| IC 443661 | 92.0 ± 2.00 b | 11.3 ± 0.85 i | 9.84 ± 0.45 e | 16.2 ± 0.58 e | 26.1 ± 0.75 g | |
| EC 277134 | 94.8 ± 1.18 ab | 10.5 ± 1.09 j | 10.5 ± 0.45 c | 19.1 ± 0.97 a | 29.6 ± 1.35 b | |
| WH 1105 | 87.7 ± 3.13 c | 14.0 ± 1.62 h | 9.56 ± 0.40 f | 17.3 ± 0.91 d | 26.9 ± 1.29 f | |
| WH 711 | 94.2 ± 1.69 ab | 15.9 ± 0.56 e | 9.31 ± 0.42 g | 18.1 ± 1.16 c | 27.4 ± 1.55 e | |
| WH 542 | 92.3 ± 1.82 b | 14.5 ± 0.91 g | 10.7 ± 0.43 c | 15.8 ± 0.98 f | 26.5 ± 1.37 f | |
| PBW 725 | 84.4 ± 3.14 d | 16.9 ± 0.63 c | 8.97 ± 0.46 h | 15.8 ± 1.15 f | 24.7 ± 1.59 h | |
| HD 3086 | 93.4 ± 1.47 ab | 16.4 ± 0.70 d | 9.93 ± 0.24 e | 17.8 ± 0.89 c | 27.7 ± 1.08 de | |
| HD 2967 | 92.3 ± 0.94 b | 19.3 ± 0.44 a | 10.2 ± 0.63 d | 18.4 ± 0.92 bc | 28.6 ± 1.07 c | |
| DBW 88 | 89.0 ± 2.26 c | 17.9 ± 0.51 b | 10.0 ± 0.45 de | 18.1 ± 1.01 c | 28.1 ± 1.05 d | |
| LSDTm | 1.49 | 0.705 | 0.12 | 0.19 | 0.23 | |
| LSDGn | 2.58 | 1.22 | 0.20 | 0.33 | 0.40 | |
| d.f. | 143 | 143 | 143 | 143 | 143 |
| Main Effects | Levels | Seedling Fresh Weight (mg) (Mean ± SE) | Seedling Dry Weight (mg) (Mean ± SE) | Percent Dry Weight in Seedlings (%) (Mean ± SE) |
|---|---|---|---|---|
| Temperature, Tm | 20 °C | 138 ± 3.88 d | 13.0 ± 0.30 c | 9.49 ± 0.18 a |
| 25 °C | 199 ± 4.30 a | 16.5 ± 0.52 a | 8.27 ± 0.16 c | |
| 30 °C | 177 ± 3.33 c | 16.5 ± 0.43 a | 9.53 ± 0.21 a | |
| 35 °C | 181 ± 3.11 b | 15.3 ± 0.39 b | 8.48 ± 0.17 b | |
| Genotype, Gn | WH 730 | 196 ± 12.7 a | 18.7 ± 0.85 b | 9.71 ± 0.28 ab |
| WH 1123 | 162 ± 7.54 e | 15.9 ± 0.58 d | 9.91 ± 0.28 a | |
| WH 1021 | 169 ± 9.92 d | 15.7 ± 0.69 d | 9.47 ± 0.42 bc | |
| IC 443661 | 151 ± 7.98 g | 13.9 ± 0.58 g | 9.42 ± 0.45 bcd | |
| EC 277134 | 194 ± 5.24 a | 15.2 ± 0.20 e | 7.89 ± 0.17 f | |
| WH 1105 | 161 ± 2.06 f | 12.6 ± 0.37 h | 7.84 ± 0.24 f | |
| WH 711 | 185 ± 4.94 b | 14.8 ± 0.46 f | 8.01 ± 0.20 f | |
| WH 542 | 135 ± 2.93 h | 12.3 ± 0.41 i | 9.06 ± 0.19 de | |
| PBW 725 | 172 ± 8.82 c | 13.6 ± 0.54 g | 8.09 ± 0.36 f | |
| HD 3086 | 164 ± 8.44 e | 14.8 ± 0.55 f | 9.14 ± 0.27 cde | |
| HD 2967 | 194 ± 5.28 a | 19.4 ± 0.66 a | 10.0 ± 0.15 a | |
| DBW 88 | 195 ± 6.32 a | 17.1 ± 0.74 c | 8.77 ± 0.24 e | |
| LSDTm | 1.60 | 0.17 | 0.13 | |
| LSDGn | 2.77 | 0.30 | 0.22 | |
| d.f. | 143 | 143 | 143 |
| Main Effects | Levels | Seedling Vigor Index-I (Mean ± SE) | Seedling Vigor Index-II (Mean ± SE) | Catalase Activity (Units min−1 g−1 F.W.) (Mean ± SE) | Peroxidase Activity (Units min−1 g−1 F.W.) (Mean ± SE) | Superoxide Dismutase Activity (Units min−1 g−1 F.W.) (Mean ± SE) |
|---|---|---|---|---|---|---|
| Temperature, Tm | 20 °C | 2364 ± 45.6 b | 1263 ± 27.8 b | 0.474 ± 0.02 c | 50.9 ± 0.55 c | 23.3 ± 1.51 d |
| 25 °C | 3207 ± 36.1 a | 1596 ± 48.7 a | 0.482 ± 0.02 c | 55.3 ± 0.42 a | 39.3 ± 1.43 b | |
| 30 °C | 2195 ± 61.1 c | 1468 ± 47.5 c | 0.511 ± 0.02 b | 54.4 ± 0.86 b | 54.1 ± 1.90 a | |
| 35 °C | 2379 ± 54.9 b | 1310 ± 47.0 d | 0.885 ± 0.01 a | 42.9 ± 0.80 d | 30.2 ± 2.11 c | |
| Genotype, G | WH 730 | 2967 ± 98.7 a | 1779 ± 79.6 a | 0.636 ± 0.04 b | 53.1 ± 1.33 c | 26.1 ± 3.36 i |
| WH 1123 | 2287 ± 102 g | 1519 ± 50.0 b | 0.608 ± 0.05 d | 51.4 ± 1.82 e | 38.5 ± 5.83 cd | |
| WH 1021 | 2738 ± 113 c | 1415 ± 45.9 cd | 0.461 ± 0.08 g | 54.0 ± 1.47 b | 38.3 ± 4.69 d | |
| IC 443661 | 2405 ± 103 f | 1275 ± 46.2 e | 0.486 ± 0.08 f | 58.2 ± 1.97 a | 41.0 ± 2.66 b | |
| EC 277134 | 2815 ± 141 b | 1441 ± 21.6 c | 0.685 ± 0.06 a | 49.7 ± 1.47 f | 40.4 ± 5.83 b | |
| WH 1105 | 2363 ± 160 f | 1098 ± 33.2 g | 0.556 ± 0.04 e | 52.0 ± 1.80 de | 33.3 ± 2.56 g | |
| WH 711 | 2587 ± 170 d | 1399 ± 60.6 cd | 0.496 ± 0.07 f | 51.9 ± 1.35 de | 27.9 ± 3.71 h | |
| WH 542 | 2440 ± 136 ef | 1130 ± 39.7 fg | 0.621 ± 0.06 c | 48.6 ± 1.50 g | 39.1 ± 5.69 c | |
| PBW 725 | 2099 ± 177 h | 1158 ± 74.9 f | 0.683 ± 0.06 a | 46.6 ± 2.05 i | 35.5 ± 4.93 f | |
| HD 3086 | 2589 ± 109 d | 1372 ± 41.0 d | 0.629 ± 0.07 c | 44.9 ± 1.83 j | 34.1 ± 4.37 f | |
| HD 2967 | 2638 ± 111 d | 1792 ± 62.6 a | 0.643 ± 0.05 b | 52.3 ± 1.65 d | 49.4 ± 3.64 a | |
| DBW 88 | 2507 ± 129 e | 1532 ± 94.1 b | 0.551 ± 0.04 e | 47.8 ± 1.86 h | 37.4 ± 2.79 e | |
| LSDTm | 41.0 | 26.8 | 0.006 | 0.359 | 0.421 | |
| LSDG | 71.0 | 46.5 | 0.011 | 0.623 | 0.729 | |
| d.f. | 143 | 143 | 143 | 143 | 143 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, S.; Singh, V.; Tanwar, H.; Mor, V.S.; Kumar, M.; Punia, R.C.; Dalal, M.S.; Khan, M.; Sangwan, S.; Bhuker, A.; et al. Impact of High Temperature on Germination, Seedling Growth and Enzymatic Activity of Wheat. Agriculture 2022, 12, 1500. https://doi.org/10.3390/agriculture12091500
Sharma S, Singh V, Tanwar H, Mor VS, Kumar M, Punia RC, Dalal MS, Khan M, Sangwan S, Bhuker A, et al. Impact of High Temperature on Germination, Seedling Growth and Enzymatic Activity of Wheat. Agriculture. 2022; 12(9):1500. https://doi.org/10.3390/agriculture12091500
Chicago/Turabian StyleSharma, Sushma, Vikram Singh, Hemender Tanwar, Virender Singh Mor, Mukesh Kumar, Ramesh Chander Punia, Mohinder Singh Dalal, Mujahid Khan, Sonali Sangwan, Axay Bhuker, and et al. 2022. "Impact of High Temperature on Germination, Seedling Growth and Enzymatic Activity of Wheat" Agriculture 12, no. 9: 1500. https://doi.org/10.3390/agriculture12091500
APA StyleSharma, S., Singh, V., Tanwar, H., Mor, V. S., Kumar, M., Punia, R. C., Dalal, M. S., Khan, M., Sangwan, S., Bhuker, A., Dagar, C. S., Yashveer, S., & Singh, J. (2022). Impact of High Temperature on Germination, Seedling Growth and Enzymatic Activity of Wheat. Agriculture, 12(9), 1500. https://doi.org/10.3390/agriculture12091500







