1. Introduction
The introduction of legumes in the cropping systems plays a key role in promoting environmental sustainability, especially within cereal-based systems, because of their symbiosis with nitrogen-fixing soil bacteria. Among legumes, in chickpea (
Cicer arietinum L.) residual soil nitrogen appears to not affect nitrogen fixation, and a starter fertilization supply is frequently adopted [
1]. Chickpea is a valuable source of proteins, dietary fiber, phytochemicals, and minerals, and as grains to cook or new flours used in addition or as an alternative to wheat [
2,
3]. Globulins represent the major fractions within storage proteins, including the cupin proteins convicilin, 7s vicilin, and 11s legumin, characterized by lower cysteine content. Lectin, lipoxygenase, 2s albumin, and other protease inhibitors represent the other minor fractions [
4,
5]. While the existence of a genetic variability is well known [
6,
7], less information is available on the effect of environment and management on legume storage protein composition and technological performance [
2,
8,
9].
Abiotic and biotic stresses can strongly affect seed production, and agronomic management can play an important role in improving yield productivity and stability [
10,
11,
12,
13]; indeed, chickpea is considered a crop adapted to semi-arid environments, with a good level of drought tolerance, and one which can be cultivated also under rainfed conditions [
14]. Water availability and drought can affect chickpea crop growth rate and yield [
15]; flowering and podding are, in particular, very sensitive to abiotic stress conditions and genetic improvement for heat and drought tolerance could be promising for chickpea [
16,
17]. On the other hand, the environment can affect grain quality in chickpea [
18] and this is particularly relevant under high rainfall variability, such as in the Mediterranean basin [
19].
The need to improve resource-use efficiency is leading to increasing adoption of precision agriculture, with the target of using the right input at the right time [
20]. Indeed, the use of vegetation indexes (VIs) on the canopy, by remote or proximal sensing, allows the assessment of crop water and nutritional status [
21]. The normalized difference vegetation index (NDVI) is commonly the most adopted VI, and is based on the higher reflectance of the crops in near-infrared (NIR) with respect to the visible (VIS) spectra; many other spectral indices are available in the literature [
22]. Major applications are generally carried out on cereals, while less information is available on the relationships of VIs on pulses. The requirement of more efficient crop systems is mandatory in a scenario of climate changes where resources, such as water, might be more and more limited. For this reason, the optimization of management, also relative to irrigation and water use efficiency, is valuable for chickpea, also considering its good ability to use a water supply, especially during flowering [
23]. Most of the studies available on chickpea are conducted in Asia, North America, and Australia [
15,
24,
25], while fewer observations are available in the Mediterranean basin, with also little information on the effect on protein quality.
In a previous study conducted in Mediterranean environment, chickpea protein composition was found to be more affected by agronomic conditions than protein content [
9]; thus, this study was carried out in order to test the hypothesis that water and nitrogen supply could affect protein composition as well as other crop physiological and agronomic traits. To this aim, the relationship between the productive response of chickpea genotypes and hyperspectral crop reflectance, including VIs, also in relation to changes in grain protein composition, was evaluated. To this purpose, two chickpea varieties were grown with and without irrigation and a starter nitrogen supply, in two locations selected for different climatic conditions; crop physiological assessments by spectral phenotyping and the changes of the main grain protein fractions were carried out.
4. Discussion
Yield production obtained by spring sowing in the two environments, characterized by different mean temperatures and climatic rainfall deficits, was in a range comparable with previous observations in the literature [
41]. In a previous study [
10] conducted in a Mediterranean environment, the same varieties, Pascià and Sultano, showed a higher yield under winter sowing with respect to spring sowing. However, this advantage is critically influenced by unfavorable climatic conditions, especially during flowering, which can lead to a higher risk of biotically stressful conditions [
25].
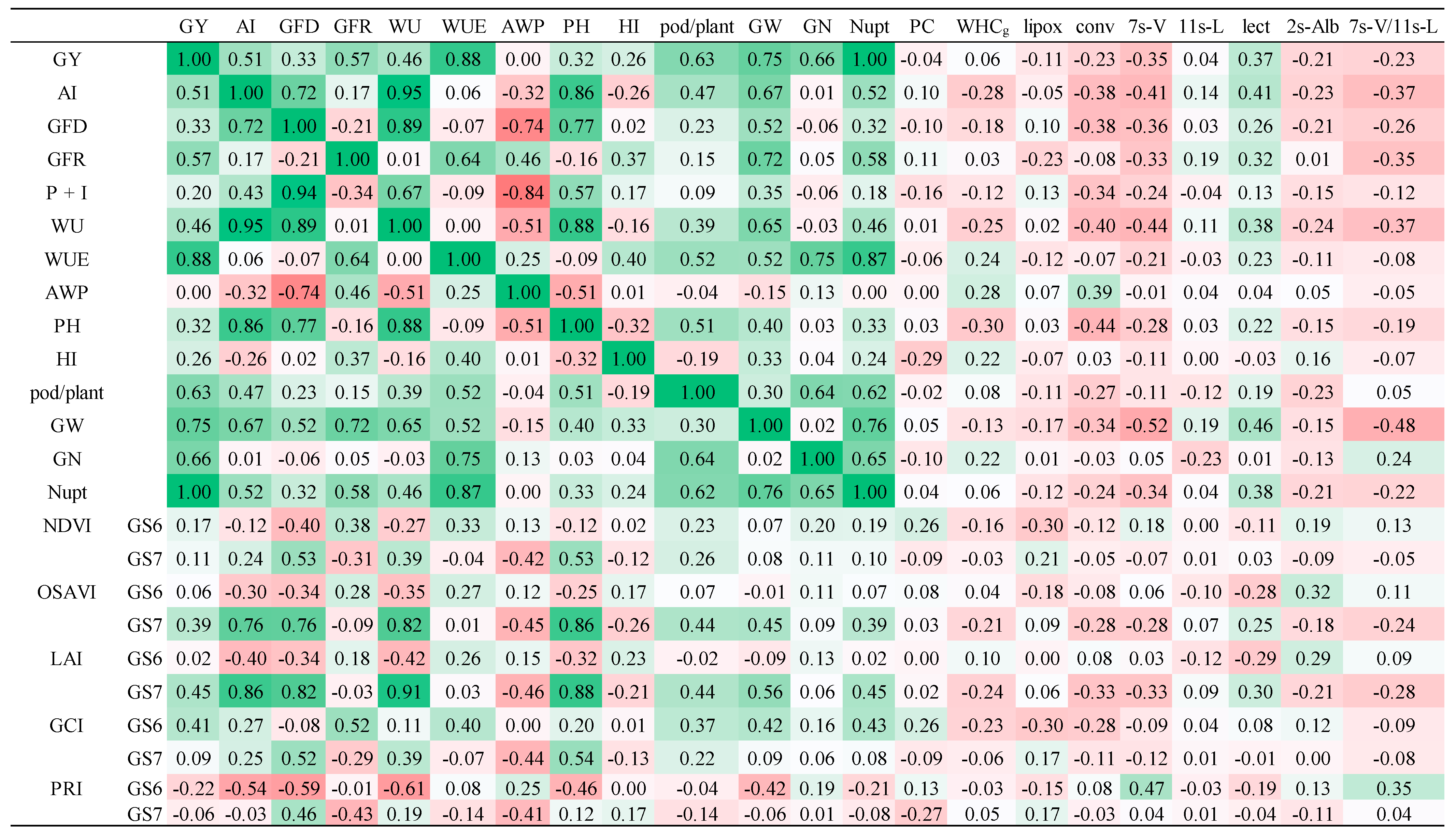
As for the role of soil water availability, the main effect was a positive relationship with grain filling duration and rate. The higher water use resulted in higher grain weight and then yield. In the absence of marked biotic stressful conditions, such as anthracnose, a positive relationship between rainfall distribution and biomass and yield traits is reported in the literature for chickpea under Mediterranean environments [
42], thus, influencing, in particular, leaf area index duration. In the current study, the significant correlation between estimated LAI and yield traits was markedly relevant for the number of pods per plant and grain weight. The influence of N supply led to a moderate advantage for chickpea growth, with increasing water use efficiency; by increasing the number of pods per plant, this had more of an effective for the large seed variety Pascià. On the other hand, grain weight, which was the most determinant yield component, was more affected by genotype and water supply than starter N fertilization. This result is in accordance with a study conducted on the chickpea genotype Sultano [
2], in which the authors observed that supplementary mineral N fertilization led to a non-significant increase in 100 seed weights.
The use of data from spectral phenotyping could be strategically useful for precision agriculture to achieve the sustainable goals of food quality and food safety promoted by climate-smart agriculture [
43].This has a great relevance for pulse crops, such as chickpea, that are strongly influenced by abiotic and biotic conditions [
44]. The outcomes of this study, in particular data from OSAVI and EVI-LAI, allowed us to describe crop water status during the key stages to determine final yields, such as flowering and grain filling. The former stage is highly determinant of seed numbers, while the latter is determinant for seed weight. This might be explained by the strong influence of water availability on crop growth rate and duration, especially for late sowing [
15]. Recently, a phenomic investigation on pulse crops, including chickpea, was carried out in the Pacific Northwest of the United States, to provide useful tools for plant breeders to accelerate breeding programs [
25]. The assessed VIs, including NDVI, correlated with agronomic traits, i.e., seed yield, flowering date, and days to physiological maturity (grain filling duration). The timing of VI measurement is fundamental in order to better predict final yield [
45]; in a study in which remote sensing was applied to chickpea subjected to biotic stress conditions, early NDVI observations produced weaker results than late ones for predicting final grain yield [
46]. The higher correlation observed with the use of the OSAVI with respect to NDVI is explained by the ability of this VI to correct the influence of soil background, and it is particularly suggested in a semi-arid area, especially for chickpea characterized by a lower soil coverage than other grasses [
47]. Furthermore, in a study conducted in India [
24], the authors reported observation data using satellite remote sensing (Landsat TM) to describe LAI variations by different VIs on wheat and chickpea; the regressions obtained were better for wheat than chickpea, and the best performances were observed in growing LAI phases; the authors also observed a better regression with the use of SAVI and RVI rather than NDVI for chickpea.
As for water use efficiency (WUE), the results of our observations are in a range reported on chickpea under a Mediterranean environment [
42]. The WUE parameters are reported to be related to crop growth length and days to maturity in chickpea [
48], and improved with irrigation only in the environment with the higher rainfall deficit; indeed, the values observed in terms of AWP were higher than that observed on common bean grown under comparable conditions and in one of the investigated environment [
38]. This result confirms the greater drought tolerance of chickpea [
14] with respect to other pulses, particularly valuable in the Mediterranean and semi-arid basins where water limitations are frequent. Furthermore, the relationship between water supply and crop production indicates the presence of a plateau value; further water supply seems to not further increase yield. Bellido et al. [
1] reported a quadratic relationship between seed yield and rainfall amount with a plateau around 400 mm, with excessive rainfall having a negative effect on chickpea. On the other hand, the higher water supply may be of interest for merceological quality, since larger seeds are generally appreciated by the market and consumers [
49]. In these terms, the significant relationship observed between VIs with grain filling rate may be useful for precision water management to achieve quality requirements. In fact, the amount of leaf chlorophyll (chl-a) estimated by GCI at flowering can influence photosynthetic rate and final dry matter accumulation in grain [
50].
Protein content in seeds is generally in the 19–24% range in chickpea [
7]; in the current study, a reduced variability was observed, in a range comparable to that observed in a set of genotypes that included Pascià and Sultano grown in South Italy [
51]. The limited variation observed confirms that protein content is a quite stable trait in chickpea, despite protein composition, which can be also influenced by environment and management [
9]. Most of the studies available on legume storage proteins are focused more on genetic diversity [
6,
7] than on agronomical factors [
52]. In a previous study conducted in a set of chickpea genotypes, including Pascià and Sultano, a strong impact of cropping systems was observed, especially for the ratio of 7s to 11s globulins [
9]. This ratio, in particular 7s vicilin, negatively correlated with grain yield; the same result was observed in the current study, confirming the association between agronomic traits and grain protein composition. The outcomes of this study underline the higher contribution of water supply rather than nitrogen on regulating the expression of the vicilin fraction. This result is of interest since the 50-kDa subunit of 7s vicilin has a putative role as an allergen [
53]. In these terms, a good agronomic management could promote an increase in yield and grain size together with a reduction in allergenic potential. Furthermore, the changes in lectin amount, negatively associated with rainfall and water use, are of particular interest because of its anti-nutritional properties [
54]. In addition, the higher lectin expression observed in the large-seed genotype Pascià with respect to Sultano is consistent with previous investigations on the same genetic material [
9]. Finally, since most of the legume storage globulins have enzymatic properties [
4], metabolic implications could be expected in relation to chickpea adaptability under contrasting environmental conditions.
5. Conclusions
The two investigated chickpea genotypes showed a differential response in terms of water use efficiency; in particular, irrigation treatment was efficient only in the low rainfall environment. Starter N fertilization, on the other hand, contributed to improving the response of different agronomic traits by improving water use efficiency, especially in combination with the irrigation supply. The hyperspectral phenotyping carried out gave interesting results, in particular in terms of individuating the optimal timing, grain filling, and vegetation indexes that best describe the physiological status of the two investigated chickpea genotypes. In particular, while GCI and PRI showed a good relationship with grain filling rate, OSAVI and EVI-LAI were better with grain yield, and also better than NDVI. These outcomes can be useful for the scientific community, field technicians, and farmers, since little information is actually available on chickpea grown in the Mediterranean basin. As for quality, reduced changes were observed in terms of protein content and grain water-holding capacity; instead, more variations were observed in terms of grain protein composition. In particular, a negative correlation with water supply and yield was observed for 7s vicilin, and a positive correlation was found with grain weight and lectin. Further proteomic investigations, also under different environments and on more genotypes, will be carried out to provide a deep insight into these effects on metabolic protein regulations. Furthermore, data from spectral phenotyping will be useful for digital farming applications, in order to assess crop physiological status in modern agricultural systems.