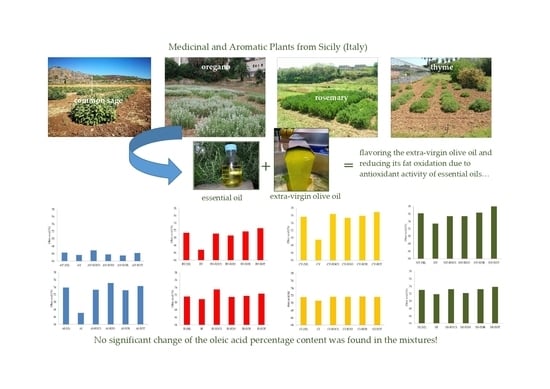
Flavouring Extra-Virgin Olive Oil with Aromatic and Medicinal Plants Essential Oils Stabilizes Oleic Acid Composition during Photo-Oxidative Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site of MAPs
2.2. Collection Fields of MAPs and Main Cultivation Practices
2.3. Morphological and Production Parameters
2.4. Chemicals
2.5. GC-MS Analyses of EOs
2.6. Photo-Oxidative Studies
2.7. Fatty Acid Determination before and after Photo-Oxidative Studies
2.8. Statistical Analysis
3. Results and Discussion
3.1. Agronomic Assessment of Common Sage, Oregano, Rosemary and Thyme Accessions
3.2. Chemical Composition of the MAPs EOs
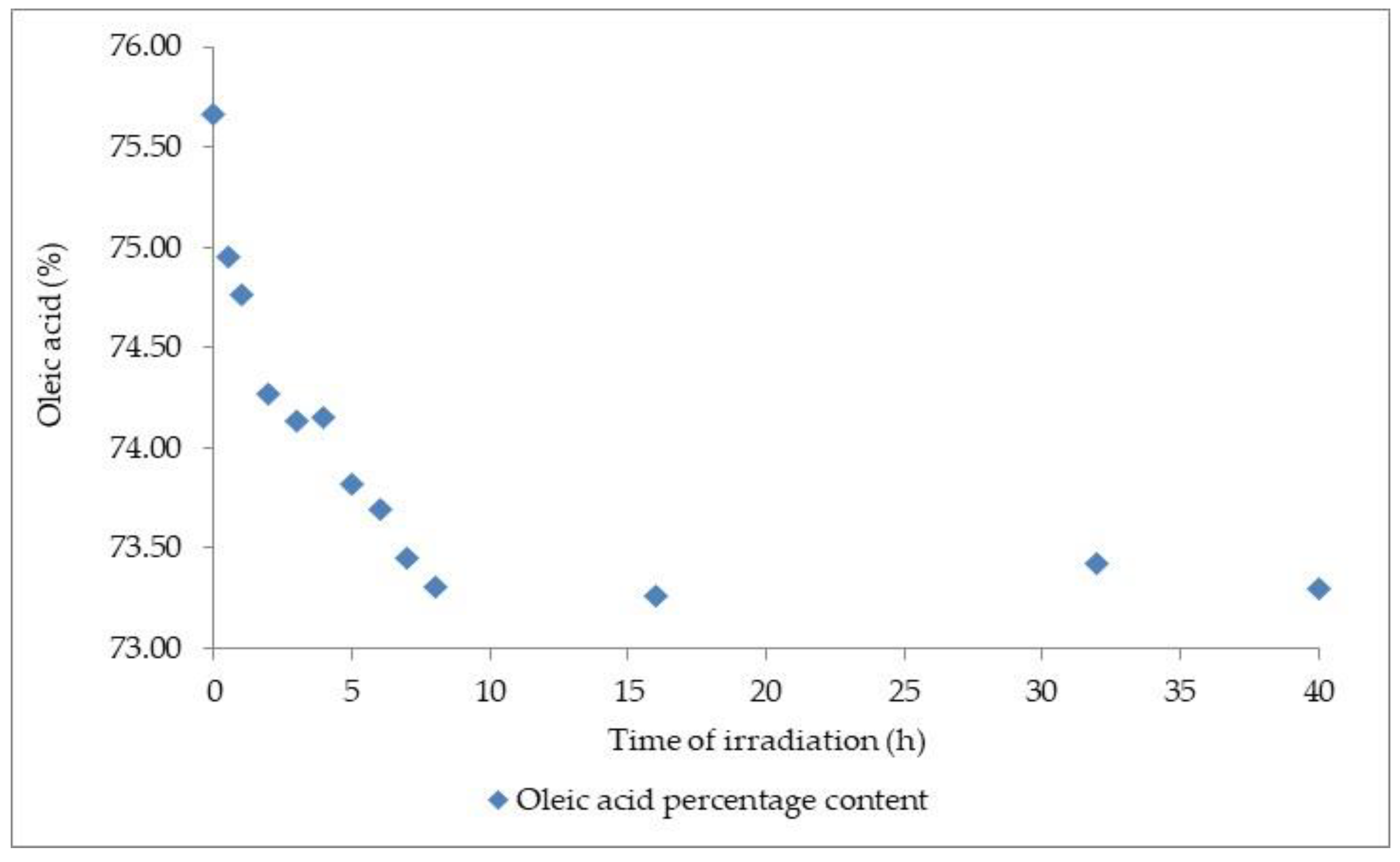
3.3. Photo-Oxidative Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caporaso, N.; Padano, A.; Nicoletti, G.; Sacchi, R. Capsaicinoids, antioxidant activity, and volatile compounds in olive oil flavoured with dried chilli pepper (Capsicum annuum). Eur. J. Lipid Sci. Technol. 2013, 10, 1434–1442. [Google Scholar] [CrossRef]
- Baiano, A.; Gambacorta, G.; La Notte, E. Aromatization of Olive Oil, 1st ed.; Transworld Research Network: Kerala, India, 2010; pp. 1–29. [Google Scholar]
- Harwood, J.L.; Yaqoob, P. Nutritional and health aspects of olive oil. Eur. J. Lipid Sci. Tech. 2002, 104, 685–697. [Google Scholar] [CrossRef]
- Vitaglione, P.; Savarese, M.; Paduano, A.; Scalfi, L.; Fogliano, V.; Sacchi, R. Healthy virgin olive oil: A matter of bitterness. Crit. Rev. Food Sci. Nutr. 2015, 55, 1808–1818. [Google Scholar] [CrossRef] [PubMed]
- Nieva-Echevarría, B.; Goicoechea, E.; Guillén, M.D. Oxidative stability of extra-virgin olive oil enriched or not with lycopene. Importance of the initial quality of the oil for its performance during in vitro gastrointestinal digestion. Food Res. Int. 2020, 130, 108987. [Google Scholar] [CrossRef] [PubMed]
- Clodoveo, M.L.; Dipalmo, T.; Crupi, P.; Durante, V.; Pesce, V.; Maiellaro, I.; Lovece, A.; Mercurio, A.; Laghezza, A.; Corbo, F.; et al. Comparison between different flavored olive oil production techniques: Healthy value and process efficiency. Plant Food Hum. Nutr. 2016, 71, 81–87. [Google Scholar] [CrossRef]
- Issaouia, M.; Flamini, G.; Souid, S.; Bendini, A.; Barbieri, S.; Gharbi, I.; Gallina Toschi, T.; Cioni, P.L.; Hammami, M. How the addition of spices and herbs to virgin olive oil to produce flavored oils affects consumer acceptance. Nat. Prod. Comm. 2016, 11, 775–780. [Google Scholar] [CrossRef]
- Caponio, F.; Durante, V.; Varva, G.; Silletti, R.; Previtali, M.a.; Viggiani, I.; Squeo, G.; Summo, C.; Pasqualone, A.; Gomes, T.; et al. Effect of infusion of spices into the oil vs. combined malaxation of olive paste and spices on quality of naturally flavoured virgin olive oils. Food Chem. 2016, 202, 221–228. [Google Scholar] [CrossRef]
- Miguel, M.G. Antioxidant activity of medicinal and aromatic plants. A review. Flavour Fragr. J. 2010, 25, 291–312. [Google Scholar] [CrossRef]
- Parham, S.; Khazari, A.Z.; Bakhsheshi-Rad, H.R.; Nur, H.; Ismail, A.F.; Sharif, S.; RamaKrishna, S.; Berto, F. Antioxidant, antimicrobial and antiviral properties of herbal materials. Antioxidants 2020, 9, 1309. [Google Scholar] [CrossRef]
- Mutlu-Ingok, A.; Devecioglu, D.; Nur Dikmetas, D.; Karbancioglu-Guler, F.; Capanoglu, E. Antibacterial, antifungal, antimycotoxigenic, and antioxidant activities of essential oils: An updated review. Molecules 2020, 25, 4711. [Google Scholar] [CrossRef]
- Asensio, C.M.; Nepote, V.; Grosso, N.R. Sensory attribute preservation in extra virgin olive oil with addition of oregano essential oil as natural antioxidant. J. Food Sci. 2012, 77, S294–S301. [Google Scholar] [CrossRef]
- Tuttolomondo, T.; Iapichino, G.; Licata, M.; Virga, G.; Leto, C.; La Bella, S. Agronomic evaluation and chemical characterization of Sicilian Salvia sclarea L. accessions. Agronomy 2020, 10, 1114. [Google Scholar] [CrossRef]
- Licata, M.; Tuttolomondo, T.; Dugo, G.; Ruberto, G.; Leto, C.; Napoli, E.M.; Rando, R.; Fede, M.R.; Virga, G.; Leone, R.; et al. Study of quantitative and qualitative variations in essential oils of Sicilian oregano biotypes. J. Essent. Oil Res. 2015, 27, 293–306. [Google Scholar] [CrossRef]
- Tuttolomondo, T.; Dugo, G.; Ruberto, G.; Leto, C.; Napoli, E.M.; Cicero, N.; Gervasi, T.; Virga, G.; Leone, R.; Licata, M.; et al. Study of quantitative and qualitative variations in essential oils of Sicilian Rosmarinus officinalis L. Nat. Prod. Res. 2015, 29, 1928–1934. [Google Scholar] [CrossRef] [PubMed]
- Tuttolomondo, T.; Dugo, G.; Leto, C.; Cicero, N.; Tropea, A.; Virga, G.; Leone, R.; Licata, M.; La Bella, S. Agronomical and chemical characterisation of Thymbra capitata (L.) Cav. biotypes from Sicily, Italy. Nat. Prod. Res. 2015, 29, 1289–1299. [Google Scholar] [CrossRef]
- La Bella, S.; Tuttolomondo, T.; Dugo, G.; Ruberto, G.; Leto, C.; Napoli, E.M.; Potortì, A.G.; Fede, M.R.; Virga, G.; Leone, R.; et al. Composition and variability of the essential oil of the flowers of Lavandula stoechas from various geographical sources. Nat. Prod. Comm. 2015, 10, 2001–2004. [Google Scholar] [CrossRef]
- Maggio, A.; Rosselli, S.; Bruno, M. Essential oils and pure volatile compounds as potential drugs in Alzheimer’s disease therapy: An updated review of the literature. Curr. Pharm. Design 2016, 22, 4011–4027. [Google Scholar] [CrossRef] [PubMed]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Yaseen, M.; Singh, M.; Ram, D.; Singh, K. Production potential, nitrogen use efficiency and economics of clary sage (Salvia sclarea L.) varieties as influenced by nitrogen levels under different locations. Ind. Crop. Prod. 2014, 54, 86–91. [Google Scholar] [CrossRef]
- Rostro-Alanis, M.J.; Báez-González, J.; Torres-Alvarez, C.; Parra-Saldívar, R.; Rodriguez-Rodriguez, J.; Castillo, S. Chemical composition and biological activities of oregano essential oil and its fractions obtained by vacuum distillation. Molecules 2019, 24, 1904. [Google Scholar] [CrossRef]
- Atti-Santos, A.C.; Rossato, M.; Pauletti, G.P.; Duarte Rota, L.; Rech, J.C.; Pansera, M.R.; Agostini, F.; Atti Serafini, L.; Moyna, P. Physico-chemical evaluation of Rosmarinus officinalis L. essential oils. Braz. Arch. Biol. Technol. 2005, 48, 1035–1039. [Google Scholar] [CrossRef]
- Barra, A. Factors affecting chemical variability of essential oils: A review of recent developments. Nat. Prod. Commun. 2009, 4, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Tsusaka, T.; Makino, B.; Ohsawa, R.; Ezura, H. Genetic and environmental factors influencing the contents of essential oil compounds in Atractylodes lancea. PLoS ONE 2019, 14, e0217522. [Google Scholar] [CrossRef]
- Bakha, M.; El Mtili, N.; Machon, N.; Aboukhalid, K.; Amchra, F.Z.; Khiraoui, A.; Gibernau, M.; Tomi, F.; Al Faiz, C. Intraspecific chemical variability of the essential oils of Moroccan endemic Origanum elongatum L. (Lamiaceae) from its whole natural habitats. Arab. J. Chem. 2020, 13, 3070–3081. [Google Scholar] [CrossRef]
- Moghaddam, M.; Farhadi, N. Influence of environmental and genetic factors on resin yield, essential oil content and chemical composition of Ferula assa-foetida L. populations. J. Appl. Res. Med. Aromat. Plants 2015, 2, 69–76. [Google Scholar] [CrossRef]
- Tuttolomondo, T.; Dugo, G.; Ruberto, G.; Leto, C.; Napoli, E.M.; Potortì, A.G.; Fede, M.R.; Virga, G.; Leone, R.; D’Anna, E.; et al. Agronomical evaluation of Sicilian biotypes of Lavandula stoechas L. spp. stoechas and analysis of the essential oils. J. Essent. Oil Res. 2015, 27, 115–124. [Google Scholar] [CrossRef]
- Yeddes, W.; Wannes, W.A.; Hammami, M.; Smida, M.; Chebbi, A.; Marzouk, B.; Tounsi, M.S. Effect of environmental conditions on the chemical composition and antioxidant activity of essential oils from Rosmarinus officinalis L. growing wild in Tunisia. J. Essent. Oil. Bear. Pl. 2018, 21, 972–986. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Mikallou, M.; Petropoulos, S.; Tzortzakis, N. Profiling of essential oils components and polyphenols for their antioxidant activity of medicinal and aromatic plants grown in different environmental conditions. Agronomy 2020, 10, 727. [Google Scholar] [CrossRef]
- Yavari, A.; Nazeri, V.; Sefidkon, F.; Hassani, M.E. Influence of some environmental factors on the essential oil variability of Thymus migricus. Nat. Prod. Commun. 2010, 5, 943–948. [Google Scholar] [CrossRef]
- Aboukhalid, K.; Al Faiz, C.; Douaik, A.; Bakha, M.; Kursa, K.; Agacka-Mołdoch, M.; Machon, N.; Tomi, F.; Lamiri, A. Influence of environmental factors on essential oil variability in Origanum compactum Benth. growing wild in Morocco. Chem. Biodivers. 2017, 14, e1700158. [Google Scholar] [CrossRef]
- Stefanaki, A.; Cook, C.M.; Lanaras, T.; Kokkini, S. The Oregano plants of Chios Island (Greece): Essential oils of Origanum onites L. growing wild in different habitats. Ind. Crop. Prod. 2016, 82, 107–113. [Google Scholar] [CrossRef]
- Kokkini, S.; Karousou, R.; Hanlidou, E.; Lanaras, T. Essential oil composition of Greek (Origanum vulgare ssp. hirtum) and Turkish (O. onites) oregano: A tool for their distinction. J. Essent. Oil Res. 2004, 16, 334–338. [Google Scholar]
- Zaouali, Y.; Messaoud, C.; Salah, A.B.; Boussaid, M. Oil composition variability among populations in relationship with their ecological areas in Tunisian Rosmarinus officinalis L. Flavour Frag. J. 2005, 20, 512–520. [Google Scholar] [CrossRef]
- Bakhy, K.; Benlhabib, O.; Al Faiz, C.; Bighelli, A.; Casanova, J.; Tomi, F. Wild Thymbra capitata from Western Rif (Morocco): Essential oil composition, chemical homogeneity and yield variability. Nat. Prod. Commun. 2013, 8, 1155–1158. [Google Scholar] [CrossRef] [PubMed]
- Couladis, M.; Tzakou, O.; Mimica-Dukíc, N.; Jančić, R.; Stojanović, D. Essential oil of Salvia officinalis L. from Serbia and Montenegro. Flavour Frag. J. 2002, 17, 119–126. [Google Scholar] [CrossRef]
- Arraiza, M.P.; Arrabal, C.; Lopez, J.V. Seasonal variation of essential oil yield and composition of sage (Salvia officinalis L.) grown in Castilla—La Mancha (Central Spain). Not. Bot. Horti. Agrobo. 2012, 40, 106–108. [Google Scholar] [CrossRef]
- Angerosa, F. Influence of volatile compounds on virgin oli.ve oil quality evaluated by analytical approaches and sensor panels. Eur. J. Lipid Sci. Tech. 2002, 104, 639–660. [Google Scholar] [CrossRef]


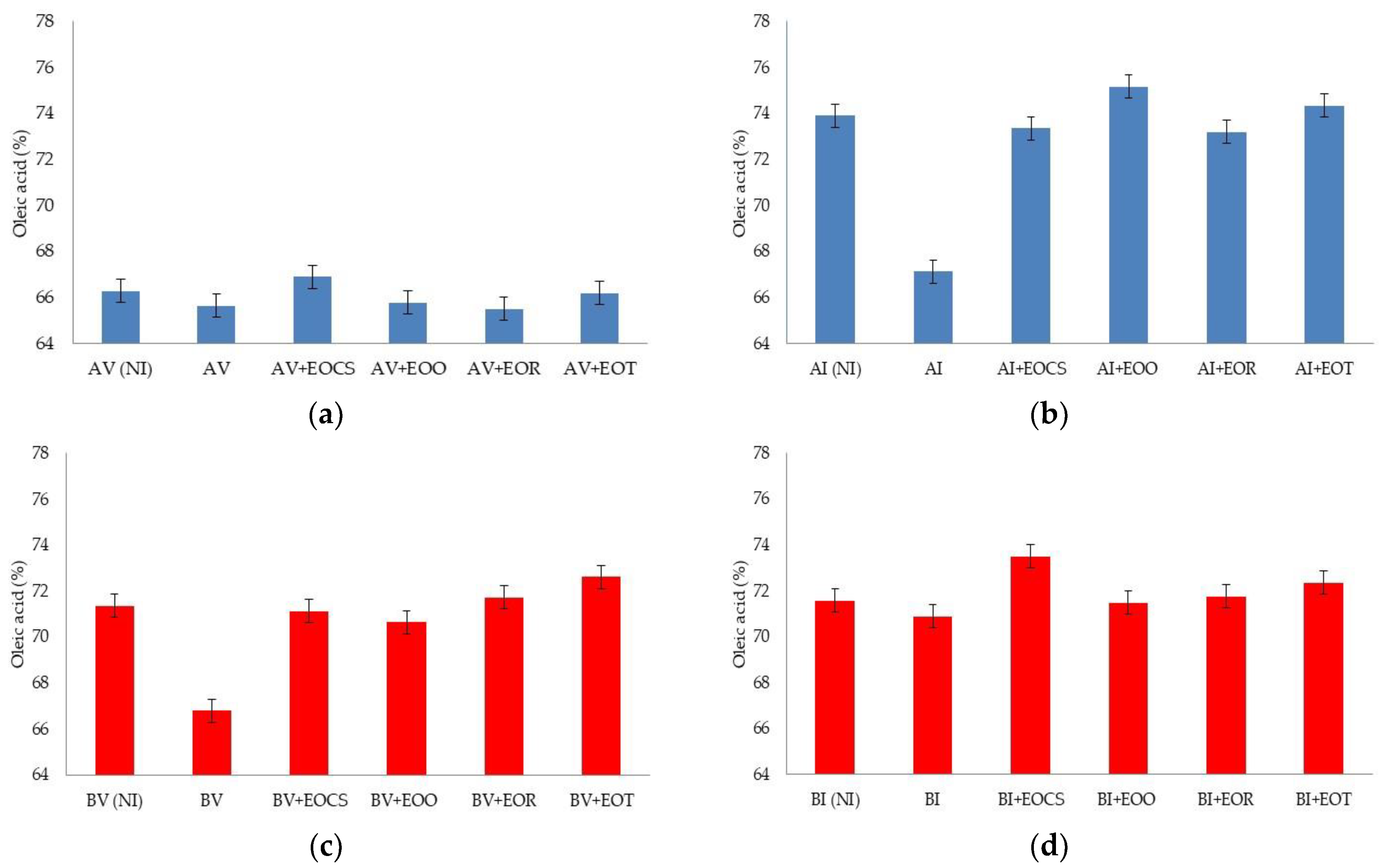
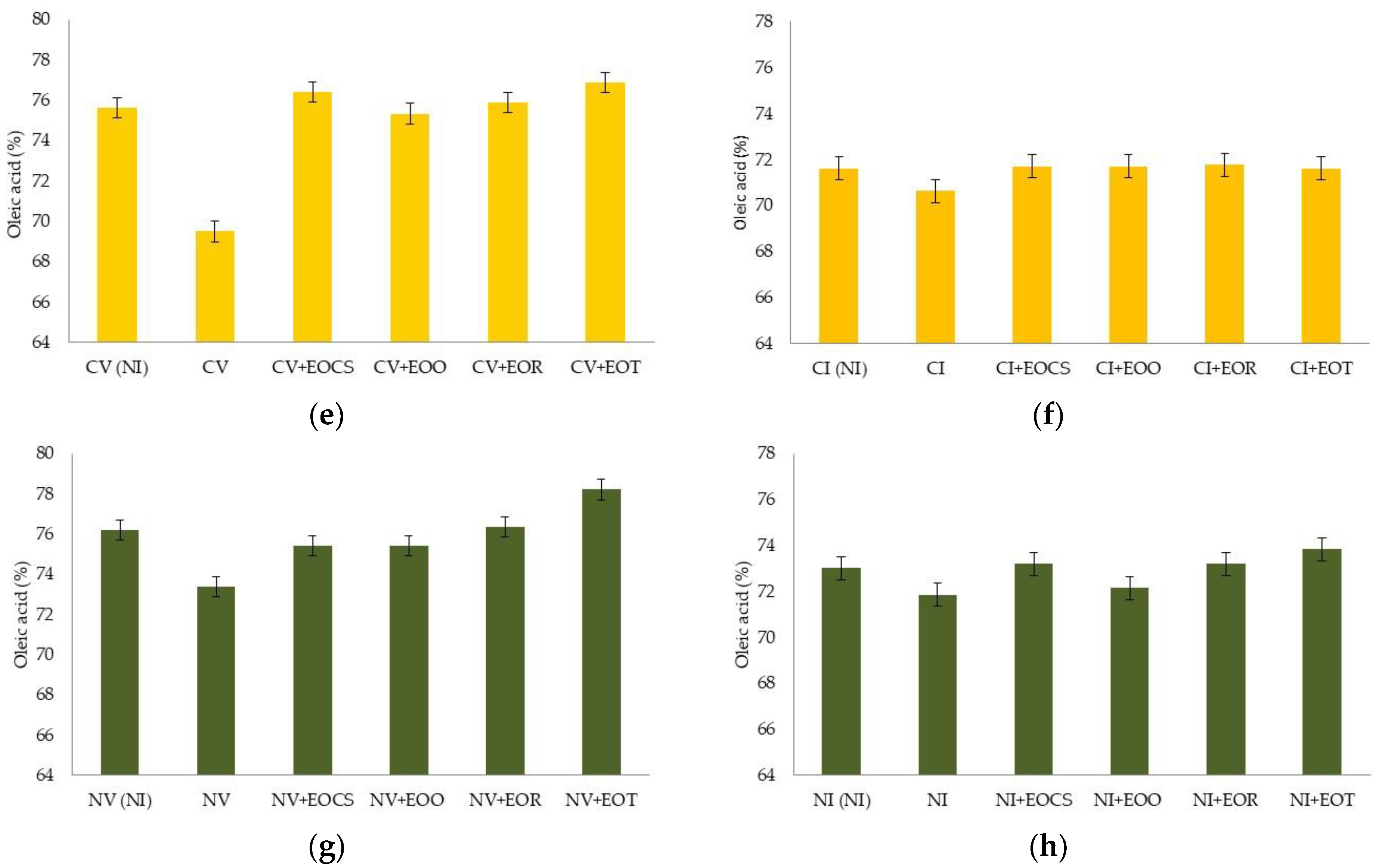
| Variety of Olive | Preveraison Stage (Green Fruit) | Veraison Stage (Brown Fruit) |
|---|---|---|
| Arbequina | AV | AI |
| Biancolilla | BV | BI |
| Cerasuola | CV | CI |
| Nocellara del Belice | NV | NI |
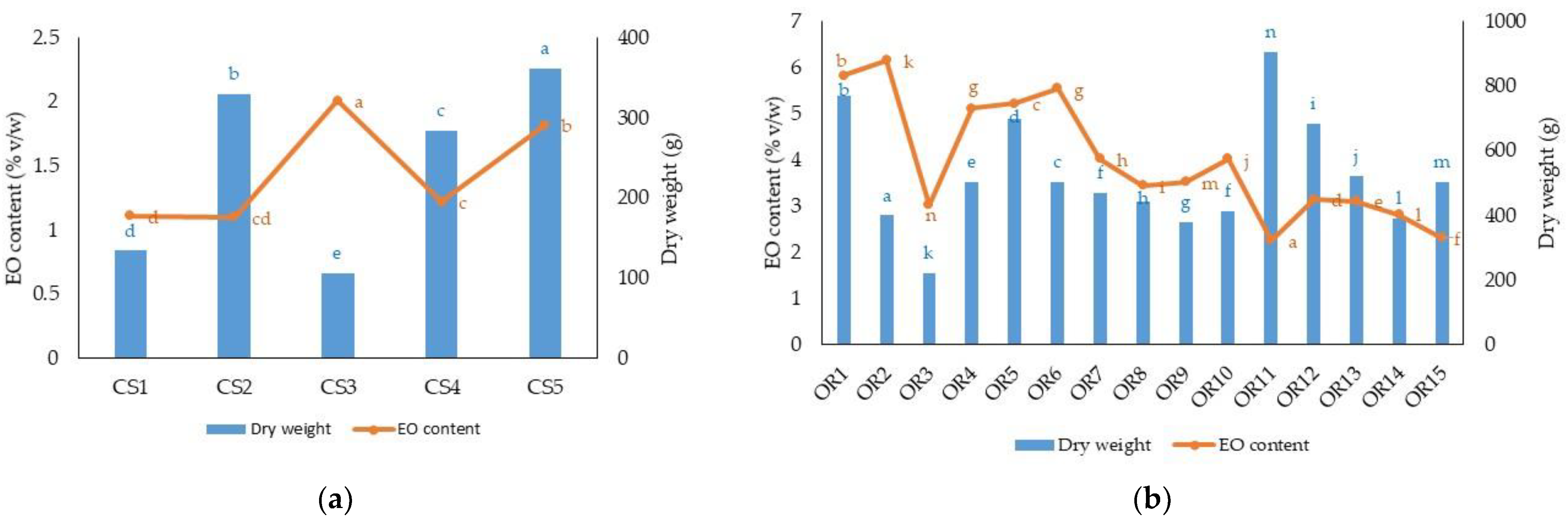
| Species | Dry Weight (g plant−1) | Dry Matter Yield (kg ha−1) | EO Content (% v/w) | EO Yield (kg ha−1) |
|---|---|---|---|---|
| Common sage | ||||
| CS1 | 134.06 d | 671.33 d | 1.08 d | 6.35 e |
| CS2 | 329.53 b | 1645.10 b | 1.10 cd | 16.32 b |
| CS3 | 105.53 e | 529.07 e | 2.04 a | 9.47 d |
| CS4 | 284.27 b | 1423.60 c | 1.20 c | 15.43 c |
| CS5 | 361.30 a | 1810.73 a | 1.80 b | 29.55 a |
| Oregano | ||||
| OR1 | 770.13 b | 5139.44 b | 5.82 b | 268.51 a |
| OR2 | 399.60 k | 2666.30 j | 6.15 a | 147.12 e |
| OR3 | 221.73 n | 1478.53 m | 3.01 k | 36.74 n |
| OR4 | 504.03 g | 3360.72 f | 5.11 e | 154.72 d |
| OR5 | 698.12 c | 4652.69 c | 5.12 d | 218.52 b |
| OR6 | 503.93 g | 3358.79 f | 5.55 c | 167.85 c |
| OR7 | 468.80 h | 3125.88 g | 4.02 f | 113.12 h |
| OR8 | 441.77 i | 2952.40 h | 3.45 h | 91.68 j |
| OR9 | 379.93 m | 2529.14 l | 3.52 g | 80.14 k |
| OR10 | 413.20 j | 2754.71 i | 4.01 f | 99.41 i |
| OR11 | 905.80 a | 6039.59 a | 2.26 n | 122.33 g |
| OR12 | 684.12 d | 4562.01 d | 3.15 i | 129.18 f |
| OR13 | 521.50 e | 3476.67 e | 3.11 j | 97.30 i |
| OR14 | 390.70 l | 2604.79 k | 2.82 l | 65.92 m |
| OR15 | 503.07 f | 3364.21 f | 2.32 m | 70.51 l |
| Rosemary | ||||
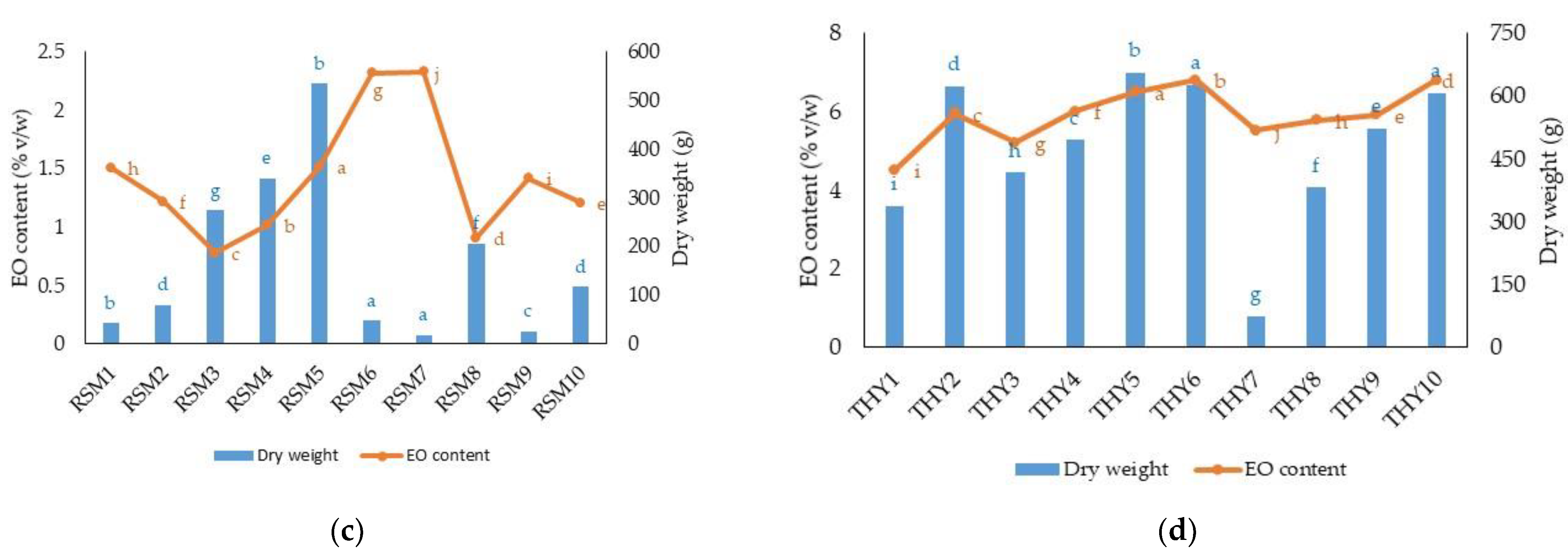
| RSM1 | 42.20 h | 211.33 h | 1.51 b | 2.62 h |
| RSM2 | 79.23 f | 396.51 f | 1.21 d | 4.20 g |
| RSM3 | 274.53 c | 1376.77 c | 0.78 g | 9.65 c |
| RSM4 | 339.20 b | 1709.11 b | 1.01 e | 15.37 b |
| RSM5 | 534.76 a | 2684.33 a | 1.52 b | 36.61 a |
| RSM6 | 47.67 g | 240.07 g | 2.32 a | 5.04 f |
| RSM7 | 18.53 j | 93.52 j | 2.31 a | 2.03 i |
| RSM8 | 205.63 d | 1034.81 d | 0.91 f | 8.42 d |
| RSM9 | 26.20 i | 131.61 i | 1.42 c | 1.66 i |
| RSM 10 | 117.23 e | 586.43 e | 1.21 d | 6.29 e |
| Thyme | ||||
| THY1 | 337.67 i | 2252.70 i | 4.51 i | 91.28 h |
| THY2 | 624.50 c | 4174.77 c | 5.96 d | 223.94 c |
| THY3 | 416.67 g | 2777.27 g | 5.20 h | 130.26 g |
| THY4 | 494.60 f | 3294.80 f | 6.02 c | 178.45 e |
| THY5 | 656.17 a | 4374.20 a | 6.52 b | 256.57 a |
| THY6 | 626.17 b | 4177.20 b | 6.80 a | 256.51 a |
| THY7 | 75.17 j | 501.73 j | 5.52 g | 25.58 i |
| THY8 | 381.30 h | 2541.93 h | 5.78 f | 133.24 f |
| THY9 | 522.60 e | 3486.93 e | 5.90 e | 185.94 d |
| THY10 | 607.20 d | 4048.20 d | 6.80 a | 249.13 b |
| RT a | KI b | Compound | Common Sage | Oregano | Rosemary | Thyme |
|---|---|---|---|---|---|---|
| Monoterpene Hydrocarbons | 27.73 | 31.31 | 31.47 | 28.86 | ||
| 10.22 | 938 | α thujene | 0.37 | 0.79 | 0.11 | 0.91 |
| 10.60 | 944 | α pinene | 4.93 | 0.90 | 11.25 | 1.04 |
| 11.59 | 957 | camphene | 6.88 | t | 6.88 | 0.41 |
| 11.92 | 961 | thuja-2.4(10)-diene | 0.32 | |||
| 13.10 | 974 | verbenene | 0.16 | |||
| 13.23 | 975 | sabinene | 0.39 | 0.16 | 0.05 | |
| 13.46 | 978 | ß pinene | 8.39 | 0.01 | 3.53 | 0.16 |
| 14.61 | 990 | ß-myrcene | 3.83 | 2.24 | 1.93 | 2.36 |
| 15.39 | 997 | Mentha-1(7),8-diene | 0.04 | |||
| 15.56 | 999 | phellandrene α | 0.04 | 0.36 | 0.28 | 0.38 |
| 15.66 | 1000 | δ-3-carene | 0.11 | 2.64 | ||
| 16.34 | 1012 | terpinene α | 0.23 | 3.42 | 0.35 | 2.87 |
| 16.60 | 1017 | ρ-cymene | 0.01 | 0.03 | ||
| 16.89 | 1022 | o-cymene | 0.22 | 9.70 | 3.09 | 8.73 |
| 17.24 | 1028 | sylvestrene | 1.65 | 0.76 | 0.75 | |
| 17.99 | 1042 | (Z)-ß-ocimene | 0.15 | 0.02 | ||
| 18.70 | 1053 | (E)-ß-ocimene | 0.02 | 0.10 | 0.05 | |
| 19.37 | 1064 | terpinene γ | 0.43 | 12.59 | 0.32 | 10.98 |
| 21.32 | 1094 | Mentha-2.4(8)-diene | 0.20 | 0.13 | 0.40 | 0.22 |
| 21.70 | 1099 | Cymenene | 0.09 | |||
| Oxygenated Monoterpenes | 51.20 | 64.06 | 61.87 | 65.00 | ||
| 17.37 | 1031 | eucalyptol | 20.58 | 0.06 | 39.38 | |
| 20.21 | 1077 | sabinene hydrate cis | 0.14 | 0.64 | 0.43 | |
| 22.43 | 1109 | Sabinene hydrate <trans-> | 0.10 | 0.12 | ||
| 22.51 | 1110 | linalool | 0.10 | |||
| 22.57 | 1111 | Pinene oxide<α-> | 0.09 | 0.36 | 2.27 | 0.22 |
| 22.79 | 1114 | thujone<cis-> | 8.25 | 0.02 | ||
| 23.62 | 1125 | thujone<trans-> | 5.78 | |||
| 23.68 | 1126 | fenchol <exo> | 0.05 | |||
| 24.29 | 1134 | Campholenal α | 0.03 | |||
| 25.14 | 1144 | Pinocarveol trans | 0.30 | |||
| 25.52 | 1149 | camphor | 13.77 | 4.81 | ||
| 26.29 | 1158 | Eucarvone | 0.09 | |||
| 26.55 | 1161 | pinocamphone trans | 0.16 | |||
| 26.69 | 1163 | pinocarvone | 0.60 | |||
| 27.29 | 1170 | isocitral<(Z)-> | 0.10 | |||
| 27.42 | 1171 | Borneol | 0.80 | 0.07 | 10.40 | 1.00 |
| 27.65 | 1174 | pinocamphone cis | 0.50 | |||
| 28.08 | 1178 | terpinen-4-ol | 0.27 | 0.32 | 0.46 | 0.61 |
| 29.18 | 1190 | α terpineol | 0.09 | 0.02 | 0.78 | |
| 29.31 | 1192 | dihydro carvone cis | 0.06 | |||
| 29.91 | 1198 | verbenone | 0.26 | |||
| 31.68 | 1228 | thymol methyl ether | 0.24 | |||
| 32.27 | 1238 | carvacrol methyl ether | 3.70 | 0.17 | ||
| 32.68 | 1245 | carvone | 0.17 | |||
| 35.29 | 1287 | Isobornyl acetate | 0.04 | 1.43 | ||
| 35.54 | 1291 | thymol | 1.09 | 58.40 | 0.23 | |
| 36.56 | 1307 | carvacrol | 0.11 | 62.35 | ||
| 39.42 | 1348 | thymol acetate | 0.03 | |||
| Sesquiterpene Hydrocarbons | 18.17 | 1.17 | 5.30 | 4.13 | ||
| 38.42 | 1334 | elemene<δ> | 0.03 | |||
| 40.80 | 1367 | ylangene α | 0.24 | 0.05 | ||
| 41.15 | 1372 | copaene α | 0.06 | |||
| 41.64 | 1379 | Bourbonene ß | 0.04 | |||
| 43.16 | 1398 | Longipinene<ß> | 1.40 | |||
| 43.34 | 1401 | Longifolene | 0.09 | |||
| 43.83 | 1409 | caryophyllene (Z) | 6.08 | 0.72 | 4.91 | 3.93 |
| 44.47 | 1419 | caryophyllene (E) | 0.31 | 0.02 | ||
| 44.93 | 1427 | Aromadendrene | 3.98 | |||
| 45.52 | 1436 | Barbatene<ß> | 0.35 | |||
| 46.05 | 1444 | caryophyllene α | 2.88 | 0.02 | 0.37 | 0.07 |
| 46.38 | 1449 | Muurola-3-5-diene cis | 0.30 | |||
| 47.21 | 1462 | Unknown | 0.03 | |||
| 47.42 | 1465 | Cadina-1(6).4-diene cis | 0.09 | 0.08 | ||
| 47.68 | 1469 | Muurola-4(14).5-diene | 0.07 | |||
| 48.16 | 1476 | Aristolochene<4.5-di-epi-> | 0.20 | |||
| 48.39 | 1480 | Muurolene (γ) | 0.84 | |||
| 48.55 | 1482 | bicyclogermacrene | 1.06 | |||
| 49.70 | 1499 | amorphene γ | 0.03 | 0.15 | 0.13 | |
| 50.02 | 1503 | amorphene δ | 0.09 | 0.15 | ||
| Other | 0.26 | 0.00 | 0.67 | 0.65 | ||
| 14.14 | 985 | octen-3-ol | 0.20 | t | 0.52 | 0.58 |
| 14.40 | 988 | octanone | 0.06 | t | 0.15 | |
| 15.36 | 997 | octan-3-ol | 0.07 | |||
| Total | 97.36 | 96.54 | 99.31 | 98.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barreca, S.; La Bella, S.; Maggio, A.; Licata, M.; Buscemi, S.; Leto, C.; Pace, A.; Tuttolomondo, T. Flavouring Extra-Virgin Olive Oil with Aromatic and Medicinal Plants Essential Oils Stabilizes Oleic Acid Composition during Photo-Oxidative Stress. Agriculture 2021, 11, 266. https://doi.org/10.3390/agriculture11030266
Barreca S, La Bella S, Maggio A, Licata M, Buscemi S, Leto C, Pace A, Tuttolomondo T. Flavouring Extra-Virgin Olive Oil with Aromatic and Medicinal Plants Essential Oils Stabilizes Oleic Acid Composition during Photo-Oxidative Stress. Agriculture. 2021; 11(3):266. https://doi.org/10.3390/agriculture11030266
Chicago/Turabian StyleBarreca, Salvatore, Salvatore La Bella, Antonella Maggio, Mario Licata, Silvestre Buscemi, Claudio Leto, Andrea Pace, and Teresa Tuttolomondo. 2021. "Flavouring Extra-Virgin Olive Oil with Aromatic and Medicinal Plants Essential Oils Stabilizes Oleic Acid Composition during Photo-Oxidative Stress" Agriculture 11, no. 3: 266. https://doi.org/10.3390/agriculture11030266
APA StyleBarreca, S., La Bella, S., Maggio, A., Licata, M., Buscemi, S., Leto, C., Pace, A., & Tuttolomondo, T. (2021). Flavouring Extra-Virgin Olive Oil with Aromatic and Medicinal Plants Essential Oils Stabilizes Oleic Acid Composition during Photo-Oxidative Stress. Agriculture, 11(3), 266. https://doi.org/10.3390/agriculture11030266