Lipopeptides Produced by Bacillus mojavensis P1709 as an Efficient Tool to Maintain Postharvest Cherry Tomato Quality and Quantity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biosurfactant Producing Bacteria
2.2. Emulsification Index Assessment
2.3. Identification of Genes Responsible for Lipopeptide Synthesis
2.4. Biosurfactant Production, Extraction and Purification
2.5. Chemical Characterization of the APF
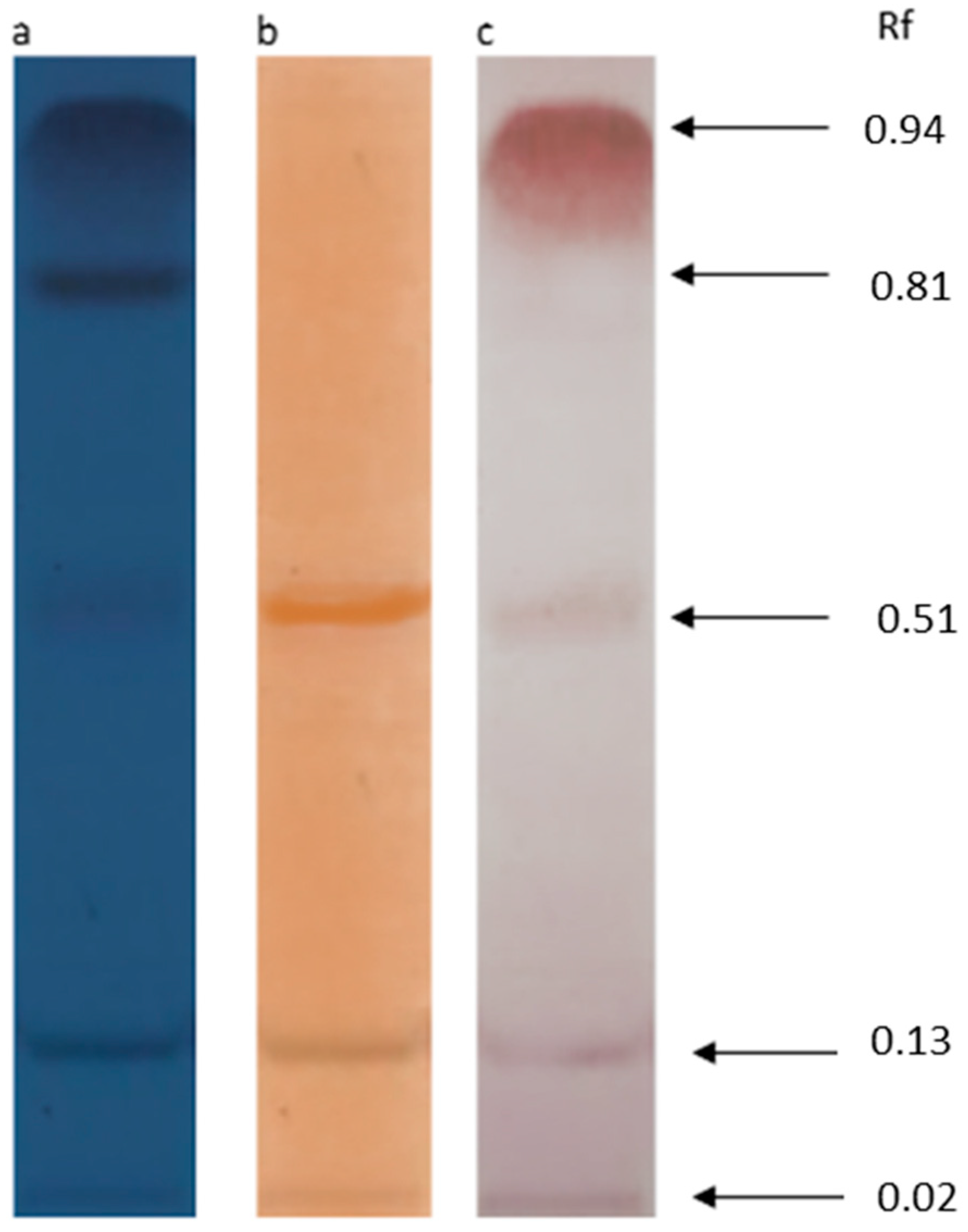
2.5.1. Thin Layer Chromatography (TLC) with Following Staining
2.5.2. Fourier Transform Infrared Spectroscopy (FTIR)
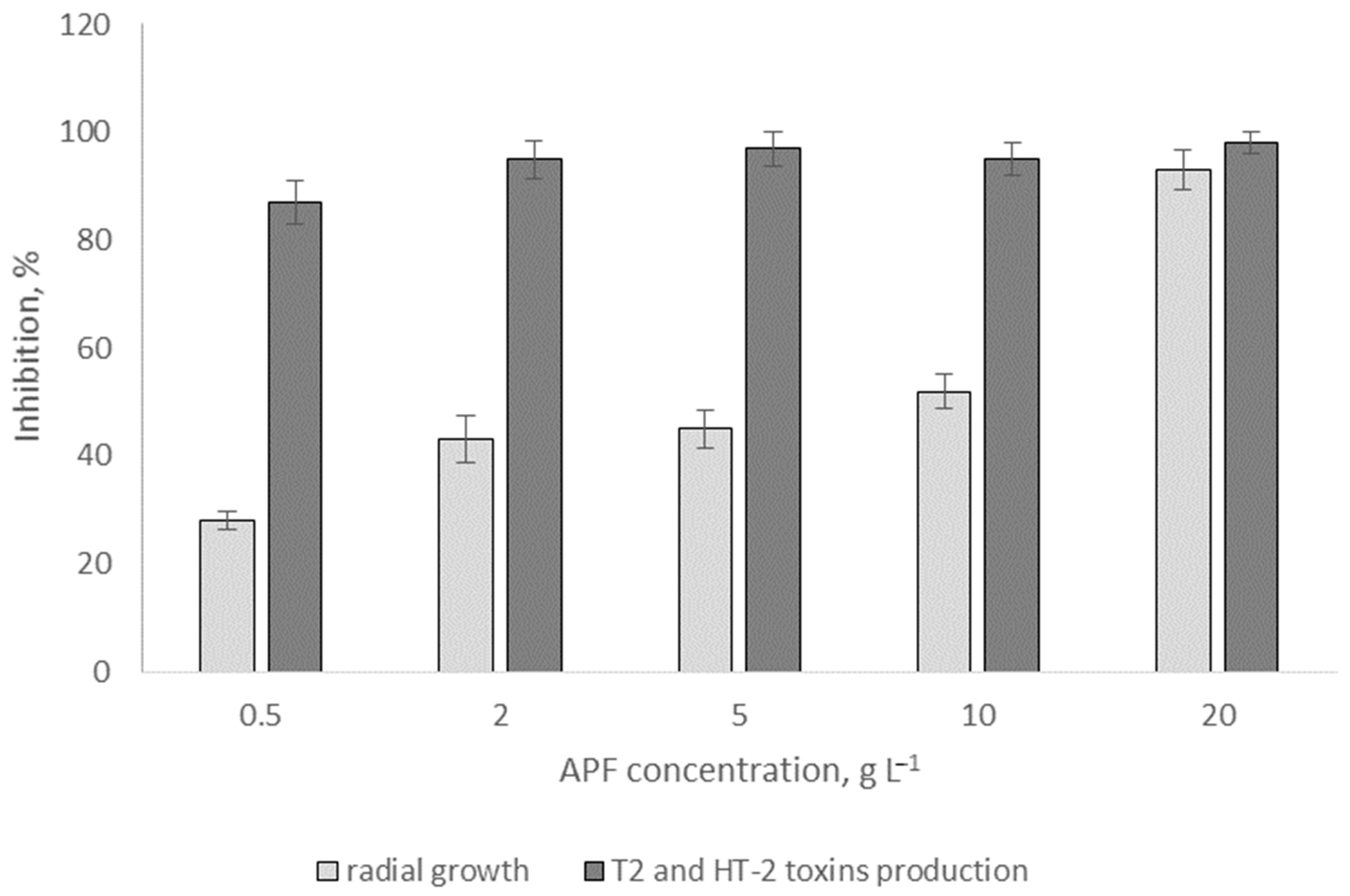
2.6. Effects of Biosurfactants on Radial Fungal Growth and Mycotoxin Production In Vitro
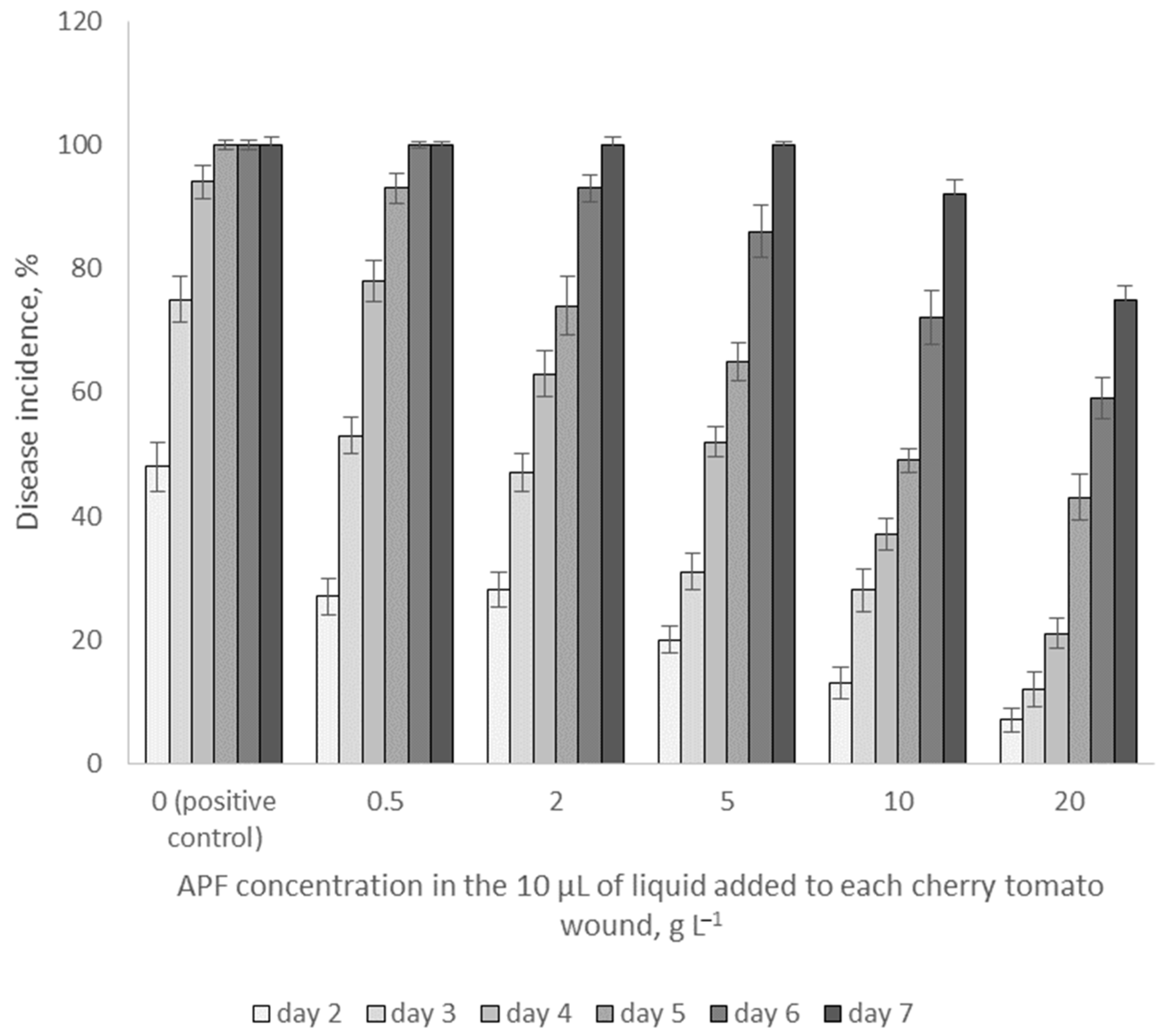
2.7. Effects of Biosurfactants on Fungal Growth on Cherry Tomatoes In Vivo
2.8. Effects of Biosurfactants on Defense-Related Enzymes of Cherry Tomatoes
2.9. Statistical Analysis
3. Results and Discussion
3.1. Emulsifying Ability of B. mojavensis P1709
3.2. Antimicrobial Peptide Synthesis Genes
3.3. TLC and FTIR Analysis
3.4. Effects of Biosurfactants on Radial Fungal Growth and Mycotoxin Production In Vitro
3.5. Effects of APF on Fungal Growth on Cherry Tomatoes In Vivo
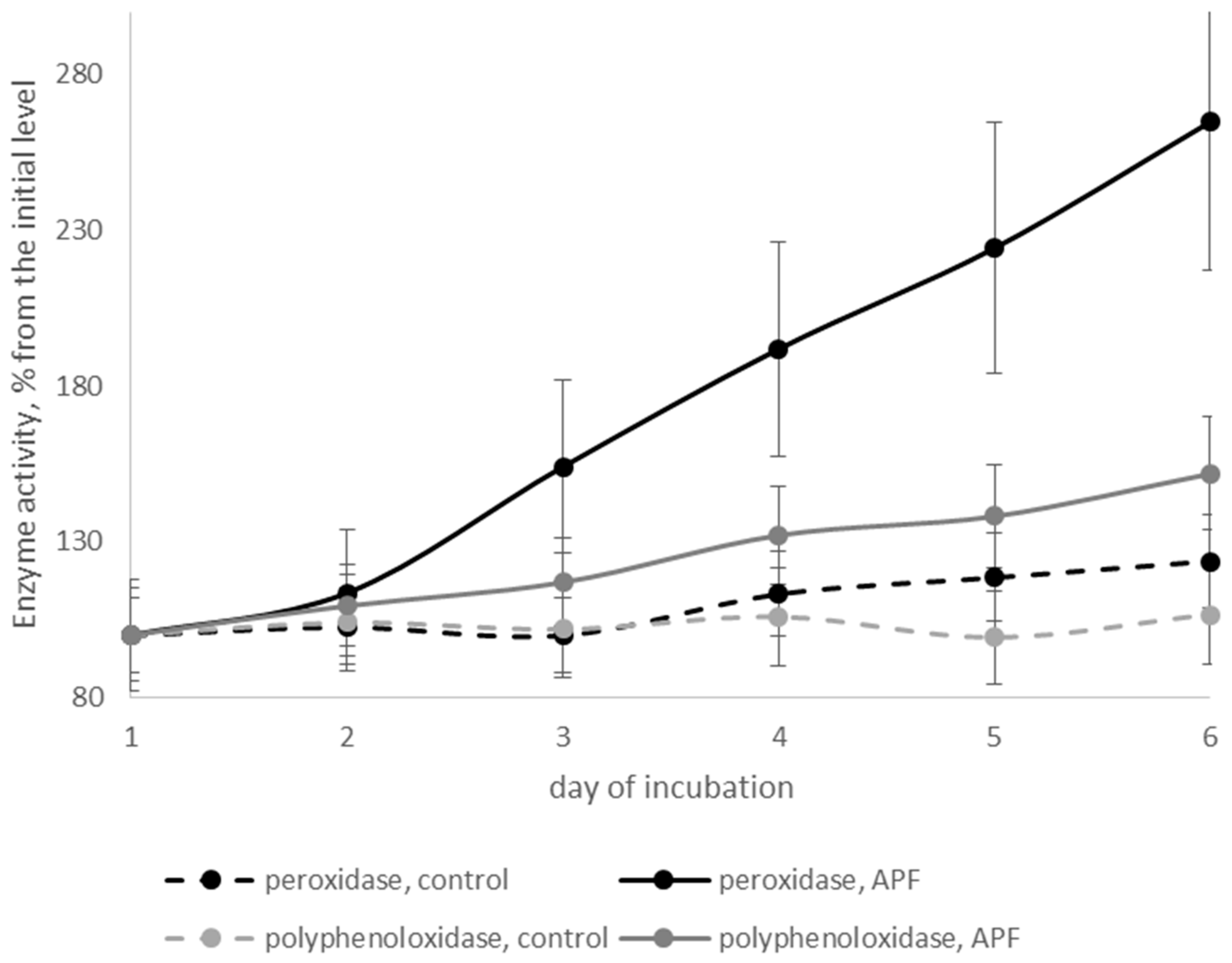
3.6. Effects of Biosurfactants on Defense-Related Enzymes of Cherry Tomatoes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Szalewski, D.A.; Hinrichs, V.S.; Zinniel, D.K.; Barletta, R.G. The pathogenicity of Aspergillus fumigatus, drug resistance, and nanoparticle delivery. Can. J. Microbiol. 2018, 64, 439–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strange, R.N.; Scott, P.R. Plant disease: A threat to global food security. Annu. Rev. Phytopathol. 2005, 43, 83–116. [Google Scholar] [CrossRef] [PubMed]
- Crouzet, J.; Arguelles-Arias, A.; Dhondt-Cordelier, S.; Cordelier, S.; Pršić, J.; Hoff, G.; Mazeyrat-Gourbeyre, F.; Baillieul, F.; Clément, C.; Ongena, M.; et al. Biosurfactants in Plant Protection Against Diseases: Rhamnolipids and Lipopeptides Case Study. Front. Bioeng. Biotechnol. 2020, 8, 1014. [Google Scholar] [CrossRef]
- Sachdev, D.P.; Cameotra, S.S. Biosurfactants in agriculture. Appl. Microbiol. Biotechnol. 2013, 97, 1005–1016. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Rabha, J.; Jha, D.K. Antagonistic activity of lipopeptide-biosurfactant producing Bacillus subtilis AKP, against Colletotrichum capsici, the causal organism of anthracnose disease of chilli. Biocatal. Agric. Biotechnol. 2021, 36, 102133. [Google Scholar] [CrossRef]
- Varvaresou, A.; Iakovou, K. Biosurfactants in cosmetics and biopharmaceuticals. Lett. Appl. Microbiol. 2015, 61, 214–223. [Google Scholar] [CrossRef]
- Köhl, J.; Medeiros, F.H.V.; Lombaers-van der Plas, C.; Groenenboom-de Haas, L.; van den Bosch, T. Efficacies of bacterial and fungal isolates in biocontrol of Botrytis cinerea and Pseudomonas syringae pv. tomato and growth promotion in tomato do not correlate. Biol. Control 2020, 150, 104375. [Google Scholar] [CrossRef]
- Rodrigues, A.I.; Gudiña, E.J.; Abrunhosa, L.; Malheiro, A.R.; Fernandes, R.; Teixeira, J.A.; Rodrigues, L.R. Rhamnolipids inhibit aflatoxins production in Aspergillus flavus by causing structural damages in the fungal hyphae and down-regulating the expression of their biosynthetic genes. Int. J. Food Microbiol. 2021, 348, 109207. [Google Scholar] [CrossRef]
- Ongena, M.; Jacques, P.; Touré, Y.; Destain, J.; Jabrane, A.; Thonart, P. Involvement of fengycin-type lipopeptides in the multifaceted biocontrol potential of Bacillus subtilis. Appl. Microbiol. Biotechnol. 2005, 69, 29–38. [Google Scholar] [CrossRef]
- Caulier, S.; Nannan, C.; Gillis, A.; Licciardi, F.; Bragard, C.; Mahillon, J. Overview of the Antimicrobial Compounds Produced by Members of the Bacillus subtilis Group. Front. Microbiol. 2019, 10, 302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudakova, M.A.; Galitskaya, P.Y.; Selivanovskaya, S.Y. Biosurfactants: Current Application Trends. Uchenye Zap. Kazan. Univ. Seriya Estestv. Nauk. 2021, 163, 177–208. [Google Scholar] [CrossRef]
- Geissler, M.; Oellig, C.; Moss, K.; Schwack, W.; Henkel, M.; Hausmann, R. High-performance thin-layer chromatography (HPTLC) for the simultaneous quantification of the cyclic lipopeptides Surfactin, Iturin A and Fengycin in culture samples of Bacillus species. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1044–1045, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Massart, S.; Perazzolli, M.; Höfte, M.; Pertot, I.; Jijakli, M.H. Impact of the omic technologies for understanding the modes of action of biological control agents against plant pathogens. BioControl 2015, 60, 725–746. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.K.; Kant, C.; Verma, H.; Kumar, D.; Singh, P.P.; Modi, A.; Droby, S.; Kesawat, M.S.; Alavilli, H.; et al. Microbial biosurfactant: A new frontier for sustainable agriculture and pharmaceutical industries. Antioxidants 2021, 10, 1472. [Google Scholar] [CrossRef]
- Seneviratne, G.; Weerasekara, M.L.M.A.W.; Seneviratne, K.A.C.N.; Zavahir, J.S.; Kecskés, M.L.; Kennedy, I.R. Importance of Biofilm Formation in Plant Growth Promoting Rhizobacterial Action. In Plant Growth and Health Promoting Bacteria; Springer: Berlin/Heidelberg, Germany, 2010; pp. 81–95. [Google Scholar] [CrossRef]
- Zeriouh, H.; de Vicente, A.; Pérez-García, A.; Romero, D. Surfactin triggers biofilm formation of Bacillus subtilis in melon phylloplane and contributes to the biocontrol activity. Environ. Microbiol. 2014, 16, 2196–2211. [Google Scholar] [CrossRef]
- Chen, L.; Wang, N.; Wang, X.; Hu, J.; Wang, S. Characterization of two anti-fungal lipopeptides produced by Bacillus amyloliquefaciens SH-B10. Bioresour. Technol. 2010, 101, 8822–8827. [Google Scholar] [CrossRef]
- Gaur, V.K.; Sharma, P.; Sirohi, R.; Varjani, S.; Taherzadeh, M.J.; Chang, J.-S.; Ng, H.Y.; Wong, J.W.; Kim, S.-H. Production of biosurfactants from agro-industrial waste and waste cooking oil in a circular bioeconomy: An overview. Bioresour. Technol. 2022, 343, 126059. [Google Scholar] [CrossRef]
- Medina-Romero, Y.M.; Roque-Flores, G.; Macías-Rubalcava, M.L. Volatile organic compounds from endophytic fungi as innovative postharvest control of Fusarium oxysporum in cherry tomato fruits. Appl. Microbiol. Biotechnol. 2017, 101, 8209–8222. [Google Scholar] [CrossRef]
- Raynaldo, F.A.; Dhanasekaran, S.; Ngea, G.L.N.; Yang, Q.; Zhang, X.; Zhang, H. Investigating the biocontrol potentiality of Wickerhamomyces anomalus against postharvest gray mold decay in cherry tomatoes. Sci. Hortic. (Amst.) 2021, 285, 110137. [Google Scholar] [CrossRef]
- Ignjatov, M.; Milosevic, D.; Nikolic, Z.; Gvozdanovic-Varga, J.; Jovicic, D.; Zdjelar, G. Fusarium oxysporum as causal agent of tomato wilt and fruit rot. Pestic. Fitomedicina 2012, 27, 25–31. [Google Scholar] [CrossRef]
- Thipe, V.C.; Bloebaum, P.; Khoobchandani, M.; Karikachery, A.R.; Katti, K.K.; Katti, K.V. Green nanotechnology: Nanoformulations against toxigenic fungi to limit mycotoxin production. In Nanomycotoxicology: Treating Mycotoxins in the Nano Way; Academic Press: Cambridge, MA, USA, 2019; pp. 155–188. [Google Scholar] [CrossRef]
- Ji, F.; He, D.; Olaniran, A.O.; Mokoena, M.P.; Xu, J.; Shi, J. Occurrence, toxicity, production and detection of Fusarium mycotoxin: A review. Food Prod. Process. Nutr. 2019, 1, 6. [Google Scholar] [CrossRef]
- Mart, P.V.; Garrigues, S.; Marcos, J.F.; Manzanares, P. Antifungal Peptides and Proteins to Control Toxigenic Fungi and Mycotoxin Biosynthesis. Int. J. Mol. Sci. 2021, 22, 13261. [Google Scholar] [CrossRef]
- Harwig, J.; Scott, P.M.; Stoltz, D.R.; Blanchfield, B.J. Toxins of molds from decaying tomato fruit. Appl. Environ. Microbiol. 1979, 38, 267–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasannath, K. Plant defense-related enzymes against pathogens: A review. AGRIEAST J. Agric. Sci. 2017, 11, 38. [Google Scholar] [CrossRef]
- Indunil Kumari, Y.S.M.A.; Vengadaramana, A. Stimulation of Defense Enzymes in Tomato (Solanum lycopersicum L.) and Chilli (Capsicum annuum L.) in Response to Exogenous Application of Different Chemical Elicitors. Univers. J. Plant Sci. 2017, 5, 10–15. [Google Scholar] [CrossRef] [Green Version]
- Lorenzo, O.; Solano, R. Molecular players regulating the jasmonate signalling network. Curr. Opin. Plant Biol. 2005, 8, 532–540. [Google Scholar] [CrossRef]
- Ralph, S.; Park, J.Y.; Bohlmann, J.; Mansfield, S.D. Dirigent proteins in conifer defense: Gene discovery, phylogeny, and differential wound- and insect-induced expression of a family of DIR and DIR-like genes in spruce (Picea spp.). Plant Mol. Biol. 2006, 60, 21–40. [Google Scholar] [CrossRef]
- Agrios, G.N. How plants defend themselves against pathogens. In Plant Pathology, 5th ed.; Academic Press: Cambridge, MA, USA, 2005; pp. 207–248. [Google Scholar] [CrossRef]
- Luzuriaga-Loaiza, W.P.; Schellenberger, R.; De Gaetano, Y.; Obounou Akong, F.; Villaume, S.; Crouzet, J.; Haudrechy, A.; Baillieul, F.; Clément, C.; Lins, L.; et al. Synthetic Rhamnolipid Bolaforms trigger an innate immune response in Arabidopsis thaliana. Sci. Rep. 2018, 8, 8534. [Google Scholar] [CrossRef] [Green Version]
- Henry, G.; Deleu, M.; Jourdan, E.; Thonart, P.; Ongena, M. The bacterial lipopeptide surfactin targets the lipid fraction of the plant plasma membrane to trigger immune-related defence responses. Cell. Microbiol. 2011, 13, 1824–1837. [Google Scholar] [CrossRef]
- Biktasheva, L.; Galitskaya, P.S.S. Potential of biosurfactant production in bacteria isolated from oil polluted soils. Int. Multidiscip. Sci. GeoConf. Surv. Geol. Min. Ecol. Manag. SGEM 2021, 225–232. [Google Scholar] [CrossRef]
- Chung, S.; Kong, H.; Buyer, J.S.; Lakshman, D.K.; Lydon, J.; Kim, S.D.; Roberts, D.P. Isolation and partial characterization of Bacillus subtilis ME488 for suppression of soilborne pathogens of cucumber and pepper. Appl. Microbiol. Biotechnol. 2008, 80, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, H.; Dowarah, B.; Baruah, P.M.; Bordoloi, K.S.; Krishnatreya, D.B.; Agarwala, N. Endophytes from Gnetum gnemon L. can protect seedlings against the infection of phytopathogenic bacterium Ralstonia solanacearum as well as promote plant growth in tomato. Microbiol. Res. 2020, 238, 126503. [Google Scholar] [CrossRef]
- Galitskaya, P.; Biktasheva, L.; Kuryntseva, P.; Selivanovskaya, S. Response of soil bacterial communities to high petroleum content in the absence of remediation procedures. Environ. Sci. Pollut. Res. 2021, 28, 9610–9627. [Google Scholar] [CrossRef]
- Alvarez, V.M.; Guimarães, C.R.; Jurelevicius, D.; de Castilho, L.V.A.; de Sousa, J.S.; da Mota, F.F.; Freire, D.M.G.; Seldin, L. Microbial enhanced oil recovery potential of surfactin-producing Bacillus subtilis AB2.0. Fue 2020, 272, 117730. [Google Scholar] [CrossRef]
- Nayarisseri, A.; Singh, P.; Singh, S.K. Screening, isolation and characterization of biosurfactant producing Bacillus subtilis strain ANSKLAB03. Bioinformation 2018, 14, 304–314. [Google Scholar] [CrossRef]
- Varadavenkatesan, T.; Murty, V.R. Production of a Lipopeptide Biosurfactant by a Novel Bacillus sp. and Its Applicability to Enhanced Oil Recovery. ISRN Microbiol. 2013, 2013, 621519. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Cao, W.; Jiang, M.; Saren, G.; Liu, J.; Cao, J.; Ali, I.; Yu, X.; Peng, C.; Naz, I. Isolation and characterization of biosurfactant-producing and diesel oil degrading Pseudomonas sp. CQ2 from Changqing oil field, China. RSC Adv. 2018, 8, 39710–39720. [Google Scholar] [CrossRef] [Green Version]
- Griffith, G.W.; Easton, G.L.; Detheridge, A.; Roderick, K.; Edwards, A.; Worgan, H.J.; Nicholson, J.; Perkins, W.T. Copper, deficiency in potato dextrose agar causes reduced pigmentation in cultures of various fungi. FEMS Microbiol. Lett. 2007, 276, 165–171. [Google Scholar] [CrossRef]
- Mendes, G.; Gonçalves, V.N.; Souza-Fagundes, E.M.; Kohlhoff, M.; Rosa, C.A.; Zani, C.L.; Cota, B.B.; Rosa, L.H.; Johann, S. Antifungal activity of extracts from Atacama Desert fungi against Paracoccidioides brasiliensis and identification of Aspergillus felis as a promising source of natural bioactive compounds. Mem. Inst. Oswaldo Cruz. 2016, 111, 209–217. [Google Scholar] [CrossRef]
- Khaliq, G.; Muda Mohamed, M.T.; Ali, A.; Ding, P.; Ghazali, H.M. Effect of gum arabic coating combined with calcium chloride on physico-chemical and qualitative properties of mango (Mangifera indica L.) fruit during low temperature storage. Sci. Hortic. (Amsterdam) 2015, 190, 187–194. [Google Scholar] [CrossRef]
- Uzoigwe, C.; Burgess, J.G.; Ennis, C.J.; Rahman, P.K.S.M. Bioemulsifiers are not biosurfactants and require different screening approaches. Front Microbiol. 2015, 6, 245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barin, R.; Talebi, M.; Biria, D.; Beheshti, M. Fast bioremediation of petroleum-contaminated soils by a consortium of biosurfactant/bioemulsifier producing bacteria. Int. J. Environ. Sci. Technol. 2014, 11, 1701–1710. [Google Scholar] [CrossRef] [Green Version]
- Pereira, J.F.B.; Gudiña, E.J.; Costa, R.; Vitorino, R.; Teixeira, J.A.; Coutinho, J.A.P.; Rodrigues, L.R. Optimization and characterization of biosurfactant production by Bacillus subtilis isolates towards microbial enhanced oil recovery applications. Fuel 2013, 111, 259–268. [Google Scholar] [CrossRef] [Green Version]
- Freitas de Oliveira, D.W.; Lima França, Í.W.; Nogueira Félix, A.K.; Lima Martins, J.J.; Aparecida Giro, M.E.; Melo, V.M.M.; Goncalves, L.R.B. study of biosurfactant production by Bacillus subtilis LAMI005 grown in clarified cashew apple juice. Colloids Surf. B Biointerfaces 2013, 101, 34–43. [Google Scholar] [CrossRef]
- Cawoy, H.; Debois, D.; Franzil, L.; De Pauw, E.; Thonart, P.; Ongena, M. Lipopeptides as main ingredients for inhibition of fungal phytopathogens by Bacillus subtilis/amyloliquefaciens. Microb. Biotechnol. 2015, 8, 281–295. [Google Scholar] [CrossRef]
- El-Sheshtawy, H.S.; Aiad, I.; Osman, M.E.; Abo-ELnasr, A.A.; Kobisy, A.S. Production of biosurfactant from Bacillus licheniformis for microbial enhanced oil recovery and inhibition the growth of sulfate reducing bacteria. Egypt. J. Pet. 2015, 24, 155–162. [Google Scholar] [CrossRef] [Green Version]
- Nayak, N.S.; Purohit, M.S.; Tipre, D.R.; Dave, S.R. Biosurfactant production and engine oil degradation by marine halotolerant Bacillus licheniformis LRK1. Biocatal. Agric. Biotechnol. 2020, 29, 101808. [Google Scholar] [CrossRef]
- Velho, R.V.; Medina, L.F.C.; Segalin, J.; Brandelli, A. Production of lipopeptides among Bacillus strains showing growth inhibition of phytopathogenic fungi. Folia Microbiol. (Praha) 2011, 56, 297–303. [Google Scholar] [CrossRef]
- Adhikari, M.; Negi, B.; Kaushik, N.; Adhikari, A.; Al-Khedhairy, A.A.; Kaushik, N.K.; Choi, E.H. T-2 mycotoxin: Toxicological effects and decontamination strategies. Oncotarget 2017, 8, 33933. Available online: www.impactjournals.com/oncotarget (accessed on 8 February 2017). [CrossRef] [Green Version]
- Yan, F.; Xu, S.; Chen, Y.; Zheng, X. Effect of rhamnolipids on Rhodotorula glutinis biocontrol of Alternaria alternata infection in cherry tomato fruit. Postharvest Biol. Technol. 2014, 97, 32–35. [Google Scholar] [CrossRef]
- Constabel, C.P.; Barbehenn, R. Defensive roles of polyphenol oxidase in plants. In Induced Plant Resistance to Herbivory; Springer: Dordrecht, The Netherlands, 2008; pp. 253–270. [Google Scholar] [CrossRef]
- García-Gutiérrez, L.; Zeriouh, H.; Romero, D.; Cubero, J.; de Vicente, A.; Pérez-García, A. The antagonistic strain Bacillus subtilisUMAF6639 also confers protection to melon plants against cucurbit powdery mildew by activation of jasmonate- and salicylic acid-dependent defence responses. Microb. Biotechnol. 2013, 6, 264–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
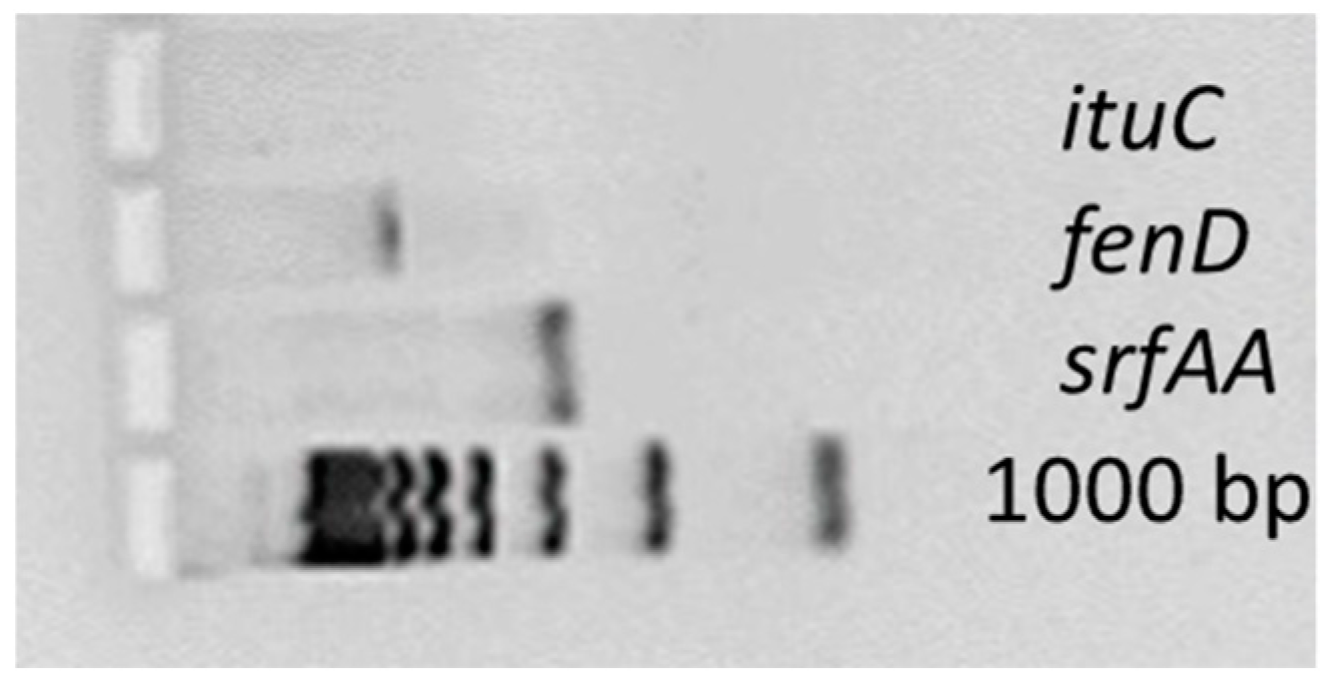

| Target Gene | Primer Sequence, 5′-3′ | Amplicon Length (bp) |
|---|---|---|
| fenD | F: GGCCCGTTCTCTAAATCCAT | 670 |
| R: GTCATGCTGACGAGAGCAAA | ||
| ituC | F: GGCTGCTGCAGATGCTTTAT | 423 |
| R: TCGCAGATAATCGCAGTGAG | ||
| srfAA | F: TCGGGACAGGAAGACATCAT | 201 |
| R: CCACTCAAACGGATAATCCTGA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galitskaya, P.; Karamova, K.; Biktasheva, L.; Galieva, G.; Gordeev, A.; Selivanovskaya, S. Lipopeptides Produced by Bacillus mojavensis P1709 as an Efficient Tool to Maintain Postharvest Cherry Tomato Quality and Quantity. Agriculture 2022, 12, 609. https://doi.org/10.3390/agriculture12050609
Galitskaya P, Karamova K, Biktasheva L, Galieva G, Gordeev A, Selivanovskaya S. Lipopeptides Produced by Bacillus mojavensis P1709 as an Efficient Tool to Maintain Postharvest Cherry Tomato Quality and Quantity. Agriculture. 2022; 12(5):609. https://doi.org/10.3390/agriculture12050609
Chicago/Turabian StyleGalitskaya, Polina, Kamalya Karamova, Liliya Biktasheva, Gulnaz Galieva, Alexander Gordeev, and Svetlana Selivanovskaya. 2022. "Lipopeptides Produced by Bacillus mojavensis P1709 as an Efficient Tool to Maintain Postharvest Cherry Tomato Quality and Quantity" Agriculture 12, no. 5: 609. https://doi.org/10.3390/agriculture12050609
APA StyleGalitskaya, P., Karamova, K., Biktasheva, L., Galieva, G., Gordeev, A., & Selivanovskaya, S. (2022). Lipopeptides Produced by Bacillus mojavensis P1709 as an Efficient Tool to Maintain Postharvest Cherry Tomato Quality and Quantity. Agriculture, 12(5), 609. https://doi.org/10.3390/agriculture12050609






