Diabetic Corneal Neuropathy
Abstract
1. Introduction
2. Pathogenesis of Diabetic Neuropathy and Diabetic Corneal Neuropathy
2.1. Accumulation of Advanced Glycation End Products (AGE)
2.2. Polyol Pathway
2.3. Oxidative Stress
2.4. Protein Kinase C (PKC) Activation Pathway
3. Diabetic Peripheral Neuropathy (DPN)
4. Diabetic Corneal Neuropathy
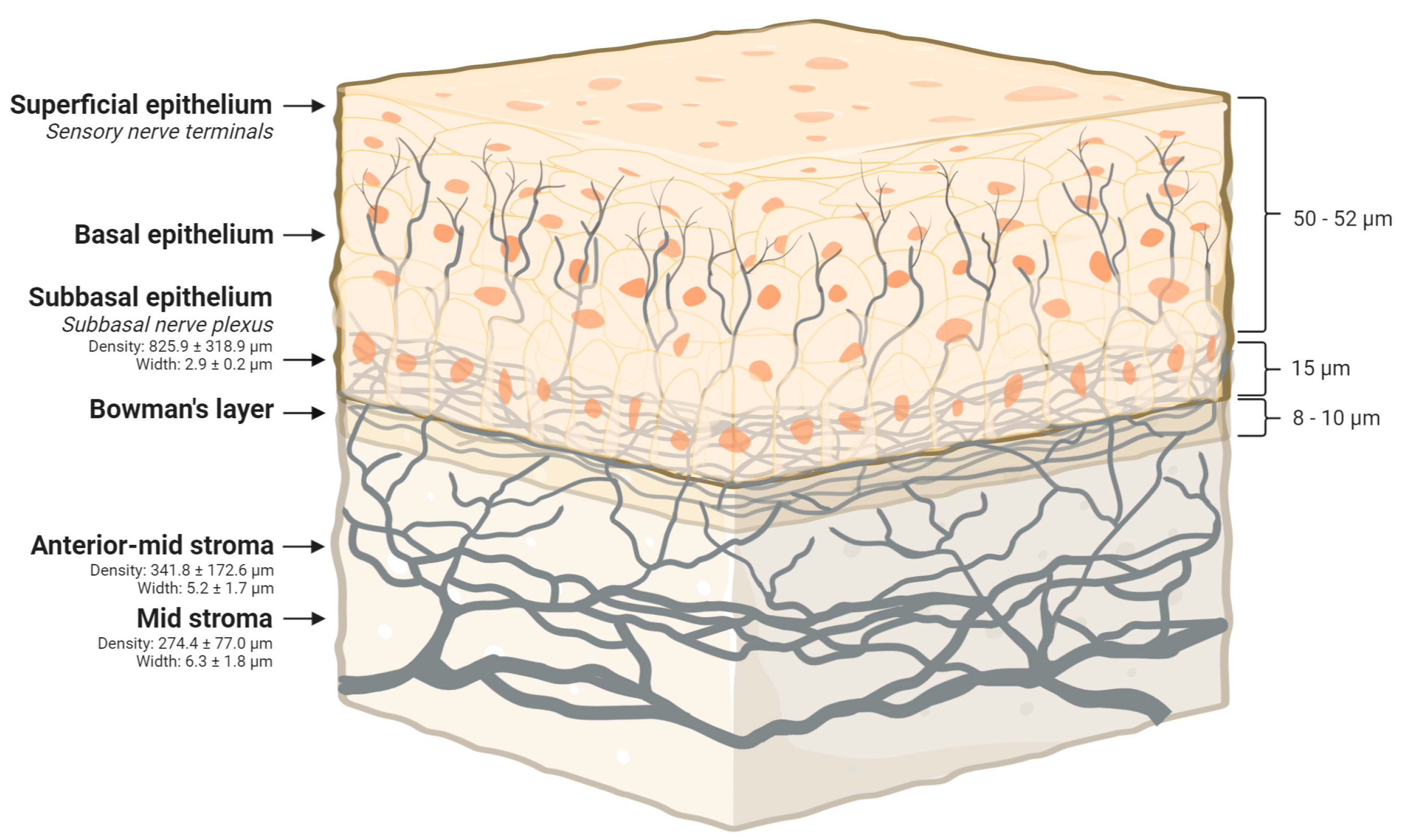
4.1. Corneal Innervation
4.2. Corneal Nerve Plexus: A Surrogate Marker of DPN and Interventional Efficacy
4.3. Neurotrophic Role of Corneal Nerves
5. Clinical Manifestations and Evaluation of Diabetic Corneal Neuropathy
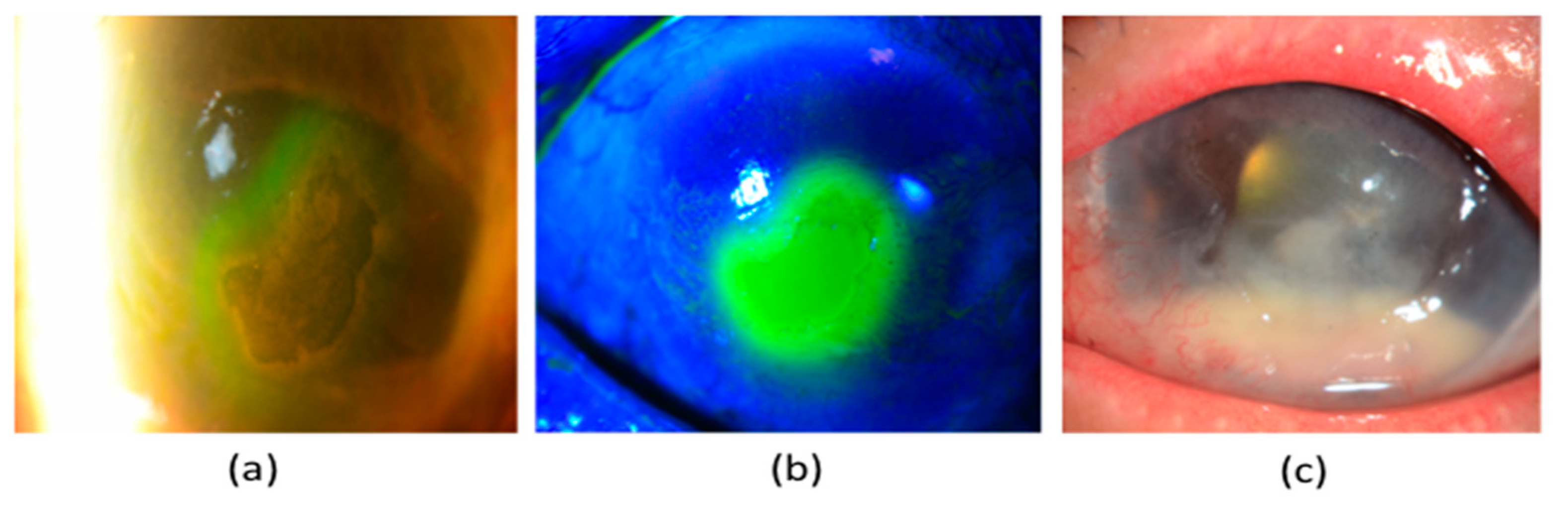
5.1. Neurotrophic Keratopathy (NK)
5.2. Evaluation of Diabetic Corneal Neuropathy
5.2.1. Clinical History
5.2.2. Corneal Sensitivity Assessment
5.2.3. Ocular Surface Staining
5.2.4. Tear Film Function
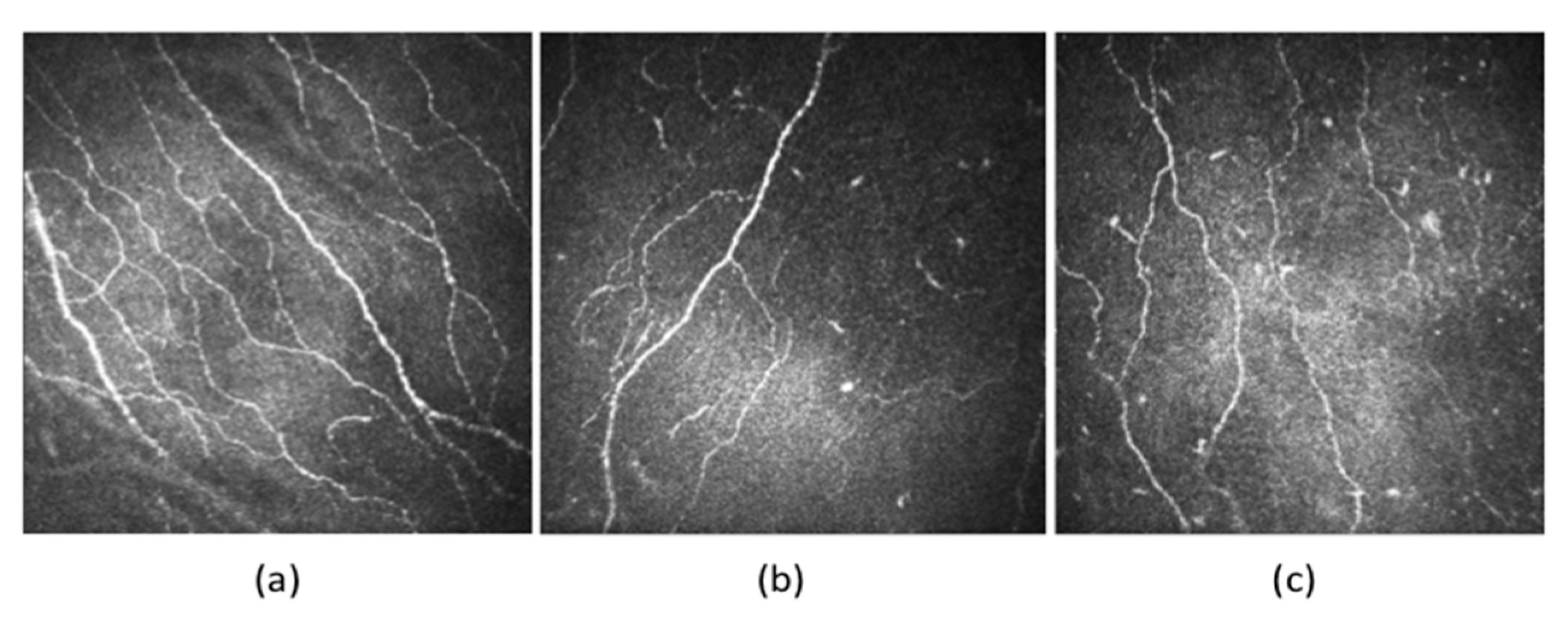
5.2.5. Corneal Confocal Microscopy
6. Management of Diabetic Keratopathy
6.1. Stage 1 NK
6.2. Stage 2 NK
6.2.1. Debridement
6.2.2. Therapeutic Contact Lenses
6.2.3. Autologous Serum Eye Drops
6.2.4. Platelet-Rich Plasma
6.2.5. Amniotic Membrane Transplantation (AMT)
6.2.6. Eyelid Closure
6.2.7. Conjunctival Flaps
6.2.8. Nerve Growth Factor (NGF) Eye Drops
6.2.9. Prevention of Infection and Stromal Lysis
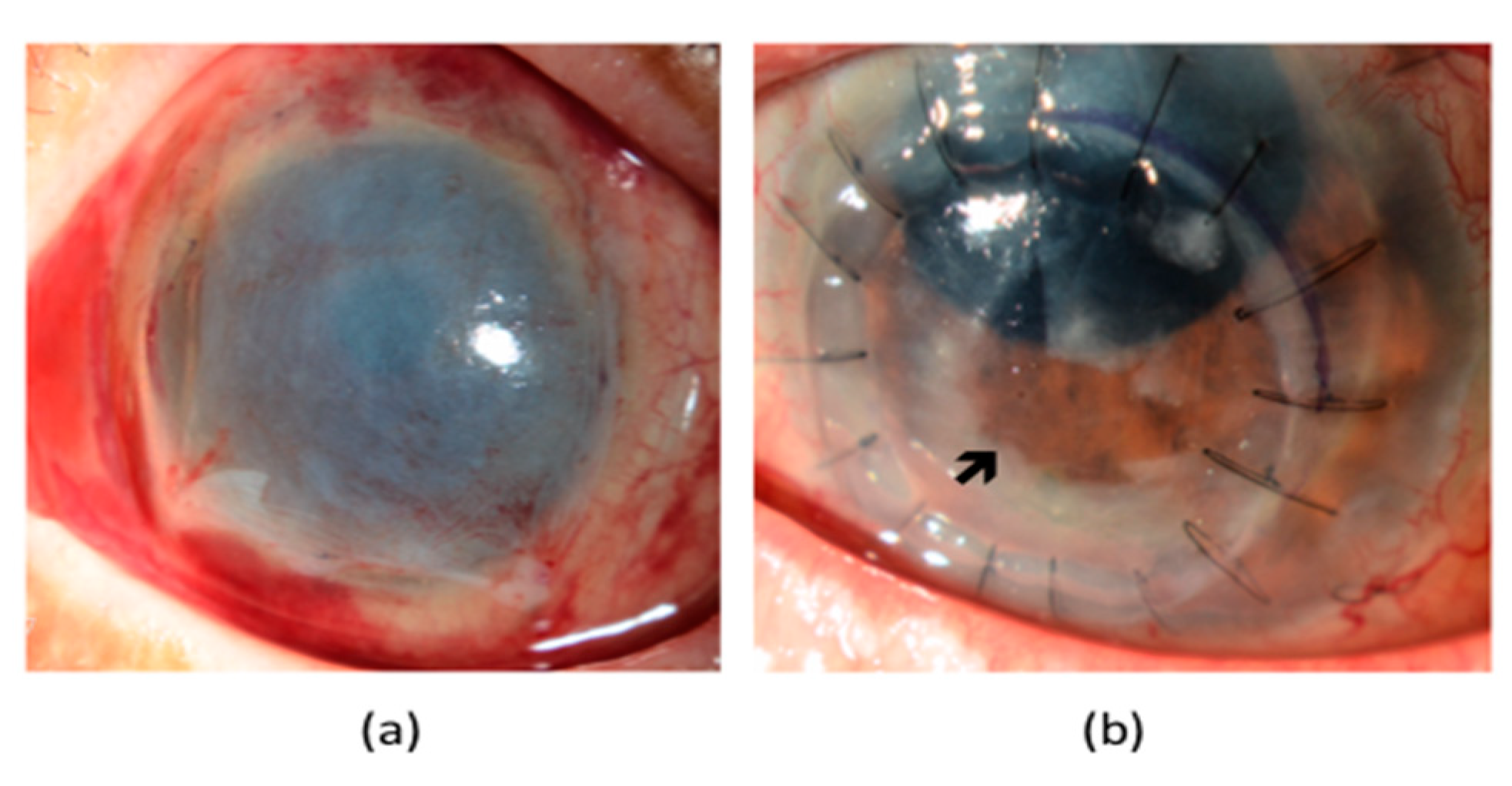
6.3. Stage 3 NK
7. Future Perspectives
7.1. Tear Biomarkers for Corneal Neuropathy
7.1.1. Insulin-Like Growth Factor 1 (IGF-1) and Insulin-Like Growth Factor Binding Protein-3 (IGFB-3)
7.1.2. Substance P (SP)
7.1.3. Inflammatory Tear Biomarkers
7.2. Emerging Therapies for Diabetic Corneal Neuropathy
7.3. Innovative Clinical Tools
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Markoulli, M.; Flanagan, J.; Tummanapalli, S.S.; Wu, J.; Willcox, M. The impact of diabetes on corneal nerve morphology and ocular surface integrity. Ocul. Surf. 2018, 16, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Bommer, C.; Sagalova, V.; Heesemann, E.; Manne-Goehler, J.; Atun, R.; Bärnighausen, T.; Davies, J.; Vollmer, S. Global Economic Burden of Diabetes in Adults: Projections From 2015 to 2030. Diabetes Care 2018, 41, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Bommer, C.; Heesemann, E.; Sagalova, V.; Manne-Goehler, J.; Atun, R.; Bärnighausen, T.; Vollmer, S. The global economic burden of diabetes in adults aged 20–79 years: A cost-of-illness study. Lancet Diabetes Endocrinol. 2017, 5, 423–430. [Google Scholar] [CrossRef]
- World Health Organization. Available online: www.who.int (accessed on 5 December 2020).
- Cade, W.T. Diabetes-Related Microvascular and Macrovascular Diseases in the Physical Therapy Setting. Phys. Ther. 2008, 88, 1322–1335. [Google Scholar] [CrossRef] [PubMed]
- Han, S.B.; Yang, H.K.; Hyon, J.Y. Influence of diabetes mellitus on anterior segment of the eye. Clin. Interv. Aging 2018, 14, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Barsegian, A.; Lee, J.; Salifu, M.O.; McFarlane, S.I. Corneal Neuropathy: An Underrated Manifestation of Diabetes Mellitus. J Clin Endocrinol Diabetes 2018, 2, JCED-111. [Google Scholar]
- Zhao, H.; He, Y.; Ren, Y.-R.; Chen, B.-H. Corneal alteration and pathogenesis in diabetes mellitus. Int. J. Ophthalmol. 2019, 12, 1939–1950. [Google Scholar] [CrossRef]
- Stanisic, S.; Marocco, A.; Gallo, A.; Rama, P.; Sacchetti, M.; Rolando, M.; Pocobelli, A.; Ceccuzzi, R.; Leonardi, A.; Mencucci, R.; et al. Epidemiology and economic impact of moderate and severe neurotrophic keratopathy in Italy. Glob. Reg. Health Technol. Assessment: Ital. North. Eur. Span. 2018. [Google Scholar] [CrossRef]
- Roszkowska, A.M.; Licitra, C.; Tumminello, G.; Postorino, E.I.; Colonna, M.R.; Aragona, P. Corneal nerves in diabetes—The role of the in vivo corneal confocal microscopy of the subbasal nerve plexus in the assessment of peripheral small fiber neuropathy. Surv. Ophthalmol. 2020. [Google Scholar] [CrossRef]
- Kim, J.; Kim, C.-S.; Sohn, E.; Jeong, I.-H.; Kim, H.; Kim, J.S. Involvement of advanced glycation end products, oxidative stress and nuclear factor-kappaB in the development of diabetic keratopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2011, 249, 529–536. [Google Scholar] [CrossRef]
- Brownlee, M.; Vlassara, H.; Cerami, A. Nonenzymatic Glycosylation and the Pathogenesis of Diabetic Complications. Ann. Intern. Med. 1984, 101, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Ryle, C.; Donaghy, M. Non-enzymatic glycation of peripheral nerve proteins in human diabetics. J. Neurol. Sci. 1995, 129, 62–68. [Google Scholar] [CrossRef]
- Sugimoto, K.; Yasujima, M.; Yagihashi, S. Role of Advanced Glycation End Products in Diabetic Neuropathy. Curr. Pharm. Des. 2008, 14, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Stavniichuk, R.; Shevalye, H.; Hirooka, H.; Nadler, J.L.; Drel, V.R. Interplay of sorbitol pathway of glucose metabolism, 12/15-lipoxygenase, and mitogen-activated protein kinases in the pathogenesis of diabetic peripheral neuropathy. Biochem. Pharmacol. 2012, 83, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Oates, P.J. Aldose Reductase, Still a Compelling Target for Diabetic Neuropathy. Curr. Drug Targets 2008, 9, 14–36. [Google Scholar] [CrossRef] [PubMed]
- Greene, D.A.; Sima, A.A.F.; Stevens, M.J.; Feldman, E.L.; Lattimer, S.A. Complications: Neuropathy, Pathogenetic Considerations. Diabetes Care 1992, 15, 1902–1925. [Google Scholar] [CrossRef]
- Betteridge, D.J. What is oxidative stress? Metabolism 2000, 49 (2 Suppl. 1), 3–8. [Google Scholar] [CrossRef]
- Babizhayev, M.A.; Strokov, I.A.; Nosikov, V.V.; Savel’Yeva, E.L.; Sitnikov, V.F.; Yegorov, Y.E.; Lankin, V.Z. The Role of Oxidative Stress in Diabetic Neuropathy: Generation of Free Radical Species in the Glycation Reaction and Gene Polymorphisms Encoding Antioxidant Enzymes to Genetic Susceptibility to Diabetic Neuropathy in Population of Type I Diabetic Patients. Cell Biochem. Biophys. 2015, 71, 1425–1443. [Google Scholar] [CrossRef]
- Duby, J.J.; Campbell, R.K.; Setter, S.M.; White, J.R.; Rasmussen, K.A. Diabetic Neuropathy: An intensive review. Am. J. Health Pharm. 2004, 61, 160–173. [Google Scholar] [CrossRef]
- Obrosova, I.G. Diabetes and the peripheral nerve. Biochim. Biophys. Acta 2009, 1792, 931–940. [Google Scholar] [CrossRef]
- Casellini, C.M.; Barlow, P.M.; Rice, A.L.; Casey, M.; Simmons, K.; Pittenger, G.; Bastyr, E.J., 3rd; Wolka, A.M.; Vinik, A.I. A 6-month, randomized, double-masked, placebo-controlled study evaluating the effects of the protein kinase C-beta inhibitor ruboxistaurin on skin microvascular blood flow and other measures of diabetic peripheral neuropathy. Diabetes Care 2007, 30, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Pirart, J. Diabetes mellitus and its degenerative complications: A prospective study of 4,400 patients observed between 1947 and 1973 (3rd and last part) (author’s transl). Diabete Metab 1977, 3, 245–256. [Google Scholar] [PubMed]
- Said, G. Diabetic neuropathy—A review. Nat. Clin. Pract. Neurol. 2007, 3, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Boulton, A.J.; Vileikyte, L.; Ragnarson-Tennvall, G.; Apelqvist, J. The global burden of diabetic foot disease. Lancet 2005, 366, 1719–1724. [Google Scholar] [CrossRef]
- Sitompul, R. Corneal Sensitivity as a Potential Marker of Diabetic Neuropathy. Acta Med. Indones. 2017, 49, 166–172. [Google Scholar]
- Banerjee, S.; Kim, E.; Parker, M.M.; Gilliam, L.K.; Dlott, R.; Adams, A.S. Clinical Response to Real-Time Patient-Reported Diabetic Peripheral Neuropathy Symptoms. Perm. J. 2019, 23. [Google Scholar] [CrossRef]
- Dyck, P.J.; Dyck, P.; Kennedy, W.R.; Kesserwani, H.; Melanson, M.; Ochoa, J.; Shy, M.E.; Stevens, J.C.; Suarez, G.A.; O’Brien, P.C. Limitations of quantitative sensory testing when patients are biased toward a bad outcome. Neurology 1998, 50, 1213. [Google Scholar] [CrossRef]
- Iqbal, Z.; Azmi, S.; Yadav, R.; Ferdousi, M.; Kumar, M.; Cuthbertson, D.J.; Lim, J.; Malik, R.A.; Alam, U. Diabetic Peripheral Neuropathy: Epidemiology, Diagnosis, and Pharmacotherapy. Clin. Ther. 2018, 40, 828–849. [Google Scholar] [CrossRef]
- Sveen, K.A.; Karimé, B.; Jørum, E.; Mellgren, S.I.; Fagerland, M.W.; Monnier, V.M.; Dahl-Jørgensen, K.; Hanssen, K.F. Small- and Large-Fiber Neuropathy After 40 Years of Type 1 Diabetes: Associations with glycemic control and advanced protein glycation: The Oslo Study. Diabetes Care 2013, 36, 3712–3717. [Google Scholar] [CrossRef]
- Quattrini, C.; Tavakoli, M.; Jeziorska, M.; Kallinikos, P.; Tesfaye, S.; Finnigan, J.; Marshall, A.; Boulton, A.J.; Efron, N.; Malik, R.A. Surrogate Markers of Small Fiber Damage in Human Diabetic Neuropathy. Diabetes 2007, 56, 2148–2154. [Google Scholar] [CrossRef]
- Hertz, P.; Bril, V.; Orszag, A.; Ahmed, A.; Ng, E.; Nwe, P.; Ngo, M.; Perkins, B.A. Reproducibility of in vivo corneal confocal microscopy as a novel screening test for early diabetic sensorimotor polyneuropathy. Diabet. Med. 2011, 28, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Messmer, E.M.; Schmid-Tannwald, C.; Zapp, D.; Kampik, A. In vivo confocal microscopy of corneal small fiber damage in diabetes mellitus. Graefe’s Arch. Clin. Exp. Ophthalmol. 2010, 248, 1307–1312. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, M.; Quattrini, C.; Abbott, C.; Kallinikos, P.; Marshall, A.; Finnigan, J.; Morgan, P.; Efron, N.; Boulton, A.J.; Malik, R.A. Corneal Confocal Microscopy: A novel noninvasive test to diagnose and stratify the severity of human diabetic neuropathy. Diabetes Care 2010, 33, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Edwards, K.; Pritchard, N.; Vagenas, D.; Russell, A.; Malik, R.A.; Efron, N. Utility of corneal confocal microscopy for assessing mild diabetic neuropathy: Baseline findings of the LANDMark study. Clin. Exp. Optom. 2012, 95, 348–354. [Google Scholar] [CrossRef]
- Müller, L.J.; Marfurt, C.F.; Kruse, F.; Tervo, T.M. Corneal nerves: Structure, contents and function. Exp. Eye Res. 2003, 76, 521–542. [Google Scholar] [CrossRef]
- Bikbova, G.; Oshitari, T.; Baba, T.; Yamamoto, S. Neuronal Changes in the Diabetic Cornea: Perspectives for Neuroprotection. BioMed Res. Int. 2016, 2016, 1–8. [Google Scholar] [CrossRef]
- Belmonte, C.; Acosta, M.C.; Gallar, J. Neural basis of sensation in intact and injured corneas. Exp. Eye Res. 2004, 78, 513–525. [Google Scholar] [CrossRef]
- Marfurt, C.F.; Cox, J.; Deek, S.; Dvorscak, L. Anatomy of the human corneal innervation. Exp. Eye Res. 2010, 90, 478–492. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Lin, M.T.-Y.; Mehta, J.S. Analysis of corneal nerve plexus in corneal confocal microscopy images. Neural Regen. Res. 2021, 16, 690–691. [Google Scholar] [CrossRef]
- Al-Aqaba, M.A.; Dhillon, V.K.; Mohammed, I.; Said, D.G.; Dua, H.S. Corneal nerves in health and disease. Prog. Retin. Eye Res. 2019, 73, 100762. [Google Scholar] [CrossRef]
- Chin, J.Y.; Yang, L.W.Y.; Ji, A.J.S.; Nubile, M.; Mastropasqua, L.; Allen, J.C.; Mehta, J.S.; Liu, Y.-C. Validation of the Use of Automated and Manual Quantitative Analysis of Corneal Nerve Plexus Following Refractive Surgery. Diagnostics (Basel) 2020, 10, 493. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, I.N.; Alam, U.; Fadavi, H.; Asghar, O.; Green, P.; Ponirakis, G.; Marshall, A.; Boulton, A.J.; Tavakoli, M.; Verjee, M.A. Corneal Nerve Loss Detected with Corneal Confocal Microscopy Is Symmetrical and Related to the Severity of Diabetic Polyneuropathy. Diabetes Care 2013, 36, 3646–3651. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, N.; Edwards, K.; Dehghani, C.; Fadavi, H.; Jeziorska, M.; Marshall, A.; Petropoulos, I.N.; Ponirakis, G.; Russell, A.W.; Sampson, G.P.; et al. Longitudinal assessment of neuropathy in type 1 diabetes using novel ophthalmic markers (LANDMark): Study design and baseline characteristics. Diabetes Res. Clin. Pr. 2014, 104, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.L.; Craig, J.P.; Patel, D.V.; McGhee, C.N.J.; Pradhan, M.; Ellyett, K.; Kilfoyle, D.; Braatvedt, G. In Vivo Confocal Microscopy of Corneal Nerves: An Ocular Biomarker for Peripheral and Cardiac Autonomic Neuropathy in Type 1 Diabetes Mellitus. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5060. [Google Scholar] [CrossRef]
- Jiang, M.-S.; Yuan, Y.; Gu, Z.-X.; Zhuang, S.-L. Corneal confocal microscopy for assessment of diabetic peripheral neuropathy: A meta-analysis. Br. J. Ophthalmol. 2015, 100, 9–14. [Google Scholar] [CrossRef]
- Tavakoli, M.; Mitu-Pretorian, M.; Petropoulos, I.N.; Fadavi, H.; Asghar, O.; Alam, U.; Ponirakis, G.; Jeziorska, M.; Marshall, A.; Efron, N.; et al. Corneal Confocal Microscopy Detects Early Nerve Regeneration in Diabetic Neuropathy After Simultaneous Pancreas and Kidney Transplantation. Diabetes 2012, 62, 254–260. [Google Scholar] [CrossRef]
- Tavakoli, M.; Kallinikos, P.; Iqbal, A.; Herbert, A.; Fadavi, H.; Efron, N.; Boulton, A.J.M.; Verjee, M.A. Corneal confocal microscopy detects improvement in corneal nerve morphology with an improvement in risk factors for diabetic neuropathy. Diabet. Med. 2011, 28, 1261–1267. [Google Scholar] [CrossRef]
- Baker, K.S.; Anderson, S.C.; Romanowski, E.G.; Thoft, R.A.; Sundarraj, N. Trigeminal ganglion neurons affect corneal epithelial phenotype. Influence on type VII collagen expression in vitro. Investig. Ophthalmol. Vis. Sci. 1993, 34, 137–144. [Google Scholar]
- Tomlinson, D.R.; Fernyhough, P.; Diemel, L.T. Role of Neurotrophins in Diabetic Neuropathy and Treatment with Nerve Growth Factors. Diabetes 1997, 46 (Suppl. 2), S43–S49. [Google Scholar] [CrossRef]
- Minnone, G.; De Benedetti, F.; Bracci-Laudiero, L. NGF and Its Receptors in the Regulation of Inflammatory Response. Int. J. Mol. Sci. 2017, 18, 1028. [Google Scholar] [CrossRef]
- Chen, J.; Chen, P.; Backman, L.J.; Zhou, Q.; Danielson, P. Ciliary Neurotrophic Factor Promotes the Migration of Corneal Epithelial Stem/progenitor Cells by Up-regulation of MMPs through the Phosphorylation of Akt. Sci. Rep. 2016, 6, 25870. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Yan, C.; Lee, P.; Sun, H.; Yu, F.-S. Dendritic cell dysfunction and diabetic sensory neuropathy in the cornea. J. Clin. Investig. 2016, 126, 1998–2011. [Google Scholar] [CrossRef] [PubMed]
- Dua, H.S.; Said, D.G.; Messmer, E.M.; Rolando, M.; Benitez-Del-Castillo, J.M.; Hossain, P.; Shortt, A.J.; Geerling, G.; Nubile, M.; Figueiredo, F.C.; et al. Neurotrophic keratopathy. Prog. Retin. Eye Res. 2018, 66, 107–131. [Google Scholar] [CrossRef] [PubMed]
- Al-Qudah, M.A.; Al-Dwairi, A. Mechanisms and regulation of neurotrophin synthesis and secretion. Neurosciences (Riyadh) 2016, 21, 306–313. [Google Scholar] [CrossRef]
- Yang, L.; Di, G.; Qi, X.; Qu, M.; Wang, Y.; Duan, H.; Danielson, P.; Xie, L.; Zhou, Q. Substance P Promotes Diabetic Corneal Epithelial Wound Healing Through Molecular Mechanisms Mediated via the Neurokinin-1 Receptor. Diabetes 2014, 63, 4262–4274. [Google Scholar] [CrossRef]
- Tran, M.T.; Ritchie, M.H.; Lausch, R.N.; Oakes, J.E. Calcitonin Gene-Related Peptide Induces IL-8 Synthesis in Human Corneal Epithelial Cells. J. Immunol. 2000, 164, 4307–4312. [Google Scholar] [CrossRef]
- Ekstrand, A.J.; Cao, R.; Björndahl, M.; Nyström, S.; Jönsson-Rylander, A.-C.; Hassani, H.; Hallberg, B.; Nordlander, M.; Cao, Y. Deletion of neuropeptide Y (NPY) 2 receptor in mice results in blockage of NPY-induced angiogenesis and delayed wound healing. Proc. Natl. Acad. Sci. USA 2003, 100, 6033–6038. [Google Scholar] [CrossRef]
- Jiang, X.; McClellan, S.A.; Barrett, R.P.; Zhang, Y.; Hazlett, L.D. Vasoactive Intestinal Peptide Downregulates Proinflammatory TLRs While Upregulating Anti-Inflammatory TLRs in the Infected Cornea. J. Immunol. 2012, 189, 269–278. [Google Scholar] [CrossRef]
- HALL, J.E. Guyton and Hall Textbook of Medical Physiology, 12th ed.; Saunders/Elsevier: Philadelphia, PA, USA, 2010; ISBN 1416045740. [Google Scholar]
- Chernyavsky, A.I.; Galitovskiy, V.; Shchepotin, I.B.; Jester, J.V.; Grando, S.A. The Acetylcholine Signaling Network of Corneal Epithelium and Its Role in Regulation of Random and Directional Migration of Corneal Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6921–6933. [Google Scholar] [CrossRef][Green Version]
- Lockwood, A.; Hopeross, M.W.; Chell, P.B. Neurotrophic keratopathy and diabetes mellitus. Eye 2005, 20, 837–839. [Google Scholar] [CrossRef]
- Lambiase, A.; Sacchetti, M. Diagnosis and management of neurotrophic keratitis. Clin. Ophthalmol. 2014, 8, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Morishige, N.; Morita, Y.; Yamada, N.; Nishida, T.; Sonoda, K.-H. Congenital hypoplastic trigeminal nerve revealed by manifestation of corneal disorders likely caused by neural factor deficiency. Case Rep. Ophthalmol. 2014, 5, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-C.; Jung, A.S.J.; Chin, J.Y.; Yang, L.W.Y.; Mehta, J.S. Cross-sectional Study on Corneal Denervation in Contralateral Eyes Following SMILE Versus LASIK. J. Refract. Surg. 2020, 36, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, W.J.; Varley, G.A. Corneal Diagnostic Technique. In Cornea: Fundamentals of Corneal and External Disease; Krachmer, J.H., Mannis, M.J., Holland, E.J., Eds.; Mosby: St Louis, MO, USA, 1997. [Google Scholar]
- Tesón, M.; Calonge, M.; Fernández, I.; Stern, M.E.; González-García, M.J. Characterization by Belmonte’s Gas Esthesiometer of Mechanical, Chemical, and Thermal Corneal Sensitivity Thresholds in a Normal Population. Investig. Ophthalmol. Vis. Sci. 2012, 53, 3154–3268. [Google Scholar] [CrossRef]
- Begley, C.; Caffery, B.; Chalmers, R.; Situ, P.; Simpson, T.; Nelson, J.D. Review and analysis of grading scales for ocular surface staining. Ocul. Surf. 2019, 17, 208–220. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, L.; Deng, S.; Sun, X.; Wang, N. Dry Eye Syndrome in Patients with Diabetes Mellitus: Prevalence, Etiology, and Clinical Characteristics. J. Ophthalmol. 2016, 2016, 1–7. [Google Scholar] [CrossRef]
- Nakata, M.; Okada, Y.; Kobata, H.; Shigematsu, T.; Reinach, P.S.; Tomoyose, K.; Saika, S. Diabetes mellitus suppresses hemodialysis-induced increases in tear fluid secretion. BMC Res. Notes 2014, 7, 78. [Google Scholar] [CrossRef]
- Ozdemir, M.; Buyukbese, M.A.; Cetinkaya, A.; Ozdemir, G. Risk factors for ocular surface disorders in patients with diabetes mellitus. Diabetes Res. Clin. Pr. 2003, 59, 195–199. [Google Scholar] [CrossRef]
- Versura, P.; Giannaccare, G.; Pellegrini, M.; Sebastiani, S.; Campos, E.C. Neurotrophic keratitis: Current challenges and future prospects. Eye Brain 2018, 10, 37–45. [Google Scholar] [CrossRef]
- Misra, S.L.; Patel, D.V.; McGhee, C.N.J.; Pradhan, M.; Kilfoyle, D.; Braatvedt, G.D.; Craig, J.P. Peripheral Neuropathy and Tear Film Dysfunction in Type 1 Diabetes Mellitus. J. Diabetes Res. 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Manaviat, M.R.; Rashidi, M.; Afkhami-Ardekani, M.; Shoja, M. Prevalence of dry eye syndrome and diabetic retinopathy in type 2 diabetic patients. BMC Ophthalmol. 2008, 8, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Heigle, T.J.; Pflugfelder, S.C. Aqueous Tear Production in Patients with Neurotrophic Keratitis. Cornea 1996, 15, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Jalbert, I.; Stapleton, F.; Papas, E.; Sweeney, D.F.; Coroneo, M. In vivo confocal microscopy of the human cornea. Br. J. Ophthalmol. 2003, 87, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Markoulli, M. Automatic analysis of corneal nerves imaged usingin vivoconfocal microscopy. Clin. Exp. Optom. 2017, 101, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, F.; Kojima, R.; Taniguchi, M.; Kosaka, A.; Uetake, H.; Tavakoli, M. The Expanded Bead Size of Corneal C-Nerve Fibers Visualized by Corneal Confocal Microscopy Is Associated with Slow Conduction Velocity of the Peripheral Nerves in Patients with Type 2 Diabetes Mellitus. J. Diabetes Res. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, I.N.; Ferdousi, M.; Marshall, A.; Alam, U.; Ponirakis, G.; Azmi, S.; Fadavi, H.; Efron, N.; Tavakoli, M.; Malik, R.A. The Inferior Whorl For Detecting Diabetic Peripheral Neuropathy Using Corneal Confocal Microscopy. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2498–2504. [Google Scholar] [CrossRef]
- Shih, K.C.; Lam, K.S.-L.; Tong, L. A systematic review on the impact of diabetes mellitus on the ocular surface. Nutr. Diabetes 2017, 7, e251. [Google Scholar] [CrossRef]
- Azmi, S.; Ferdousi, M.; Petropoulos, I.N.; Ponirakis, G.; Fadavi, H.; Tavakoli, M.; Alam, U.; Jones, W.; Marshall, A.; Jeziorska, M.; et al. Corneal Confocal Microscopy Shows an Improvement in Small-Fiber Neuropathy in Subjects With Type 1 Diabetes on Continuous Subcutaneous Insulin Infusion Compared With Multiple Daily Injection. Diabetes Care 2014, 38, e3–e4. [Google Scholar] [CrossRef]
- Azmi, S.; Ferdousi, M.; Petropoulos, I.N.; Ponirakis, G.; Alam, U.; Fadavi, H.; Asghar, O.; Marshall, A.; Atkinson, A.J.; Jones, W.; et al. Corneal Confocal Microscopy Identifies Small-Fiber Neuropathy in Subjects with Impaired Glucose Tolerance Who Develop Type 2 Diabetes. Diabetes Care 2015, 38, 1502–1508. [Google Scholar] [CrossRef]
- Jia, X.; Wang, X.; Wang, X.; Pan, Q.; Xian, T.; Yu, X.; Guo, L. In Vivo Corneal Confocal Microscopy Detects Improvement of Corneal Nerve Parameters following Glycemic Control in Patients with Type 2 Diabetes. J. Diabetes Res. 2018, 2018, 1–8. [Google Scholar] [CrossRef]
- Boucek, P. ‘Observing’ diabetic neuropathy with corneal confocal microscopy: The effect of improvement of risk factors. Expert Rev. Endocrinol. Metab. 2011, 6, 773–775. [Google Scholar] [CrossRef] [PubMed]
- Hindman, H.B.; Patel, S.B.; Jun, A.S. Rationale for Adjunctive Topical Corticosteroids in Bacterial Keratitis. Arch. Ophthalmol. 2009, 127, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Guidera, A.C.; Luchs, J.I.; Udell, I.J. Keratitis, ulceration, and perforation associated with topical nonsteroidal anti-inflammatory drugs. Ophthalmology 2001, 108, 936–944. [Google Scholar] [CrossRef]
- Guzey, M.; Kilic, A.; Basar, E.; Dogan, Z.; Satici, A.; Karadede, S.; Ozardali, I. The treatment of severe trachomatous dry eye with canalicular silicone plugs. Eye 2001, 15, 297–303. [Google Scholar] [CrossRef]
- Demartelaere, S.L.; Blaydon, S.M.; Tovilla-Canales, J.L.; Shore, J.W. A Permanent and Reversible Procedure to Block Tear Drainage for the Treatment of Dry Eye. Ophthalmic Plast. Reconstr. Surg. 2006, 22, 352–355. [Google Scholar] [CrossRef]
- Kim, B.M.; Osmanović, S.S.; Edward, D.P. Pyogenic granulomas after silicone punctal plugs: A clinical and histopathologic study. Am. J. Ophthalmol. 2005, 139, 678–684. [Google Scholar] [CrossRef]
- Tai, M.-C.; Cosar, C.B.; Cohen, E.J.; Rapuano, C.J.; Laibson, P.R. The Clinical Efficacy of Silicone Punctal Plug Therapy. Cornea 2002, 21, 135–139. [Google Scholar] [CrossRef]
- Tong, L.; Beuerman, R.; Simonyi, S.; Hollander, D.A.; Stern, M.E. Effects of Punctal Occlusion on Clinical Signs and Symptoms and on Tear Cytokine Levels in Patients with Dry Eye. Ocul. Surf. 2016, 14, 233–241. [Google Scholar] [CrossRef]
- Katzman, L.R.; Jeng, B.H. Management strategies for persistent epithelial defects of the cornea. Saudi J. Ophthalmol. 2014, 28, 168–172. [Google Scholar] [CrossRef]
- Liu, L.; Hartwig, D.; Harloff, S.; Herminghaus, P.; Wedel, T.; Geerling, G. An optimised protocol for the production of autologous serum eyedrops. Graefe’s Arch. Clin. Exp. Ophthalmol. 2005, 243, 706–714. [Google Scholar] [CrossRef]
- Jeng, B.H.; Dupps, W.J. Autologous Serum 50% Eyedrops in the Treatment of Persistent Corneal Epithelial Defects. Cornea 2009, 28, 1104–1108. [Google Scholar] [CrossRef] [PubMed]
- Young, A.L.; Cheng, A.C.O.; Ng, H.K.; Cheng, L.L.; Leung, G.Y.S.; Lam, D.S.C. The use of autologous serum tears in persistent corneal epithelial defects. Eye 2004, 18, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Schrader, S.; Wedel, T.; Moll, R.; Geerling, G. Combination of serum eye drops with hydrogel bandage contact lenses in the treatment of persistent epithelial defects. Graefe’s Arch. Clin. Exp. Ophthalmol. 2006, 244, 1345–1349. [Google Scholar] [CrossRef] [PubMed]
- Soni, N.G.; Jeng, B.H. Blood-derived topical therapy for ocular surface diseases. Br. J. Ophthalmol. 2015, 100, 22–27. [Google Scholar] [CrossRef]
- Cooper, L.J.; Kinoshita, S.; German, M.J.; Koizumi, N.; Nakamura, T.; Fullwood, N.J. An Investigation into the Composition of Amniotic Membrane Used for Ocular Surface Reconstruction. Cornea 2005, 24, 722–729. [Google Scholar] [CrossRef]
- Khokhar, S.; Natung, T.; Sony, P.; Sharma, N.; Agarwal, N.; Vajpayee, R. Amniotic membrane transplantation in refractory neurotrophic corneal ulcers. A randomized, controlled clinical trial. Am. J. Ophthalmol. 2005, 140, 1175. [Google Scholar] [CrossRef]
- Kruse, F.E.; Rohrschneider, K.; Völcker, H.E. Multilayer amniotic membrane transplantation for reconstruction of deep corneal ulcers. Ophthalmology 1999, 106, 1504–1511. [Google Scholar] [CrossRef]
- Chen, H.-J.; Pires, R.T.F.; Tseng, S.C.G. Amniotic membrane transplantation for severe neurotrophic corneal ulcers. Br. J. Ophthalmol. 2000, 84, 826–833. [Google Scholar] [CrossRef]
- Cosar, C.B.; Cohen, E.J.; Rapuano, C.J.; Maus, M.; Penne, R.P.; Flanagan, J.C.; Laibson, P.R. Tarsorrhaphy: Clinical experience from a cornea practice. Cornea 2001, 20, 787–791. [Google Scholar] [CrossRef]
- Schilimow, A.; Wiechens, B. Botulinum toxin A induced protective ptosis for the treatment of recurrent epithelial defects in neurotrophic keratopathy. Ophthalmologe 2017, 114, 745–747. [Google Scholar] [CrossRef]
- Allen, V.D.; Malinovsky, V. Management of Neurotrophic Keratopathy. Contact Lens Anterior Eye 2003, 26, 161–165. [Google Scholar] [CrossRef]
- Tuli, S.; Schultz, G.S.; Downer, D.M. Science and Strategy for Preventing and Managing Corneal Ulceration. Ocul. Surf. 2007, 5, 23–39. [Google Scholar] [CrossRef]
- Alino, A.M.; Perry, H.D.; Kanellopoulos, A.J.; Donnenfeld, E.D.; Rahn, E.K. Conjunctival flaps. Ophthalmology 1998, 105, 1120–1123. [Google Scholar] [CrossRef]
- Oostra, T.D.; Mauger, T.F. Conjunctival Flaps: A Case Series and Review of the Literature. Eye Contact Lens 2020, 46, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Lugo, M.; Arentsen, J.J. Treatment of Neurotrophic Ulcers with Conjunctival Flaps. Am. J. Ophthalmol. 1987, 103, 711–712. [Google Scholar] [CrossRef]
- Lim, L.S.; How, A.C.; Ang, L.P.K.; Tan, D.T.H. Gundersen Flaps in the Management of Ocular Surface Disease in an Asian Population. Cornea 2009, 28, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Jhanji, V. Conjunctival flap cover surgery: 10-year review. Ann. Eye Sci. 2017, 2, 25. [Google Scholar] [CrossRef]
- Han, S.B.; Liu, Y.-C.; Mohamed-Noriega, K.; Mehta, J.S. Application of Novel Drugs for Corneal Cell Regeneration. J. Ophthalmol. 2018, 2018, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lambiase, A.; Sacchetti, M.; Bonini, S. Nerve growth factor therapy for corneal disease. Curr. Opin. Ophthalmol. 2012, 23, 296–302. [Google Scholar] [CrossRef]
- Bonini, S.; Lambiase, A.; Rama, P.; Caprioglio, G.; Aloe, L. Topical treatment with nerve growth factor for neurotrophic keratitis. Ophthalmology 2000, 107, 1347–1351. [Google Scholar] [CrossRef]
- Park, J.H.; Kang, S.-S.; Kim, J.Y.; Tchah, H. Nerve Growth Factor Attenuates Apoptosis and Inflammation in the Diabetic Cornea. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6767. [Google Scholar] [CrossRef] [PubMed]
- Pflugfelder, S.C.; Massaro-Giordano, M.; Perez, V.L.; Hamrah, P.; Deng, S.X.; Espandar, L.; Foster, C.S.; Affeldt, J.; Seedor, J.A.; Afshari, N.A.; et al. Topical Recombinant Human Nerve Growth Factor (Cenegermin) for Neurotrophic Keratopathy: A Multicenter Randomized Vehicle-Controlled Pivotal Trial. Ophthalmology 2020, 127, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Mastropasqua, L.; Lanzini, M.; Dua, H.S.; Uffizi, A.D.; Di Nicola, M.; Calienno, R.; Bondì, J.; Said, D.G.; Nubile, M. In Vivo Evaluation of Corneal Nerves and Epithelial Healing After Treatment with Recombinant Nerve Growth Factor for Neurotrophic Keratopathy. Am. J. Ophthalmol. 2020, 217, 278–286. [Google Scholar] [CrossRef]
- Stern, G.A.; Schemmer, G.B.; Farber, R.D.; Gorovoy, M.S. Effect of Topical Antibiotic Solutions on Corneal Epithelial Wound Healing. Arch. Ophthalmol. 1983, 101, 644–647. [Google Scholar] [CrossRef] [PubMed]
- Ogut, D.; Reel, B.; Korkmaz, C.G.; Arun, M.Z.; Micili, S.C.; Ergur, B.U. Doxycycline down-regulates matrix metalloproteinase expression and inhibits NF-kappaB signaling in LPS-induced PC3 cells. Folia Histochem. Cytobiol. 2017, 54, 171–180. [Google Scholar] [CrossRef]
- Sekundo, W.; Augustin, A.J.; Strempel, I. Topical allopurinol or corticosteroids and acetylcysteine in the early treatment of experimental corneal alkali burns: A pilot study. Eur. J. Ophthalmol. 2002, 12, 366–372. [Google Scholar] [CrossRef]
- Parker, A.V.; Williams, R.N.; Paterson, C.A. The effect of sodium citrate on the stimulation of polymorphonuclear leukocytes. Investig. Ophthalmol. Vis. Sci. 1985, 26, 1257–1261. [Google Scholar]
- Guerra, M.; Marques, S.; Gil, J.Q.; Campos, J.; Ramos, P.; Rosa, A.M.; Quadrado, M.J.; Murta, J.N. Neurotrophic Keratopathy: Therapeutic Approach Using a Novel Matrix Regenerating Agent. J. Ocul. Pharmacol. Ther. 2017, 33, 662–669. [Google Scholar] [CrossRef]
- Tummanapalli, S.S.; Willcox, M.D.; Issar, T.; Yan, A.; Pisarcikova, J.; Kwai, N.; Poynten, A.M.; Krishnan, A.V.; Markoulli, M. Tear film substance P: A potential biomarker for diabetic peripheral neuropathy. Ocul. Surf. 2019, 17, 690–698. [Google Scholar] [CrossRef]
- Stuard, W.L.; Titone, R.; Robertson, D.M. Tear Levels of Insulin-Like Growth Factor Binding Protein 3 Correlate With Subbasal Nerve Plexus Changes in Patients With Type 2 Diabetes Mellitus. Investig. Ophthalmol. Vis. Sci. 2017, 58, 6105–6112. [Google Scholar] [CrossRef]
- Zheng, W.-H.; Kar, S.; Doré, S.; Quirion, R. Insulin-like growth factor-1 (IGF-1): A neuroprotective trophic factor acting via the Akt kinase pathway. J. Neural. Transm. Suppl. 2000, 60, 261–272. [Google Scholar]
- Wu, Y.-C.; Buckner, B.R.; Zhu, M.; Cavanagh, H.D.; Robertson, D.M. Elevated IGFBP3 Levels in Diabetic Tears: A Negative Regulator of IGF-1 Signaling in the Corneal Epithelium. Ocul. Surf. 2012, 10, 100–107. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Iyengar, M.F.; Soto, L.F.; Requena, D.; Ruiz-Alejos, A.O.; Huaylinos, Y.; Velasquez, R.; Bernabe-Ortiz, A.; Gilman, R.H. Tear biomarkers and corneal sensitivity as an indicator of neuropathy in type 2 diabetes. Diabetes Res. Clin. Pr. 2020, 163, 108143. [Google Scholar] [CrossRef]
- Ueno, H.; Hattori, T.; Kumagai, Y.; Suzuki, N.; Ueno, S.; Takagi, H. Alterations in the Corneal Nerve and Stem/Progenitor Cells in Diabetes: Preventive Effects of Insulin-Like Growth Factor-1 Treatment. Int. J. Endocrinol. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhou, Q.; Luo, Y.; Nguyen, T.; Rosenblatt, M.I.; Guaiquil, V.H. Semaphorin3A induces nerve regeneration in the adult cornea-a switch from its repulsive role in development. PLoS ONE 2018, 13, e0191962. [Google Scholar] [CrossRef]
- Namavari, A.; Chaudhary, S.; Ozturk, O.; Chang, J.-H.; Yco, L.; Sonawane, S.; Katam, N.; Khanolkar, V.; Hallak, J.; Sarkar, J.; et al. Semaphorin 7a Links Nerve Regeneration and Inflammation in the Cornea. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4575–4585. [Google Scholar] [CrossRef] [PubMed]
- Davidson, E.P.; Coppey, L.J.; Yorek, M. Early Loss of Innervation of Cornea Epithelium in Streptozotocin-Induced Type 1 Diabetic Rats: Improvement with Ilepatril Treatment. Investig. Ophthalmol. Vis. Sci. 2012, 53, 8067–8074. [Google Scholar] [CrossRef][Green Version]
- Davidson, E.P.; Holmes, A.; Coppey, L.J.; Yorek, M. Effect of combination therapy consisting of enalapril, α-lipoic acid, and menhaden oil on diabetic neuropathy in a high fat/low dose streptozotocin treated rat. Eur. J. Pharmacol. 2015, 765, 258–267. [Google Scholar] [CrossRef]
- Shevalye, H.; Yorek, M.S.; Coppey, L.J.; Holmes, A.; Harper, M.M.; Kardon, R.H.; Yorek, M. Effect of enriching the diet with menhaden oil or daily treatment with resolvin D1 on neuropathy in a mouse model of type 2 diabetes. J. Neurophysiol. 2015, 114, 199–208. [Google Scholar] [CrossRef]
- Zagon, I.S.; Sassani, J.W.; Immonen, J.A.; McLaughlin, P.J. Ocular surface abnormalities related to type 2 diabetes are reversed by the opioid antagonist naltrexone. Clin. Exp. Ophthalmol. 2013, 42, 159–168. [Google Scholar] [CrossRef]
- Zagon, I.S.; Klocek, M.S.; Sassani, J.W.; McLaughlin, P.J. Dry Eye Reversal and Corneal Sensation Restoration With Topical Naltrexone in Diabetes Mellitus. Arch. Ophthalmol. 2009, 127, 1468. [Google Scholar] [CrossRef] [PubMed]
- Matlock, H.G.; Qiu, F.; Malechka, V.; Zhou, K.; Cheng, R.; Benyajati, S.; Whelchel, A.; Karamichos, D.; Ma, J.X. Pathogenic Role of PPARalpha Downregulation in Corneal Nerve Degeneration and Impaired Corneal Sensitivity in Diabetes. Diabetes 2020, 69, 1279–1291. [Google Scholar] [CrossRef] [PubMed]
- Winkler, M.A.; Dib, C.; Ljubimov, A.V.; Saghizadeh, M. Targeting miR-146a to Treat Delayed Wound Healing in Human Diabetic Organ-Cultured Corneas. PLoS ONE 2014, 9, e114692. [Google Scholar] [CrossRef] [PubMed]
- Saghizadeh, M.; Epifantseva, I.; Hemmati, D.M.; Ghiam, C.A.; Brunken, W.J.; Ljubimov, A.V. Enhanced Wound Healing, Kinase and Stem Cell Marker Expression in Diabetic Organ-Cultured Human Corneas Upon MMP-10 and Cathepsin F Gene Silencing. Investig. Ophthalmol. Vis. Sci. 2013, 54, 8172–8180. [Google Scholar] [CrossRef]
- Saghizadeh, M.; Dib, C.M.; Brunken, W.J.; Ljubimov, A.V. Normalization of wound healing and stem cell marker patterns in organ-cultured human diabetic corneas by gene therapy of limbal cells. Exp. Eye Res. 2014, 129, 66–73. [Google Scholar] [CrossRef]
- Di, G.; Du, X.; Qingjun, Z.; Zhao, X.; Duan, H.; Xianli, D.; Xie, L.; Zhou, Q. Mesenchymal Stem Cells Promote Diabetic Corneal Epithelial Wound Healing Through TSG-6–Dependent Stem Cell Activation and Macrophage Switch. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4344–4354. [Google Scholar] [CrossRef]
- Lee, B.H.; Lee, G.J.; Park, Y.J.; Lee, K.W. Clinical Research on Surgical Treatment for Double-Head Pterygium. J. Korean Ophthalmol. Soc. 2010, 51, 642–650. [Google Scholar] [CrossRef]
- Williams, B.M.; Borroni, D.; Liu, R.; Zhao, Y.; Zhang, J.; Lim, J.; Ma, B.; Romano, V.; Qi, H.; Ferdousi, M.; et al. An artificial intelligence-based deep learning algorithm for the diagnosis of diabetic neuropathy using corneal confocal microscopy: A development and validation study. Diabetol. 2019, 63, 419–430. [Google Scholar] [CrossRef]

| Categories | Neuromediator | Mechanism of Action | Functions [1,37,54] |
|---|---|---|---|
| Neurotrophins | NGF | The binding of NGF with TrkA decreases NF-κB nuclear translocation, inhibits GSK3 activity, as well as enhances the activation of the PI3K/Akt pathway to elicit downstream events [51]. | (1) Maintains neural homeostasis and promotes corneal neuronal regeneration. (2) Reduction of neuroinflammation. (3) Maintains corneal nerve density and modulates corneal sensitivity. (4) Corneal nociception. (5) Enhances corneal wound healing by stimulating epithelial cell migration, colony formation and proliferation. |
| NT-3 | NT-3 interacts with TrkC and activates GTPase, Ras, phosphatidylinositol 3-kinase and PLC-g1 to regulate neuronal survival and differentiation via activation of mitogen-activated protein kinases [55]. | (1) Essential factor of sensory and sympathetic corneal neurons. (2) Regulates corneal neuronal regeneration. | |
| Neuropeptides | SP | SP acts on Neurokinin-1 receptor and activates EGFR together with Sirt1 and Akt-signalling pathways to promote corneal epithelial wound healing [56]. | (1) Modulator of corneal epithelial proliferation, migration and adhesion. (2) Inhibits apoptosis of corneal epithelial cells. (3) Recovery of corneal sensation. (4) Improvement of mitochondrial function in corneal epithelial cells. |
| CGRP | CGRP is released from corneal sensory neurons in response to pain stimulus, and it upregulates the expression IL-8, which is a neutrophil chemotactic protein [57]. | (1) Maintenance of corneal epithelial integrity by facilitating epithelial cell migration and differentiation. (2) Corneal nociception. (3) Vasoactive effects. (4) Modulator of innate immunity. | |
| NPY | NPY mediates its response by binding to NPY 2 receptor that inhibits adenylate cyclase activity and increases intracellular Ca2+ levels through pertussis toxin-sensitive G proteins [58]. | (1) Stimulator of angiogenesis and angiogenesis-dependent wound healing. (2) Anti-inflammatory effects on the cornea. | |
| VIP | VIP performs its functions by downregulating pro-inflammatory and upregulating anti-inflammatory Toll-like receptors in a cAMP-dependent fashion [59]. | (1) Promotes corneal neuronal regeneration. (2) Anti-inflammatory effects on the cornea. (3) Promotes neurotrophin production. | |
| Neurotransmitters | Catecholamines (epinephrine and norepinephrine) | Catecholamines bind to adrenergic receptors present on the plasma membrane of effector cells. This would either promote or inhibit adenylyl cyclase-mediated intracellular cAMP production, or influence PKC signalling mechanism, to modulate downstream events [60]. | (1) Corneal epithelial cell migration and proliferation. (2) Transcellular transport. |
| Ach | Ach activates both muscarinic and nicotinic receptors, allowing gated ion channels to open for the influx of Na+ and Ca2+ and efflux of K+. It also regulates the activities of phosphatases and protein kinases [61]. | (1) Maintains an ionic gradient during nerve impulse conduction. (2) Corneal epithelial cell DNA synthesis and epithelial cell migration. (3) Corneal stromal keratocytes proliferation. (4) Inhibits corneal fibrosis and apoptosis. |
| 1. Genetic Causes [64] |
| Familial corneal hypoaesthesia |
| Mobius syndrome |
| Goldenhar-Gorlin syndrome |
| Familial dysautonomia (Riley-Day Syndrome) |
| 2. Ocular Causes |
| Chemical burns |
| Post-herpetic infections |
| Acanthamoeba infections |
| Toxicity of topical anaesthetics and topical drugs (e.g., diclofenac sodium, etc.) |
| Chronic ocular surface inflammation |
| Contact lens wear |
| Corneal dystrophies (Lattice or granular dystrophies) |
| Orbital Neoplasia |
| 3. Systemic Causes |
| DM (diabetes mellitus) |
| Multiple sclerosis |
| Alzheimer’s disease |
| Parkinsonism |
| Leprosy |
| Vitamin A Deficiency |
| Amyloidosis |
| Neoplasms and aneurysms of the central nervous system |
| Cerebral vascular accidents |
| 4. Surgical Causes [54] |
| Refractive surgery [65] |
| Corneal cross-linking |
| Panretinal photocoagulation for diabetic retinopathy |
| Post-cataract surgery |
| Post-vitrectomy |
| Post-neurosurgical procedures (e.g., surgery for trigeminal neuralgia, acoustic neuroma etc.) |
| Iatrogenic injury to the trigeminal nerve |
| Therapeutic Agent | Mechanism of Action | Route of Administration | Experimental Evidence |
|---|---|---|---|
| IGF-1 | Growth factor | Topical | IGF-1 treatment protected against the corneal nerve damage and improved corneal subbasal nerve density in type 2 diabetic mice [127]. |
| Sema3a | Membrane-bound axon-guidance protein | Intrastromal Sema3a pellet implantation | Sema3a induced neuronal regeneration in the injured mice corneas [128]. |
| Sema7a | Membrane-bound axonal growth promoter and immune regulator | Sema7a pellet implantation under the corneal flap after lamellar nerve-transection surgery | Sema7a supplementation promoted nerve regeneration and influenced inflammatory processes in the mice cornea [129]. |
| Ilepatril | Vasopeptidase inhibitor | Systemic | Ilepatril prevented the loss of motor and sensory nerve conduction velocity, as well as the decrease in intraepidermal nerve density, in type 1 diabetic rats [130]. |
| Enalapril | ACE inhibitor | Systemic | Treating diabetic rats with the combination of enalapril, α-lipoic acid and menhaden oil reversed diabetic corneal and peripheral neuropathy [131]. |
| Resolvin-D1 | Anti-inflammatory eicosanoid | Systemic | Resolvin-D1, together with menhaden (fish) oil supplementation, reduced the degeneration of corneal and peripheral nerves in diabetic rats [132]. |
| Naltrexone | Long-acting opioid antagonist | Topical/Systemic | Naltrexone promoted corneal epithelial wound repair and restored corneal sensitivity in type 1 and 2 diabetic rats [133,134]. |
| Fenofibrate | PPARα agonist | Systemic | Oral administration of fenofibrate protected the corneal nerves from degeneration in streptozotocin-induced diabetic rats and mice [135]. |
| miR-146Aa | Regulates gene expression by repressing translation | Topical | Targeted inhibition of miR-146Aa promoted wound healing by enhancing the migration of limbal epithelial cells in human diabetic corneas; however, this has been observed in organ cultures only [136]. |
| Experimental Gene Therapy | c-met proto-oncogene overexpression and/or cathepsin F and MMP-10 gene silencing | Vector delivery | Adenoviral gene therapy enhanced corneal epithelial wound healing and normalised stem cell marker expression in organ-cultured human diabetic corneas [137,138]. |
| MSCs | TSG-6 mediated corneal epithelial wound healing | Subconjunctival injections of bone marrow-derived MSCs | Locally transplanted MSCs promoted the healing of corneal epithelial wounds in diabetic mice via activation of corneal epithelial stem/progenitor cells [139]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansoor, H.; Tan, H.C.; Lin, M.T.-Y.; Mehta, J.S.; Liu, Y.-C. Diabetic Corneal Neuropathy. J. Clin. Med. 2020, 9, 3956. https://doi.org/10.3390/jcm9123956
Mansoor H, Tan HC, Lin MT-Y, Mehta JS, Liu Y-C. Diabetic Corneal Neuropathy. Journal of Clinical Medicine. 2020; 9(12):3956. https://doi.org/10.3390/jcm9123956
Chicago/Turabian StyleMansoor, Hassan, Hong Chang Tan, Molly Tzu-Yu Lin, Jodhbir S. Mehta, and Yu-Chi Liu. 2020. "Diabetic Corneal Neuropathy" Journal of Clinical Medicine 9, no. 12: 3956. https://doi.org/10.3390/jcm9123956
APA StyleMansoor, H., Tan, H. C., Lin, M. T.-Y., Mehta, J. S., & Liu, Y.-C. (2020). Diabetic Corneal Neuropathy. Journal of Clinical Medicine, 9(12), 3956. https://doi.org/10.3390/jcm9123956





