Incidence and Management of Hypertriglyceridemia-Associated Acute Pancreatitis: A Prospective Case Series in a Single Australian Tertiary Centre
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Comparison of the HTGAP and Non-HTGAP Cohorts
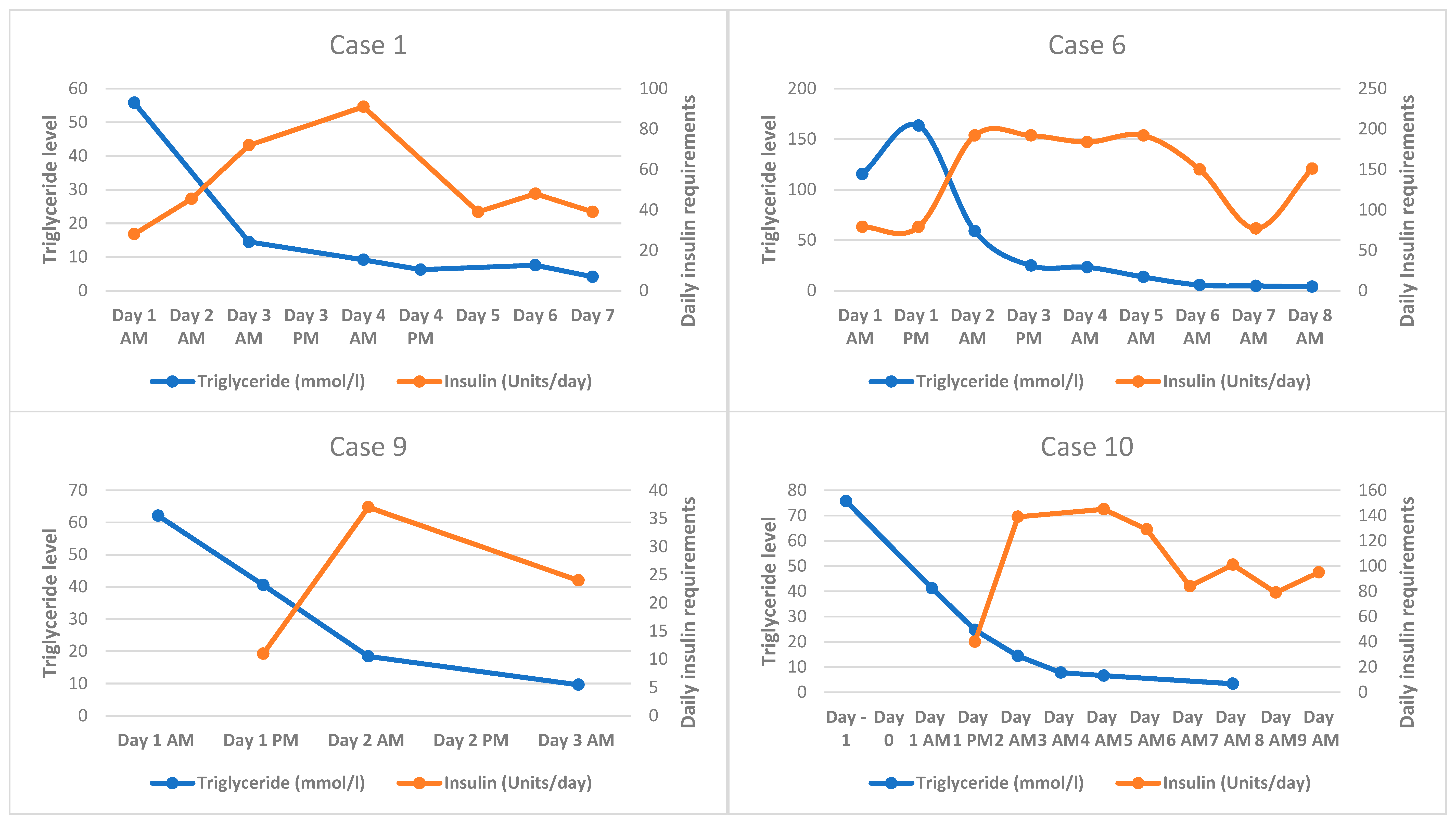
3.2. HTGAP Case Series
4. Discussion
4.1. Incidence
4.2. Clinical Factors
4.3. Severity
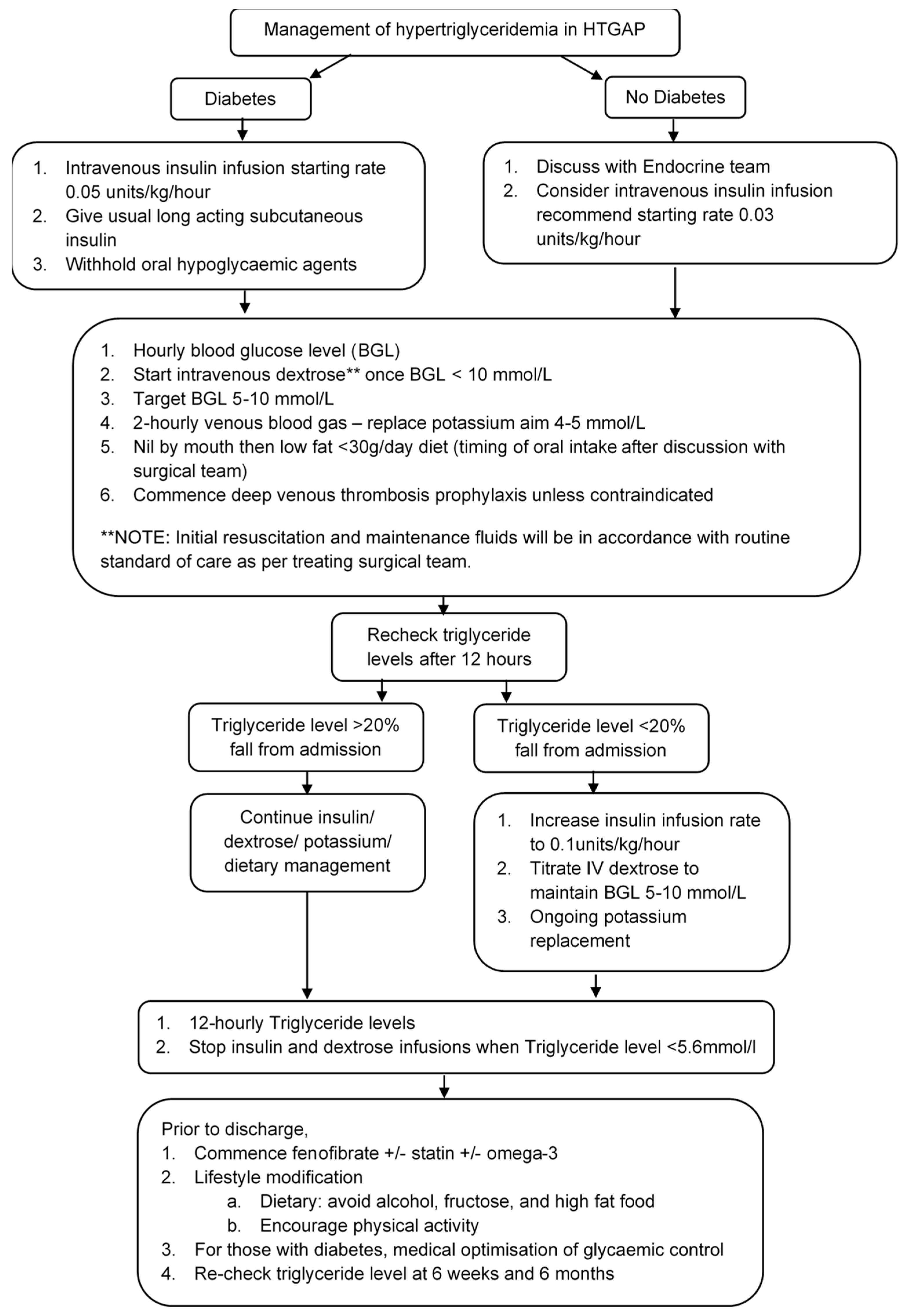
4.4. Acute Management of HTGAP
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Valdivielso, P.; Ramirez-Bueno, A.; Ewald, N. Current knowledge of hypertriglyceridemic pancreatitis. Eur. J. Intern. Med. 2014, 25, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Vipperla, K.; Somerville, C.; Furlan, A.; Koutroumpakis, E.; Saul, M.; Chennat, J.; Rabinovitz, M.; Whitcomb, D.C.; Slivka, A.; Papachristou, G.I.; et al. Clinical Profile and Natural Course in a Large Cohort of Patients with Hypertriglyceridemia and Pancreatitis. J. Clin. Gastroenterol. 2017, 51, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Lindkvist, B.; Appelros, S.; Regner, S.; Manjer, J. A prospective cohort study on risk of acute pancreatitis related to serum triglycerides, cholesterol and fasting glucose. Pancreatology 2012, 12, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Fortson, R.M.; Freedman, S.N.; Webster, P.D., 3rd. Clinical assessment of hyperlipidemic pancreatitis. Am. J. Gastroenterol. 1995, 90, 2134–2139. [Google Scholar] [PubMed]
- Saligram, S.; Lo, D.; Saul, M.; Yadav, D. Analyses of hospital administrative data that use diagnosis codes overestimate the cases of acute pancreatitis. Clin. Gastroenterol. Hepatol. 2012, 10, 805–811 e1. [Google Scholar] [CrossRef]
- Kloer, H.U.; Hauenschild, A. 3P-0788 Severe chylomicronemia: Clinical epidemiology and recommendations for treatment. Atheroscler. Suppl. 2003, 4, 234–235. [Google Scholar] [CrossRef]
- Lloret Linares, C.; Pelletier, A.L.; Czernichow, S.; Vergnaud, A.C.; Bonnefont-Rousselot, D.; Levy, P.; Ruszniewski, P.; Bruckert, E. Acute pancreatitis in a cohort of 129 patients referred for severe hypertriglyceridemia. Pancreas 2008, 37, 13–22. [Google Scholar] [CrossRef]
- Sandhu, S.; Al-Sarraf, A.; Taraboanta, C.; Frohlich, J.; Francis, G.A. Incidence of pancreatitis, secondary causes, and treatment of patients referred to a specialty lipid clinic with severe hypertriglyceridemia: A retrospective cohort study. Lipids Health Dis. 2011, 10, 157. [Google Scholar] [CrossRef]
- Anderson, F.; Mbatha, S.Z.; Thomson, S.R. The early management of pancreatitis associated with hypertriglyceridaemia. S. Afr. J. Surg. 2011, 49, 82–84. [Google Scholar]
- Carr, R.A.; Rejowski, B.J.; Cote, G.A.; Pitt, H.A.; Zyromski, N.J. Systematic review of hypertriglyceridemia-induced acute pancreatitis: A more virulent etiology? Pancreatology 2016, 16, 469–476. [Google Scholar] [CrossRef]
- Toskes, P.P. Hyperlipidemic pancreatitis. Gastroenterol. Clin. North Am. 1990, 19, 783–791. [Google Scholar] [PubMed]
- Ewald, N.; Hardt, P.D.; Kloer, H.U. Severe hypertriglyceridemia and pancreatitis: Presentation and management. Curr. Opin. Lipidol. 2009, 20, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Kimura, W.; Mossner, J. Role of hypertriglyceridemia in the pathogenesis of experimental acute pancreatitis in rats. Int. J. Pancreatol. 1996, 20, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Saharia, P.; Margolis, S.; Zuidema, G.D.; Cameron, J.L. Acute pancreatitis with hyperlipemia: Studies with an isolated perfused canine pancreas. Surgery 1977, 82, 60–67. [Google Scholar] [PubMed]
- Havel, R.J. Pathogenesis, differentiation and management of hypertriglyceridemia. Adv. Intern. Med. 1969, 15, 117–154. [Google Scholar]
- Chang, Y.T.; Chang, M.C.; Su, T.C.; Liang, P.C.; Su, Y.N.; Kuo, C.H.; Wei, S.C.; Wong, J.M. Association of cystic fibrosis transmembrane conductance regulator (CFTR) mutation/variant/haplotype and tumor necrosis factor (TNF) promoter polymorphism in hyperlipidemic pancreatitis. Clin. Chem. 2008, 54, 131–138. [Google Scholar] [CrossRef]
- Ivanova, R.; Puerta, S.; Garrido, A.; Cueto, I.; Ferro, A.; Ariza, M.J.; Cobos, A.; Gonzalez-Santos, P.; Valdivielso, P. Triglyceride levels and apolipoprotein E polymorphism in patients with acute pancreatitis. Hepatobiliary Pancreat. Dis. Int. 2012, 11, 96–101. [Google Scholar] [CrossRef]
- Laufs, U.; Parhofer, K.G.; Ginsberg, H.N.; Hegele, R.A. Clinical review on triglycerides. Eur. Heart. J. 2020, 41, 99–109c. [Google Scholar] [CrossRef]
- Tenner, S.; Baillie, J.; DeWitt, J.; Vege, S.S. American College of Gastroenterology guideline: Management of acute pancreatitis. Am. J. Gastroenterol. 2013, 108, 1400–1415. [Google Scholar] [CrossRef]
- Berglund, L.; Brunzell, J.D.; Goldberg, A.C.; Goldberg, I.J.; Sacks, F.; Murad, M.H.; Stalenhoef, A.F. Evaluation and treatment of hypertriglyceridemia: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2012, 97, 2969–2989. [Google Scholar] [CrossRef]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Australian Government Department of Health. How much alcohol is safe to drink? 22 April 2020. Available online: https://www.health.gov.au/health-topics/alcohol/about-alcohol/how-much-alcohol-is-safe-to-drink#:~:text=If%20you’re%20a%20healthy,risk%20of%20alcohol%2Drelated%20harm (accessed on 12 October 2020).
- Speck, L. Fall von lipamia. Arch. Verin Wissenschaftl Heilkunde 1865; 1: 232. In Lipidoses, Diseases of the Intracellular Lipid Metabolism; Thannhauser, S.J., Ed.; Grune & Stratton: New York, NY, USA, 1958. [Google Scholar]
- Adiamah, A.; Psaltis, E.; Crook, M.; Lobo, D.N. A systematic review of the epidemiology, pathophysiology and current management of hyperlipidaemic pancreatitis. Clin. Nutr. 2018, 37, 1810–1822. [Google Scholar] [CrossRef] [PubMed]
- He, W.H.; Yu, M.; Zhu, Y.; Xia, L.; Liu, P.; Zeng, H.; Zhu, Y.; Lv, N.H. Emergent Triglyceride-lowering Therapy With Early High.-volume Hemofiltration Against Low-Molecular-Weight Heparin Combined With Insulin in Hypertriglyceridemic Pancreatitis: A Prospective Randomized Controlled Trial. J. Clin. Gastroenterol. 2016, 50, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, S.B.; Varbo, A.; Langsted, A.; Nordestgaard, B.G. Chylomicronemia risk factors ranked by importance for the individual and community in 108 711 women and men. J. Intern. Med. 2018, 283, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Tada, H.; Kawashiri, M.A.; Nakahashi, T.; Yagi, K.; Chujo, D.; Ohbatake, A.; Mori, Y.; Mori, S.; Kometani, M.; Fujii, H.; et al. Clinical characteristics of Japanese patients with severe hypertriglyceridemia. J. Clin. Lipidol. 2015, 9, 519–524. [Google Scholar] [CrossRef]
- Ford, E.S.; Li, C.; Zhao, G.; Pearson, W.S.; Mokdad, A.H. Hypertriglyceridemia and its pharmacologic treatment among US adults. Arch. Intern. Med. 2009, 169, 572–578. [Google Scholar] [CrossRef]
- Esparza, M.I.; Li, X.; Adams-Huet, B.; Vasandani, C.; Vora, A.; Das, S.R.; Garg, A.; Ahmad, Z. Very Severe Hypertriglyceridemia in a Large US County Health Care System: Associated Conditions and Management. J. Endocr. Soc. 2019, 3, 1595–1607. [Google Scholar] [CrossRef]
- Xiao, C.; Dash, S.; Morgantini, C.; Hegele, R.A.; Lewis, G.F. Pharmacological Targeting of the Atherogenic Dyslipidemia Complex.: The Next Frontier in CVD Prevention Beyond Lowering LDL Cholesterol. Diabetes 2016, 65, 1767–1778. [Google Scholar] [CrossRef]
- Xiao, C.; Dash, S.; Morgantini, C.; Lewis, G.F. Intravenous Glucose Acutely Stimulates Intestinal Lipoprotein Secretion in Healthy Humans. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1457–1463. [Google Scholar] [CrossRef]
- Paquette, M.; Bernard, S.; Hegele, R.A.; Baass, A. Chylomicronemia: Differences between familial chylomicronemia syndrome and multifactorial chylomicronemia. Atherosclerosis 2019, 283, 137–142. [Google Scholar] [CrossRef]
- Dominguez-Munoz, J.E.; Malfertheiner, P.; Ditschuneit, H.H.; Blanco-Chavez, J.; Uhl, W.; Buchler, M.; Ditschuneit, H. Hyperlipidemia in acute pancreatitis. Relationship with etiology, onset, and severity of the disease. Int. J. Pancreatol. 1991, 10, 261–267. [Google Scholar] [PubMed]
- Yadav, D.; Pitchumoni, C.S. Issues in hyperlipidemic pancreatitis. J. Clin. Gastroenterol. 2003, 36, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.S.; Langsted, A.; Nordestgaard, B.G. Nonfasting Mild-to-Moderate Hypertriglyceridemia and Risk of Acute Pancreatitis. JAMA Intern. Med. 2016, 176, 1834–1842. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.J.; Sheng, X.; MacDonald, T.M.; Wei, L. Hypertriglyceridemia and acute pancreatitis. JAMA Intern. Med. 2013, 173, 162–164. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Munoz, E.J.; Junemann, F.; Malfertheiner, P. Hyperlipidemia in acute pancreatitis. Cause or epiphenomenon? Int. J. Pancreatol. 1995, 18, 101–106. [Google Scholar] [PubMed]
- Kiss, L.; Fur, G.; Matrai, P.; Hegyi, P.; Ivany, E.; Cazacu, I.M.; Szabo, I.; Habon, T.; Alizadeh, H.; Gyongyi, Z.; et al. The effect of serum triglyceride concentration on the outcome of acute pancreatitis: Systematic review and meta-analysis. Sci. Rep. 2018, 8, 14096. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, G.; Qiu, Z.; He, X.; Liu, C. Elevated Serum Triglycerides in the Prognostic Assessment of Acute Pancreatitis: A Systematic Review and Meta-Analysis of Observational Studies. J. Clin. Gastroenterol. 2017, 51, 586–593. [Google Scholar] [CrossRef]
- Czako, L.; Szabolcs, A.; Vajda, A.; Csati, S.; Venglovecz, V.; Rakonczay, Z., Jr.; Hegyi, P.; Tiszlavicz, L.; Csont, T.; Posa, A. Hyperlipidemia induced by a cholesterol-rich diet aggravates necrotizing pancreatitis in rats. Eur. J. Pharmacol. 2007, 572, 74–81. [Google Scholar] [CrossRef]
- Hofbauer, B.; Friess, H.; Weber, A.; Baczako, K.; Kisling, P.; Schilling, M.; Uhl, W.; Dervenis, C.; Buchler, M.W. Hyperlipaemia intensifies the course of acute oedematous and acute necrotising pancreatitis in the rat. Gut 1996, 38, 753–758. [Google Scholar] [CrossRef]
- Ponzo, O.; Schreier, L.; Resnik, R.; Negri, G.; Scacchi, P.; Cresta, M.A.; Wikinski, R. Endogenous hypertriglyceridemia intensifies the course of cerulein-induced pancreatitis in rat: Relation with changes in the VLDL composition. Ann. Nutr. Metab. 2006, 50, 37–44. [Google Scholar] [CrossRef]
- Working Party of the British Society of Gastroenterology. Association of Surgeons of Great Britain and Ireland; Pancreatic Society of Great Britain and Ireland; Association of Upper GI Surgeons of Great Britain and Ireland. UK guidelines for the management of acute pancreatitis. Gut 2005, 54 (Suppl. 3), iii1–iii9. [Google Scholar]
- Sadur, N.C.; Eckel, R.H. Insulin stimulation of adipose tissue lipoprotein lipase. Use of the euglycemic clamp technique. J. Clin. Investig. 1982, 69, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Eckel, R.H. Lipoprotein lipase. A multifunctional enzyme relevant to common metabolic diseases. N. Engl. J. Med. 1989, 320, 1060–1068. [Google Scholar] [PubMed]
- Whayne, F.T., Jr.; Felts, J.M.; Harris, P.A. Effect of heparin on the inactivation of serum lipoprotein lipase by the liver in unanesthetized dogs. J. Clin. Investig. 1969, 48, 1246–1251. [Google Scholar] [CrossRef][Green Version]
- Whayne, F.T., Jr.; Felts, J.M. Activation of lipoprotein lipase. Comparative study of man and other mammals. Circ. Res. 1970, 26, 545–551. [Google Scholar] [CrossRef]
- Nasstrom, B.; Olivecrona, G.; Olivecrona, T.; Stegmayr, B.G. Lipoprotein lipase during continuous heparin infusion: Tissue stores become partially depleted. J. Lab. Clin. Med. 2001, 138, 206–213. [Google Scholar] [CrossRef]
- Nasstrom, B.; Stegmayr, B.G.; Olivecrona, G.; Olivecrona, T. Lower plasma levels of lipoprotein lipase after infusion of low molecular weight heparin than after administration of conventional heparin indicate more rapid catabolism of the enzyme. J. Lab. Clin. Med. 2003, 142, 90–99. [Google Scholar] [CrossRef]
- Whayne, T.F., Jr. Concerns about heparin therapy for hypertriglyceridemia. Arch. Intern. Med. 2010, 170, 108–109. [Google Scholar] [CrossRef]
- Thuzar, M.; Shenoy, V.V.; Malabu, U.H.; Schrale, R.; Sangla, K.S. Extreme hypertriglyceridemia managed with insulin. J. Clin. Lipidol. 2014, 8, 630–634. [Google Scholar] [CrossRef]
- Coskun, A.; Erkan, N.; Yakan, S.; Yildirim, M.; Carti, E.; Ucar, D.; Oymaci, E. Treatment of hypertriglyceridemia-induced acute pancreatitis with insulin. Prz. Gastroenterol. 2015, 10, 18–22. [Google Scholar] [CrossRef]
- Mikhail, N.; Trivedi, K.; Page, C.; Wali, S.; Cope, D. Treatment of severe hypertriglyceridemia in nondiabetic patients with insulin. Am. J. Emerg. Med. 2005, 23, 415–417. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.T.; Tsai, T.Y.; Liao, H.Y.; Chang, C.M.; Jheng, J.S.; Huang, W.H.; Chou, C.Y.; Chen, C.J. Double Filtration Plasma Apheresis Shortens Hospital Admission Duration of Patients with Severe Hypertriglyceridemia-Associated Acute Pancreatitis. Pancreas 2016, 45, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.L.E.; Goodsall, T.; McDonald, G.; Joshi, T.; Acharya, S.; Wynne, K. Hypertriglyceridemia and Acute Pancreatitis in a Cohort of Overweight and Obese Patients. In Proceedings of the Australian & New Zealand Obesity Society 2016 Annual Scientific Meeting, Brisbane, Australia, 19–21 October 2016. [Google Scholar]
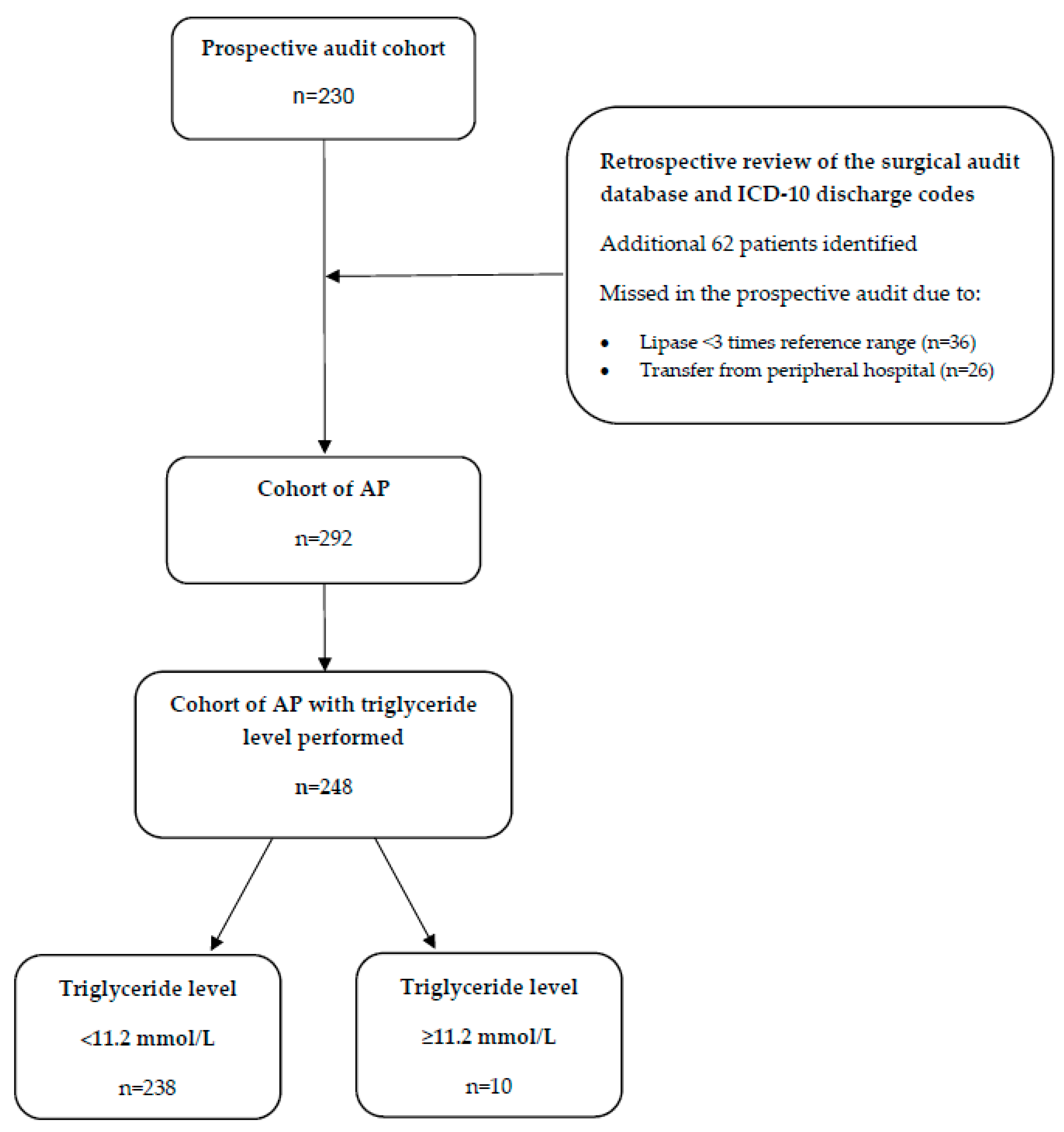
| Parameter | Overall | Non-HTGAP | HTGAP | p-Value |
|---|---|---|---|---|
| n | 248 | 238 | 10 | |
| Age | ||||
| Years ± SEM | 53.9 ± 1.2 | 54.2 ± 1.2 | 48.7 ± 2.4 | 0.37 |
| Male | ||||
| n (%) | 117 (47.2) | 111 (46.6) | 6 (60) | 0.50 |
| Body mass index | ||||
| n/total (%) + | 80/248 (32) | 74/238 (31) | 6/10 (60) | 0.86 |
| mean ± SEM, kg/m2 | 31.0 ± 0.8 | 31.1 ± 0.9 | 30.6 ± 1.6 | |
| Smoker * | ||||
| n/total (%) | 68/190 (36) | 64/180 (34) | 4/10 (40) | 0.75 |
| Alcohol risk | ||||
| High ** | 57(33) | 52 (32) | 5 (50) | 0.42 |
| Low | 41 (25) | 40 (25) | 1 (10) | |
| Non-drinker | 72 (42) | 68 (43) | 4 (40) | |
| Known dyslipidemia ‡ | ||||
| n (%) | 54 (22) | 49 (21) | 5 (50) | 0.04 |
| Diabetes ‡ | ||||
| Type 1 | 5 (2.0) | 5 (2.1) | 0 (0.0) | 0.01 |
| Type 2 | 42 (17.1) | 36 (15.3) | 6 (60.0) | |
| Gestational | 1 (0.4) | 1 (0.4) | 0 (0.0) | |
| Nil | 198 (80.5) | 194 (82.2) | 4 (40.0) | |
| Lipase level, IU/L | 1154 | 1145 | 1731 | 0.67 |
| Median (IQR) | (2915) | (3065) | (1725) | |
| Triglyceride level, mmol/L | 1.3 | 1.3 | 51 | <0.001 |
| Median (IQR) | (1.1) | (0.9) | (46.8) | |
| Length of stay, days | ||||
| Median (IQR) | 2.9 (3.7) | 2.8 (3.7) | 6.7 (9.8) | 0.46 |
| ICU admission | ||||
| n (%) | 19 (8) | 14 (6) | 5 (50) | <0.001 |
| Mortality | ||||
| n (%) | 7 (3) | 6 (3) | 1 (10) | 0.25 |
| Gender | Age (Years) | Triglyceride (TG) Level on Admission (mmol/L) | Pre-Admission Lipid Treatment | Previous AP | BMI kg/m2 | T2DM (HbA1c mmol/mol, %) | Smoker | Concurrent Etiology | ICU Admission (APACHE II, Charlson Comorbidity Index) | Endocrine Input | In-Patient Management of HTGAP | TG Level after 24 h, mmol/L (% Decline from Admission) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gallstones on Imaging | Alcohol ** | |||||||||||||
| 1 | Female | 53 | 55.8 | Statin Fenofibrate | Y | 32 | Y (45, 6.3) | Y | Y | No risk | Y (16, 2) | Day 3 of admission | Inotropic support NBM, then <30 g fat daily Intravenous insulin infusion increased from 3 to 4 units/h (0.05 units/kg/h) after 48 h Statin, fenofibrate Laparoscopic cholecystectomy Subcutaneous enoxaparin DVT prophylaxis | NA † |
| 2 ∞ | Male | 55 | 75.6 | Statin | Y | -NA | N | N | Previous resection | High risk | Y (14, 3) | N | Inotropic support Nasogastric feeding Supportive therapy for multi-organ failure Subcutaneous heparin DVT prophylaxis | NA |
| 3 | Female | 52 | 15.3 | N | N | 30 | N | N | N | High risk | Y (8, 2) | N | NBM, then <30 g fat diet Nil specific therapy for triglycerides Subcutaneous heparin DVT prophylaxis | NA |
| 4 | Female | 59 | 11.3 | Ceased statin 1 month ago | N | 26 | Y ‡ (46, 6.4) | Y | Previous resection | No risk | N (NA, 7) | Y | NBM, then <30 g fat diet Subcutaneous basal-bolus insulin Statin Fenofibrates not commenced due to end-stage kidney disease (peritoneal dialysis). Omega-3 tablets ceased due to dyspepsia Subcutaneous heparin DVT prophylaxis | 8.9 (21%) |
| 5 | Male | 34 | 52.4 | N | N | 37 | Y (NA) | N | N | High risk | N (NA, 1) | N | NBM, then <30 g fat diet Intravenous fluids Subcutaneous enoxaparin DVT prophylaxis | NA |
| 6 | Male | 48 | 115.5 ^ | Statin Gemfibrozil Ezetimibe | N | NA | Y (75, 9.0) | Y | Possible | No risk | Y (10, 2) | Y | NBM, then <30 g fat daily Intravenous fluids Intravenous insulin infusion increased from 4 to 8 units/h (0.1 units/kg/h) after 5 h Statin, fenofibrate, ezetimibe Subcutaneous enoxaparin DVT prophylaxis | 37.0 (77%) # |
| 7 | Female | 50 | 49.6 | N | N | NA | N | Y | Possible | High risk | N (NA, 1) | Y | NBM, then <30 g fat daily Intravenous fluids and electrolyte replacement Statin not commenced due to liver derangement and spontaneous improvement of TG after 24 h | 5.9 (88%) |
| 8 | Male | 52 | 11.2 | Self-ceased statin and fenofibrate 4 years ago | Y | NA | Y (52, 6.9) | N | N | Low risk | N (NA, 2) | Y | NBM, then <30 g fat daily Intravenous fluids Statin, fenofibrate, omega-3 | 3.6 (68%) |
| 9 | Male | 38 | 62.1 | N | N | 27 | N (38, 5.6) | N | N | High risk | N (NA, 0) | Y | NBM, then <30 g fat daily Intravenous fluids Intravenous insulin infusion 2 units/h (0.02 units/kg/h) Statin, fenofibrate Subcutaneous enoxaparin DVT prophylaxis | 18.4 (70%) |
| 10 | Male | 49 | 41.2 | N | N | 31 | Y * (104, 11.7) | N | N | No risk | Y (9, 1) | Y | NBM, then <30 g fat daily Intravenous fluids Intravenous insulin infusion increased from 4 to 6 units/h (0.06 units/kg/h) after 24 h Statin, fenofibrate Subcutaneous enoxaparin DVT prophylaxis | 14.4 (65%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, H.L.E.; McDonald, G.; Payne, A.; Yu, W.; Ismadi, Z.; Tran, H.; Gani, J.; Wynne, K. Incidence and Management of Hypertriglyceridemia-Associated Acute Pancreatitis: A Prospective Case Series in a Single Australian Tertiary Centre. J. Clin. Med. 2020, 9, 3954. https://doi.org/10.3390/jcm9123954
Tan HLE, McDonald G, Payne A, Yu W, Ismadi Z, Tran H, Gani J, Wynne K. Incidence and Management of Hypertriglyceridemia-Associated Acute Pancreatitis: A Prospective Case Series in a Single Australian Tertiary Centre. Journal of Clinical Medicine. 2020; 9(12):3954. https://doi.org/10.3390/jcm9123954
Chicago/Turabian StyleTan, Hong Lin Evelyn, Georgina McDonald, Alexander Payne, William Yu, Zahrul Ismadi, Huy Tran, Jon Gani, and Katie Wynne. 2020. "Incidence and Management of Hypertriglyceridemia-Associated Acute Pancreatitis: A Prospective Case Series in a Single Australian Tertiary Centre" Journal of Clinical Medicine 9, no. 12: 3954. https://doi.org/10.3390/jcm9123954
APA StyleTan, H. L. E., McDonald, G., Payne, A., Yu, W., Ismadi, Z., Tran, H., Gani, J., & Wynne, K. (2020). Incidence and Management of Hypertriglyceridemia-Associated Acute Pancreatitis: A Prospective Case Series in a Single Australian Tertiary Centre. Journal of Clinical Medicine, 9(12), 3954. https://doi.org/10.3390/jcm9123954






