Abstract
Infections are among the most frequent and challenging events in diabetic foot ulcers (DFUs). Pathogenic bacteria growing in biofilms within host tissue are highly tolerant to environmental and chemical agents, including antibiotics. The present study was aimed at assessing the use of silver sulfadiazine (SSD) for wound healing and infection control in 16 patients with DFUs harboring biofilm-growing Staphylococcus aureus and Pseudomonas aeruginosa. All patients received a treatment based on a dressing protocol including disinfection, cleansing, application of SSD, and application of nonadherent gauze, followed by sterile gauze and tibio-breech bandage, in preparation for toilet surgery after 30 days of treatment. Clinical parameters were analyzed by the T.I.M.E. classification system. In addition, the activity of SSD against biofilm-growing S. aureus and P. aeruginosa isolates was assessed in vitro. A total of 16 patients with S. aureus and P. aeruginosa infected DFUs were included in the study. Clinical data showed a statistically significant (p < 0.002) improvement of patients’ DFUs after 30 days of treatment with SSD with significant amelioration of all the parameters analyzed. Notably, after 30 days of treatment, resolution of infection was observed in all DFUs. In vitro analysis showed that both S. aureus and P. aeruginosa isolates developed complex and highly structured biofilms. Antibiotic susceptibility profiles indicated that biofilm cultures were significantly (p ≤ 0.002) more tolerant to all tested antimicrobials than their planktonic counterparts. However, SSD was found to be effective against fully developed biofilms of both S. aureus and P. aeruginosa at concentrations below those normally used in clinical preparations (10 mg/mL). These results strongly suggest that the topical administration of SSD may represent an effective alternative to conventional antibiotics for the successful treatment of DFUs infected by biofilm-growing S. aureus and P. aeruginosa.
1. Introduction
Diabetic foot ulcer (DFU) is the most common manifestation of diabetes [1,2]. Treatments available for DFUs include debridement of wound necrotic tissues, wound dressings, administration of systemic and topical antimicrobial agents, and amputation as the last treatment option [3,4]. The number of diabetic patients undergoing major foot amputations has increased in recent years [5,6]. Besides, after a lower extremity amputation, half of the patients die or lose the contralateral limb within five years [2,7].
Infections in DFUs are the primary cause of lower-extremity amputation, and although most infections remain superficial, approximately 25% will spread from the skin to deeper subcutaneous tissues and bone [8,9]. Treatment of DFU infections are particularly challenging due to the presence of comorbidities, poor vascularization (determining reduced drug distribution in the lesional area), and microbial cell growth within a biofilm [10,11,12,13]. Indeed, biofilm acts as an important predisposing factor to the chronicity of a nonhealing ulcer by providing a protective environment against phagocytosis and decreasing the diffusion of antibiotics and antimicrobial agents [14,15,16,17,18]. Besides, previous studies have shown that the biofilm lifestyle promotes the horizontal transfer of virulence genes and the development of multidrug-resistant (MDR) organisms [19,20,21,22,23].
Directly related to the ability to form biofilms is the issue of antimicrobial tolerance [24,25]. Antibiotic tolerance is not mediated by the acquisition of antibiotic resistance genes [26] but rather is a transient phenotype that renders the bacterial cells within a biofilm highly refractory to antimicrobial therapy, allowing a subpopulation of cells to persist in the wound environment [27]. Despite the growing interest, biofilm production is not routinely analyzed in clinical microbiology testing [28,29,30,31]. Thus, considering the refractory nature of biofilm, the clinical management of infections caused by biofilm-growing bacteria, such those generally found in DFUs, requires the introduction of more targeted antimicrobial strategies [32,33].
Silver has a broad-spectrum antimicrobial activity against multidrug-susceptible and multidrug-resistant strains like Pseudomonas aeruginosa, extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli, methicillin-resistant Staphylococcus aureus (MRSA), and vancomycin-resistant S. aureus (VRSA) [34,35,36,37,38]. Silver is toxic to microorganisms, affecting respiratory enzymes and components of the microbial electron transport system [39,40,41]. Besides, silver ions exert a bactericidal mechanism by binding to bacterial DNA and interfering with the transcription and replication processes [39,42]. Different formulations containing silver have been shown to eradicate bacterial biofilm in burns and slow-healing wounds [29,43,44,45,46]. Specifically, silver sulfadiazine (SSD) has proved to be an effective alternative to conventional antimicrobials, mainly when used topically in high concentrations directly at the site of infection [29,44,46].
The present study was aimed at evaluating the use of SSD for wound healing and infection control in patients with chronic DFUs infected with S. aureus and P. aeruginosa biofilms.
2. Experimental Section
2.1. Ethics, Patients, and Samples
The Central Ethics Committee I.R.C.C.S Lazio granted ethical approval for this study (Prot. CE/1016/15—15 December 2015, trials registry N. 730/15). Adult patients (9 females and 7 males; average age 63) with type 2 diabetes mellitus, all under insulin therapy, according to individual regimens, with a chronic DFU (>3 months) infected by S. aureus or P. aeruginosa, were recruited over a period between January 2017 and December 2019 at the Department of Plastic and Reconstructive Surgery, University of Rome “Tor Vergata”, and the IRCCS San Gallicano Dermatological Institute of Rome, Italy. Certain cases were complicated by diabetic nephropathy, retinopathy renal failure, and cardiovascular disease. The presence of the infection was defined according to specified guidelines [47]. All patients underwent vascular evaluation with Doppler vascular ultrasound in the absence of an indication of immediate surgical intervention. The exclusion criteria were as follows: patients diagnosed for cancer, patients who required vascular surgery at the time of admission or had received vascular surgery < 6 month before hospital admission, patient who had received immunosuppressive therapy, systemic or topical antimicrobial therapy 2 weeks before enrolment, patients who were discharged early or discontinued the therapy, were excluded from the study. One swab per patient was collected aseptically at baseline (T0—untreated patient) and after 30 days of treatment. Swabs were immediately transported to the microbiology laboratory and processed within 2 hours from collection [47].
All patients were treated with the complex dressing operative protocol in preparation for toilet surgery performed after 30 days of treatment. The protocol included disinfection; cleansing; application of SSD 1%; and application of nonadherent gauze, sterile gauze, and tibio-breech bandage. The dressing was changed every 72 h.
During the treatment period, different parameters including nonviable tissue (T), infection and/or inflammation (I), moisture imbalance (M), and nonadvancing edge of wound (E) were evaluated, according to the T.I.M.E. protocol [48,49,50], which allows for a systematic review of the characteristics of the lesion.
We used a modified T.I.M.E. protocol aimed at identifying some objective changes in tissue regeneration within each criterion and assigning them a score to classify the improvement to allow for a statistical assessment. Criterion T (bottom of the lesion): (1) presence of necrotic areas, (2) fibrinous bottom, (3) granulating bottom, (4) fund reduction with initial re-epithelialization. Criterion I (presence of clinically evident infection and inflammation): (1) present, (2) absent. Criterion M (moisture balance): (1) absent, (2) low, (3) medium, (4) high. Criterion E (edge of wound): (1) hyperkeratotic, (2) excoriated, (3) macerated, (4) undermined, (5) integrate.
2.2. Histological Evaluation
Incisional punch biopsies of ulcers (3 mm in diameter) were obtained at baseline (pretreatment, T0) and after 7 and 30 days of treatment with SSD. Microscopic evaluation of routinely hematoxylin–eosin-stained paraffin sections was performed to verify the healing process, and images were acquired using a digital camera (E600 Eclipse, Nikon, Tokyo, Japan).
2.3. Microbiological Assessment
Specimen collection and bacterial isolation from DFUs were performed as previously described [14]. S. aureus or P. aeruginosa were identified by the automatic VITEK 2 system (bioMérieux, Marcy-l’Étoile, France) and by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF, Bruker Daltonics, Bremen, Germany) [14,51].
2.4. Biofilm Production
S. aureus or P. aeruginosa strains, isolated from DFUs, were analyzed for their ability to produce biofilm by the clinical BioFilm Ring Test (cBRT) (Biofilm Control, Saint Beauzire, France) as previously described [31]. Each strain was analyzed in duplicate, and experiments were repeated three times.
2.5. Antimicrobial Susceptibility of Planktonic- and Biofilm-Grown Strains
The antimicrobial susceptibility testing (AST) was performed by the broth microdilution test (Thermo Scientific, Waltham, MA, USA) for the definition of the minimum inhibitory concentration (MIC) criteria, according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST Clinical Breakpoint Table v 10.0). Biofilms of S. aureus or P. aeruginosa were grown in 96-well plates (Corning Inc., Corning, NY, USA) for the definition of the minimal biofilm-eradication concentration (MBEC) [25,52]. Results were interpreted according to the EUCAST Clinical Breakpoint (Table v 10.0). Briefly, wells were inoculated with approximately 1 × 105 cells in 100 μL of BHI medium and incubated for 22 h at 37 °C to allow biofilm formation. Subsequently, the medium was removed, and the wells were washed with 100 μL of sterile distilled water to remove nonadherent cells. The preformed biofilms were treated with different antibiotics and SSD at predefined concentrations in fresh BHI medium and incubated for 22 h at 37 °C.
The metabolic activity of the treated planktonic and biofilm cultures was evaluated using the CellTiter-Blue staining (Promega Corporation, Madison, WI, USA) [53,54,55]. After 60 min of incubation, resorufin production was measured with the plate reader PhD lx System (Bio-Rad Laboratories, Hercules, CA, USA) using an excitation peak wavelength of 550 nm and an emission wavelength of 620 nm. Controls were carried out by replacing the culture medium with fresh BHI without SSD, and wells with the noninoculated medium were used as blanks. Percent resazurin reduction was calculated using the following formula: (experimental well absorbance – negative control absorbance)/positive control absorbance) × 100.
Tolerance factor (TF) calculation for SSD was adapted from Stewart [56] and measured according to the following equation:
where RF refers to a calculated reduction of relative fluorescence according to the resazurin viability assays for S. aureus (Sa) and P. aeruginosa (Pa) at different concentrations of SSD.
TF = (RFSa/RFPa)
Measurements were performed in triplicate for at least three independent experiments, and the results are expressed as mean and standard deviation.
2.6. Biofilm Imaging
Biofilms were grown in μ-Slide (Ibidi, Gräfelfing, Germany) inoculated with ~1 × 105 cells in 500 μL of fresh BHI medium and incubated for 48 h at 37 °C. The culture medium was changed after 24 h of biofilm growth. Then, biofilms were treated with different concentrations of SSD in fresh BHI medium and incubated for an additional 22 h at 37 °C. Biofilms were stained using the LIVE/DEAD BacLight Bacterial Viability Kit (Life Technologies, New York, NY, USA), according to supplier specifications and examined with Apotome Fluorescence Microscope (Carl Zeiss International, Oberkochen, Germany). Data were analyzed with the AxioVision 4.8 software [57].
2.7. Statistical Analysis
Statistical analysis was performed using the chi-square test when applicable. The Wilcoxon test was used to compare the distributions of cases at T0 and T1, and the Mann–Whitney U-test was used for comparing the features of patients with S. aureus with those of patients with P. aeruginosa. Statistical analyses were carried out using IBM SPSS v.21 statistics software. Differences were considered statistically significant for values of p < 0.05 (*), p < 0.01 (**), and p < 0.001 (***).
3. Results
A total of 16 participants with infected DFUs and matching the study inclusion criteria were enrolled within the 2017–2019 timeframe. The study group included 7 (44%) males and 9 (56%) females with a mean age of 63.6 years (SD: ±13.7). The most common location for DFUs was on the forefoot area (N6; 37.4%), followed by the heel (N4; 25%), the mid-foot area (N3; 18.8%), and the toe tips (N3; 18.8%). Ulcer characteristics, according to the T.I.M.E. classification system, are summarized in Table 1. All the study participants were treated with SSD after careful disinfection and cleansing of the wound bed and application of nonadherent gauze, sterile gauze, and tibio-breech bandage every 72 h.

Table 1.
Clinical characteristics of the diabetic foot ulcers (DFUs) reported as absolute frequencies and percentages (%) at baseline (T0) and 30 days. The distributions were compared for each variable using the Wilcoxon test, and the relevant p-values are reported.
Data showed a statistically significant improvement of patients’ ulcers after 30 days of treatment according to the T.I.M.E. (Table 1). The wound healing and the decrease in wound size are also shown in Figure 1. Notably, the resolution of infection was observed after 30 days of treatment with SSD in all the DFUs. Furthermore, no significant differences were found between patients with DFUs infected by S. aureus and P. aeruginosa at T0 in terms of tissue (p = 0.264), infection (p = 0.999), exudate (p = 0.777), and the edge of the wound (p = 0.375). Similarly, no significant differences were found after 30 days among patients for tissue (p = 0.288), infection (p = 0.999), exudate (p = 0.814), and the edge of the wound (p = 0.535). No adverse events related to the SSD treatment were reported during the study period. A representative image of wound healing and decrease in wound size is shown in Figure 1.
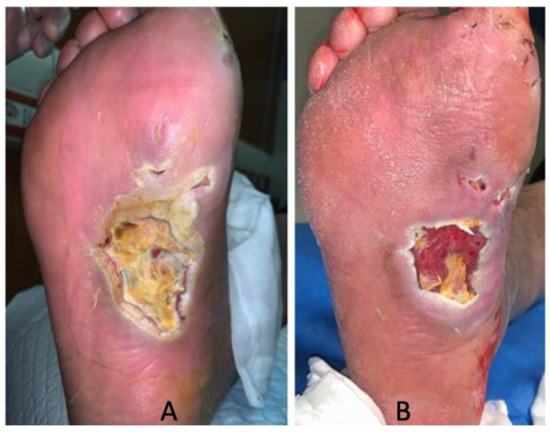
Figure 1.
DFU healing. (A) Wound at baseline (T0) and (B) after 30 days of treatment.
3.1. Microscopic Evaluation
Representative microphotographs of hematoxylin–eosin staining, describing the evolution of the healing process of an infected DFU, are shown in Figure 2. An evident healing process was documented after 7 and 30 days of treatment as compared with the pretreatment biopsy, which showed an intense inflammatory infiltrate, cellular debris, and edema. Microbial clusters were visible along the epithelial borderline. After treatment, the healing process was accompanied by a reduction of the inflammatory infiltrate and microbial agents and an increase of newly formed dermal tissue.
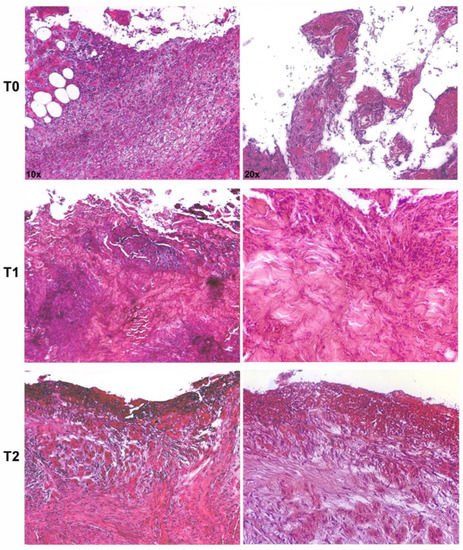
Figure 2.
Representative microphotographs of hematoxylin–eosin-stained paraffin sections of skin biopsies show progressive healing of an infected ulcer treated with silver sulfadiazine (SSD). The pretreatment biopsy (T0) presented an intense inflammatory infiltrate in the dermis and evident clusters of microbial agents along the epithelial borderline. After 7 (T1) and 30 days (T2) of treatment, a significant reduction of the inflammatory infiltrate and microbial agents were observed, with the deposition of new collagen (magnification: 10× and 20×).
3.2. Biofilm Production
A total of 16 strains, including eight S. aureus and eight P. aeruginosa, isolated from patients with DFUs, were analyzed. All the isolates were found to be strong biofilm-producers by the cBRT. Confocal microscopy analysis of the biofilms (Figure 3) was performed after 48 h of incubation to develop a mature biofilm. S. aureus isolates (Figure 3A,B) formed a uniform layer of biofilm of 25–40 μm. P. aeruginosa isolates (Figure 3C,D) formed either pronounced mushroom-shaped structures of 40–60 μm or a uniform layer of cells of 25–40 μm.
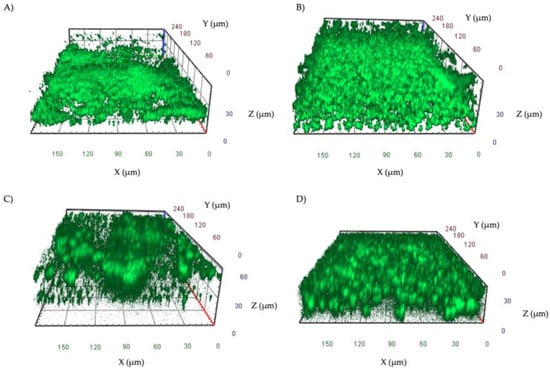
Figure 3.
Representative images of biofilms of S. aureus (A,B) and P. aeruginosa (C,D) isolates grown in IBIDI μ-slides for 48 h at 37 °C. Orthogonal sections displaying horizontal (z) and side views (x and y) of reconstructed 3D biofilm images are shown.
3.3. Antimicrobial Susceptibility Testing of Planktonic and Biofilm Cells
The antibiotic susceptibility profiles (AST) of S. aureus and P. aeruginosa isolates, determined according to EUCAST breakpoint guidelines, are summarized in Table 2. All the S. aureus isolates were found susceptible to daptomycin (MIC 0.25 to 1 mg/L), fusidic acid (MIC ≤0.5 mg/L), gentamicin (MIC ≤0.5 mg/L), linezolid (MIC 2 mg/L), teicoplanin (MIC ≤0.12 mg/L), tigecycline (MIC ≤0.12 to 0.25 mg/L), trimethoprim/sulfamethoxazole (TMP/SMX) (MIC ≤0.25 to 1 mg/L), and vancomycin (MIC ≤0.5 to 1 mg/L). Besides, seven (87.5%) S. aureus isolates were susceptible to oxacillin (MIC ≤0.25 mg/L), six (75%) to clindamycin (MIC ≤0.25 mg/L), five to erythromycin (MIC ≤0.25 to 1 mg/L), and one to benzylpenicillin (MIC 0.25 mg/L). Only one strain was classified as MRSA with an oxacillin MIC of 4. Notably, all P. aeruginosa isolates were susceptible to colistin (MIC ≤0.25 mg/L) and piperacillin/tazobactam (PIT) (MIC ≤4 to 8 mg/L), while seven (87.5%) isolates were susceptible to imipenem (MIC 0.25 to 1 mg/L) and six (75%) isolates were susceptible to amikacin (MIC ≤2 mg/L), cefepime (MIC ≤1 mg/L), ceftazidime (MIC 2 to 4 mg/L), ciprofloxacin (MIC ≤0.25 to 0.5 mg/L), and gentamicin (MIC ≤ 1 to 2 mg/L). Only one strain was classified as MDR, resulting as susceptible only to colistin (MIC ≤0.25 mg/L) and PIT (MIC ≤4 mg/L).

Table 2.
Antibiotic susceptibility profile (% of susceptible strains) of S. aureus and P. aeruginosa as obtained by the antimicrobial susceptibility testing (AST) and the anti-biofilm test (ABT). PIT, piperacillin/tazobactam; TMP/SMX, trimethoprim/sulfamethoxazole.
The antibiotic susceptibility of S. aureus and P. aeruginosa isolates in biofilm significantly (p < 0.001) differed from those gathered by AST (Table 1). In particular, of the S. aureus isolates analyzed in biofilm, two (25.0%) were susceptible to fusidic acid (MBEC 0.5 mg/mL), oxacillin (MBEC 0.25 to 1 mg/L), and teicoplanin (MBEC 1 mg/L), but all the isolates were found to be resistant to benzylpenicillin (MBEC >8 mg/L), TMP/SMX (MBEC >4 mg/L), and vancomycin (MBEC >4 mg/L). The P. aeruginosa isolates in the biofilm were found susceptible in two cases to amikacin (MBEC 4 mg/L) and gentamicin (MBEC 4 mg/L). Notably, all isolates were resistant to cefepime (MBEC >32 mg/L), ceftazidime (MBEC ≥32 mg/L), ciprofloxacin (MBEC 1 to >2 mg/L), and PIT (MBEC >128 mg/L).
3.4. Silver Sulfadiazine Susceptibility Testing of Planktonic and Biofilm Cells
The S. aureus and P. aeruginosa isolates in biofilm exhibited a considerable increase of antibiotic tolerance as compared to their planktonic counterparts. SSD has been suggested as an effective alternative for local treatment of wound infections, even against biofilm [43,48,58]. In this study, the SSD activity was initially evaluated by the broth microdilution test on planktonic isolates. Notably, all the S. aureus and P. aeruginosa isolates showed MIC ≤0.16 mg/L. The antimicrobial activity of SSD was further assessed in biofilm-growing cells. S. aureus isolates exhibited MBEC ranging from 1.25 to 2.5 mg/L, while P. aeruginosa showed MBEC values ranging from 0.16 to 0.31 mg/L. The results are summarized in Figure 3.
Planktonic cells of S. aureus and P. aeruginosa isolates exhibited a significant (p < 0.001) decrease in cell viability at 0.16 mg/mL of SSD when compared to the untreated controls. The viability of S. aureus in biofilm was significantly (p = 0.01) reduced by 36.8% in the presence of 1.25 mg/mL of SSD and experienced a severe (p < 0.001) reduction of 94.7% when exposed to a concentration of 2.5 mg/mL (Figure 4a). Notably, the resazurin assay showed that the viability of P. aeruginosa isolates was significantly (p < 0.001) reduced by 92.6% when biofilms were exposed to a concentration of 0.31 mg/mL of SSD (Figure 4b).
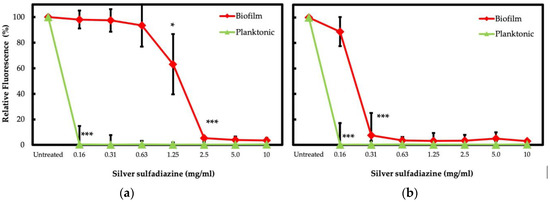
Figure 4.
Viability of bacterial isolates treated with SSD. S. aureus (a) and P. aeruginosa (b) were exposed to different concentrations of SSD for 24 h. Bacterial cells were incubated with the BHI medium in the presence of resazurin. The resorufin production was quantified by measuring fluorescence (relative fluorescence), relative to the untreated control, after 60 min of incubation for S. aureus and P. aeruginosa. p < 0.05 (*) and p < 0.001 (***).
The rate of killing by SSD was lower for S. aureus than P. aeruginosa (Table 3). This analysis was performed by adapting the calculation of tolerance factor (TF) described by Stewart [56]. Biofilm-growing S. aureus and P. aeruginosa isolates were found to be equally tolerant to SSD at 0.16 mg/mL. Conversely, S. aureus resulted in 26.7 and 20.0 times more tolerant than P. aeruginosa at 0.63 and 1.25 mg/mL. However, S. aureus and P. aeruginosa were both found highly susceptible at concentrations of SSD between 2.5 and 10 mg/mL.

Table 3.
Tolerance factor (TF) between S. aureus (Sa) and P. aeruginosa (Pa), calculated at different concentrations of SSD.
4. Discussion
DFU is a severe and frequent complication of diabetes mellitus worldwide and the most common cause of hospitalization in diabetic patients. Approximately half of DFUs become infected [8], and amputation is required in more than 15% of cases [9,59]. Systemic antibiotics are prescribed in the presence of clinical signs of DFU infection [60,61,62]. However, the resolution of infection after antibiotic treatment varies widely, with values ranging between 5.6% and 77.8% [63].
The presence of a microbial biofilm within the host tissue poses a significant clinical complication. Biofilm-associated infections exhibit high resistance to host defenses, often contributing to an excessive or inappropriate inflammatory response that, in turn, leads to further tissue damage and spreading of the infection [64,65]. The present study was aimed at assessing the use of SSD on DFUs infected by biofilm-producing S. aureus and P. aeruginosa in 16 diabetic patients. Clinical data showed that the application SSD after careful disinfection and cleansing of the wound bed allowed a significant reduction of the exudate and local infection signs, along with the preservation of the structural and anatomical characteristics of the treated areas. Notably, after 30 days of treatment, wound sampling gave negative microbial cultures in all patients, suggesting that SSD may represent a useful prophylactic and a broad-spectrum antimicrobial agent [35,36,37,40]. The histological images showed that the presurgery period was characterized by the presence of an intense inflammatory infiltrate, cellular debris, and edema in the dermis with evident groups of microbial aggregates along the epithelial border. After about two weeks of treatment, a reduction in the inflammatory infiltrate and microbial aggregates was observed, along with the deposition of new collagen. These results confirmed that the beneficial effects of the SSD on wound management are mostly correlated to both antimicrobial and anti-inflammatory activity [38].
The conventional AST revealed that the S. aureus and P. aeruginosa isolates were highly susceptible to most antibiotics tested. Specifically, S. aureus was found highly susceptible to daptomycin, fusidic acid, gentamicin, linezolid, teicoplanin, tigecycline, TMP/SMX, and vancomycin, with MIC values comparable to previous studies [66,67,68,69]. Notably, the MIC value for methicillin-susceptible S. aureus (MSSA) was ≤0.25 mg/L, and one strain was found resistant to oxacillin with MIC ≥4 mg/L. The increased prevalence of MRSA in DFUs has promoted a return to non-β-lactam antimicrobial agents, such as rifampicin, fusidic acid, and TMP/SMX [68]. Although these agents have proven to be effective in treating DFUs, they are believed to enhance antibiotic resistance [2]. Besides, dalbavancin showed good antimicrobial activity in diabetic foot infections, showing higher activity than vancomycin, daptomycin, and linezolid against MRSA and MSSA [66,70].
Colistin and PIT were found to be the most effective drugs against P. aeruginosa isolate, with MIC ≤0.25 mg/L and ≤4 to 8 mg/L, respectively. Imipenem was active against 87.5% of strains. Besides, 75% of P. aeruginosa isolates were susceptible to cefepime and ceftazidime. These findings are also consistent with the results reported by other studies from different countries showing that colistin, β-lactams, and aminoglycosides were effective against P. aeruginosa isolated from patients with diabetic foot infection [14,69,71,72]. Likewise, aminoglycosides and ciprofloxacin were effective against 75% of the isolates. A microbiological survey conducted in Italy on patients with DFUs showed comparable results in antibiotic susceptibility rates. Specifically, colistin was found to be the most effective antibiotic, with a susceptibility rate above 90% against P. aeruginosa isolates [68]. Besides, approximately 80% of P. aeruginosa strains were found to be susceptible to PIT, cefepime, and ceftazidime [68]. Previous studies also reported a comparable trend for PIT on P. aeruginosa isolates from DFUs [72,73,74]. Aminoglycosides were found effective in more than 74% of cases [68]. However, studies from Pakistan found that P. aeruginosa was more susceptible to quinolones but less susceptible to β-lactams [69,75]. Ciprofloxacin was considered to be effective against P. aeruginosa infections [74]. However, more recent studies showed that approximately 50% of P. aeruginosa isolates from diabetic wounds were resistant to this antibiotic [72]. This result may reflect substantial geographical variations in the use of antibiotics and antibiotic prescriptions.
The extraordinary tolerance to antimicrobial agents observed in vivo in patients with chronic DFU infections is apparently in contrast with the AST profiles gathered in this study. Biofilm formation by pathogenic bacteria is a characteristic hallmark of chronic DFU infections [76,77,78]. Biofilm has been reported in 77% of patients with DFUs, and biofilm-embedded cells were found to be more tolerant to antibiotic treatments than planktonic cells. Thus, antibiotic treatments based on planktonic cells’ susceptibility profiles may lead to recurrent and difficult-to-treat wound infections [29,79,80]. Despite its recognized importance, biofilm is not assessed in chronic wound infection, and the detection of the biofilm remains a difficult task in routine clinical laboratories.
The cBRT and confocal microscopy analysis showed that all the clinical isolates analyzed in this study developed complex, three-dimensional biofilm structures. Notably, the confocal microscopy images were highly consistent with the results obtained by the cBRT, revealing a biofilm matrix between 25 and 60 μm in height with all isolates (Figure 2). This result is in accordance with previous studies showing a high level of biofilm production for S. aureus and P. aeruginosa isolated from chronic DFUs [31,76,81].
The antibiotic concentration required to eradicate biofilm bacteria can be several orders of magnitude higher than that required for the same microorganism in the planktonic state [25,82,83,84]. The antibiotic susceptibility profiles do not consider the presence of biofilm-growing microorganisms and might not represent the bacterial drug susceptibility in vivo [85]. Thus, using the criteria recommended by the EUCAST for the determination of MIC, we evaluated the antimicrobial susceptibility profile of the biofilm-growing isolates. In this study, biofilm cultures of S. aureus and P. aeruginosa were found to be significantly (p < 0.001) more tolerant than their planktonic counterparts to all antibiotics tested. Specifically, S. aureus isolates were found to be fully tolerant to vancomycin and TMP/SMX when assessed in a biofilm. Fusidic acid, oxacillin, and teicoplanin were the most active drugs against S. aureus biofilm. However, MBEC values remained below breakpoints in only 25% of cases. The most effective antibiotics against and P. aeruginosa biofilm were amikacin and gentamicin, with MBEC values below the breakpoints in 25% of cases. The efficacy of gentamicin was previously demonstrated against S. aureus and P. aeruginosa biofilm in an in vitro model studying the effectiveness of different treatments for infected DFUs. However, this study concluded that gentamicin was active against biofilm as a topical antibiotic but inadequate when administered systemically [86]. After systemic administration, the wound’s antibiotic concentration is lower than that detected in the serum at any given time [87]. A previous study reported that vancomycin penetration into soft tissue is reduced in diabetic patients [88]. Besides, other reports have described a variable penetration of antibiotics into the soft tissue of diabetic patients [86,89,90,91]. Peripheral artery disease (PAD) is a significant risk factor in chronic wounds caused by reduced blood flow and immune involvement at the site of infection [92,93]. Studies of antibiotic concentrations in DFIs generally do not include patients with PAD; thus, the concentration of antibiotics reaching tissue may be even lower than reported [13]. The reduced penetration of antibiotics in the site of infection may offer selective pressure to promote antibiotic resistance [94,95]. Thus, in the presence of high-risk infected DFUs, it has been proposed that topical antimicrobial therapy may represent a more appropriate option to reduce bacterial bioburden and accelerate healing [96]. Local administration of SSD is considered effective in treating infected wounds [43,58,97]. Our data confirm the efficacy of SSD against planktonic S. aureus and P. aeruginosa isolates at MIC of ≤0.16 mg/L. Previous in vitro tests have demonstrated a strong antibacterial activity of SSD against S. aureus and P. aeruginosa strains at concentrations lower than those generally used in clinical preparations (10 mg/mL) [98]. The antimicrobial activity of SSD was further assessed in biofilm-growing cells. S. aureus isolates exhibited MBEC ranging from 1.25 to 2.5 mg/L, while P. aeruginosa showed MBEC values ranging from 0.16 to 0.31 mg/L. Previous works have proved that SSD is effective at eliminating S. aureus and P. aeruginosa biofilms at concentrations below 10 mg/mL [48,99,100]. Besides, it has also been observed that SSD concentrations between 5 and 10 mg/mL are effective against mature biofilms of P. aeruginosa [58]. The rate of killing by SSD was lower for S. aureus than P. aeruginosa. Biofilm-growing S. aureus and P. aeruginosa isolates were found to be equally tolerant to SSD at 0.16 mg/mL. Notably, S. aureus resulted in 26.7 and 20.0 times more tolerant than P. aeruginosa at 0.63 and 1.25 mg/mL.
Most studies have demonstrated that SSD is nontoxic. However, the overuse of SSD and silver derivatives can accumulate in the skin, causing skin irritation and argyria. Allergic contact dermatitis to SSD has been reported, although most of the toxic effects were related to the excipients. After absorption, silver has been found in different tissues, including the liver, kidney, heart, brain, eye, and other organs. Burn patients treated with SSD cream showed elevated serum silver (over 20 mg L−1). However, this occurred after prolonged exposure of leg ulcers and acute burns to 1% SSD [101]. Different in vitro studies have also described the concentration-dependent toxicity of silver in mammalian cell lines such as keratinocytes or fibroblasts [101]. This evidence suggests that the judicious use of silver-containing dressings is essential to limit toxicity and optimize wound healing.
A potential limitation of this study is the use of monocultures of S. aureus or P. aeruginosa that may not reflect the polymicrobial nature of most DFU infections [2,18,102,103,104]. Indeed, a polymicrobial population, particularly when embedded in a biofilm, may have a more structured biofilm and a significantly increased tolerance to antibiotics [56,105,106,107,108]. Besides, our study would also benefit from validation in a larger cohort of patients and from studying other types of bacteria in mono- or polymicrobial cultures. The diversity of the bacterial populations in DFUs is considered an important contributor to the chronicity of the ulcers [2,18,103,104]. However, in patients with chronic DFUs and under antibiotic therapy, like those enrolled in this study, monomicrobial infection is common. S. aureus and P. aeruginosa represent the most prevalent and clinically relevant pathogens associated with severe or even fatal infections [47,104,109,110,111,112,113]. Taken together, the findings presented in this study may provide relevant information for avoiding unnecessary or prolonged antibiotic therapy and addressing an appropriate targeting of therapeutic intervention in chronic DFUs.
5. Conclusions
The therapeutic protocol presented in this study was based on topical SSD application in preparation for the surgical toilet. Results showed a significant improvement after 30 days of treatment in all the T.I.M.E. parameters with a reduction of the local infection signs and optimal infection control. Besides, this study revealed that S. aureus and P. aeruginosa isolated from infected DFUs developed complex and highly structured biofilms in vitro. The choice of antibiotic therapy is generally based on the causative pathogens. However, in the presence of highly tolerant biofilm-growing bacteria, the antibiotic susceptibility profiles might not be representative of the bacterial drug susceptibility in vivo. SSD was found to be effective against fully developed biofilms of both S. aureus and P. aeruginosa at concentrations below those normally used in clinical preparations (10 mg/mL). The recognized importance of the microbial biofilm in chronic wound infections has led to the proposal of biofilm-based wound care (BBWC) [80]. This clinical guideline suggests a combination of treatment with a broad-spectrum antibiotic and application of a local antibiofilm agent accompanied by sharp debridement of the wound [80]. Our results further support the introduction of a BBWC protocol and provide the basis for the clinical validation of a novel diagnostic approach aimed at defining biofilm-specific eradication strategies for the management of chronic DFU infections in a personalized manner.
Author Contributions
Conceptualization, E.G.D.D., B.D.A., and L.T.; methodology, I.C., F.S., F.O., M.F.L.M.D., C.D.S., P.G., M.G.S., A.O., D.K., and G.C.; validation, G.P., F.P., and I.L.L.P.; data analysis, M.D., G.D., E.T., A.S., and T.K.; writing—original draft preparation, E.D., B.D., and L.T.; writing—review and editing, V.C. and F.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
This work was supported by the L’Associazione Nazionale Contro le Infezioni Ospedaliere (L’ANCIO).
Conflicts of Interest
Luigi Toma has consulted for SOFAR S.p.A. All other authors have no conflicts of interest to declare.
References
- Hamdy, O.; Ashrafzadeh, S.; Mottalib, A. Weight Management in Patients with Type 2 Diabetes: A Multidisciplinary Real-world Approach. Curr. Diab. Rep. 2018, 18, 66. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, B.A.; Berendt, A.R.; Cornia, P.B.; Pile, J.C.; Peters, E.J.; Armstrong, D.G.; Deery, H.G.; Embil, J.M.; Joseph, W.S.; Karchmer, A.W.; et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin. Infect Dis. 2012, 54, e132–e173. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Kim, W.H.; Lee, J.H.; Choi, M.S.S. Risk factors of treatment failure in diabetic foot ulcer patients. Arch. Plast. Surg. 2013, 40, 123–128. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brechow, A.; Slesaczeck, T.; Münch, D.; Nanning, T.; Paetzold, H.; Schwanebeck, U.; Bornstein, S.; Weck, M. Improving major amputation rates in the multicomplex diabetic foot patient: Focus on the severity of peripheral arterial disease. Ther. Adv. Endocrinol. Metab. 2013, 4, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.; Collier, A.; Townsend, E.M.; O’Donnell, L.E.; Abhijit, B.M.; Butcher, J.; Mackay, W.G.; Ramage, G.; Williams, C. One step closer to understanding the role of bacteria in diabetic foot ulcers: Characterizing the microbiome of ulcers. BMC Microbiol. 2016, 16, 54. [Google Scholar] [CrossRef] [PubMed]
- Sargen, M.R.; Hoffstad, O.; Margolis, D.J. Geographic variation in Medicare spending and mortality for diabetic patients with foot ulcers and amputations. J. Diabetes Complicat. 2013, 27, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Gemechu, F.W.; Seemant, F.; Curley, C.A. Diabetic foot infections. Am. Fam. Physician 2013, 88, 177–184. [Google Scholar]
- Lavery, L.A.; Armstrong, D.G.; Wunderlich, R.P.; Boulton, A.J.M.; Tredwell, J.L. Diabetic foot syndrome: Evaluating the prevalence and incidence of foot pathology in Mexican Americans and non-Hispanic whites from a diabetes disease management cohort. Diabetes Care 2003, 26, 1435–1438. [Google Scholar] [CrossRef]
- Glaudemans, A.W.; Uçkay, I.; Lipsky, B.A. Challenges in diagnosing infection in the diabetic foot. Diabet. Med. 2015, 32, 748–759. [Google Scholar] [CrossRef]
- Dowd, S.E.; Wolcott, R.D.; Sun, Y.; McKeehan, T.; Smith, E.; Rhoads, D. Polymicrobial nature of chronic diabetic foot ulcer biofilm infections determined using bacterial tag encoded FLX amplicon pyrosequencing (bTEFAP). PLoS ONE 2008, 3, e3326. [Google Scholar] [CrossRef]
- Adler, A.I.; Boyko, E.J.; Ahroni, J.H.; Smith, D.G. Lower-extremity amputation in diabetes. The independent effects of peripheral vascular disease, sensory neuropathy, and foot ulcers. Diabetes Care 1999, 22, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Uccioli, L.; Izzo, V.; Meloni, M.; Vainieri, E.; Ruotolo, V.; Giurato, L. Non-healing foot ulcers in diabetic patients: General and local interfering conditions and management options with advanced wound dressings. J. Wound Care 2015, 24, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Barwell, N.D.; Devers, M.C.; Kennon, B.; Hopkinson, H.E.; McDougall, C.; Young, M.J.; Robertson, H.M.A.; Stang, D.; Dancer, S.J.; Seaton, A.; et al. Diabetic foot infection: Antibiotic therapy and good practice recommendations. Int. J. Clin. Pract. 2017, 71, e13006. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, E.G.; Farulla, I.; Prignano, G.; Gallo, M.T.; Vespaziani, M.; Cavallo, I.; Sperduti, I.; Pontone, M.; Bordignon, V.; Cilli, L.; et al. Biofilm is a Major Virulence Determinant in Bacterial Colonization of Chronic Skin Ulcers Independently from the Multidrug Resistant Phenotype. Int. J. Mol. Sci. 2017, 18, 1077. [Google Scholar] [CrossRef]
- Suryaletha, K.; John, J.; Radhakrishnan, M.P.; George, S.; Thomas, S. Metataxonomic approach to decipher the polymicrobial burden in diabetic foot ulcer and its biofilm mode of infection. Int. Wound J. 2018, 15, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.L.; Hill, K.E.; Williams, D.W.; Hooper, S.J.; Thomas, D.W.; Costerton, J.W. A review of the scientific evidence for biofilms in wounds. Wound Repair Regen. 2012, 20, 647–657. [Google Scholar] [CrossRef]
- Wilson, M.; Devine, D.A. Medical Implications of Biofilms; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Malone, M.; Bjarnsholt, T.; McBain, A.J.; James, G.A.; Stoodley, P.; Leaper, D.; Tachi, M.; Schultz, G.; Swanson, T.; Wolcott, R.D. The prevalence of biofilms in chronic wounds: A systematic review and meta-analysis of published data. J. Wound Care 2017, 26, 20–25. [Google Scholar] [CrossRef]
- Kwon, A.S.; Park, G.C.; Ryu, S.Y.; Lim, D.H.; Lim, D.Y.; Choi, C.H.; Park, Y.; Lim, Y. Higher biofilm formation in multidrug-resistant clinical isolates of Staphylococcus aureus. Int. J. Antimicrob. Agents 2008, 32, 68–72. [Google Scholar] [CrossRef]
- Reiter, K.C.; Da Silva Paim, T.G.; De Oliveira, C.F.; D’Azevedo, P.A. High biofilm production by invasive multiresistant staphylococci. APMIS 2011, 119, 776–781. [Google Scholar] [CrossRef]
- Sanchez, C.J., Jr.; Mende, K.; Beckius, M.L.; Akers, K.S.; Romano, D.R.; Wenke, J.C.; Murray, C.K. Biofilm formation by clinical isolates and the implications in chronic infections. BMC Infect. Dis. 2013, 13, 47. [Google Scholar] [CrossRef]
- Qi, L.; Li, H.; Zhang, C.; Liang, B.; Li, J.; Wang, L.; Du, X.; Liu, X.; Qiu, S.; Song, H. Relationship between Antibiotic Resistance, Biofilm Formation, and Biofilm-Specific Resistance in Acinetobacter baumannii. Front. Microbiol. 2016, 7, 483. [Google Scholar] [CrossRef] [PubMed]
- Katongole, P.; Nalubega, F.; Florence, N.C.; Asiimwe, B.; Andia, I. Biofilm formation, antimicrobial susceptibility and virulence genes of Uropathogenic Escherichia coli isolated from clinical isolates in Uganda. BMC Infect. Dis. 2020, 20, 453. [Google Scholar] [CrossRef] [PubMed]
- Penesyan, A.; Gillings, M.; Paulsen, I.T. Antibiotic discovery: Combatting bacterial resistance in cells and in biofilm communities. Molecules 2015, 20, 5286–5298. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, E.G.; Rimoldi, S.G.; Cavallo, I.; D’Agosto, G.; Trento, E.; Cagnoni, G.; Palazzin, A.; Pagani, C.; Romeri, F.; De Vecchi, E.; et al. Microbial biofilm correlates with an increased antibiotic tolerance and poor therapeutic outcome in infective endocarditis. BMC Microbiol. 2019, 19, 228. [Google Scholar] [CrossRef]
- Keren, I.; Kaldalu, N.; Spoering, A.; Wang, Y.; Lewis, K. Persister cells and tolerance to antimicrobials. FEMS Microbiol. Lett. 2004, 230, 13–18. [Google Scholar] [CrossRef]
- Clinton, A.; Carter, T. Chronic Wound Biofilms: Pathogenesis and Potential Therapies. Lab. Med. 2015, 46, 277–284. [Google Scholar] [CrossRef]
- Kaeberlein, T.; Lewis, K.; Epstein, S.S. Isolating “uncultivable” microorganisms in pure culture in a simulated natural environment. Science 2002, 296, 1127–1129. [Google Scholar] [CrossRef]
- Bjarnsholt, T.; Kirketerp-Møller, K.; Jensen, P.Ø.; Madsen, K.G.; Phipps, R.; Krogfelt, K.; Høiby, N.; Givskov, M. Why chronic wounds will not heal: A novel hypothesis. Wound Repair Regen. 2008, 16, 2–10. [Google Scholar] [CrossRef]
- Macià, M.D.; Rojo-Molinero, E.; Oliver, A. Antimicrobial susceptibility testing in biofilm—Growing bacteria. Clin. Microbiol. Infect. 2014, 20, 981–990. [Google Scholar] [CrossRef]
- Di Domenico, E.G.; Toma, L.; Provot, C.; Ascenzioni, F.; Sperduti, I.; Prignano, G.; Gallo, M.T.; Pimpinelli, F.; Bordignon, V.; Bernardi, T.; et al. Development of an in vitro assay, based on the Biofilm Ring Test®, for rapid profiling of biofilm—Growing bacteria. Front. Microbiol. 2016, 7, 1429. [Google Scholar] [CrossRef]
- Kennedy, P.; Brammah, S.; Wills, E. Burns, biofilm and a new appraisal of burn wound sepsis. Burns 2010, 36, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.A.; Bjarnsholt, T.; Alhede, M. Biofilms in wounds: A review of present knowledge. J. Wound Care 2014, 23, 570–582. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.K.; Deshmukh, S.D.; Ingle, A.P.; Gade, A.K. Silver nanoparticles: The powerful nanoweapon against multidrug-resistant bacteria. J. Appl. Microbiol. 2012, 112, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Paddock, H.N.; Schultz, G.S.; Perrin, K.J.U.; Moldawer, L.L.; Wright, B.; Burrell, R.E.; Mozingo, D.W. Clinical assessment of silver-coated antimicrobial dressing on MMPs and cytokine levels in non-healing wounds. Wound Rep. Reg. 2002, 10, A45. [Google Scholar]
- Ulkur, E.; Oncul, O.; Karagoz, H.; Celikoz, B.; Cavuslu, S. Comparison of silver-coated dressing (Acticoat), chlorhexidine acetate 0.5% (Bactigrass), and silver sulfadiazine 1% (Silverdin) for topical antibacterial effect in Pseudomonas aeruginosa-contaminated, full-skin thickness burn wounds in rats. J. Burn Care Rehabil. 2005, 5, 430–433. [Google Scholar]
- Chu, C.S.; McManus, A.T.; Mason, A.D.; Pruitt, B.A., Jr. Topical silver treatment after escharectomy of infected full thickness burn wounds in rats. J. Trauma 2005, 58, 1040–1046. [Google Scholar] [CrossRef]
- Atiyeh, B.S.; Costagliola, M.; Hayek, S.N.; Dibo, S.A. Effect of silver on burn wound infection control and healing: Review of the literature. Burns 2007, 33, 139–148. [Google Scholar] [CrossRef]
- Chaloupka, K.; Malam, Y.; Seifalian, A.M. Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol. 2010, 28, 580–588. [Google Scholar] [CrossRef]
- Wright, J.B.; Lam, K.; Burrell, R.E. Wound management in an era of increasing bacterial antibiotic resistance: A role for topical silver treatment. Am. J. Inf. Control 1998, 26, 572–577. [Google Scholar] [CrossRef]
- Cervantes, C.; Silver, S. Metal resistance in pseudomonas: Genes and mechanisms. In Molecular Biology of Pseudomonads; Nakazawa, T., Furukawa, K., Haas, D., Silver, S., Eds.; American Society for Microbiology: Washington, DC, USA, 1996. [Google Scholar]
- Russell, A.D.; Hugo, W.B. Antimicrobial activity and action of silver. Prog. Med. Chem. 1994, 31, 351–370. [Google Scholar]
- Marx, D.E.; Barillo, D.J. Silver in medicine: The basic science. Burns 2014, 40, S9–S18. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.L.; Mayer, D.; Salisbury, A.M. Efficacy of a surfactant-based wound dressing on biofilm control. Wound Repair Regen. 2017, 25, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.C.; Li, J.; Gil, J.; Valdes, J.; Solis, M.; Higa, A.; Bowler, P. The wound-healing effects of a next-generation anti-biofilm silver Hydrofiber wound dressing on deep partial-thickness wounds using a porcine model. Int. Wound J. 2018, 15, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Moyano, A.J.; Mas, C.R.; Colque, C.A.; Smania, A.M. Dealing with biofilms of Pseudomonas aeruginosa and Staphylococcus aureus: In vitro evaluation of a novel aerosol formulation of silver sulfadiazine. Burns 2020, 46, 128–135. [Google Scholar] [CrossRef]
- Lipsky, B.A.; Aragón-Sánchez, J.; Diggle, M.; Embil, J.; Kono, S.; Lavery, L.; International Working Group on the Diabetic Foot. IWGDF guidance on the diagnosis and management of foot infections in persons with diabetes. Diabetes Metab. Res. Rev. 2016, 32, 45–74. [Google Scholar] [CrossRef]
- Schultz, G.; Mozingo, D.; Romanelli, M.; Claxton, K. Wound healing and TIME; new concepts and scientific applications. Wound Repair Regen. 2005, 13, S1–S11. [Google Scholar] [CrossRef]
- European Wound Management Association (EWMA). Position Document: Wound Bed Preparation in Practice. Available online: http://woundsinternational.com (accessed on 1 March 2013).
- Wang, A.; Lv, G.; Cheng, X.; Ma, X.; Wang, W.; Gui, J.; Hu, J.; Lu, M.; Chu, G.; Chen, J.; et al. Guidelines on multidisciplinary approaches for the prevention and management of diabetic foot disease (2020 edition). Burn. Trauma 2020, 8, tkaa017. [Google Scholar] [CrossRef]
- Lucarelli, C.; Di Domenico, E.G.; Toma, L.; Bracco, D.; Prignano, G.; Fortunati, M.; Pelagalli, L.; Ensoli, F.; Pezzotti, P.; García-Fernández, A.; et al. Ralstonia mannitolilytica infections in an oncologic day ward: Description of a cluster among high-risk patients. Antimicrob. Resist. Infect. Control 2017, 6, 20. [Google Scholar] [CrossRef]
- Ceri, H.; Olson, M.E.; Stremick, C.; Read, R.R.; Morck, D.; Buret, A. The Calgary Biofilm Device: New technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J. Clin. Microbiol. 1999, 37, 1771–1776. [Google Scholar] [CrossRef]
- Peeters, E.; Nelis, H.J.; Coenye, T. Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J. Microbiol. Methods 2007, 72, 157–165. [Google Scholar] [CrossRef]
- Van den Driessche, F.; Rigole, P.; Brackman, G.; Coenye, T. Optimization of resazurin-based viability staining for quantification of microbial biofilms. J. Microbiol. Methods 2013, 98, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Vogeleer, P.; Tremblay, Y.D.N.; Jubelin, G.; Jacques, M.; Harel, J. Biofilm-Forming Abilities of Shiga Toxin-Producing Escherichia coli Isolates Associated with Human Infections. Appl. Environ. Microbiol. 2015, 82, 1448–1458. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S. Antimicrobial Tolerance in Biofilms. Microbiol. Spectr. 2015, 3, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, E.G.; Petroni, G.; Mancini, D.; Geri, A.; Di Palma, L.; Ascenzioni, F. Development of Electroactive and Anaerobic Ammonium-Oxidizing (Anammox) Biofilms from Digestate in Microbial Fuel Cells. Biomed. Res. Int. 2015, 2015, 351014. [Google Scholar] [CrossRef]
- Bjarnsholt, T.; Kirketerp-Møller, K.; Kristiansen, S.; Phipps, R.; Nielsen, A.K.; Jensen, P.Ø.; Høiby, N.; Givskov, M. Silver against Pseudomonas aeruginosa biofilms. APMIS 2007, 115, 921–928. [Google Scholar] [CrossRef]
- Ramsey, S.D.; Newton, K.; Blough, D.; McCulloch, D.K.; Sandhu, N.; Reiber, G.E.; Wagner, E.H. Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care 1999, 22, 382–387. [Google Scholar] [CrossRef]
- Maple-Brown, L.J.; Cunningham, J.; Dunne, K.; Whitbread, C.; Howard, D.; Weeramanthri, T.S.; Tatipata, S.; Dunbar, T.; Harper, C.A.; Taylor, H.R.; et al. Complications of diabetes in urban Indigenous Australians: The DRUID study. Diabetes Res. Clin. Pract. 2008, 80, 455–462. [Google Scholar] [CrossRef]
- Minges, K.E.; Zimmet, P.; Magliano, D.J.; Dunstan, D.W.; Brown, A.; Shaw, J.E. Diabetes prevalence and determinants in Indigenous Australian populations: A systematic review. Diabetes Res. Clin. Pract. 2011, 93, 139–149. [Google Scholar] [CrossRef]
- O’Dea, K.; Cunningham, J.; Maple-Brown, L.; Weeramanthri, T.; Shaw, J.; Dunbar, T.; Zimmet, P. Diabetes and cardiovascular risk factors in urban Indigenous adults: Results from the DRUID study. Diabetes Res. Clin. Pract. 2008, 80, 483–489. [Google Scholar] [CrossRef]
- Lazzarini, P.A.; Gurr, J.M.; Rogers, J.R.; Schox, A.; Bergin, S.M. Diabetes foot disease: The Cinderella of Australian diabetes management? J. Foot Ankle Res. 2012, 5, 24. [Google Scholar] [CrossRef]
- Høiby, N.; Ciofu, O.; Johansen, H.K.; Song, Z.; Moser, C.; Jensen, P.Ø.; Molin, S.; Givskov, M.; Tolker-Nielsen, T.; Bjarnsholt, T. The clinical impact of bacterial biofilms. Int. J. Oral Sci. 2011, 2, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.Ø.; Givskov, M.; Bjarnsholt, T.; Moser, C. The immune system vs. Pseudomonas aeruginosa biofilms. FEMS Immunol. Med. Microbiol. 2010, 59, 292–305. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, E.J.; Citron, D.M.; Warren, Y.A.; Tyrrell, K.L.; Merriam, C.V.; Fernandez, H.T. In vitro activities of dalbavancin and 12 other agents against 329 aerobic and anaerobic gram-positive isolates recovered from diabetic foot infections. Antimicrob. Agents Chemother. 2006, 50, 2875–2879. [Google Scholar] [CrossRef] [PubMed]
- Raja, N.S. Microbiology of diabetic foot infections in a teaching hospital in Malaysia: A retrospective study of 194 cases. J. Microbiol. Immunol. Infect 2007, 40, 39–44. [Google Scholar] [PubMed]
- Tascini, C.; Piaggesi, A.; Tagliaferri, E.; Iacopi, E.; Fondelli, S.; Tedeschi, A.; Rizzo, L.; Leonildi, A.; Menichetti, F. Microbiology at first visit of moderate-to-severe diabetic foot infection with antimicrobial activity and a survey of quinolone monotherapy. Diabetes Res. Clin. Pract. 2011, 94, 133–139. [Google Scholar] [CrossRef]
- Wu, M.; Pan, H.; Leng, W.; Lei, X.; Chen, L.; Liang, Z. Distribution of Microbes and Drug Susceptibility in Patients with Diabetic Foot Infections in Southwest China. J. Diabetes Res. 2018, 2018, 9817308. [Google Scholar] [CrossRef]
- Petrakis, V.; Panagopoulos, P.; Papanas, N. Dalbavancin for the Treatment of Complicated Gram-Positive Skin and Soft Tissue Infections. Int. J. Low Extrem. Wounds 2020, 19, 236–241. [Google Scholar] [CrossRef]
- Sabir, R.; Alvi, S.F.; Fawwad, A.; Basit, A. Antibiogram of Pseudomonas aeruginosa and Methicillin-resistant Staphylococcus aureus in patients with diabetes. Pak. J. Med. Sci. 2014, 30, 814–818. [Google Scholar] [CrossRef]
- Murali, T.S.; Kavitha, S.; Spoorthi, J.; Bhat, D.V.; Prasad, A.S.B.; Upton, Z.; Ramachandra, L.; Acharya, R.V.; Satyamoorthy, K. Characteristics of microbial drug resistance and its correlates in chronic diabetic foot ulcer infections. J. Med. Microbiol. 2014, 63, 1377–1385. [Google Scholar] [CrossRef]
- Jones, R.N. Resistance patterns among nosocomial pathogens: Trends over the past few years. Chest 2001, 119, 397S–404S. [Google Scholar] [CrossRef]
- Paterson, D.L.; Rossi, F.; Baquero, F.; Hsueh, P.-R.; Woods, G.L.; Satishchandran, V.; Snyder, T.A.; Harvey, C.M.; Teppler, H.; DiNubile, M.J.; et al. In vitro susceptibilities of aerobic and facultative Gram-negative bacilli isolated from patients with intraabdominal infections worldwide: The 2003 Study for Monitoring Antimicrobial Resistance Trends (SMART). J. Antimicrob. Chemother. 2005, 55, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Najjad, M.K.; Idrees, Z.; Zamir, M.; Zeeshan, S.; Shah, S.A. Pseudomonas as trespassers in diabetic foot infections: More questions and fewer answers. JPMA 2014, 64, S112–S115. [Google Scholar]
- James, G.A.; Swogger, E.; Wolcott, R. Biofilms in chronic wounds. Wound Repair Regen. 2008, 16, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Wolcott, R.D.; Gontcharova, V.; Sun, Y.; Zischkau, A.M.; Dowd, S.E. Bacterial diversity in surgical site infections: Not just aerobic cocci anymore. J. Wound Care 2009, 18, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Mah, T.F. Biofilm-specific antibiotic resistance. Future Microbiol. 2012, 7, 1061–1072. [Google Scholar] [CrossRef]
- Bianchi, T.; Wolcott, R.D.; Peghetti, A.; Leaper, D.; Cutting, K.; Polignano, R.; Rosa Rita, Z.; Moscatelli, A.; Greco, A.; Romanelli, M.; et al. Recommendations for the management of biofilm: A consensus document. J. Wound Care 2016, 25, 305–317. [Google Scholar] [CrossRef]
- Schultz, G.; Bjarnsholt, T.; James, G.A.; Leaper, D.J.; McBain, A.J.; Malone, M.; Stoodley, P.; Swanson, T.; Tachi, M.; Wolcott, R.D. Global Wound Biofilm Expert Panel. Consensus guidelines for the identification and treatment of biofilms in chronic nonhealing wounds. Wound Repair Regen. 2017, 25, 744–757. [Google Scholar] [CrossRef]
- Vatan, A.; Saltoglu, N.; Yemisen, M.; Balkan, I.I.; Surme, S.; Demiray, T.; Mete, B.; Tabak, F.; Cerrahpasa Diabetic Foot Study Group. Association between biofilm and multi/extensive drug resistance in diabetic foot infection. Int. J. Clin. Pract. 2018, 72, e13060. [Google Scholar] [CrossRef]
- Girard, L.P.; Ceri, H.; Gibb, A.P.; Olson, M.; Sepandj, F. MIC versus MBEC to determine the antibiotic sensitivity of Staphylococcus aureus in peritoneal dialysis peritonitis. Perit. Dial. Int. 2010, 30, 652–656. [Google Scholar] [CrossRef]
- Castaneda, P.; McLaren, A.; Tavaziva, G.; Overstreet, D. Biofilm antimicrobial susceptibility increases with antimicrobial exposure time. Clin. Orthop. Relat. Res. 2016, 474, 1659–1664. [Google Scholar] [CrossRef]
- Di Domenico, E.G.; Cavallo, I.; Bordignon, V.; Prignano, G.; Sperduti, I.; Gurtner, A.; Trento, E.; Toma, L.; Pimpinelli, F.; Capitanio, B.; et al. Inflammatory cytokines and biofilm production sustain Staphylococcus aureus outgrowth and persistence: A pivotal interplay in the pathogenesis of Atopic Dermatitis. Sci. Rep. 2018, 8, 9573. [Google Scholar] [CrossRef] [PubMed]
- Algburi, A.; Comito, N.; Kashtanov, D.; Dicks, L.M.T.; Chikindas, M.L. Control of biofilm formation: Antibiotics and beyond. Appl. Environ. Microbiol. 2017, 83, e02508-16. [Google Scholar] [CrossRef] [PubMed]
- Price, B.L.; Morley, R.; Bowling, F.L.; Lovering, A.M.; Dobson, C.B. Susceptibility of monomicrobial or polymicrobial biofilms derived from infected diabetic foot ulcers to topical or systemic antibiotics in vitro. PLoS ONE 2020, 15, e0228704. [Google Scholar] [CrossRef] [PubMed]
- Naresh-Babu, J.; Arun-Kumar, V. Do Prophylactic Antibiotics Reach the Operative Site Adequately?: A Quantitative Analysis of Serum and Wound Concentrations of Systemic and Local Prophylactic Antibiotics in Spine Surgery. Spine 2020, 45, E196–E202. [Google Scholar] [CrossRef]
- Skhirtladze, K.; Hutschala, D.; Fleck, T.; Thalhammer, F.; Ehrlich, M.; Vukovich, T.; Müller, M.; Tschernko, E.M. Impaired target site penetration of vancomycin in diabetic patients following cardiac surgery. Antimicrob. Agents Chemother. 2006, 50, 1372–1375. [Google Scholar] [CrossRef]
- Legat, F.J.; Krause, R.; Zenahlik, P.; Hoffmann, C.; Scholz, S.; Salmhofer, W.; Tscherpel, J.; Kerl, H.; Dittrich, P.; Tscherpel, T. Penetration of piperacillin and tazobactam into inflamed soft tissue of patients with diabetic foot infection. Antimicrob. Agents Chemother. 2005, 49, 4368–4371. [Google Scholar] [CrossRef]
- Kim, S.H.; Opdahl, A.; Marmo, C.; Somorjai, G.A. AFM and SFG studies of pHEMA-based hydrogel contact lens surfaces in saline solution: Adhesion, friction, and the presence of non-crosslinked polymer chains at the surface. Biomaterials 2002, 23, 1657–1666. [Google Scholar] [CrossRef]
- Traunmüller, F.; Schintler, M.V.; Metzler, J.; Spendel, S.; Mauric, O.; Popovic, M.; Konz, K.H.; Scharnagl, E.; Joukhadar, C. Soft tissue and bone penetration abilities of daptomycin in diabetic patients with bacterial foot infections. J. Antimicrob. Chemother. 2010, 65, 1252–1257. [Google Scholar] [CrossRef]
- Hobizal, K.B.; Wukich, D.K. Diabetic foot infections: Current concept review. Diabet Foot Ankle 2012, 3, S1. [Google Scholar] [CrossRef]
- Brownrigg, J.R.W.; Apelqvist, J.; Bakker, K.; Schaper, N.C.; Hinchliffe, R.J. Evidence-based management of PAD & the diabetic foot. Eur. J. Vasc. Endovasc. Surg. 2013, 45, 673–681. [Google Scholar]
- Gullberg, E.; Cao, S.; Berg, O.G.; Ilbäck, C.; Sandegren, L.; Hughes, D.; Andersson, D.I. Selection of Resistant Bacteria at Very Low Antibiotic Concentrations. PLoS Pathog. 2011, 7, e1002158. [Google Scholar] [CrossRef] [PubMed]
- Blondeau, J.M.; Hansen, G.; Metzler, K.; Hedlin, P. The Role of PK/PD Parameters to Avoid Selection and Increase of Resistance: Mutant Prevention Concentration. J. Chemother. 2016, 16, 1–19. [Google Scholar] [CrossRef]
- Dumville, J.C.; Lipsky, B.A.; Hoey, C.; Cruciani, M.; Fiscon, M.; Xia, J. Topical antimicrobial agents for treating foot ulcers in people with diabetes. Cochrane Database Syst. Rev. 2017, 6, CD011038. [Google Scholar] [CrossRef]
- Koo, D.S.; Zhen, S.; Zhen, Z.D.; Shi, X.W.; Xiang, S.J. Assessment of topical therapy of the burn wound with silver sulphadiazine after its use for 15 years in a burn unit. Burns 1989, 15, 193–196. [Google Scholar] [PubMed]
- Marone, P.; Monzillo, V.; Perversi, L.; Carretto, E. Comparative in vitro activity of silver sulfadiazine, alone and in combination with cerium nitrate, against staphylococci and gram-negative bacteria. J. Chemother. 1998, 10, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Yamasaki, O.; Kanzaki, H.; Tada, J.; Arata, J. Effects of sucrose and silver on Staphylococcus aureus biofilms. J. Antimicrob. Chemother. 1998, 42, 629–634. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schuenck, R.P.; Dadalti, P.; Silva, M.G.; Fonseca, L.S.; Santos, K.R. Oxacillin- and mupirocin-resistant Staphylococcus aureus: In vitro activity of silver sulphadiazine and cerium nitrate in hospital strains. J. Chemother. 2004, 16, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Lansdown, A.B.; Williams, A. How safe is silver in wound care? J Wound Care 2004, 13, 131–136. [Google Scholar] [CrossRef]
- Sun, Y.; Dowd, S.E.; Smith, E.; Rhoads, D.D.; Wolcott, R.D. In vitro multispecies Lubbock chronic wound biofilm model. Wound Repair Regen. 2008, 16, 805–813. [Google Scholar] [CrossRef]
- Loesche, M.; Gardner, S.E.; Kalan, L.; Horwinski, J.; Zheng, Q.; Hodkinson, B.P.; Tyldsley, A.S.; Franciscus, C.L.; Hillis, S.L.; Mehta, S.; et al. Temporal Stability in Chronic Wound Microbiota Is Associated With Poor Healing. J. Investig. Dermatol. 2017, 137, 237–244. [Google Scholar] [CrossRef]
- Gardiner, M.; Vicaretti, M.; Sparks, J.; Bansal, S.; Bush, S.; Liu, M.; Darling, A.; Harry, E.; Burke, C.M. A longitudinal study of the diabetic skin and wound microbiome. PeerJ 2017, 5, e3543. [Google Scholar] [CrossRef] [PubMed]
- Dalton, T.; Dowd, S.E.; Wolcott, R.D.; Sun, Y.; Watters, C.; Griswold, J.A.; Rumbaugh, K.P. An in vivo polymicrobial biofilm wound infection model to study interspecies interactions. PLoS ONE 2011, 6, e27317. [Google Scholar] [CrossRef] [PubMed]
- Howlin, R.P.; Brayford, M.J.; Webb, J.S.; Cooper, J.J.; Aiken, S.S.; Stoodley, P. Antibiotic-loaded synthetic calcium sulfate beads for prevention of bacterial colonization and biofilm formation in periprosthetic infections. Antimicrob. Agents Chemother. 2015, 59, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Mottola, C.; Mendes, J.J.; Cristino, J.M.; Cavaco-Silva, P.; Tavares, L.; Oliveira, M. Polymicrobial biofilms by diabetic foot clinical isolates. Folia Microbiol. 2016, 61, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Omar, A.; Wright, J.; Schultz, G.; Burrell, R.; Nadworny, P. Microbial Biofilms and Chronic Wounds. Microorganisms 2017, 5, 9. [Google Scholar] [CrossRef]
- Armstrong, D.G.; Liswood, P.J.; Todd, W.F. Prevalence of mixed infections in the diabetic pedal wound. A retrospective review of 112 infections. J. Am. Podiatr. Med. Assoc. 1995, 85, 533–537. [Google Scholar] [CrossRef]
- Clokie, M.; Greenway, A.L.; Harding, K.; Jones, N.J.; Vedhara, K.; Game, F.; Dhatariya, K.K. New horizons in the understanding of the causes and management of diabetic foot disease: Report from the 2017 Diabetes UK Annual Professional Conference Symposium. Diabet. Med. 2017, 34, 305–315. [Google Scholar] [CrossRef]
- Noor, S.; Zubair, M.; Ahmad, J. Diabetic foot ulcer-A review on pathophysiology, classification and microbial etiology. Diabetes Metab. Syndr. 2015, 9, 192–199. [Google Scholar] [CrossRef]
- Lipsky, B.A.; Dryden, M.; Gottrup, F.; Nathwani, D.; Seaton, R.A.; Stryja, J. Antimicrobial stewardship in wound care: A position paper from the British Society for antimicrobial chemotherapy and European wound management association. J. Antimicrob. Chemother. 2016, 71, 3026–3035. [Google Scholar] [CrossRef]
- Wolcott, R.D.; Hanson, J.D.; Rees, E.J.; Koenig, L.D.; Phillips, C.D.; Wolcott, R.A.; Cox, S.B.; White, J.S. Analysis of the chronic wound microbiota of 2,963 patients by 16S rDNA pyrosequencing. Wound Repair Regen. 2016, 24, 163174. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).