Serum Metabolomic Profiling of Patients with Non-Infectious Uveitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Diagnosis
2.2. Serum Sample Collection
2.3. Sample Processing
2.4. LC-MS Systems
2.5. Data Processing
2.6. Statistical Analysis
3. Results
3.1. Summary of Quantified Metabolic Profiles
3.2. Discrimination Abilities of Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Durrani, O.M.; Tehrani, N.N.; Marr, J.E.; Moradi, P.; Stavrou, P.; Murray, P.I. Degree, duration, and causes of visual loss in uveitis. Br. J. Ophthalmol. 2004, 88, 1159–1162. [Google Scholar] [CrossRef] [PubMed]
- Dandona, L.; Dandona, R.; John, R.K.; McCarty, C.A.; Rao, G.N. Population based assessment of uveitis in an urban population in southern India. Br. J. Ophthalmol. 2000, 84, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Kunimi, K.; Usui, Y.; Tsubota, K.; Mitsuhashi, R.; Umazume, A.; Kezuka, T.; Sakai, J.; Goto, H. Changes in Etiology of Uveitis in a Single Center in Japan. Ocul. Immunol. Inflamm. 2020, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Goto, H.; Mochizuki, M.; Yamaki, K.; Kotake, S.; Usui, M.; Ohno, S. Epidemiological survey of intraocular inflammation in Japan. Jpn. J. Ophthalmol. 2007, 51, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Nashtaei, E.M.; Soheilian, M.; Herbort, C.P.; Yaseri, M. Patterns of uveitis in the middle East and Europe. J. Ophthalmic. Vis. Res. 2011, 6, 233–240. [Google Scholar] [PubMed]
- Miserocchi, E.; Fogliato, G.; Modorati, G.; Bandello, F. Review on the worldwide epidemiology of uveitis. Eur. J. Ophthalmol. 2013, 23, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Tong, B.; Liu, X.; Xiao, J.; Su, G. Immunopathogenesis of Behcet’s Disease. Front. Immunol. 2019, 10, 665. [Google Scholar] [CrossRef]
- Ziegenhagen, M.W.; Muller-Quernheim, J. The cytokine network in sarcoidosis and its clinical relevance. J. Intern. Med. 2003, 253, 18–30. [Google Scholar] [CrossRef]
- Sakthivel, P.; Bruder, D. Mechanism of granuloma formation in sarcoidosis. Curr. Opin. Hematol. 2017, 24, 59–65. [Google Scholar] [CrossRef]
- Liang, L.; Peng, X.Y.; Wang, H. Th lymphocyte subsets in patients with Vogt-Koyanagi-Harada disease. Int. J. Ophthalmol. 2019, 12, 207–211. [Google Scholar]
- Wu, L.; Wen, H.; Zhou, Y.; Yu, H.; Liu, Y.; Bai, L.; Kijlstra, A.; Yang, P. Activation of the liver X receptor inhibits Th17 and Th1 responses in Behcet’s disease and Vogt-Koyanagi-Harada disease. Curr. Mol. Med. 2014, 14, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Gundlach, E.; Hoffmann, M.M.; Prasse, A.; Heinzelmann, S.; Ness, T. Interleukin-2 Receptor and Angiotensin-Converting Enzyme as Markers for Ocular Sarcoidosis. PLoS ONE 2016, 11, e0147258. [Google Scholar] [CrossRef] [PubMed]
- Groen-Hakan, F.; Eurelings, L.; ten Berge, J.C.; van Laar, J.; Ramakers, C.R.B.; Dik, W.A.; Rothova, A. Diagnostic Value of Serum-Soluble Interleukin 2 Receptor Levels vs Angiotensin-Converting Enzyme in Patients With Sarcoidosis-Associated Uveitis. JAMA Ophthalmol. 2017, 135, 1352–1358. [Google Scholar] [CrossRef] [PubMed]
- Kirino, Y.; Ideguchi, H.; Takeno, M.; Suda, A.; Higashitani, K.; Kunishita, Y.; Takase-Minegishi, K.; Tamura, M.; Watanabe, T.; Asami, Y.; et al. Continuous evolution of clinical phenotype in 578 Japanese patients with Behcet’s disease: A retrospective observational study. Arthritis Res. Ther. 2016, 18, 217. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, M.; Takami, K.; Kitaichi, N.; Namba, K.; Kitamei, H.; Kotake, S.; Ohno, S. Comparative study of two sets of criteria for the diagnosis of Vogt-Koyanagi-Harada’s disease. Am. J. Ophthalmol. 2005, 139, 1080–1085. [Google Scholar] [CrossRef]
- Barbosa-Breda, J.; Himmelreich, U.; Ghesquiere, B.; Rocha-Sousa, A.; Stalmans, I. Clinical Metabolomics and Glaucoma. Ophthalmic. Res. 2018, 59, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Young, S.P.; Nessim, M.; Falciani, F.; Trevino, V.; Banerjee, S.P.; Scott, R.A.; Murray, P.I.; Wallace, G.R. Metabolomic analysis of human vitreous humor differentiates ocular inflammatory disease. Mol. Vis. 2009, 15, 1210–1217. [Google Scholar]
- Ahn, J.K.; Kim, J.; Hwang, J.; Song, J.; Kim, K.H.; Cha, H.S. Urinary Metabolomic Profiling to Identify Potential Biomarkers for the Diagnosis of Behcet’s Disease by Gas Chromatography/Time-of-Flight-Mass Spectrometry. Int. J. Mol. Sci. 2017, 18, 2309. [Google Scholar] [CrossRef]
- Geamanu, A.; Gupta, S.V.; Bauerfeld, C.; Samavati, L. Metabolomics connects aberrant bioenergetic, transmethylation, and gut microbiota in sarcoidosis. Metabolomics 2016, 12. [Google Scholar] [CrossRef]
- Chen, L.; Chang, R.; Pan, S.; Xu, J.; Cao, Q.; Su, G.; Zhou, C.; Kijlstra, A.; Yang, P. Plasma metabolomics study of Vogt-Koyanagi-Harada disease identifies potential diagnostic biomarkers. Exp. Eye Res. 2020, 196, 108070. [Google Scholar] [CrossRef]
- Health Science Study: Research on Behcet’s Disease, Final Report for 2002. In Diagnostic Criteria of Behcet’s Disease; Ministry of Health, Labour and Welfare Designated Disease Study Group: Japanese Ministry of Health, Labour and Welfare, Tokyo, Japan, 2003; pp. 11–13.
- Mochizuki, M.; Smith, J.R.; Takase, H.; Kaburaki, T.; Acharya, N.R.; Rao, N.A.; International Workshop on Ocular Sarcoidosis Study Group. Revised criteria of International Workshop on Ocular Sarcoidosis (IWOS) for the diagnosis of ocular sarcoidosis. Br. J. Ophthalmol. 2019, 103, 1418–1422. [Google Scholar] [CrossRef] [PubMed]
- Read, R.W.; Holland, G.N.; Rao, N.A.; Tabbara, K.F.; Ohno, S.; Arellanes-Garcia, L.; Pivetti-Pezzi, P.; Tessler, H.H.; Usui, M. Revised diagnostic criteria for Vogt-Koyanagi-Harada disease: Report of an international committee on nomenclature. Am. J. Ophthalmol. 2001, 131, 647–652. [Google Scholar] [CrossRef]
- Fuse, S.; Sugimoto, M.; Kurosawa, Y.; Kuroiwa, M.; Aita, Y.; Tomita, A.; Yamaguchi, E.; Tanaka, R.; Endo, T.; Kime, R.; et al. Relationships between plasma lipidomic profiles and brown adipose tissue density in humans. Int. J. Obes. 2020, 44, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Tomita, A.; Mori, M.; Hiwatari, K.; Yamaguchi, E.; Itoi, T.; Sunamura, M.; Soga, T.; Tomita, M.; Sugimoto, M. Effect of storage conditions on salivary polyamines quantified via liquid chromatography-mass spectrometry. Sci. Rep. 2018, 8, 12075. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Roszik, J.; Grimm, E.A.; Ekmekcioglu, S. Impact of l-Arginine Metabolism on Immune Response and Anticancer Immunotherapy. Front. Oncol. 2018, 8, 67. [Google Scholar] [CrossRef] [PubMed]
- Kedia-Mehta, N.; Finlay, D.K. Competition for nutrients and its role in controlling immune responses. Nat. Commun. 2019, 10, 2123. [Google Scholar] [CrossRef]
- Geiger, R.; Rieckmann, J.C.; Wolf, T.; Basso, C.; Feng, Y.; Fuhrer, T.; Kogadeeva, M.; Picotti, P.; Meissner, F.; Mann, M.; et al. L-Arginine Modulates T Cell Metabolism and Enhances Survival and Anti-tumor Activity. Cell 2016, 167, 829–842. [Google Scholar] [CrossRef]
- Pearce, E.L.; Pearce, E.J. Metabolic pathways in immune cell activation and quiescence. Immunity 2013, 38, 633–643. [Google Scholar] [CrossRef]
- Ganeshan, K.; Chawla, A. Metabolic regulation of immune responses. Annu Rev. Immunol. 2014, 32, 609–634. [Google Scholar] [CrossRef]
- Buck, M.D.; Sowell, R.T.; Kaech, S.M.; Pearce, E.L. Metabolic Instruction of Immunity. Cell 2017, 169, 570–586. [Google Scholar] [CrossRef]
- Goto, H.; Usui, Y.; Umazume, A.; Uchida, K.; Eishi, Y. Propionibacterium acnes as a possible pathogen of granuloma in patients with ocular sarcoidosis. Br. J. Ophthalmol. 2017, 101, 1510–1513. [Google Scholar] [CrossRef] [PubMed]
- Kambouchner, M.; Pirici, D.; Uhl, J.F.; Mogoanta, L.; Valeyre, D.; Bernaudin, J.F. Lymphatic and blood microvasculature organisation in pulmonary sarcoid granulomas. Eur. Respir. J. 2011, 37, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, D.A., Jr.; Cummins, C.S. Nutritional requirements of anaerobic coryneforms. J. Bacteriol. 1978, 135, 858–867. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.K.; Kim, S.; Kim, J.; Hwang, J.; Kim, K.H.; Cha, H.S. A Comparative Metabolomic Evaluation of Behcet’s Disease with Arthritis and Seronegative Arthritis Using Synovial Fluid. PLoS ONE 2015, 10, e0135856. [Google Scholar] [CrossRef]
- Bonanno, G.; Raiteri, L.; Milanese, M.; Zappettini, S.; Melloni, E.; Pedrazzi, M.; Passalacqua, M.; Tacchetti, C.; Usai, C.; Sparatore, B. The high-mobility group box 1 cytokine induces transporter-mediated release of glutamate from glial subcellular particles (gliosomes) prepared from in situ-matured astrocytes. Int. Rev. Neurobiol. 2007, 82, 73–93. [Google Scholar]
- Ahn, J.K.; Cha, H.S.; Bae, E.K.; Lee, J.; Koh, E.M. Extracellular high-mobility group box 1 is increased in patients with Behcet’s disease with intestinal involvement. J. Korean. Med. Sci. 2011, 26, 697–700. [Google Scholar] [CrossRef]
- Ungerstedt, J.S.; Blomback, M.; Soderstrom, T. Nicotinamide is a potent inhibitor of proinflammatory cytokines. Clin. Exp. Immunol. 2003, 131, 48–52. [Google Scholar] [CrossRef]
- Niren, N.M. Pharmacologic doses of nicotinamide in the treatment of inflammatory skin conditions: A review. Cutis 2006, 77, 11–16. [Google Scholar]
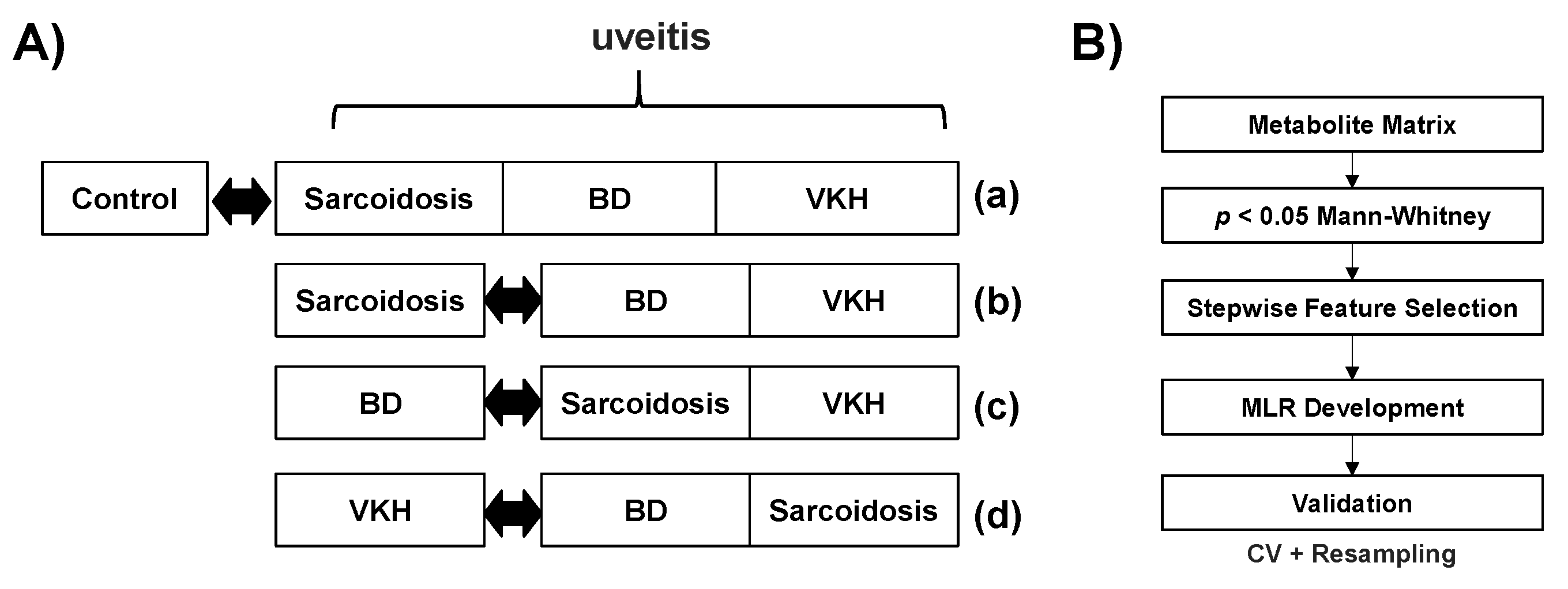
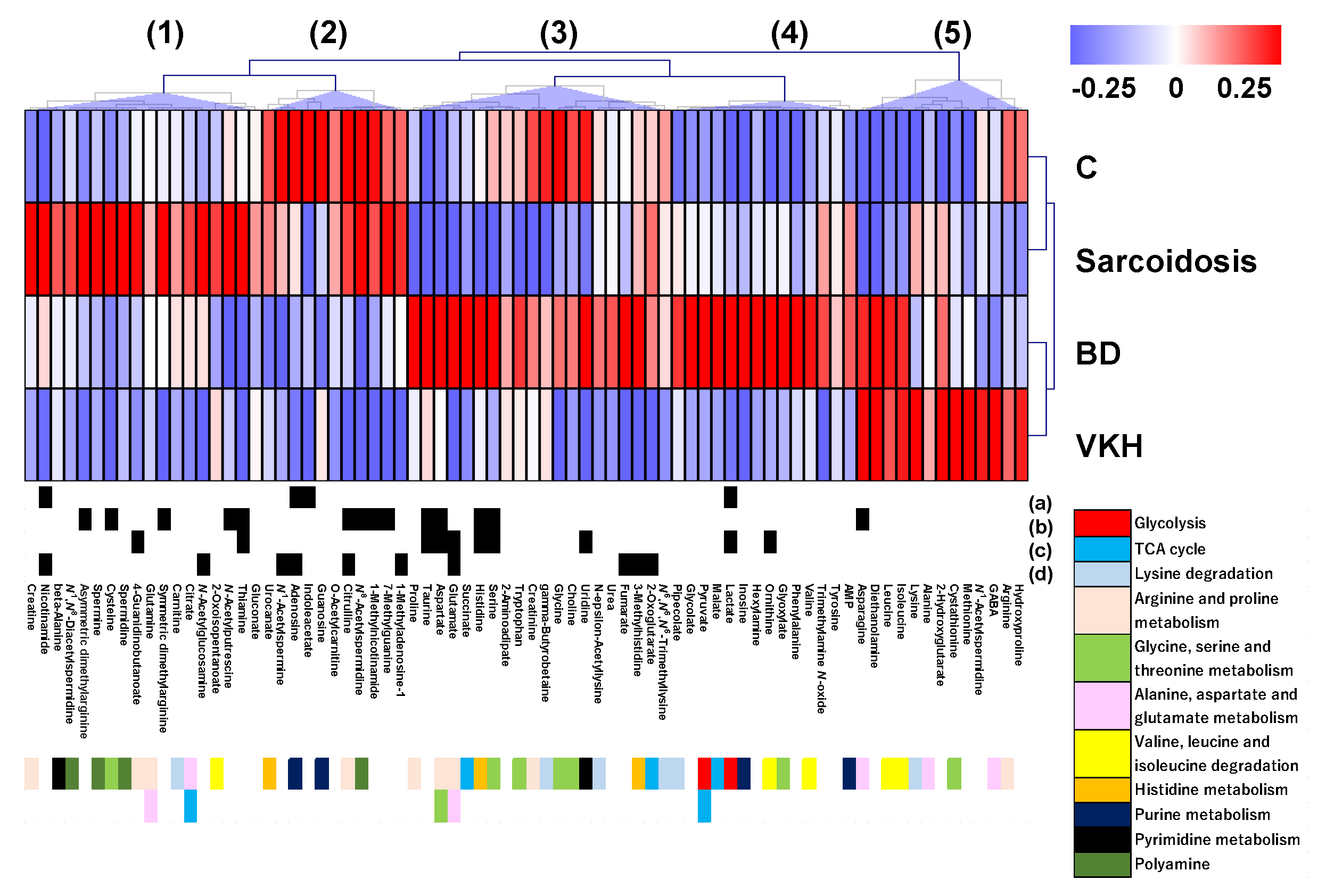

| BD | Sarcoidosis | VKH | Control | |
|---|---|---|---|---|
| Number | 19 | 20 | 15 | 16 |
| Sex (male/female) | 9/10 | 6/14 | 8/7 | 8/8 |
| Age (year) ± SD (range) | 42.7 ± 16.8 (15–71) | 69.0 ± 11.3 (45–86) | 46.4 ± 15.5 (12–71) | 53.8 ± 18.5 (27–83) |
| WBC (/μL) | 6307.5 | 6204.2 | 6508.3 | - |
| CRP (mg/dL) | 0.11 | 0.09 | 0.08 | - |
| CH50 (U/mL) | 62.1 | 63.8 | 59.1 | - |
| sIL-2R (U/mL) | 410.3 | 810.2 | - | - |
| ACE (IU/L) | 12.1 | 18.4 | 9.2 | - |
| HLA-B51 | 7 (36.8%) | - | - | - |
| HLA-A26 | 8 (42.1%) | - | - | - |
| Metabolite | Parameters | 95% CI | p-Value | Odds Ratio | 95% CI | ||
|---|---|---|---|---|---|---|---|
| (Sarcoidosis + BD + VKH) from C | |||||||
| (Intercept) | 0.614 | −0.617 | 1.40 | 0.123 | |||
| Adenosine | −39.6 | −78.1 | −1.06 | 0.0440 | 6.44 × 10−18 | 1.20 × 10−34 | 0.346 |
| Nicotinamide | 3.41 | 0.469 | 6.35 | 0.0230 | 30.2 | 1.60 | 1.20 × 102 |
| Sarcoidosis from (BD + VKH) | |||||||
| (Intercept) | 0.916 | −2.21 | 4.04 | 0.566 | |||
| Thiamine | 16.2 | 6.3 | 26.2 | 1.40 × 10−3 | 1.14 × 107 | 5.46 × 102 | 2.37 × 1011 |
| Asparagine | −0.0812 | −0.157 | −5.06 × 10−3 | 0.0366 | 0.922 | 0.854 | 0.995 |
| BD from (Sarcoidosis + VKH) | |||||||
| (Intercept) | −0.810 | −2.25 | 0.633 | 0.271 | |||
| Thiamine | −14.6 | −25.3 | −3.91 | 7.40 × 10−3 | 4.51 × 10−7 | 1.02 × 10−11 | 0.0200 |
| Uridine | 0.801 | 0.215 | 1.39 | 7.40 × 10−3 | 2.23 | 1.24 | 4.00 |
| VKH from (BD + Sarcoidosis) | |||||||
| (Intercept) | 1.67 | −0.541 | 3.88 | 0.139 | |||
| Citrulline | −0.0947 | −0.176 | −0.0134 | 0.0244 | 0.91 | 0.839 | 0.987 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimizu, H.; Usui, Y.; Asakage, M.; Nezu, N.; Wakita, R.; Tsubota, K.; Sugimoto, M.; Goto, H. Serum Metabolomic Profiling of Patients with Non-Infectious Uveitis. J. Clin. Med. 2020, 9, 3955. https://doi.org/10.3390/jcm9123955
Shimizu H, Usui Y, Asakage M, Nezu N, Wakita R, Tsubota K, Sugimoto M, Goto H. Serum Metabolomic Profiling of Patients with Non-Infectious Uveitis. Journal of Clinical Medicine. 2020; 9(12):3955. https://doi.org/10.3390/jcm9123955
Chicago/Turabian StyleShimizu, Hiroyuki, Yoshihiko Usui, Masaki Asakage, Naoya Nezu, Ryo Wakita, Kinya Tsubota, Masahiro Sugimoto, and Hiroshi Goto. 2020. "Serum Metabolomic Profiling of Patients with Non-Infectious Uveitis" Journal of Clinical Medicine 9, no. 12: 3955. https://doi.org/10.3390/jcm9123955
APA StyleShimizu, H., Usui, Y., Asakage, M., Nezu, N., Wakita, R., Tsubota, K., Sugimoto, M., & Goto, H. (2020). Serum Metabolomic Profiling of Patients with Non-Infectious Uveitis. Journal of Clinical Medicine, 9(12), 3955. https://doi.org/10.3390/jcm9123955






