Maximum Entropy Niche-Based Modeling for Predicting the Potential Suitable Habitats of a Traditional Medicinal Plant (Rheum nanum) in Asia under Climate Change Conditions
Abstract
:1. Introduction
2. Materials and Methods
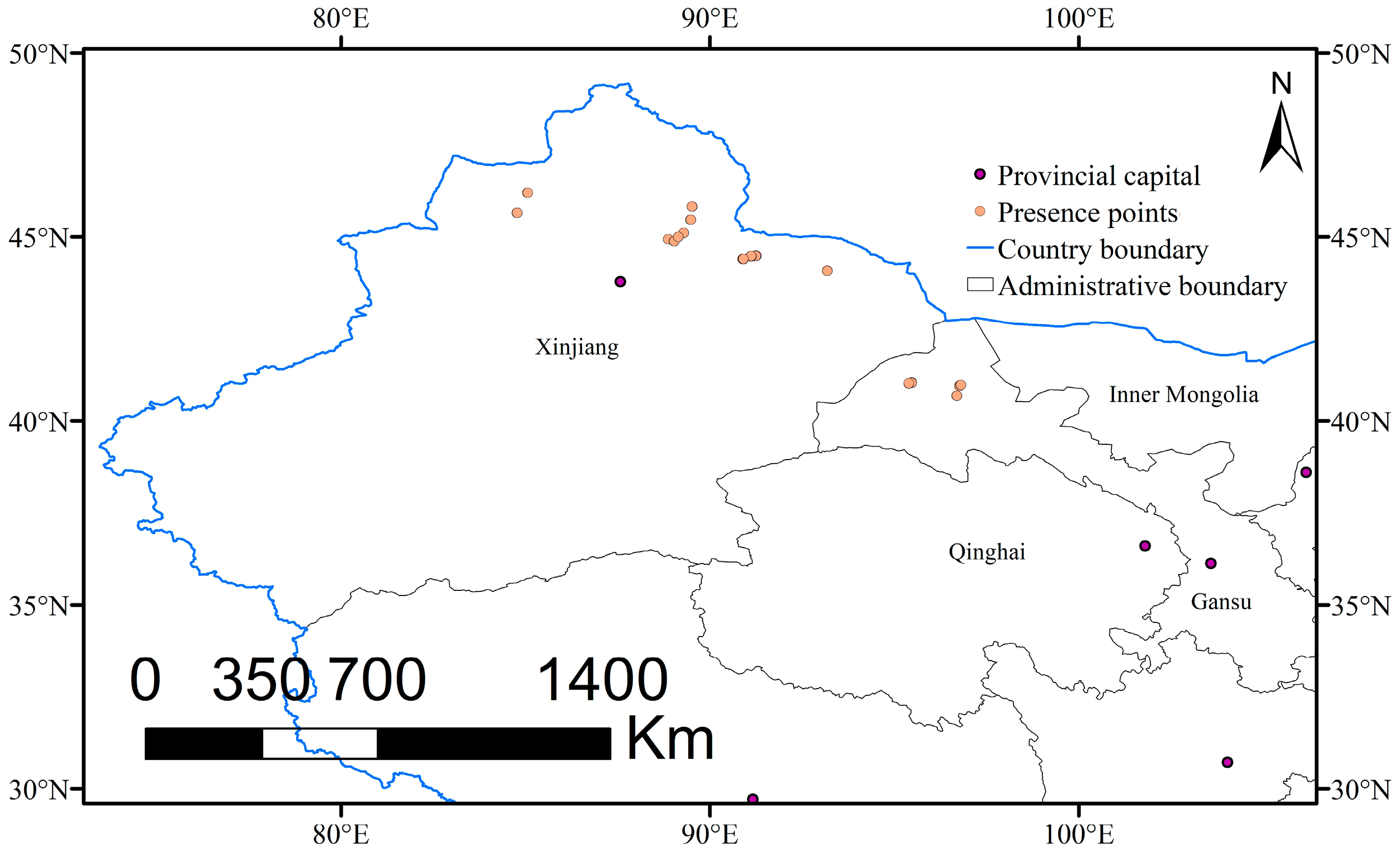
2.1. Occurrence Data and Study Area
2.2. Environmental Variables
2.3. Model Processing and Evaluation
3. Results
3.1. Contributions and Importance of Environmental Variable in the MaxEnt Model
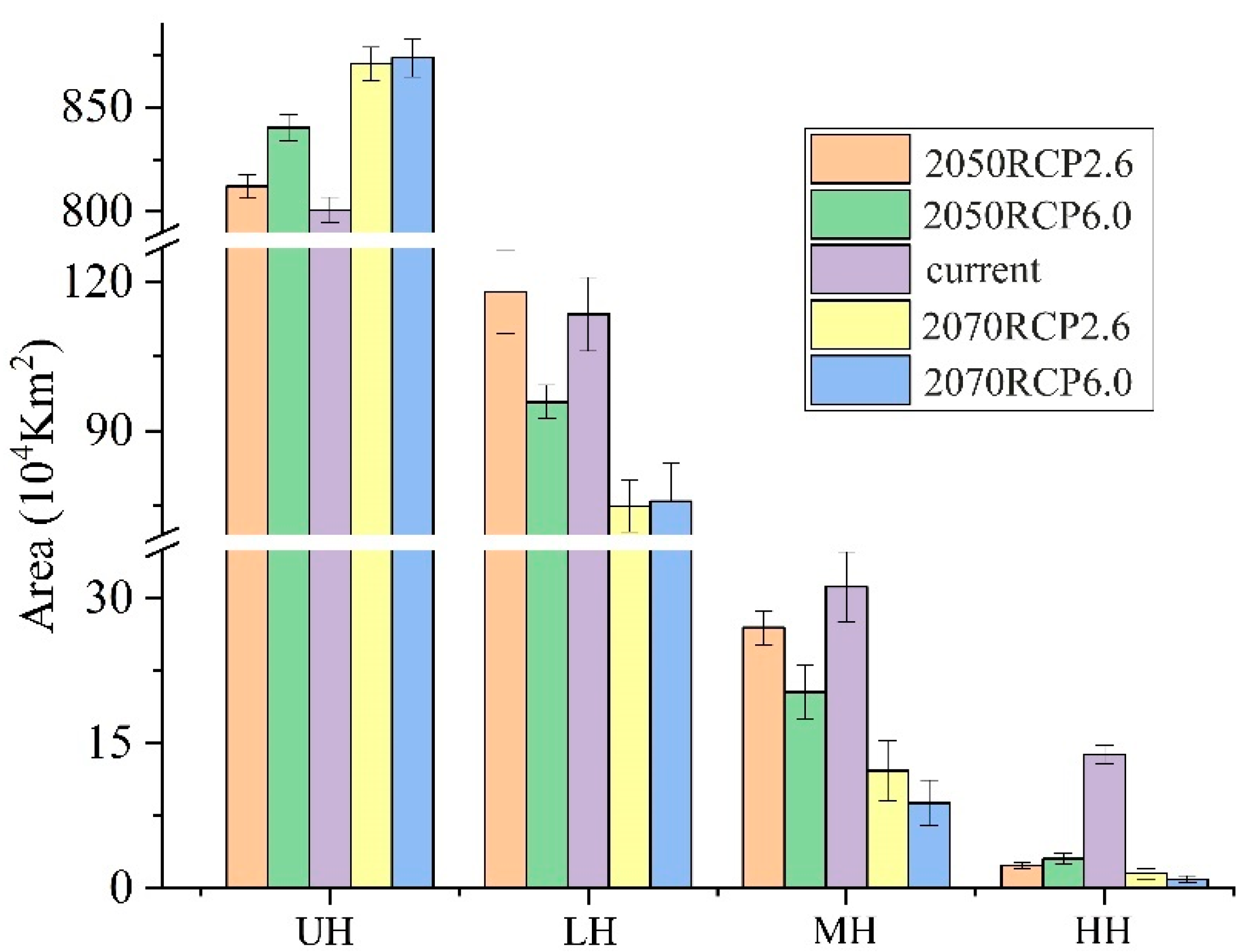
3.2. Potential Geographic Distribution and Suitable Habitat Area of R. nanum
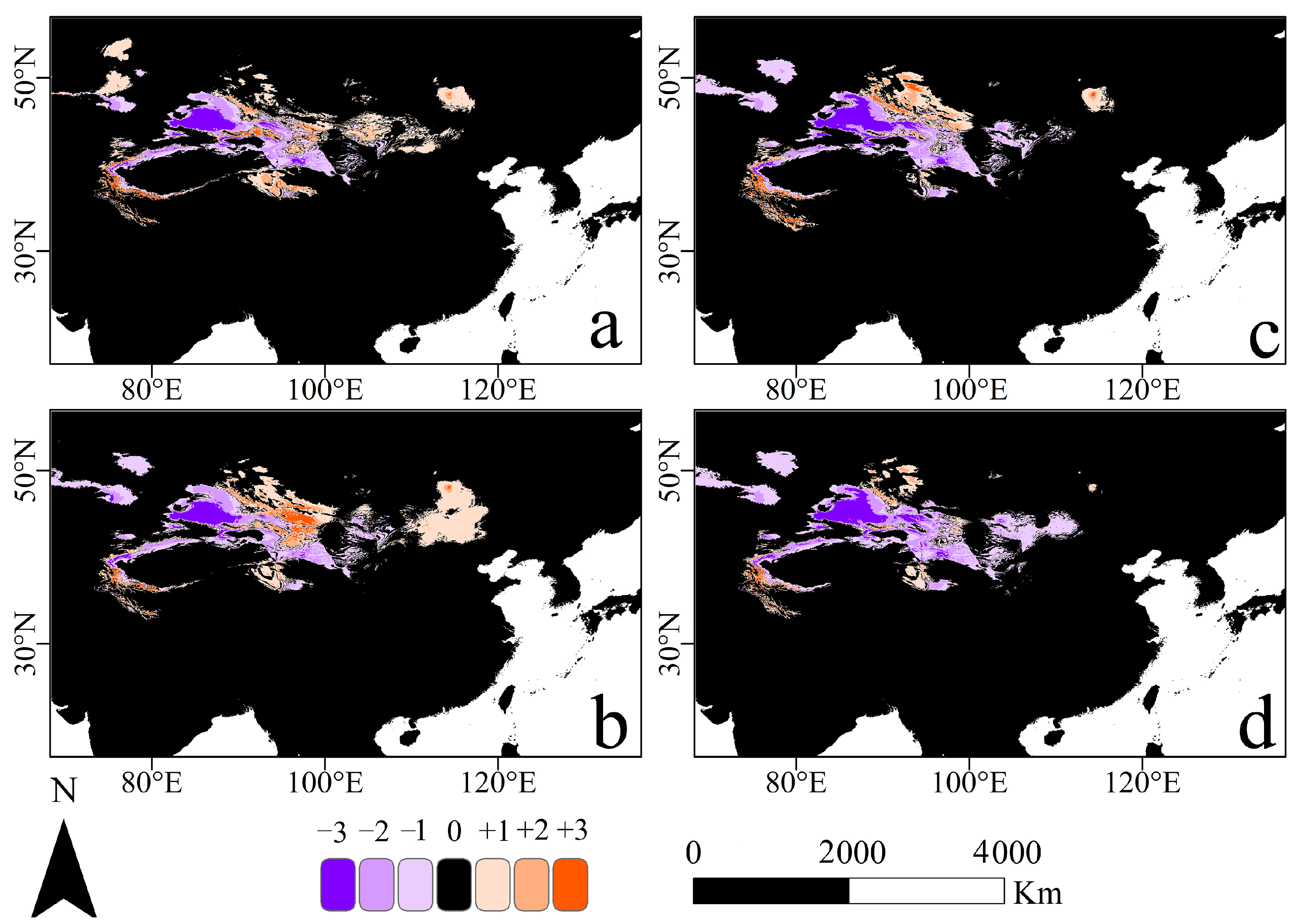
3.3. Changes of Potential Suitable Habitats in the Future Distribution Pattern
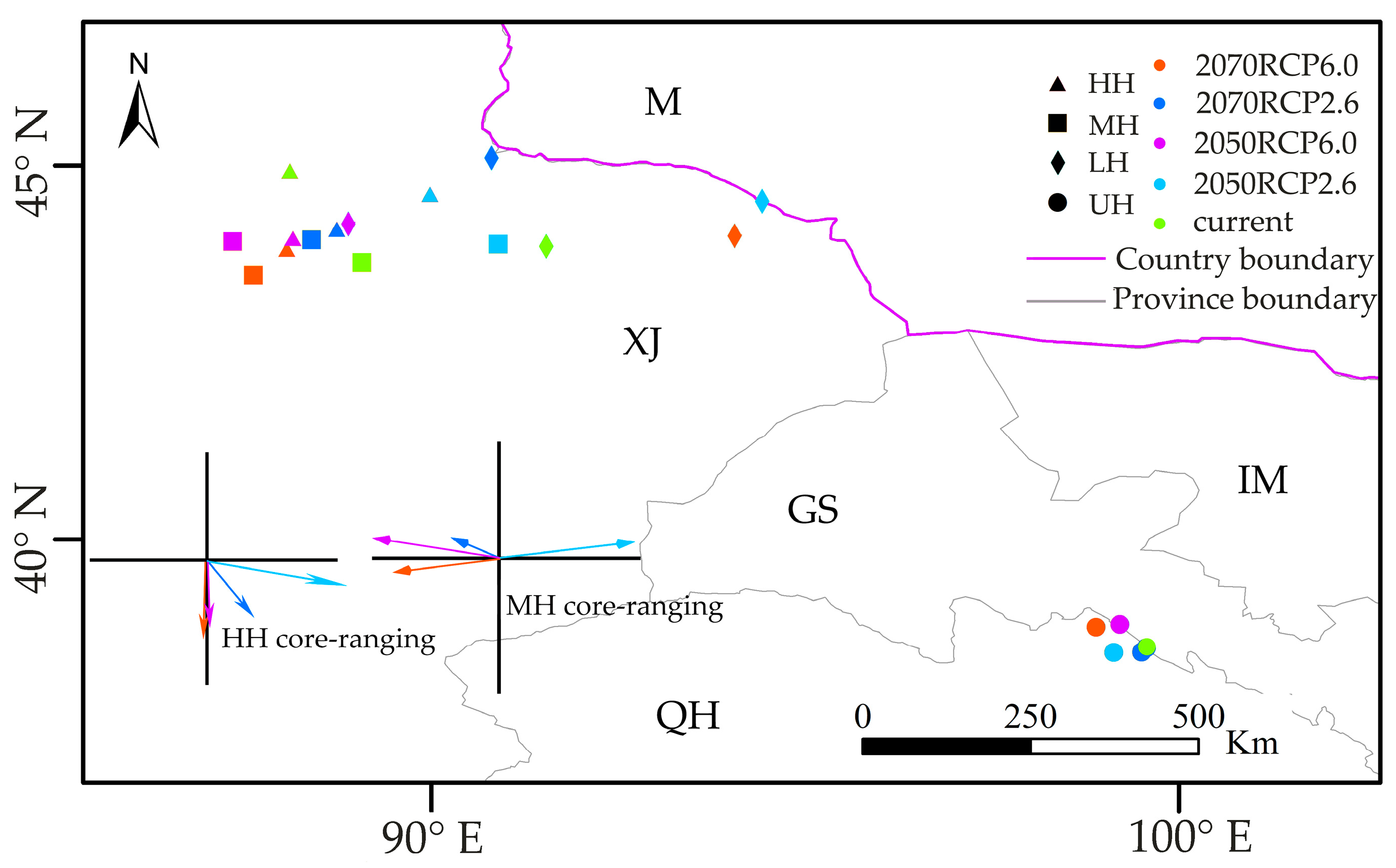
3.4. Range Shifts of Suitable Habitat Cores under Two Climate Scenarios
4. Discussion
4.1. Effects of Climate Change on Suitable Habitat Range
4.2. Conservation of Species in Ecologically Fragile Areas
4.3. Dominant Environmental Factors and Limitations in Predicting Species-Distribution Ranges
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xie, Z. Ecogeographical distribution of the species from Rheum.L (polygonaceae) in China. In Proceedings of the Third National Symposium on the Conservation and Sustainable Use of Biological Diversity, Kunming, China, 11−13 December 1998. [Google Scholar]
- Zhu, R. The Survey of Rhubarb Resources in Guyuan Country and Its Geographical Distribution. Master’s Thesis, Lanzhou University, Lanzhou, China, 2011. [Google Scholar]
- Zhe, W. A Study on the Pharmacophylogeny of the Rheum L. in China. Ph.D. Thesis, Chinese Academy of Medical Scienses & Peking Union Medical College, Beijing, China, 2011. [Google Scholar]
- Shang, X.-F.; Zhao, Z.-M.; Li, J.-C.; Yang, G.-Z.; Liu, Y.-Q.; Dai, L.-X.; Zhang, Z.-J.; Yang, Z.-G.; Miao, X.-L.; Yang, C.-J.; et al. Insecticidal and antifungal activities of Rheum palmatum L. anthraquinones and structurally related compounds. Ind. Crops Prod. 2019, 137, 508–520. [Google Scholar] [CrossRef]
- Yuan, S.; Jian, T.; Li, W.; Huang, Y. Extraction process optimization and activity assays of antioxidative substances from Rheum officinale. J. Food Meas. Charact. 2019, 14, 176–184. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, F.; Guo, F.; Zhou, Q.; Xiang, H.; Shang, D. Emodin attenuates adenosine triphosphate-induced pancreatic ductal cell injury in vitro via the inhibition of the P2X7/NLRP3 signaling pathway. Oncol. Rep. 2019, 42, 1589–1597. [Google Scholar] [CrossRef] [PubMed]
- Kalkan, S.; Otag, M.R.; Engin, M.S. Physicochemical and bioactive properties of edible methylcellulose films containing Rheum ribes L. extract. Food Chem. 2020, 307, 125524. [Google Scholar] [CrossRef]
- Qin, T.; Wu, L.; Hua, Q.; Song, Z.; Pan, Y.; Liu, T. Prediction of the mechanisms of action of Shenkang in chronic kidney disease: A network pharmacology study and experimental validation. J. Ethnopharmacol. 2020, 246, 112128. [Google Scholar] [CrossRef]
- Li, A.; Gao, Z.; Mao, Z. (Eds.) Flora of China; Science Press: Beijing, China, 1998; Volume 25. [Google Scholar]
- Kwon, H.C.; Kim, T.Y.; Lee, C.M.; Lee, K.S.; Lee, K.K. Active compound chrysophanol of Cassia tora seeds suppresses heat-induced lipogenesis via inactivation of JNK/p38 MAPK signaling in human sebocytes. Lipids Health 2019, 18, 135. [Google Scholar] [CrossRef] [Green Version]
- Dai, G.; Ding, K.; Cao, Q.; Xu, T.; He, F.; Liu, S.; Ju, W. Emodin suppresses growth and invasion of colorectal cancer cells by inhibiting VEGFR2. Eur. J. Pharmacol. 2019, 859, 172525. [Google Scholar] [CrossRef]
- Sun, J.; Luo, J.-W.; Yao, W.-J.; Luo, X.-T.; Wei, Y.-H. Effect of emodin on gut microbiota of rats with acute kidney failure. China J. Chin. Mater. Med. 2019, 44, 758–764. [Google Scholar] [CrossRef]
- Guo, Y.; Li, X.; Zhao, Z.; Wei, H.; Gao, B.; Gu, W. Prediction of the potential geographic distribution of the ectomycorrhizal mushroom Tricholoma matsutake under multiple climate change scenarios. Sci. Rep. 2017, 7, 46221. [Google Scholar] [CrossRef] [Green Version]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef] [Green Version]
- Abolmaali, S.M.-R.; Tarkesh, M.; Bashari, H. MaxEnt modeling for predicting suitable habitats and identifying the effects of climate change on a threatened species, Daphne mucronata, in central Iran. Ecol. Inform. 2018, 43, 116–123. [Google Scholar] [CrossRef]
- Papes, M.; Gaubert, P. Modelling ecological niches from low numbers of occurrences: Assessment of the conservation status of poorly known viverrids (Mammalia, Carnivora) across two continents. Divers. Distrib. 2007, 13, 890–902. [Google Scholar] [CrossRef]
- Tang, J.; Cheng, Y.; Luo, L.; Zhang, L.; Jiang, X. Maxent-based prediction of overwintering areas of Loxostege sticticalis in China under different climate changes scenarios. Acta Ecol. Sin. 2017, 37, 4852–4863. [Google Scholar] [CrossRef]
- Araujo, M.B.; Pearson, R.G.; Thuiller, W.; Erhard, M. Validation of species-climate impact models under climate change. Glob. Chang. Biol. 2005, 11, 1504–1513. [Google Scholar] [CrossRef] [Green Version]
- Peterson, A.T. Uses and Requirements of Ecological Niche Models and Related Distributional Models. Biodivers. Inform. 2006, 3, 59–72. [Google Scholar] [CrossRef] [Green Version]
- Phillips, S.J.; Dudik, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Guisan, A.; Zimmermann, N.E. Predictive habitat distribution models in ecology. Ecol. Model. 2000, 135, 147–186. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudik, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef] [Green Version]
- Garcia, K.; Lasco, R.; Ines, A.; Lyon, B.; Pulhin, F. Predicting geographic distribution and habitat suitability due to climate change of selected threatened forest tree species in the. Philippines. Appl. Geogr. 2013, 44, 12–22. [Google Scholar] [CrossRef]
- Illoldi-Rangel, P.; Sanchez-Cordero, V.; Peterson, A.T. Predicting distributions of Mexican mammals using ecological niche modeling. J. Mammal. 2004, 85, 658–662. [Google Scholar] [CrossRef] [Green Version]
- Remya, K.; Ramachandran, A.; Jayakumar, S. Predicting the current and future suitable habitat distribution of Myristica dactyloides Gaertn. using MaxEnt model in the Eastern Ghats, India. Ecol. Eng. 2015, 82, 184–188. [Google Scholar] [CrossRef]
- Zhang, K.; Yao, L.; Meng, J.; Tao, J. Maxent modeling for predicting the potential geographical distribution of two peony species under climate change. Sci. Total Environ. 2018, 634, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Sun, J. Predicting the distribution of Stipa purpurea across the Tibetan Plateau via the MaxEnt model. BMC Ecol. 2018, 18, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciss, M.; Biteye, B.; Fall, A.G.; Fall, M.; Gahn, M.C.B.; Leroux, L.; Apolloni, A. Ecological niche modelling to estimate the distribution of Culicoides, potential vectors of bluetongue virus in Senegal. BMC Ecol. 2019, 19, 45. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.L.; Li, X.; Zhao, Z.F.; Nawaz, Z. Predicting the impacts of climate change, soils and vegetation types on the geographic distribution of Polyporus umbellatus in China. Sci. Total Environ. 2019, 648, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Qin, A.; Liu, B.; Guo, Q.; Bussmann, R.W.; Ma, F.; Jian, Z.; Xu, G.; Pei, S. Maxent modeling for predicting impacts of climate change on the potential distribution of Thuja sutchuenensis Franch., an extremely endangered conifer from southwestern China. Glob. Ecol. Conserv. 2017, 10, 139–146. [Google Scholar] [CrossRef]
- Root, T.L.; Price, J.T.; Hall, K.R.; Schneider, S.H.; Rosenzweig, C.; Pounds, J.A. Fingerprints of global warming on wild animals and plants. Nature 2003, 421, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Jin, J.; Cheng, J. Predicting the Potential Geographic Distribution and Habitat Suitability of Two Economic Forest Trees on the Loess Plateau, China. Forests 2021, 12, 747. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, H.; Yue, J.; Xie, T.; Xu, Y.; Tian, Y. Predicting Shifts in the Suitable Climatic Distribution of Walnut (Juglans regia L.) in China: Maximum Entropy Model Paves the Way to Forest Management. Forests 2018, 9, 103. [Google Scholar] [CrossRef] [Green Version]
- Bracegirdle, T.J.; Krinner, G.; Tonelli, M.; Haumann, F.A.; Naughten, K.A.; Rackow, T.; Roach, L.A.; Wainer, I. Twenty first century changes in Antarctic and Southern Ocean surface climate in CMIP6. Atmos. Sci. Lett. 2020, 14, e984. [Google Scholar] [CrossRef]
- Kellermann, V.; van Heerwaarden, B. Terrestrial insects and climate change: Adaptive responses in key traits. Physiol. Entomol. 2019, 44, 99–115. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Sun, Y.; Yao, S.R.; Akram, M.A.; Hu, W.G.; Dong, L.W.; Li, H.L.; Wei, M.H.; Gong, H.Y.; Xie, S.B.; et al. Impact of climate change on plant species richness across drylands in China: From past to present and into the future. Ecol. Indic. 2021, 132, 108288. [Google Scholar] [CrossRef]
- Sun, J.; Qin, X.; Yang, J. The response of vegetation dynamics of the different alpine grassland types to temperature and precipitation on the Tibetan Plateau. Environ. Monit. Assess. 2016, 188, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, L. Biological consequences of global warming: Is the signal already apparent? Trends Ecol. Evol. 2000, 15, 56–61. [Google Scholar] [CrossRef]
- Piao, S.L.; Liu, Q.; Chen, A.P.; Janssens, I.A.; Fu, Y.S.; Dai, J.H.; Liu, L.L.; Lian, X.; Shen, M.G.; Zhu, X.L. Plant phenology and global climate change: Current progresses and challenges. Glob. Chang. Biol. 2019, 25, 1922–1940. [Google Scholar] [CrossRef]
- Iannella, M.; De Simone, W.; D’Alessandro, P.; Biondi, M. Climate change favours connectivity between virus-bearing pest and rice cultivations in sub-Saharan Africa, depressing local economies. Peerj 2021, 9, e12387. [Google Scholar] [CrossRef]
- Alexander, J.M.; Chalmandrier, L.; Lenoir, J.; Burgess, T.I.; Essl, F.; Haider, S.; Kueffer, C.; McDougall, K.; Milbau, A.; Nunez, M.A.; et al. Lags in the response of mountain plant communities to climate change. Glob. Chang. Biol. 2018, 24, 563–579. [Google Scholar] [CrossRef]
- Pepin, N.; Bradley, R.S.; Diaz, H.F.; Baraer, M.; Caceres, E.B.; Forsythe, N.; Fowler, H.; Greenwood, G.; Hashmi, M.Z.; Liu, X.D.; et al. Elevation-dependent warming in mountain regions of the world. Nat. Clim. Chang. 2015, 5, 424–430. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wang, W.J.; Wu, Z.F.; Du, H.B.; Zong, S.W.; Ma, S. Potential Distribution Shifts of Plant Species under Climate Change in Changbai Mountains, China. Forests 2019, 10, 498. [Google Scholar] [CrossRef] [Green Version]
- Shankhwar, R.; Bhandari, M.S.; Meena, R.K.; Shekhar, C.; Pandey, V.V.; Saxena, J.; Kant, R.; Barthwal, S.; Naithani, H.B.; Pandey, S.; et al. Potential eco-distribution mapping of Myrica esculenta in northwestern Himalayas. Ecol. Eng. 2019, 128, 98–111. [Google Scholar] [CrossRef]
- Wang, S.; Xu, X.; Shrestha, N.; Zimmermann, N.E.; Wang, Z. Response of spatial vegetation distribution in China to climate changes since the Last Glacial Maximum (LGM). PLoS ONE 2017, 12, e0175742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.M.; Shen, X.L.; Tong, L.; Lei, F.W.; Zhang, Z.X. Impact of Past and Future Climate Change on the Potential Distribution of an Endangered Montane Shrub Lonicera oblata and Its Conservation Implications. Forests 2021, 12, 125. [Google Scholar] [CrossRef]
- Tian, B.J.; Dong, X.Y. The Double-ITCZ Bias in CMIP3, CMIP5, and CMIP6 Models Based on Annual Mean Precipitation. Geophys. Res. Lett. 2020, 47, 11. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, B.; Tsiftsis, S.; Chapagain, D.J.; Khadka, C.; Bhattarai, P.; Kayastha Shrestha, N.; Alicja Kolanowska, M.; Kindlmann, P. Suitability of Habitats in Nepal for Dactylorhiza hatagirea Now and under Predicted Future Changes in Climate. Plants 2021, 10, 467. [Google Scholar] [CrossRef]
- van Vuuren, D.P.; Stehfest, E.; den Elzen, M.G.J.; Kram, T.; van Vliet, J.; Deetman, S.; Isaac, M.; Goldewijk, K.K.; Hof, A.; Beltran, A.M.; et al. RCP2.6: Exploring the possibility to keep global mean temperature increase below 2 degrees C. Clim. Chang. 2011, 109, 95–116. [Google Scholar] [CrossRef]
- O’Neill, B.C.; Tebaldi, C.; van Vuuren, D.P.; Eyring, V.; Friedlingstein, P.; Hurtt, G.; Knutti, R.; Kriegler, E.; Lamarque, J.F.; Lowe, J.; et al. The Scenario Model Intercomparison Project (ScenarioMIP) for CMIP6. Geosci. Model Dev. 2016, 9, 3461–3482. [Google Scholar] [CrossRef] [Green Version]
- Rana, S.K.; Rana, H.K.; Ghimire, S.K.; Shrestha, K.K.; Ranjitkar, S. Predicting the impact of climate change on the distribution of two threatened Himalayan medicinal plants of Liliaceae in Nepal. J. Mt. Sci. 2017, 14, 558–570. [Google Scholar] [CrossRef]
- Xin, X.G.; Zhang, L.; Zhang, J.; Wu, T.W.; Fang, Y.J. Climate Change Projections over East Asia with BCC_CSM1.1 Climate Model under RCP Scenarios. J. Meteorol. Soc. Jpn. 2013, 91, 413–429. [Google Scholar] [CrossRef] [Green Version]
- Breiner, F.T.; Guisan, A.; Bergamini, A.; Nobis, M.P. Overcoming limitations of modelling rare species by using ensembles of small models. Methods Ecol. Evol. 2015, 6, 1210–1218. [Google Scholar] [CrossRef]
- Hu, X.; Wu, F.; Guo, W.; Liu, N. Identification of Potential Cultivation Region fie Santalum album in China by the MaxEnt Ecologic Niche Model. Sci. Silvae Sin. 2014, 50, 27–33. [Google Scholar] [CrossRef]
- Fu, G.Q.; Xu, X.Y.; Ma, J.P.; Xu, M.S.; Jiang, L.; Ding, A.Q. Responses of Haloxylon ammodendron potential geographical distribution to the hydrothermal conditions under MaxEnt model. Pratacult. Sci. 2016, 33, 2173–2179. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudik, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Lv, W.; Li, Z.; Wu, X.; Ni, W.; Qv, W. Maximum Entropy Niche-Based Modeling (Maxent) of Potential Geographical Distributions of Lobesia Botrana (Lepidoptera: Tortricidae) in China; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Hernandez, P.A.; Graham, C.H.; Master, L.L.; Albert, D.L. The effect of sample size and species characteristics on performance of different species distribution modeling methods. Ecography 2006, 29, 773–785. [Google Scholar] [CrossRef]
- Guisan, A.; Graham, C.H.; Elith, J.; Huettmann, F.; Distri, N.S. Sensitivity of predictive species distribution models to change in grain size. Divers. Distrib. 2007, 13, 332–340. [Google Scholar] [CrossRef]
- Graham, M.H. Confronting multicollinearity in ecological multiple regression. Ecology 2003, 84, 2809–2815. [Google Scholar] [CrossRef] [Green Version]
- Vaughan, I.P.; Ormerod, S.J. The Continuing Challenges of Testing Species Distribution Models. J. Appl. Ecol. 2005, 42, 720–730. [Google Scholar] [CrossRef]
- Xu, W.; Sun, H.; Jin, J.; Cheng, J. Predicting the Potential Distribution of Apple Canker Pathogen (Valsa mali) in China under Climate Change. Forests 2020, 11, 1126. [Google Scholar] [CrossRef]
- Rana, R.S.; Singh, M.; Pathania, R.; Upadhyay, S.K.; Kalia, V. Impact of changes in climatic conditions on temperate fruit production of Himachal Pradesh. Mausam 2017, 68, 655–662. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, Q.; Yan, X.; Wang, S.; Ren, X.; Zhao, F. An Overview of Distribution Characteristics and Formation Mechanisms in Global Arid Areas. Adv. Earth Sci. 2019, 34, 826–841. [Google Scholar] [CrossRef]
- Ribeiro, M.M.; Roque, N.; Ribeiro, S.; Gavinhos, C.; Castanheira, I.; Quinta-Nova, L.; Albuquerque, T.; Gerassis, S. Bioclimatic modeling in the Last Glacial Maximum, Mid-Holocene and facing future climatic changes in the strawberry tree (Arbutus unedo L.). PLoS ONE 2019, 14, 15. [Google Scholar] [CrossRef] [Green Version]
- Murray, K.A.; Rosauer, D.; McCallum, H.; Skerratt, L.F. Integrating species traits with extrinsic threats: Closing the gap between predicting and preventing species declines. Proc. Biol. Sci. 2011, 278, 1515–1523. [Google Scholar] [CrossRef] [Green Version]
- Lenoir, J.; Gegout, J.C.; Marquet, P.A.; de Ruffray, P.; Brisse, H. A Significant Upward Shift in Plant Species Optimum Elevation During the 20th Century. Science 2008, 320, 1768–1771. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.Y.; Wang, T.J.; Groen, T.A.; Skidmore, A.K.; Yang, X.F.; Ma, K.P.; Wu, Z.F. Climate and land use changes will degrade the distribution of Rhododendrons in China. Sci. Total Environ. 2019, 659, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.C.; Hill, J.K.; Ohlemuller, R.; Roy, D.B.; Thomas, C.D. Rapid Range Shifts of Species Associated with High Levels of Climate Warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; McCarthy, T.M.; Wang, H.; Weckworth, B.V.; Schaller, G.B.; Mishra, C.; Lu, Z.; Beissinger, S.R. Climate refugia of snow leopards in High Asia. Biol. Conserv. 2016, 203, 188–196. [Google Scholar] [CrossRef]
- Hu, Z.-j.; Zhang, Y.-l.; Yu, H.-b. Simulation of Stipa purpurea disttibution pattern on Tibetan Plateau based on MaxEnt model. Chin. J. Appl. Ecol. 2015, 26, 505–511. [Google Scholar] [CrossRef]
- Sato, T.; Kimura, F.; Kitoh, A. Projection of global warming onto regional precipitation over Mongolia using a regional climate model. J. Hydrol. 2007, 333, 144–154. [Google Scholar] [CrossRef]
- Brown, S.C.; Wigley, T.M.L.; Otto-Bliesner, B.L.; Rahbek, C.; Fordham, D.A. Persistent Quaternary climate refugia are hospices for biodiversity in the Anthropocene. Nat. Clim. Change 2020, 10, 244–248. [Google Scholar] [CrossRef]
- Ron, R.; Fragman-Sapir, O.; Kadmon, R. The role of species pools in determining species diversity in spatially heterogeneous communities. J. Ecol. 2018, 106, 1023–1032. [Google Scholar] [CrossRef]
- Albuquerque, F.S.; Olalla-Tarraga, M.A.; Montoya, D.; Rodriguez, M.A. Environmental determinants of woody and herb plant species richness patterns in Great Britain. Ecoscience 2011, 18, 394–401. [Google Scholar] [CrossRef]
- Kaky, E.; Gilbert, F. Using species distribution models to assess the importance of Egypt’s protected areas for the conservation of medicinal plants. J. Arid. Environ. 2016, 135, 140–146. [Google Scholar] [CrossRef] [Green Version]
- Bhattarai, P.; Pandey, B.; Gautam, R.K.; Chhetri, R. Ecology and Conservation Status of Threatened Orchid Dactylorhiza hatagirea (D. Don) Soo in Manaslu Conservation Area, Central Nepal. Am. J. Plant Sci. 2014, 5, 3483–3491. [Google Scholar] [CrossRef] [Green Version]
- Guisan, A.; Thuiller, W. Predicting species distribution: Offering more than simple habitat models. Ecol. Lett. 2005, 8, 993–1009. [Google Scholar] [CrossRef] [PubMed]

| Code | Bioclimatic Data | Unit | Contribution (%) | Importance (%) |
|---|---|---|---|---|
| bio1 | Annual mean temperature | °C | 13.9 ± 0.8 | 54.1 ± 2.7 |
| bio9 | Mean temperature of driest quarter | °C | 7.4 ± 0.6 | 3.1 ± 0.6 |
| bio14 | Precipitation of driest month | mm | 9.7 ± 1.8 | 0.1 ± 0.1 |
| bio15 | Precipitation seasonality | 6.2 ± 1.1 | 9.3 ± 2.1 | |
| bio16 | Precipitation of wettest quarter | mm | 55.9 ± 2.1 | 20.0 ± 5.3 |
| bio19 | Precipitation of coldest quarter | mm | 6.9 ± 2.2 | 13.4 ± 2.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Zhu, S.; Yang, T.; Cheng, J.; Jin, J. Maximum Entropy Niche-Based Modeling for Predicting the Potential Suitable Habitats of a Traditional Medicinal Plant (Rheum nanum) in Asia under Climate Change Conditions. Agriculture 2022, 12, 610. https://doi.org/10.3390/agriculture12050610
Xu W, Zhu S, Yang T, Cheng J, Jin J. Maximum Entropy Niche-Based Modeling for Predicting the Potential Suitable Habitats of a Traditional Medicinal Plant (Rheum nanum) in Asia under Climate Change Conditions. Agriculture. 2022; 12(5):610. https://doi.org/10.3390/agriculture12050610
Chicago/Turabian StyleXu, Wei, Shuaimeng Zhu, Tianli Yang, Jimin Cheng, and Jingwei Jin. 2022. "Maximum Entropy Niche-Based Modeling for Predicting the Potential Suitable Habitats of a Traditional Medicinal Plant (Rheum nanum) in Asia under Climate Change Conditions" Agriculture 12, no. 5: 610. https://doi.org/10.3390/agriculture12050610
APA StyleXu, W., Zhu, S., Yang, T., Cheng, J., & Jin, J. (2022). Maximum Entropy Niche-Based Modeling for Predicting the Potential Suitable Habitats of a Traditional Medicinal Plant (Rheum nanum) in Asia under Climate Change Conditions. Agriculture, 12(5), 610. https://doi.org/10.3390/agriculture12050610







