Genotype by Environment Interaction Analysis for Grain Yield of Wheat (Triticum aestivum (L.) em.Thell) Genotypes
Abstract
:1. Introduction
2. Material and Methods
2.1. Field Experimentation
2.2. Plant Materials
2.3. Experimental Design and Layout
2.4. Statistical Analysis
3. Results
3.1. Eberhart and Russell Model
3.2. Environmental Indices
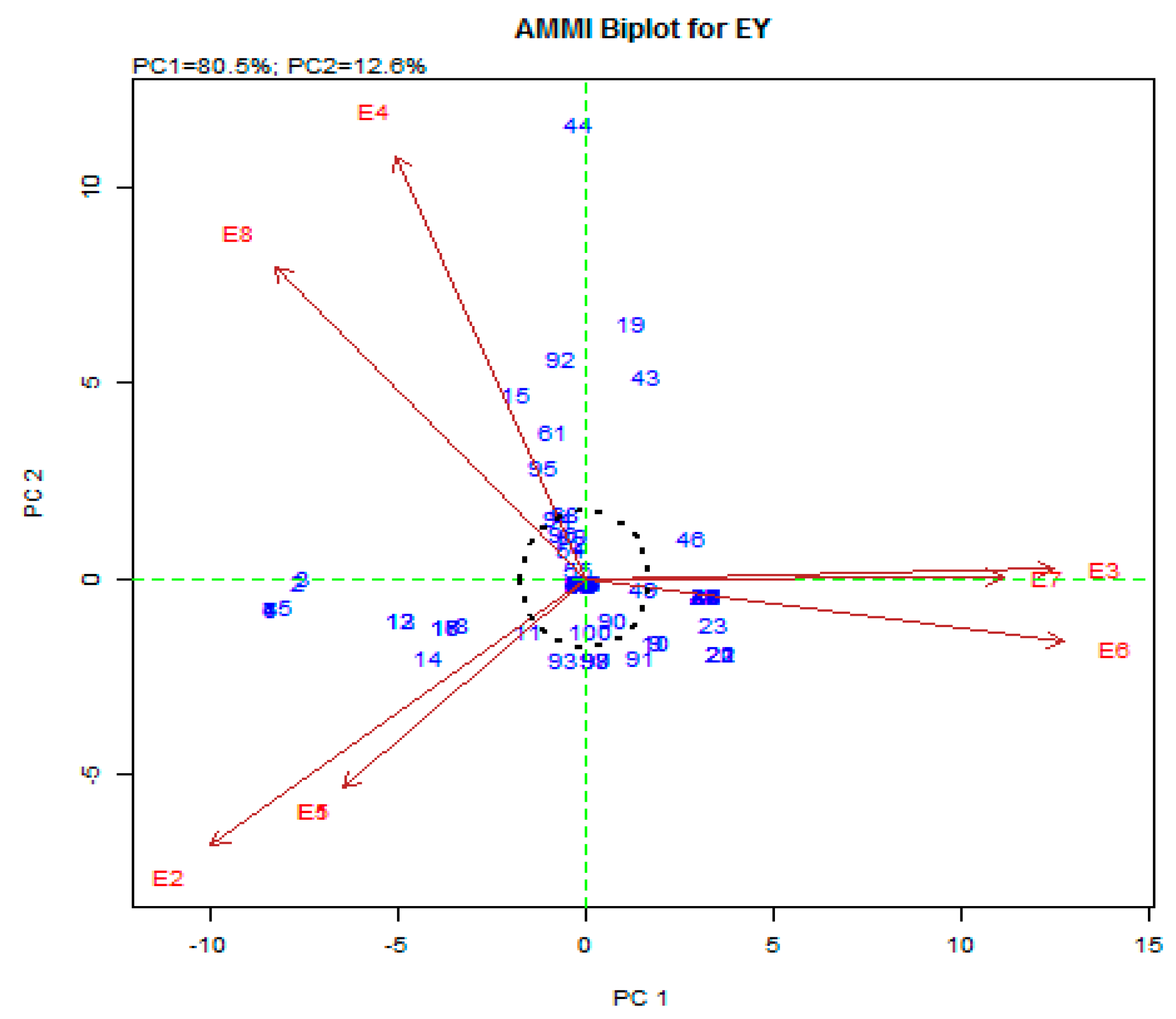
3.3. AMMI Biplot Analysis
3.4. The AMMI 1 Model
3.5. The AMMI2 Model
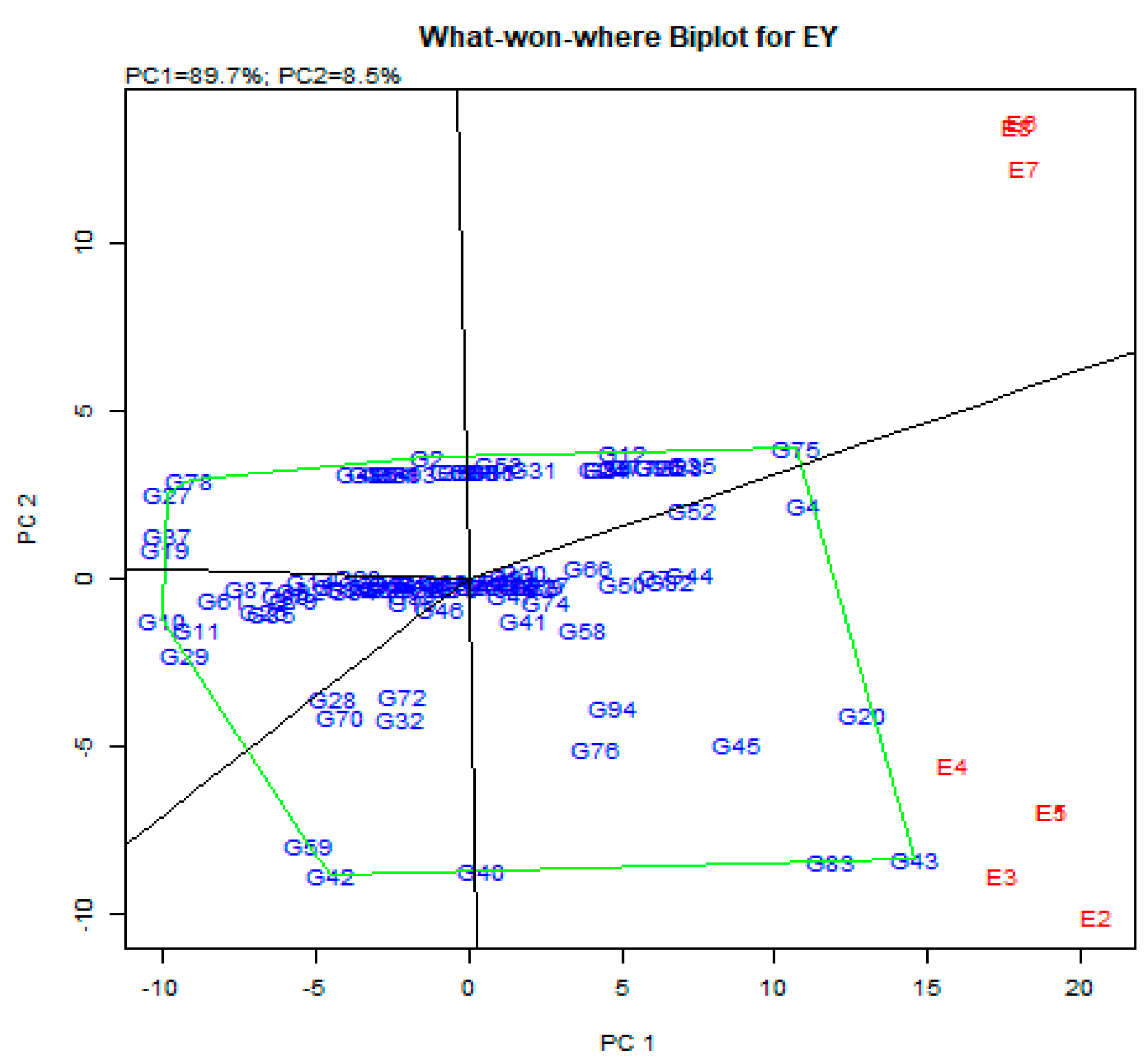
3.6. GGE Biplot Analysis
Which-Won-Where Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Singh, B.; Snehdeep, U.S.; Arya, C. Statistical assessment of rainfall trend and distribution on wheat yield in western Uttar Pradesh. In Proceedings of the Virtual National Conference on Strategic Reorientation for Climate Smart Agriculture (V-AGMET 2021), Ludhiana, India, 17–19 March 2021; Volume 2, p. 163. [Google Scholar]
- Anonymous (2021) Annual Report of Department of Agriculture, Cooperation & Farmers Welfare 2020–2021, Ministry of Agriculture & Farmers Welfare Government of India. Available online: www.agricoop.nic.in (accessed on 20 January 2022).
- Bocianowski, J.; Prażak, R. Genotype by year interaction for selected quantitative traits in hybrid lines of Triticum aestivum L. with Aegilops kotschyi Boiss. and Ae. variabilis Eig. using the additive main effects and multiplicative interaction model. Euphytica 2022, 218, 11. [Google Scholar] [CrossRef]
- Hassan, M.A.; Xiang, C.; Farooq, M.; Muhammad, N.; Yan, Z.; Hui, X.; Yuanyuan, K.; Bruno, A.K.; Lele, Z.; Jincai, L. Cold Stress in Wheat: Plant Acclimation Responses and Management Strategies. Front. Plant. Sci. 2021, 12, 676884. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.V.V.; Djanaguiraman, M.; Jagadish, S.V.K.; Ciampitti, I.A. Drought and high temperature stress and traits associated with tolerance. Sorghum A State Art Future Perspetives 2019, 58, 241–265. [Google Scholar]
- Regmi, D.; Poudel, M.R.; Bishwas, K.C.; Poudel, P.B. Yield Stability of Different Elite Wheat Lines under Drought and Irrigated Environments using AMMI and GGE Biplots. Int. J. Appl. Sci. Biotechnol. 2021, 9, 98–106. [Google Scholar] [CrossRef]
- Bhartiya, A.; Aditya, J.P.; Kumari, V.; Kishore, N.; Purwar, J.P.; Agrawal, A.; Kant, L. Stability analysis of soybean [Glycine max (L.) Merrill] genotypes under multi-environments rainfed condition of North Western Himalayan hills. Indian J. Genet. Plant Breed. 2018, 78, 342–347. [Google Scholar] [CrossRef]
- Crossa, J.; Cornelius, P.L. Sites regression and shifted multiplicative model clustering of cultivar trial sites under heterogeneity of error variances. Crop Sci. 1997, 37, 406–415. [Google Scholar] [CrossRef]
- Lewis, D. Gene-environment interaction: A relationship between dominance, heterosis, phenotypic stability and variability. Heredity 1954, 8, 333–356. [Google Scholar] [CrossRef] [Green Version]
- Finlay, K.W.; Wilkinson, G.N. The analysis of adaptation in a plant-breeding programme. Aust. J. Agric. Res. 1963, 14, 742–754. [Google Scholar] [CrossRef] [Green Version]
- Eberhart, S.A.; Russell, W.A. Stability Parameters for Comparing Varieties. Crop Sci. 1966, 6, 36–40. [Google Scholar] [CrossRef] [Green Version]
- Perkins, J.M.; Jinks, J.L. Environmental and genotype-environmental components of variability. Heredity 1968, 23, 339–356. [Google Scholar] [CrossRef] [Green Version]
- Freeman, G.H.; Perkins, J.M. Environmental and genotype environmental components of variability VIII. Relations Genotypes Grown Differ. Environ. Meas. These Environ. Hered. 1971, 27, 15. [Google Scholar]
- Gauch, H.G., Jr. Model selection and validation for yield trials with interaction. Biometrics 1988, 44, 705–715. [Google Scholar] [CrossRef]
- Yan, W.; Hunt, L.A.; Sheng, Q.; Szlavnics, Z. Cultivar evaluation and mega-environment investigation based on the GGE biplot. Crop Sci. 2000, 40, 597–605. [Google Scholar] [CrossRef]
- Awaad, H.A. Performance, adaptability and stability of promising bread wheat lines across different environments. In Mitigating Environmental Stresses for Agricultural Sustainability in Egypt; Springer: Cham, Switzerland, 2021; pp. 187–213. [Google Scholar] [CrossRef]
- Verma, A.; Singh, G.P. GxE Interactions Analysis of Wheat Genotypes Evaluated Under Peninsular Zone of the Country by AMMI Model. Am. J. Agric. For. 2021, 9, 29–36. [Google Scholar] [CrossRef]
- Gauch, H.G.; Zobel, R.W. Predictive and postdictive success of statistical analyses of yield trials. Theor. Appl. Genet. 1988, 76, 1–10. [Google Scholar] [CrossRef]
- Heidari, S.; Aziinezhad, R.; Haghparast, R. Yield stability analysis in advanced durum wheat genotypes by using AMMI and GGE biplot models under diverse environment. Indian J. Genet. Plant Breed. 2016, 76, 274–283. [Google Scholar] [CrossRef]
- Kumar, S.; Kumari, J.; Bansal, R.; Upadhyay, D.; Srivastava, A.; Rana, B.; Yadav, M.K.; Sengar, R.S.; Singh, A.K.; Singh, R. Multi-environmental evaluation of wheat genotypes for drought tolerance. Indian J. Genet. 2018, 78, 26–35. [Google Scholar] [CrossRef]
- Hagos, H.G.; Abay, F. AMMI and GGE biplot analysis of bread wheat genotypes in the northern part of Ethiopia. J. Plant. Breed. Genet. 2013, 1, 12–18. [Google Scholar]
- Dhiwar, K.; Sharma, D.J.; Agrawal, A.P.; Pandey, D. Stability analysis in wheat (Triticum aestivum L.). J. Pharmacogn. Phytochem. 2020, 9, 295–298. [Google Scholar]
- Abd El-Hammed Attia, M.; EL-Ghany, F.; El-Sadek, A.; Nawar, A.; Dessouky, A.; Shaalan, A. Genotype by environment interaction and yield stability in bread wheat cultivars under rainfed conditions. Sci. J. Agric. Sci. 2021, 3, 56–65. [Google Scholar] [CrossRef]
- Ibrahim, H.A.; Ibrahim, A.E.S.; Ali, K.A. Genotype by environment interaction and stability analyses of grain yield of selected maize (Zea mays L.) genotypes in eastern and central Sudan. Gezira J. Agric. Sci. 2021, 17, 294–312. [Google Scholar]
- Suresh, S.P.; Munjal, R. Selection of wheat genotypes under variable sowing conditions based on stability analysis. J. Cereal Res. 2020, 12, 109–113. [Google Scholar]
- Kumar, A.; Chand, P.; Thapa, R.S.; Singh, T. Assessment of stability performance and GXE interaction for yield and its attributing characters in bread wheat (Triticum aestivum L.). Electron. J. Plant Breed. 2021, 12, 235–241. [Google Scholar]
- Naheed, H.; Rahman, H.U. Stability Analysis of Bread Wheat Lines using Regression Models. Sarhad J. Agric. 2021, 37, 1450–1457. [Google Scholar] [CrossRef]
- Kant, S.; Lamba, R.A.S.; Arya, R.K.; Panwar, I.S. Effect of terminal heat stress on stability of yield and quality parameters in bread wheat in southwest Haryana. J. Wheat Res. 2014, 6, 64–73. [Google Scholar]
- Crossa, J.; Gauch, H.G.; Zobel, R.W. Additive main effects and multiplicative interaction analysis of two international maize cultivar trials. Crop Sci. 1990, 30, 493–500. [Google Scholar] [CrossRef]
- Zobel, R.W.; Wright, M.J.; Gauch, H.G., Jr. Statistical analysis of a yield trial. Agron. J. 1988, 80, 388–393. [Google Scholar] [CrossRef]
- Ljubičić, N.; Popović, V.; Ćirić, V.; Kostić, M.; Ivošević, B.; Popović, D.; Pandžić, M.; El Musafah, S.; Janković, S. Multivariate Interaction Analysis of Winter Wheat Grown in Environment of Limited Soil Conditions. Plants 2021, 10, 604. [Google Scholar] [CrossRef]
- Hanif, U.; Gul, A.; Amir, R.; Munir, F.; Sorrells, M.E.; Gauch, H.G.; Mahmood, Z.; Subhani, A.; Imtiaz, M.; Alipour, H.; et al. Genetic gain and G X E interaction in bread wheat cultivars representing 105 years of breeding in Pakistan. Crop Sci. 2022, 62, 178–191. [Google Scholar] [CrossRef]
- Anuada, A.M.; Cruz, P.C.S.; De Guzman, L.E.P.; Sanchez, P.B. Grain yield variability and stability of corn varieties in rainfed areas in the Philippines. J. Crop Sci. Biotechnol. 2022, 25, 133–147. [Google Scholar] [CrossRef]
- Ali, M.B.; El-Sadek, A.N. Ammi Biplot Analysis of Genotype × environment interaction in wheat in Egypt. Egypt. J. Plant Breed. 2015, 19, 1889–1901. [Google Scholar] [CrossRef]
- Dabi, A.; Alemu, G.; Geleta, N.; Delessa, A.; Solomon, T.; Zegaye, H.; Asnake, D.; Asefa, B.; Duga, R.; Getamesay, A.; et al. Genotype × environment interaction and stability analysis for grain yield of bread wheat (Triticum aestivum) genotypes under low moisture stress areas of Ethiopia. Am. J. Plant Biol. 2021, 6, 44. [Google Scholar] [CrossRef]
- Bishwas, K.C.; Poudel, M.R.; Regmi, D. AMMI and GGE biplot analysis of yield of different elite wheat line under terminal heat stress and irrigated environments. Heliyon 2021, 7, e07206. [Google Scholar] [CrossRef]
- Hebbache, H.; Benkherbache, N.; Mefti, M.; Bendada, H.; Achouche, A.; Kenzi, L.H. Genotype by environment interaction analysis of barley grain yield in the rain-fed regions of Algeria using AMMI model. Acta Fytotech. Zootech. 2021, 24, 117–123. [Google Scholar] [CrossRef]
- Mohiy, M.; Eissa, S. Performance and Stability of some bread wheat genotypes under heat stress conditions in new land at middle and upper Egypt. Sci. J. Agric. Sci. 2021, 3, 79–91. [Google Scholar] [CrossRef]
- Alemu, G.; Dabi, A.; Geleta, N.; Duga, R.; Solomon, T.; Zegaye, H.; Getamesay, A.; Delesa, A.; Asnake, D.; Asefa, B.; et al. Genotype × environment interaction and selection of high yielding wheat genotypes for different wheat-growing areas of Ethiopia. Am. J. Biosci. 2021, 9, 63–71. [Google Scholar] [CrossRef]
- Adil, N.; Wani, S.H.; Rafiqee, S.; Mehrajuddin, S.O.F.I.; Sofi, N.R.; Shikari, A.B.; Hussain, A.; Mohiddin, F.; Jehangir, I.A.; Khan, G.H.; et al. Deciphering genotype × environment interaction by AMMI and GGE biplot analysis among elite wheat (Triticum aestivum L. ) genotypes of himalayan region. Ekin J. Crop Breed. Genetic. 2022, 8, 41–52. [Google Scholar]
- Reddy, P.S.; Satyavathi, C.T.; Khandelwal, V.; Patil, H.T.; Gupta, P.C.; Sharma, L.D.; Mungra, K.D.; Singh, S.P.; Narasimhulu, R.; Bhadarge, H.H.; et al. Performance and stability of pearl millet varieties for grain yield and micronutrients in arid and semi-arid regions of India. Front. Plant Sci. 2021, 12, 985. [Google Scholar] [CrossRef]
- Singh, C.; Gupta, A.; Gupta, V.; Kumar, P.; Sendhil, R.; Tyagi, B.S.; Singh, G.; Chatrath, R.; Singh, G.P. Genotype × environment interaction analysis of multi-environment wheat trials in India using AMMI and GGE biplot models. Crop Breed. Appl. Biotechnol. 2019, 19, 309–318. [Google Scholar] [CrossRef]
- Oral, E.; Kendal, E.; Dogan, Y. Selection the best barley genotypes to multi and special environments by AMMI and GGE biplot models. Fresenius Environ. Bull. 2018, 27, 5179–5187. [Google Scholar]
- Shojaei, S.H.; Mostafavi, K.; Bihamta, M.R.; Omrani, A.; Mousavi, S.M.N.; Illés, Á.; Bojtor, C.; Nagy, J. Stabilityon maize hybrids based on GGE biplot graphical technique. Agronomy 2022, 12, 394. [Google Scholar] [CrossRef]

| Timely Sown (18 November) | Late Sown (22 December) | |||
|---|---|---|---|---|
| Irrigated | Rainfed | Irrigated | Rainfed | |
| 2019–2020 | E1 | E2 | E3 | E4 |
| 2020–2021 | E5 | E6 | E7 | E8 |
| Serial No. | Name of Genotype | Serial No. | Name of Genotype | Serial No. | Name of Genotype | Serial No. | Name of Genotype |
|---|---|---|---|---|---|---|---|
| 1 | WH1182 | 26 | PBW693 | 51 | WH1184 | 76 | WH789 |
| 2 | PBW725 | 27 | WH1188 | 52 | WH1021 | 77 | PBW750 |
| 3 | WH1061 | 28 | WH714 | 53 | PBW503 | 78 | DPW621-50 |
| 4 | PBW729 | 29 | PBW698 | 54 | WH1158 | 79 | WH542 |
| 5 | PBW560 | 30 | WH1062 | 55 | WH1164 | 80 | PBW486 |
| 6 | PBW728 | 31 | WH1105 | 56 | WH1129 | 81 | WH147 |
| 7 | PBW721 | 32 | DBW88 | 57 | UP2902 | 82 | WH1120 |
| 8 | WH1139 | 33 | PBW527 | 58 | WH1166 | 83 | PBW769 |
| 9 | UP2565 | 34 | PBW676 | 59 | WH711 | 84 | PB934 |
| 10 | DBW136 | 35 | WH283 | 60 | WH1181 | 85 | WH1192 |
| 11 | WH1025 | 36 | WH1138 | 61 | DBW90 | 86 | HD3086 |
| 12 | WH1152 | 37 | WH1153 | 62 | WH1140 | 87 | PBW163 |
| 13 | PBW752 | 38 | WH1175 | 63 | WH1132 | 88 | PBW712 |
| 14 | PBW475 | 39 | WH1235 | 64 | PBW158 | 89 | DBW129 |
| 15 | PBW621 | 40 | DBW233 | 65 | PBW502 | 90 | WH1124 |
| 16 | PBW730 | 41 | PBW528 | 66 | UP2338 | 91 | WH1264 |
| 17 | WH1136 | 42 | PBW88 | 67 | DBW17 | 92 | PBW762 |
| 18 | WH730 | 43 | PBW706 | 68 | PBW123 | 93 | WH1142 |
| 19 | PBW343 | 44 | WH1063 | 69 | UP2906 | 94 | WH1186 |
| 20 | DBW116 | 45 | WH1157 | 70 | PBW681 | 95 | DBW95 |
| 21 | HD2967 | 46 | PBW550 | 71 | PBW677 | 96 | PBW540 |
| 22 | WH1151 | 47 | UP2473 | 72 | PBW763 | 97 | PBW542 |
| 23 | UP2660 | 48 | UP2865 | 73 | WH1123 | 98 | PBW661 |
| 24 | PBW695 | 49 | PBW726 | 74 | WH1080 | 99 | WH1131 |
| 25 | PBW709 | 50 | C306 | 75 | DBW16 | 100 | PB533 |
| Source | Df | MSS | F |
|---|---|---|---|
| Total | (ger-1) | ||
| Treatment | (ge-1) | ||
| Genotypes | (g-1) | MS1 | MS1/MS3 |
| Environment | (e-1) | MS2 | MS2/MS3 |
| Genotype Environment | (g-1)(e-1) | MS3 | MS3/Mse |
| IPCA1 | (G + E-1-2n) | MS4 | MS4/Mse |
| IPCA2 | (G + E-1-2n) | ||
| Residual | |||
| Blocks | (r-1) | ||
| Error | (r-1)(ge-1) | Mse |
| Source of Variation | Df | MS | F Value |
|---|---|---|---|
| Genotype (G) | (g-1) | MS1 | MS1/MS3 |
| Environment (E) | (n-1) | MS2 | MS2/MS3 |
| G × E | (g-1) (n-1) | ||
| Environment (linear) | 1 | ||
| Genotype × Environment (linear) | (g-1) | MS3 | MS3/MS4 |
| Pooled Deviation | g (n-2) | ||
| Genotype 1 | (n-2) | ||
| Genotype 2 | (n-2) | ||
| Pooled error | n(g-1)(r-1) | MS4 | |
| Total | (ng-1) |
| S. No | Genotypes | Grain Yield per Plot | ||
|---|---|---|---|---|
| Mean | bi | S2di | ||
| 1 | C 306 | 521.30 | 0.788 | −1431.7637 |
| 2 | HD 2967 | 668.40 | 0.968 | 700.1297 |
| 3 | HD 3086 | 591.10 | 1.002 | −1423.8511 |
| 4 | DBW 16 | 562.30 | 1.008 | −1431.7638 |
| 5 | DBW 17 | 606.70 | 1.108 ** | −1431.9366 |
| 6 | DBW 88 | 664.10 | 0.965 ** | 44.9539 |
| 7 | DBW 90 | 394.30 | 0.897 | 155.1777 |
| 8 | DBW 95 | 415.10 | 0.990 | −265.7533 |
| 9 | DBW 116 | 551.80 | 1.008 | 703.1385 |
| 10 | DBW 129 | 538.50 | 0.977 | −1378.6330 |
| 11 | DBW 136 | 776.30 | 0.965 | −245.7541 |
| 12 | DBW 233 | 655.10 | 0.945 | 44.9539 |
| 13 | DPW 621-50 | 688.50 | 1.002 | −1423.8439 |
| 14 | PB 533 | 482.10 | 1.109 * | −1208.4167 |
| 15 | PB 934 | 498.50 | 1.002 | −1423.8433 |
| 16 | PBW 88 | 511.60 | 0.965 | 44.9544 |
| 17 | PBW 123 | 543.20 | 0.957 | −1207.4127 |
| 18 | PBW 158 | 622.30 | 1.008 | −1431.7640 |
| 19 | PBW 163 | 545.00 | 1.002 | −1423.8487 |
| 20 | PBW 343 | 396.50 | 0.767 * | −472.6532 |
| 21 | PBW 475 | 810.80 | 1.105 ** | 1056.6122 |
| 22 | PBW 486 | 595.90 | 1.002 | −1423.8325 |
| 23 | PBW 502 | 599.60 | 1.007 | −1432.1440 |
| 24 | PBW 503 | 528.70 | 1.008 | −1431.8938 |
| 25 | PBW 527 | 656.50 | 0.965 | 54.0440 |
| 26 | PBW 528 | 691.80 | 0.966 | 36.5229 |
| 27 | PBW 540 | 454.00 | 1.039 | −858.3855 |
| 28 | PBW 542 | 569.50 | 1.130 ** | −1299.3752 |
| 29 | PBW 550 | 396.20 | 0.924 | −411.0996 |
| 30 | PBW 560 | 482.40 | 1.092 | 7855.5913 *** |
| 31 | PBW 621 | 408.10 | 0.823 | 659.4150 |
| 32 | PBW 661 | 609.50 | 1.130 ** | −1299.3753 |
| 33 | PBW 676 | 616.10 | 0.965 | 44.9540 |
| 34 | PBW 677 | 550.70 | 1.008 | −1431.9195 |
| 35 | PBW 681 | 614.60 | 1.008 | −1432.0705 |
| 36 | PBW 693 | 659.60 | 0.965 | 44.9539 |
| 37 | PBW 695 | 710.70 | 0.935 | 40.9461 |
| 38 | PBW 698 | 589.60 | 0.932 | 44.9541 |
| 39 | PBW 706 | 398.80 | 0.806 * | −706.5090 |
| 40 | PBW 709 | 702.70 | 0.965 | 41.7894 |
| 41 | PBW 712 | 562.50 | 1.002 | −1423.8435 |
| 42 | PBW 721 | 582.10 | 1.104 | 8679.9017 *** |
| 43 | PBW 725 | 517.40 | 1.061 | 7269.3556 *** |
| 44 | PBW 726 | 704.90 | 0.986 | −1093.2270 |
| 45 | PBW 728 | 491.60 | 1.104 | 8679.9020 *** |
| 46 | PBW 729 | 841.60 | 1.104 | 8679.9007 *** |
| 47 | PBW 730 | 512.10 | 1.101 | 484.4683 |
| 48 | PBW 750 | 735.10 | 1.060 | 2215.0743 * |
| 49 | PBW 752 | 708.50 | 1.002 | −1423.8440 |
| 50 | PBW 762 | 560.90 | 0.875 | 198.7622 |
| 51 | PBW 763 | 601.60 | 0.978 | −1372.5130 |
| 52 | PBW 769 | 549.10 | 1.002 | −1423.8500 |
| 53 | UP 2338 | 669.40 | 1.008 | −1431.4475 |
| 54 | UP 2473 | 534.60 | 0.965 | 44.9543 |
| 55 | UP 2565 | 708.80 | 0.965 | −245.7539 |
| 56 | UP 2660 | 594.10 | 0.987 ** | 341.7076 |
| 57 | UP 2865 | 528.20 | 0.965 | 42.4225 |
| 58 | UP 2902 | 588.30 | 1.008 | −1431.7639 |
| 59 | UP 2906 | 524.60 | 1.008 | −1432.0702 |
| 60 | WH 147 | 522.90 | 1.002 | −1423.8077 |
| 61 | WH 283 | 689.60 | 0.965 | 44.9538 |
| 62 | WH 542 | 636.50 | 1.002 | −1423.8437 |
| 63 | WH 711 | 475.20 | 1.008 | −1431.9700 |
| 64 | WH 714 | 542.20 | 0.965 | 43.0552 |
| 65 | WH 730 | 661.10 | 1.101 | 484.4678 |
| 66 | WH 789 | 503.90 | 1.005 | −1431.1050 |
| 67 | WH 1021 | 582.20 | 1.008 | −1432.0206 |
| 68 | WH 1025 | 644.20 | 1.012 | −695.7495 |
| 69 | WH 1061 | 478.80 | 1.053 | 6991.1393 *** |
| 70 | WH 1062 | 572.10 | 0.965 | 44.9542 |
| 71 | WH 1063 | 432.80 | 0.623 * | 1789.4185 * |
| 72 | WH 1080 | 556.40 | 1.008 | −1431.4750 |
| 73 | WH 1105 | 567.50 | 0.965 | 50.8702 |
| 74 | WH 1120 | 530.00 | 1.002 | −1423.8434 |
| 75 | WH 1123 | 545.80 | 1.008 | −1431.7638 |
| 76 | WH 1124 | 751.20 | 1.047 | −1279.7099 |
| 77 | WH 1129 | 571.10 | 1.007 | −1432.2321 |
| 78 | WH 1131 | 509.00 | 1.130 ** | −1299.5106 |
| 79 | WH 1132 | 618.30 | 1.008 | −1431.7640 |
| 80 | WH 1136 | 498.60 | 1.101 | 484.4683 |
| 81 | WH 1138 | 532.70 | 0.966 | 338.6289 |
| 82 | WH 1139 | 535.90 | 1.035 | 388.8467 |
| 83 | WH 1140 | 568.10 | 1.007 | −1432.1681 |
| 84 | WH 1142 | 622.00 | 1.158 ** | −1135.9830 |
| 85 | WH 1151 | 772.40 | 1.008 | 700.1294 |
| 86 | WH 1152 | 651.50 | 1.060 | 2199.6990 * |
| 87 | WH 1153 | 674.60 | 0.965 | 44.9539 |
| 88 | WH 1157 | 409.60 | 0.965 | 44.9547 |
| 89 | WH 1158 | 471.80 | 0.983 | −1389.8129 |
| 90 | WH 1164 | 467.50 | 0.997 | −1405.2532 |
| 91 | WH 1166 | 545.40 | 1.008 | −1431.3627 |
| 92 | WH 1175 | 589.60 | 0.965 | 44.9541 |
| 93 | WH 1181 | 697.30 | 1.008 | −1431.7643 |
| 94 | WH 1182 | 791.60 | 1.104 | 8679.9009 *** |
| 95 | WH 1184 | 507.30 | 1.008 | −1431.7637 |
| 96 | WH 1186 | 459.20 | 1.028 | −746.4416 |
| 97 | WH 1188 | 518.50 | 0.965 | 55.5269 |
| 98 | WH 1192 | 444.70 | 1.002 | −1423.8488 |
| 99 | WH 1235 | 579.60 | 0.965 | 44.9542 |
| 100 | WH 1264 | 504.80 | 1.092 | −882.0992 |
| MEAN | 576.30 | |||
| STANDARD ERROR | 0.10 | |||
| Source | DF | Grain Yield per Plot (g) |
|---|---|---|
| Genotype (Gen.) | 99 | 77,289.410 *** |
| Environment (Env.) | 7 | 2,906,548.000 *** |
| Gen. × Env. | 693 | 1410.637 *** |
| Env. + (Gen. × Env.) | 700 | 30,462.010 *** |
| Env. (Linear) | 1 | 20,345,830.000 *** |
| Env. × Gen. (Linear) | 99 | 1112.672 ** |
| Pooled Deviation | 600 | 1445.695 ** |
| Pooled Error | 792 | 891.864 |
| Total | 799 | 36,264.150 |
| Trait | Environmental Index | Mean | |||||||
|---|---|---|---|---|---|---|---|---|---|
| E1 | E2 | E3 | E4 | E5 | E6 | E7 | E8 | ||
| GYP | 225.028 | −23.472 | −144.559 | −199.442 | 260.028 | 70.524 | −68.416 | −119.691 | 576.27 |
| Source | Degree of Freedom | Grain Yield Per Plot | % Explained |
|---|---|---|---|
| Trials | 799 | 36,264.17 *** | |
| Genotypes | 99 | 77,289.54 *** | 26.41 |
| Environments | 7 | 2,906,549.42 *** | 70.22 |
| G × E interaction | 693 | 1410.62 *** | 3.37 |
| PCA I | 105 | 7496.20 *** | 80.52 |
| PCA II | 103 | 1197.47 * | 12.62 |
| PCA III | 101 | 443.23 | 4.58 |
| Pooled error | 800 | 935.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, V.; Kumar, M.; Singh, V.; Chaudhary, L.; Yashveer, S.; Sheoran, R.; Dalal, M.S.; Nain, A.; Lamba, K.; Gangadharaiah, N.; et al. Genotype by Environment Interaction Analysis for Grain Yield of Wheat (Triticum aestivum (L.) em.Thell) Genotypes. Agriculture 2022, 12, 1002. https://doi.org/10.3390/agriculture12071002
Gupta V, Kumar M, Singh V, Chaudhary L, Yashveer S, Sheoran R, Dalal MS, Nain A, Lamba K, Gangadharaiah N, et al. Genotype by Environment Interaction Analysis for Grain Yield of Wheat (Triticum aestivum (L.) em.Thell) Genotypes. Agriculture. 2022; 12(7):1002. https://doi.org/10.3390/agriculture12071002
Chicago/Turabian StyleGupta, Vijeta, Mukesh Kumar, Vikram Singh, Lakshmi Chaudhary, Shikha Yashveer, Ravika Sheoran, Mohinder Singh Dalal, Ashish Nain, Kavita Lamba, Nikhil Gangadharaiah, and et al. 2022. "Genotype by Environment Interaction Analysis for Grain Yield of Wheat (Triticum aestivum (L.) em.Thell) Genotypes" Agriculture 12, no. 7: 1002. https://doi.org/10.3390/agriculture12071002
APA StyleGupta, V., Kumar, M., Singh, V., Chaudhary, L., Yashveer, S., Sheoran, R., Dalal, M. S., Nain, A., Lamba, K., Gangadharaiah, N., Sharma, R., & Nagpal, S. (2022). Genotype by Environment Interaction Analysis for Grain Yield of Wheat (Triticum aestivum (L.) em.Thell) Genotypes. Agriculture, 12(7), 1002. https://doi.org/10.3390/agriculture12071002






