Pre-Sowing Laser Light Stimulation Increases Yield and Protein and Crude Fat Contents in Soybean
Abstract
1. Introduction
2. Material and Methods
2.1. Experimental Design
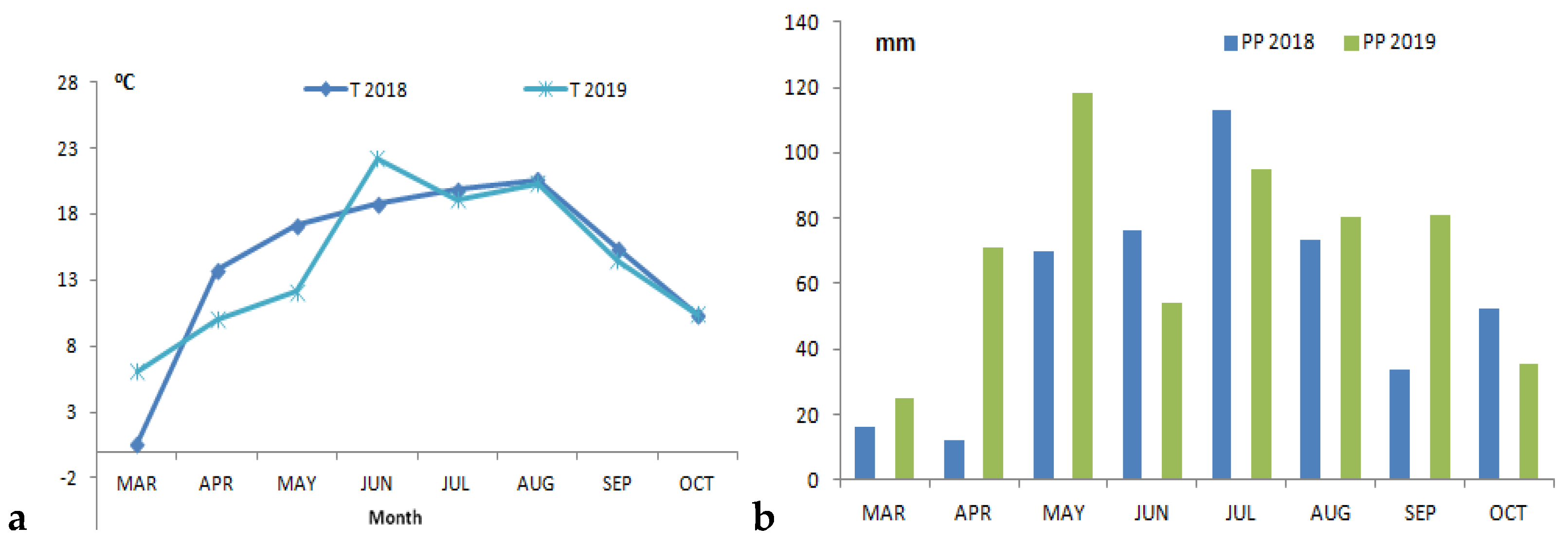
2.2. Environmental Conditions
3. Results
3.1. Yield Depending on the Type of Light Stimulation
3.2. Plant Biology
3.3. Chemical Composition of Seeds
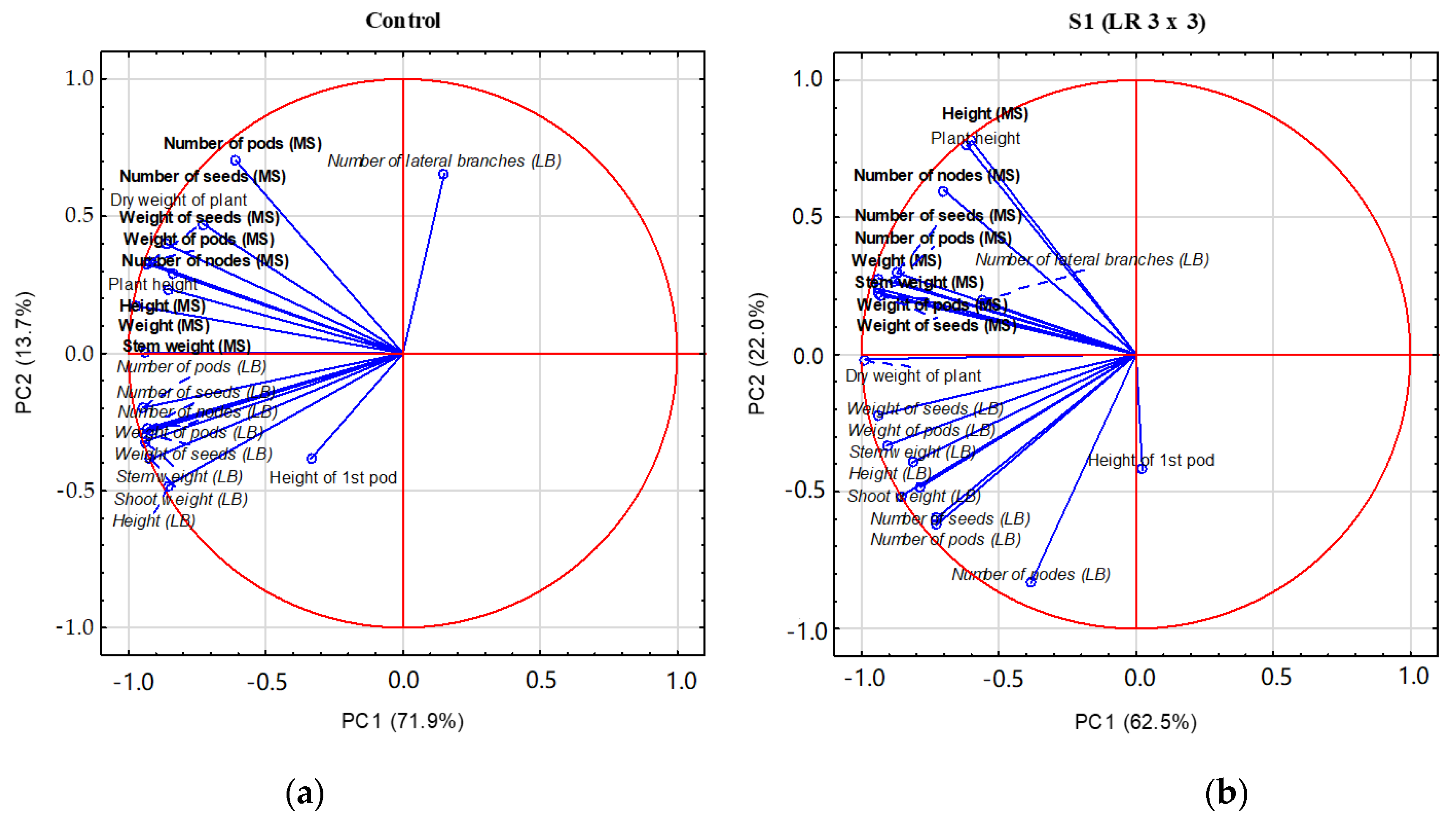
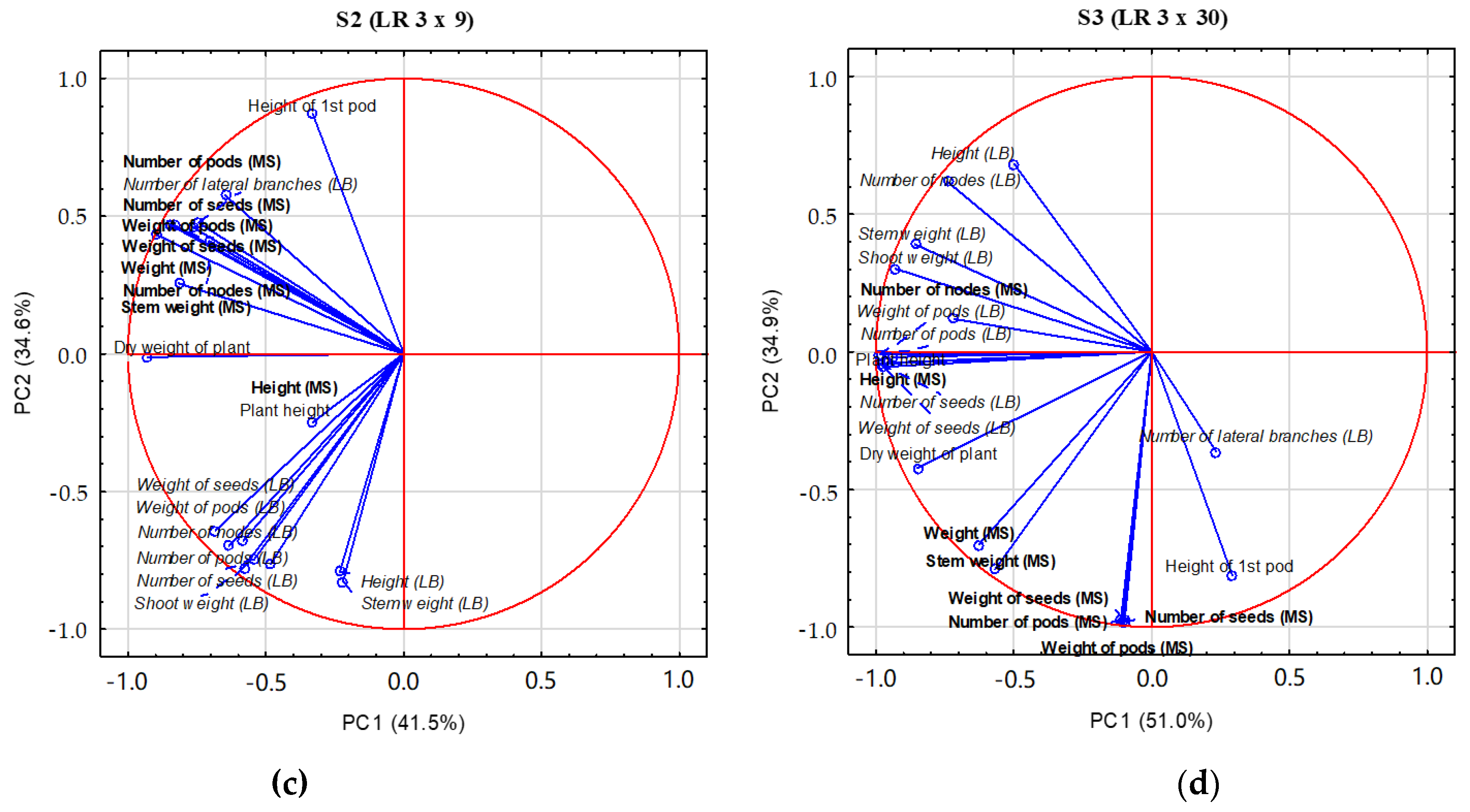
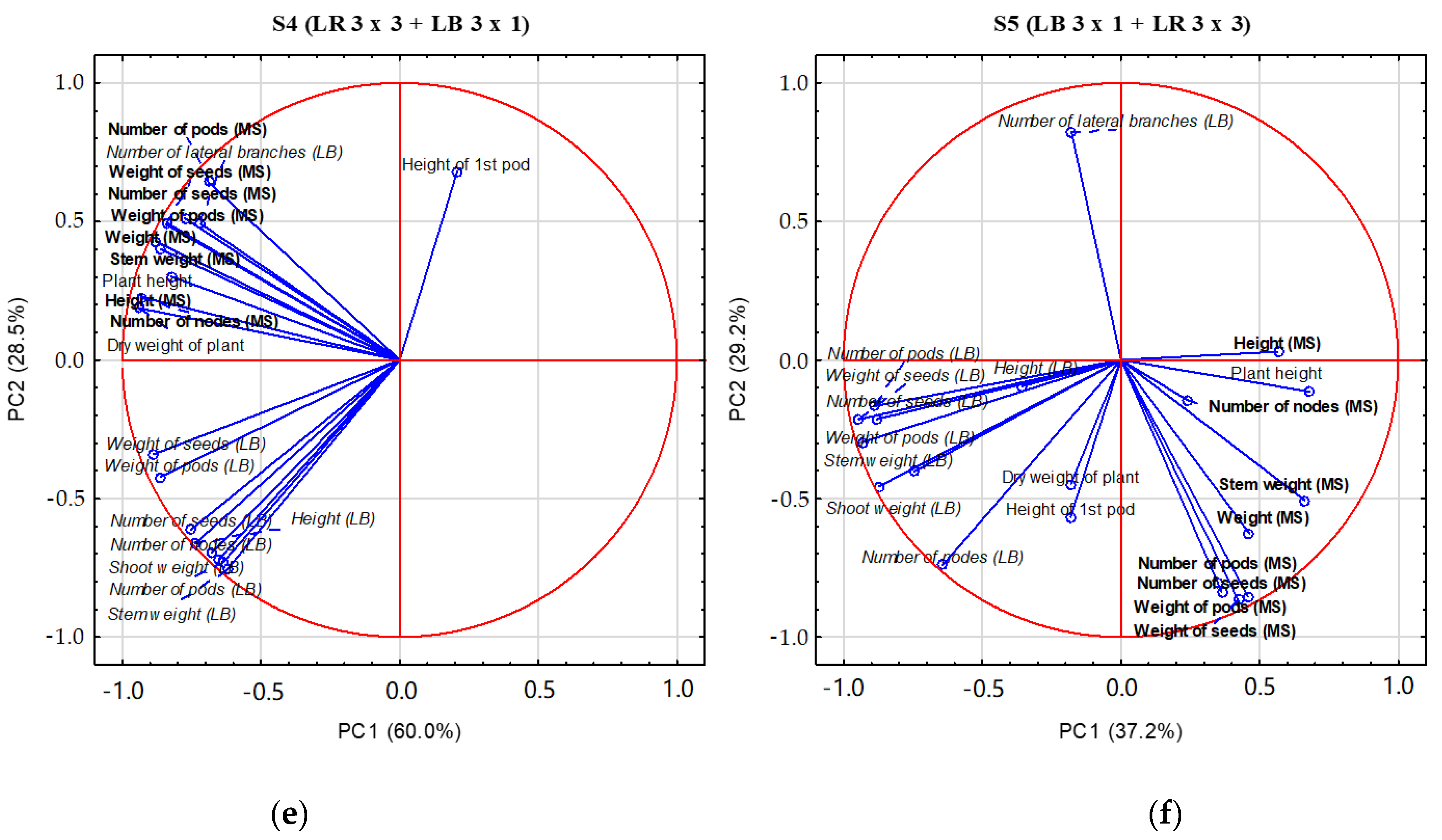
3.4. PCA Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Obaidi, J.R.; Halabi, M.F.; AlKhalifah, N.S.; Asanar, S.; AI-Soqeer, A.A.; Attia, M.F. A review on plant importance, biotechnological aspects, and cultivation challenges of jojoba plant. Biol. Res. 2017, 50, 25. [Google Scholar] [CrossRef] [PubMed]
- Msanne, J.; Kim, H.; Cahoon, E.B. Biotechnology tools and applications for development of oilseed crops with healthy vegetable oils. Biochimie 2020, 178, 4–14. [Google Scholar] [CrossRef]
- Rębilas, K.; Klimek-Kopyra, A.; Bacior, M.; Zając, T. A model for the yield losses estimation in an early soybean (Glycine max (L.) Merr.) cultivar depending on the cutting height at harvest. Field Crop. Res. 2020, 254, 107846. [Google Scholar] [CrossRef]
- Gotor, A.A.; Marraccini, E. Innovative Pulses for Western European Temperate Regions: A Review. Agronomy 2022, 12, 170. [Google Scholar] [CrossRef]
- Asghar, T.; Jamil, Y.; Iqbal, M.; Zia-ul-Haq; Abbas, M. Laser light and magnetic field stimulation effect on biochemical, enzymes activities and chlorophyll contents in soybean seeds and seedlings during early growth stages. J. Phot. Photobiol. Biol. 2016, 165, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Grzesik, M.; Janas, R. Wpływ hydrokondycjonowania na aktywność metaboliczną oraz kiełkowanie nasion i wschody siewek marchwi. J. Res. Appl. Agric. Eng. 2011, 56, 127–132. [Google Scholar]
- Klimek-Kopyra, A.; Dobrowolski, J.W.; Czech, T.; Neugschwandtner, R.W.; Gambuś, F.; Kot, D. The Use of Laser Biotechnology in Agri-environment as a Significant Agronomical Advance Increasing Crop Yield and Quality. Adv. Agron. 2021, 170, 1–33. [Google Scholar]
- Aladjadjiyan, A. Physical Factors for Plant Growth Stimulation Improve Food Quality. Food Prod. Appr. Challen. Inotech 2012, 9, 145–168. [Google Scholar]
- Jakubiak, M.; Śliwka, M. Environmental Protection Technologies—Laser Biotechnology Application; Wydawnictwa AGH: Kraków, Poland, 2013; pp. 23–30. [Google Scholar]
- Klejman, H. Lasery; Polskie Wydawnictwo Naukowe (PWN): Warszawa, Poland, 1979; p. 473. [Google Scholar]
- Klimek-Kopyra, A.; Czech, T. Complementary photostimulation of seeds and plants as an effective tool for increasing crop productivity and quality in light of new challenges facing agriculture in the 21st century—A case study. Plants 2022, 11, 1649. [Google Scholar] [CrossRef]
- Dziwulska, A.; Koper, R. Wpływ przedsiewnej biostymulacji laserowej na kiełkowanie nasion lucerny siewnej. Acta Agrophysica 2003, 82, 33–39. [Google Scholar]
- Dziwulska, A.; Koper, R.; Wilczek, M. Ocena wpływu światła lasera He-Ne na zdolność kiełkowania nasion koniczyny białej odmiany Anda. Acta Agrophysica 2004, 3, 435–441. [Google Scholar]
- Hernandez-Aguilar, C.; Dominguez-Pacheco, A.; Cruz-Orea, A.; Podleśna, A.; Ivanov, R.; Carballo, A.; Perez-Reyes, M.C.; Sanchez-Hernandez, G.; Zepeda Bautista, R.; Lopez-Bonilla, J. Bioestimulación láser en semillas y plantas. Gayana Bot. 2016, 73, 132–149. [Google Scholar] [CrossRef][Green Version]
- Ivanova, R. Influence of Pre-sowing Laser Irradiation of seeds of Introduced Flax Varieties for Linseed Oil on Yield Quality. Bulg. J. Agricult. Sci. 1998, 4, 49–53. [Google Scholar]
- Aladzhadzhiyan, A.; Khadzhiatanosov, D.; Georgiev, V. Seed coat transparency of maize, soybean and bean. Bulg. J. Agric. Sci. 1995, 1, 23–26. [Google Scholar]
- Gieroba, J.; Koper, R.; Matyka, S. The influence of pre-sowing laser biostimulation of maize seeds on the crop and nutritive value of the com. In Proceedings of the 45th Australian Cereal Chemistry Conference, Adelaide, South Australia, 10–14 September 1995; pp. 30–33. [Google Scholar]
- Wilczek, M.; Koper, R.; Ćwintal, M.; Korniłłowicz-Kowalska, T. Germination capacity and health status of alfalfa seeds after laser treatment. Int. Agrophysics 2005, 19, 85–89. [Google Scholar]
- Prośba-Białczyk, U.; Szajner, H.; Grzyś, E.; Demczuk, A.; Sacała, E.; Bąk, K. Effect of seed stimulation on germination and sugar beet yield. Int. Agrophysics 2013, 27, 195–201. [Google Scholar] [CrossRef]
- Krawiec, M.; Dziwulska-Hunek, A.; Sujak, A.; Palonka, S. Laser irradiation effects on scorzonera (Scorzonera hispanica L.) seed germination and seedling emergence. Acta Sci. Pol. Hortorum Cultus 2015, 14, 145–158. [Google Scholar]
- Podleśny, J.; Stochmal, A. Wpływ przedsiewnego traktowania nasion światłem laserowym na niektóre procesy biochemiczne i fizjologiczne w nasionach i roślinach łubinu białego i bobiku. Acta Agrophysica 2004, 4, 149–160. [Google Scholar]
- Podleśny, J. Studia nad oddziaływaniem światła laserowego na nasiona, wzrost i rozwój roślin oraz plonowanie łubinu białego (Lupinus albus L.). Monogr. I Rozpr. Nauk. Puławy 2002, 3, 7–48. [Google Scholar]
- Podleśny, J. Wpływ stymulacji magnetycznej na wzrost, rozwój i plonowanie roślin uprawnych. Acta Agrophysica 2004, 4, 459–473. [Google Scholar]
- Podleśny, J. Wpływ promieniowania nasion laserem i desykacji roślin na plonowanie i cechy jakościowe nasion łubinu białego. Acta Agrophysica 2007, 9, 733–745. [Google Scholar]
- Podleśny, J.; Stochmal, A.; Podleśna, A.; Misiak, L.E. Effect of laser light treatment on some biochemical and physiological processes in seeds and seedlings of white lupine and faba bean. Plant Growth Reg. 2012, 67, 227–233. [Google Scholar] [CrossRef]
- Dłużniewska, J.; Klimek-Kopyra, A.; Czech, T.; Dobrowolski, J.W.; Dacewicz, E. The use of coherent laser stimulation of seeds and a fungal inoculum to increase the productivity and health of soybean plants. Agronomy 2021, 11, 1923. [Google Scholar] [CrossRef]
- Chen, J.; Ren, X.; Zhang, Q.; Diao, X.; Shen, Q. Determination of protein, total carbohydrates and crude fat contents of foxtail millet using effective wavelengths in NIR spectroscopy. J. Cereal Sci. 2013, 58, 241–247. [Google Scholar] [CrossRef]
- Dobrowolski, J.W.; Sliwka, M.; Mazur, R. Laser biotechnology for more efficient bioremediation, protection of aquatic ecosystems and reclamation of contaminated areas. J. Chem. Technol. Biotechnol. 2012, 87, 1354–1359. [Google Scholar] [CrossRef]
- Lanoue, J.; Leonardos, E.D.; Grodzinski, B. Artificial Lighting Technologies for Agricultural Production. In Comprehensive Biotechnology, 3rd ed.; Moo-Young, M., Ed.; Pergamon: Oxford, UK, 2019; Volume 4, pp. 818–832. ISBN 9780444640475. [Google Scholar] [CrossRef]
- Śliwka, M. Assessment of impact of coherent light on resistance of plant growing in unfavourable environmental conditions. J. Ecol. Eng. 2014, 15, 112–118. [Google Scholar]
- Podleśna, A.; Gładyszewska, B.; Podlesny, J.; Zgrajka, W. Changes in the germination process and growth of pea in effect of laser seed irradiation. Int. Agrophysics 2015, 29, 485–492. [Google Scholar] [CrossRef]
- Dziamba, S.; Wielgo, B.; Leszek, M.; Cebula, M. Wpływ przedsiewnej biostymulacji nasion odmian owsa na plonowanie i elementy struktury plonu. Żywność 1999, 1, 113–118. [Google Scholar]
- Dziamba, S.; Dziamba, M.; Machaj, H.; Klimek, A. Wpływ przedsiewnej biostymulacji nasion światłem na plonowanie niektórych odmian kukurydzy. Biul. Inst. Hod. I Aklim. Roślin 2004, 231, 445–451. [Google Scholar]
- Ćwintal, M.; Sowa, P. Efekty przedsiewnej stymulacji nasion lucerny światłem lasera w roku siewu i latach pełnego użytkowania. Act. Sci. Pol. Agric. 2006, 5, 11–23. [Google Scholar]
- Wilczek, M.; Ćwintal, M.; Kornas-Czuczwar, B.; Koper, R. Wpływ laserowej stymulacji nasion na plonowanie di- i tetraploidalnej koniczyny czerwonej w latach pełnego użytkowania. Acta Agrophysica 2006, 8, 735–743. [Google Scholar]
- Ćwintal, M.; Olszewski, J. Wpływ przedsiewnej laserowej stymulacji nasion na intensywność fotosyntezy i transpiracji oraz plonowanie lucerny. Acta Agrophysica 2007, 9, 345–352. [Google Scholar]
- Wilczek, M.; Ćwintal, M. Wpływ przedsiewnej stymulacji laserowej nasion i dokarmiania mikroelementami (B, Mo) na plonowanie nasiennej, tetraploidalnej koniczyny czerwonej w czteroletnim użytkowaniu. Acta Agrophysica 2011, 17, 207–217. [Google Scholar]
- Podleśny, J. Wpływ przedsiewnego traktowania nasion światłem laserowym na kształtowanie cech morfologicznych i plonowanie bobiku. Zesz. Probl. Postepow Nauk. Rol. 1997, 446, 435–439. [Google Scholar]
- Pasternakiewicz, A.; Dżugan, M. Ocena zawartości podstawowych makroskładników w nasionach soi. Zeszyt Nauk Południowo-Wschodnich. 2009, 1, 217–222. [Google Scholar]
- Dziamba, J.; Dziamba, S. Wpływ przedsiewnej stymulacji nasion światłem czerwonym na zawartość składników pokarmowych w nasionach pokolenia potomnego rzepaku ozimego. Oilseed Crop. 2011, 32, 240–247. [Google Scholar]

| Treatment of Seed Light Stimulation Before Sowing (S) | Laser Type | Laser Exposure Time of Light Stimulation |
|---|---|---|
| S0 Control | - | - |
| S1 (LR 3 × 3) | He-Ne | 3 × 3 s * |
| S2 (LR 3 × 9) | He-Ne | 3 × 9 s |
| S3 (LR 3 × 30) | He-Ne | 3 × 30 s |
| S4 (LR 3 × 3 + LD 3 × 1) | He-Ne + Argon | 3 × 3 s + 3 × 1 s |
| S5 (LD 3 × 1 + LR 3 × 3) | Argon + He-Ne | 3 × 1 s + 3 × 3 s |
| Treatment | Total Yield * (g m−2) | Yield (g m−2) | TSW (g) | ||
|---|---|---|---|---|---|
| Main Shoot | Lateral Branches | Main Shoot | Lateral Branches | ||
| S0 Control | 213a | 133a | 80a | 129a | 81c |
| S1 (LR 3 × 3) | 423ab | 199a | 223ab | 150ab | 129ab |
| S2 (LR 3 × 9) | 291a | 157a | 134a | 142a | 127abc |
| S3 (LR 3 × 30) | 648b | 270a | 378bc | 177b | 159b |
| S4 (LR 3 × 3 + LD 3 × 1) | 249a | 148a | 101a | 131a | 108ac |
| S5 (LD 3 × 1 + LR 3 × 3) | 689b | 268a | 420c | 180b | 144ab |
| Treatment | Plant Height (cm) | Height of First Pod (cm) | Dry Weight of Plant (g) | Number of Lateral Branches |
|---|---|---|---|---|
| S0 Control | 69.8a | 6.57a | 62.4a | 4.43a |
| S1 (LR 3 × 3) | 83.4ab | 7.86a | 119.9ab | 7.29a |
| S2 (LR 3 × 9) | 92.4b | 5.21a | 86.2a | 5.29a |
| S3 (LR 3 × 30) | 83.3ab | 5.57a | 162.6b | 7.86a |
| S4 (LR 3 × 3 + LD 3 × 1) | 84.1ab | 8.36a | 75.2a | 7.00a |
| S5 (LD 3 × 1 + LR 3 × 3) | 82.4ab | 6.79a | 176.1b | 7.86a |
| Treatment | Shoot Weight (g) | Stem Weight (g) | Height (cm) | Number of Fruiting Nodes |
|---|---|---|---|---|
| S0 Control | 40.3a | 9.1a | 69.9a | 13.3a |
| S1 (LR 3 × 3) | 59.0a | 14.9a | 83.1a | 13.9a |
| S2 (LR 3 × 9) | 49.8a | 12.7a | 92.4a | 14.7a |
| S3 (LR 3 × 30) | 72.1a | 15.0a | 83.9a | 15.4a |
| S4 (LR 3 × 3 + LD 3 × 1) | 45.3a | 10.7a | 84.1a | 14.7a |
| S5 (LD 3 × 1 + LR 3 × 3) | 70.1a | 12.9a | 72.8a | 15.7a |
| Treatment | Shoot Weight (g) | Stem Weight (g) | Height (cm) | Number of Fruiting Nodes |
|---|---|---|---|---|
| S0 Control | 4.63a | 0.59a | 21.2c | 3.46a |
| S1 (LR 3 × 3) | 9.40ab | 1.71bcd | 37.9ab | 6.46bc |
| S2 (LR 3 × 9) | 6.69a | 1.24abc | 35.2a | 5.11ab |
| S3 (LR 3 × 30) | 14.1bc | 2.09cd | 47.7b | 7.49cd |
| S4 (LR 3 × 3 + LD 3 × 1) | 4.20a | 0.80ab | 31.9ac | 4.48 a |
| S5 (LD 3 × 1 + LR 3 × 3) | 18.0c | 2.39d | 47.1b | 9.43d |
| Treatment | Pod Number | Pod Weight (g) | Seed Number | Seed Weight (g) |
|---|---|---|---|---|
| S0 Control | 4.13a | 2.47a | 10.7a | 1.53a |
| S1 (LR 3 × 3) | 5.67bc | 3.59bc | 14.7bc | 2.35b |
| S2 (LR 3 × 9) | 4.66ab | 2.74ab | 11.6ab | 1.73ab |
| S3 (LR 3 × 30) | 6.33c | 4.56c | 16.3c | 3.12c |
| S4 (LR 3 × 3 + LD 3 × 1) | 4.73ab | 2.57ab | 11.5ab | 1.61a |
| S5 (LD 3 × 1 + LR 3 × 3) | 6.11c | 4.58c | 16.1c | 3.16c |
| Treatment | Pod Number | Pod Weight (g) | Seed Number | Seed Weight (g) |
|---|---|---|---|---|
| S0 Control | 1.94a | 0.95ab | 4.57a | 0.58ab |
| S1 (LR 3 × 3) | 2.01ab | 1.02a | 4.91a | 0.66a |
| S2 (LR 3 × 9) | 2.03ab | 1.04a | 4.89a | 0.65a |
| S3 (LR 3 × 30) | 2.40bc | 1.51c | 6.31b | 1.04c |
| S4 (LR 3 × 3 + LD 3 × 1) | 1.74a | 0.73b | 3.94a | 0.45a |
| S5 (LD 3 × 1 + LR 3 × 3) | 2.44c | 1.34c | 6.00b | 0.90c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klimek-Kopyra, A.; Neugschwandtner, R.W.; Ślizowska, A.; Kot, D.; Dobrowolski, J.W.; Pilch, Z.; Dacewicz, E. Pre-Sowing Laser Light Stimulation Increases Yield and Protein and Crude Fat Contents in Soybean. Agriculture 2022, 12, 1510. https://doi.org/10.3390/agriculture12101510
Klimek-Kopyra A, Neugschwandtner RW, Ślizowska A, Kot D, Dobrowolski JW, Pilch Z, Dacewicz E. Pre-Sowing Laser Light Stimulation Increases Yield and Protein and Crude Fat Contents in Soybean. Agriculture. 2022; 12(10):1510. https://doi.org/10.3390/agriculture12101510
Chicago/Turabian StyleKlimek-Kopyra, Agnieszka, Reinhard W. Neugschwandtner, Anna Ślizowska, Dominika Kot, Jan Wincenty Dobrowolski, Zbigniew Pilch, and Ewa Dacewicz. 2022. "Pre-Sowing Laser Light Stimulation Increases Yield and Protein and Crude Fat Contents in Soybean" Agriculture 12, no. 10: 1510. https://doi.org/10.3390/agriculture12101510
APA StyleKlimek-Kopyra, A., Neugschwandtner, R. W., Ślizowska, A., Kot, D., Dobrowolski, J. W., Pilch, Z., & Dacewicz, E. (2022). Pre-Sowing Laser Light Stimulation Increases Yield and Protein and Crude Fat Contents in Soybean. Agriculture, 12(10), 1510. https://doi.org/10.3390/agriculture12101510









