Cellular Proliferation, Dermal Repair, and Microbiological Effectiveness of Ultrasound-Assisted Wound Debridement (UAW) Versus Standard Wound Treatment in Complicated Diabetic Foot Ulcers (DFU): An Open-Label Randomized Controlled Trial
Abstract
1. Introduction
2. Methods
2.1. Trial Design
2.2. Participant
- Male and female patients over 18 years old
- Type 1 or type 2 diabetes with levels of HbA1c ≤ 85.8 mmol/mol (10%) within 30 days of the beginning of the study, based on a previous international, multicenter, randomized controlled trial [11]
- Wound stages IB, IIB, ID, and IID according to the University of Texas Diabetic Wound Classification [12]
- Wound duration of 1–24 months
- Wound size among 1–30 cm2 after debridement
- Chronic kidney disease (glomerular filtration rate < 60mL/min per 1.73 m2 during at least three months) or dialysis [17]
- Non-treated osteomyelitis
- Necrotizing soft tissue infections
- Life expectancy < 6 months due to malignant DFU
- Pregnancy and lactation
- Patients diagnosed with human immunodeficiency virus (HIV) or hepatitis
- Patients showing local or systemic conditions that could impair tissue regeneration
2.3. DFU Assessment
2.4. Intervention
2.4.1. DFU Debridement and Wound Management
2.4.2. Analysis of Tissue Samples
2.5. Outcome Measures
2.5.1. Main Outcome Measure: Cellular Proliferation Analysis of Wound Tissue Samples
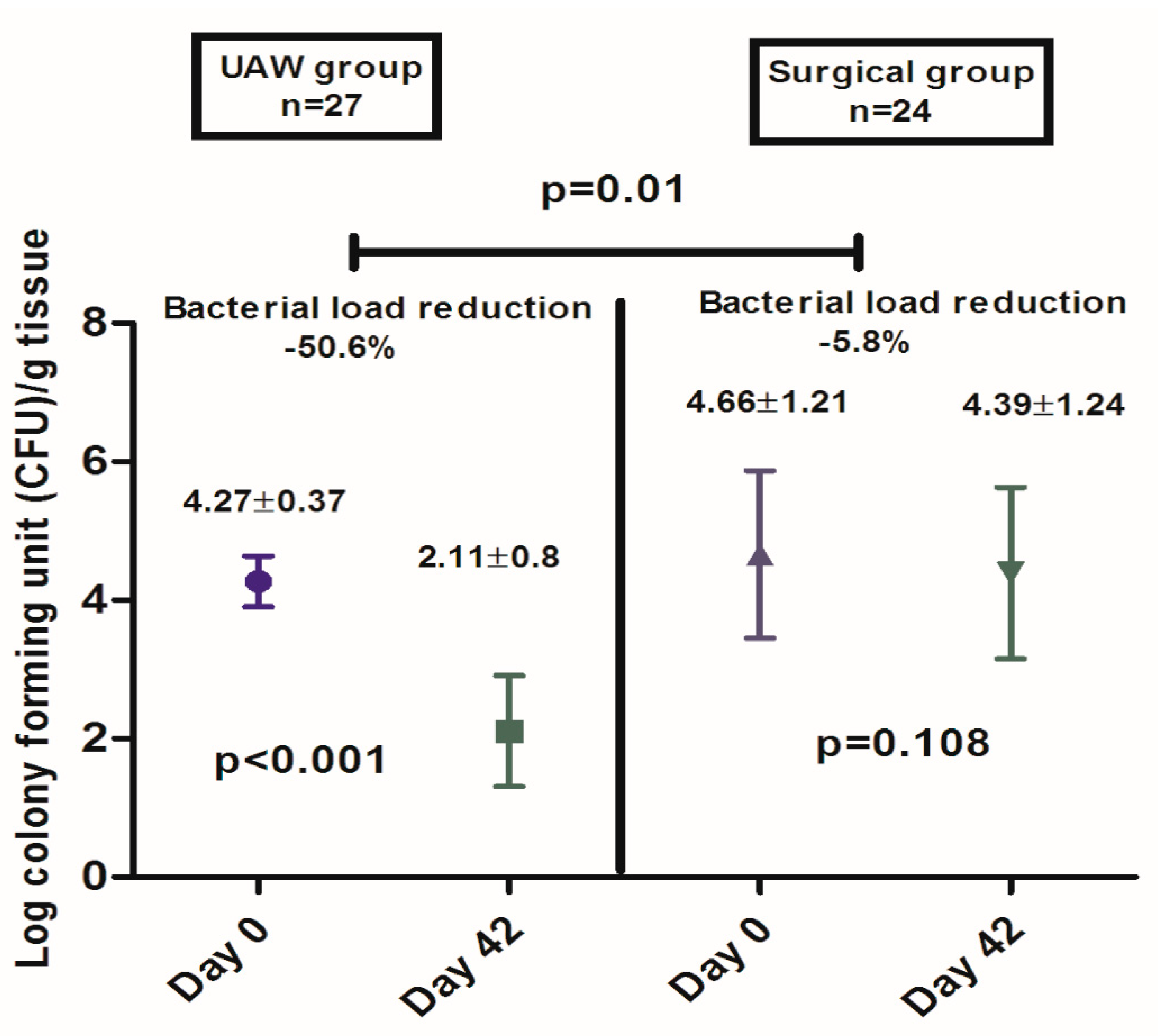
2.5.2. Secondary Outcome Measure: Microbiological Analysis of Wound Tissue Samples
2.5.3. Third Outcome Measure: Evaluation of Wound Conditions
2.6. Follow-Up
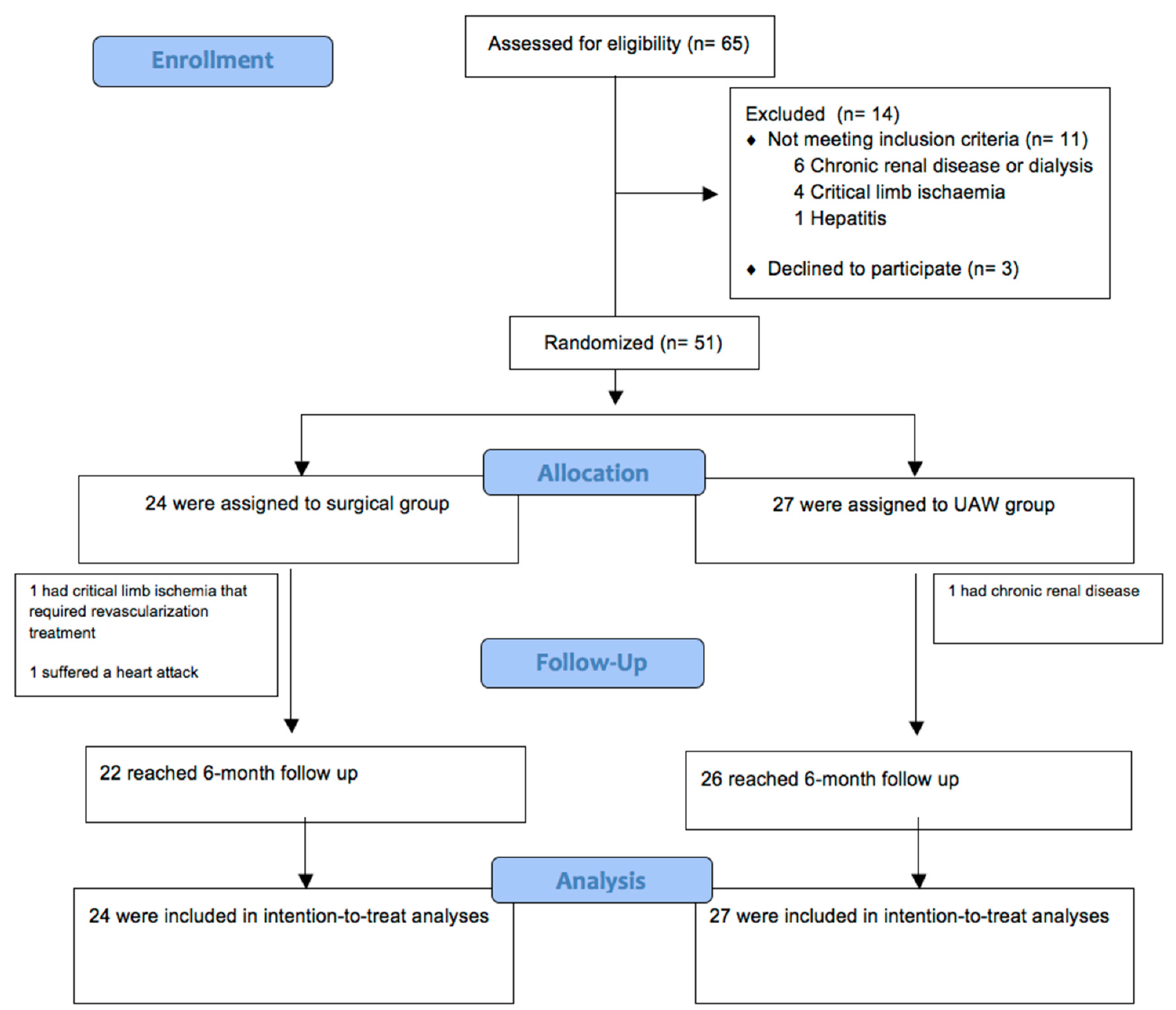
2.7. Sample Size
2.8. Randomization
2.9. Blinding
2.10. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schaper, N.C.; Van Netten, J.J.; Apelqvist, J.; Bus, S.; Hinchliffe, R.J.; Lipsky, B.A. Practical Guidelines on the prevention and management of diabetic foot disease (IWGDF 2019 update). Diabetes Metab. Res. Rev. 2020, 36 (Suppl. 1), e3266. [Google Scholar] [CrossRef]
- Kim, P.J.; Steinberg, J.S. Wound Care: Biofilm and Its Impact on the Latest Treatment Modalities for Ulcerations of the Diabetic Foot. Semin. Vasc. Surg. 2012, 25, 70–74. [Google Scholar] [CrossRef]
- Kingsley, A.; Lewis, T. Debridement and wound biofilms. J. Wound Care 2011, 20, 284. [Google Scholar] [CrossRef]
- Swanson, T.; Lázaro-Martínez, J.L.; Braumann, C.; Kirchhoff, J.-B.; Gächter, B.; Van Acker, K. Ultrasonic-assisted wound debridement: Report from a closed panel meeting. J. Wound Care 2020, 29, 128–135. [Google Scholar] [CrossRef]
- Rayman, G.; Vas, P.; Dhatariya, K.; Driver, V.; Hartemann, A.; Londahl, M.; Piaggesi, A.; Apelqvist, J.; Attinger, C.; Game, F.; et al. Guidelines on use of interventions to enhance healing of chronic foot ulcers in diabetes (IWGDF 2019 update). Diabetes Metab. Res. Rev. 2020, 36 (Suppl. 1), e3283. [Google Scholar] [CrossRef]
- Lázaro-Martínez, J.L.; Álvaro-Afonso, J.F.; Álvarez, Y.G.; Molines-Barroso, R.J.; García-Morales, E.; Fernández, A. Ultrasound-assisted debridement of neuroischaemic diabetic foot ulcers, clinical and microbiological effects: A case series. J. Wound Care 2018, 27, 278–286. [Google Scholar] [CrossRef]
- Chang, Y.-J.R.; Perry, J.; Cross, K. Low-Frequency Ultrasound Debridement in Chronic Wound Healing: A Systematic Review of Current Evidence. Plast. Surg. 2017, 25, 21–26. [Google Scholar] [CrossRef]
- Driver, V.R.; Yao, M. Discussion: Current Status of the Use of Modalities in Wound Care: Electrical Stimulation and Ultrasound Therapy. Plast. Reconstr. Surg. 2011, 127 (Suppl. 1), 103S–104S. [Google Scholar] [CrossRef]
- Ennis, W.J.; Foremann, P.; Mozen, N.; Massey, J.; Conner-Kerr, T.; Meneses, P. Ultrasound therapy for recalcitrant diabetic foot ulcers: Results of a randomized, double-blind, controlled, multicenter study. Ostomy Wound Manag. 2005, 51, 24–39. [Google Scholar]
- Driver, V.R.; Yao, M.; Miller, C.J. Noncontact low-frequency ultrasound therapy in the treatment of chronic wounds: A meta-analysis. Wound Repair Regen. 2011, 19, 475–480. [Google Scholar] [CrossRef]
- Edmonds, M.E.; Lázaro-Martínez, J.L.; Alfayate-García, J.M.; Martini, J.; Petit, J.-M.; Rayman, G.; Lobmann, R.; Uccioli, L.; Sauvadet, A.; Bohbot, S.; et al. Sucrose octasulfate dressing versus control dressing in patients with neuroischaemic diabetic foot ulcers (Explorer): An international, multicentre, double-blind, randomised, controlled trial. Lancet Diabetes Endocrinol. 2018, 6, 186–196. [Google Scholar] [CrossRef]
- Armstrong, D.G.; A Lavery, L.; Harkless, L.B. Validation of a Diabetic Wound Classification System: The contribution of depth, infection, and ischemia to risk of amputation. Diabetes Care 1998, 21, 855–859. [Google Scholar] [CrossRef]
- European Wound Management Association (EWMA). Position Document: Identifying Criteria for Wound Infection; MEP Ltd.: London, UK, 2005. [Google Scholar]
- Lipsky, B.A.; Berendt, A.R.; Cornia, P.B.; Pile, J.C.; Peters, E.J.G.; Armstrong, D.G.; Deery, H.G.; Embil, J.M.; Joseph, W.S.; Karchmer, A.W.; et al. 2012 Infectious Diseases Society of America Clinical Practice Guideline for the Diagnosis and Treatment of Diabetic Foot Infections. Clin. Infect. Dis. 2012, 54, e132–e173. [Google Scholar] [CrossRef]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G.R. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J. Vasc. Surg. 2007, 45, S5–S67. [Google Scholar] [CrossRef]
- Hinchliffe, R.; Forsythe, R.O.; Apelqvist, J.; Boyko, E.J.; Fitridge, R.; Hong, J.P.; Katsanos, K.; Mills, J.L.; Nikol, S.; Reekers, J.; et al. Guidelines on diagnosis, prognosis, and management of peripheral artery disease in patients with foot ulcers and diabetes (IWGDF 2019 update). Diabetes Metab. Res. Rev. 2020, 36 (Suppl. 1), e3276. [Google Scholar] [CrossRef]
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic Kidney Disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef]
- Boulton, A.J.M.; Armstrong, D.G.; Albert, S.F.; Frykberg, R.G.; Hellman, R.; Kirkman, M.S.; Lavery, L.A.; LeMaster, J.W.; Mills, J.L.; Mueller, M.J.; et al. Comprehensive Foot Examination and Risk Assessment: A report of the Task Force of the Foot Care Interest Group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care 2008, 31, 1679–1685. [Google Scholar] [CrossRef]
- Tan, T.; Shaw, E.J.; Siddiqui, F.; Kandaswamy, P.; Barry, P.W.; Baker, M. Inpatient management of diabetic foot problems: Summary of NICE guidance. BMJ 2011, 342. [Google Scholar] [CrossRef]
- Bus, S.; Armstrong, D.G.; Gooday, C.; Jarl, G.; Caravaggi, C.; Viswanathan, V.; Lazzarini, P.A. On behalf of the International Working Group on the Diabetic Foot (IWGDF) Guidelines on offloading foot ulcers in persons with diabetes (IWGDF 2019 update). Diabetes Metab. Res. Rev. 2020, 36 (Suppl. 1), e3274. [Google Scholar] [CrossRef]
- Tardáguila-García, A.; Lázaro-Martínez, J.L.; Sanz-Corbalán, I.; Álvarez, Y.G.; Álvaro-Afonso, J.F.; García-Morales, E. Correlation between Empirical Antibiotic Therapy and Bone Culture Results in Patients with Osteomyelitis. Adv. Ski. Wound Care 2019, 32, 41–44. [Google Scholar] [CrossRef]
- Wang, D.; Stockard, C.R.; Harkins, L.; Lott, P.; Salih, C.; Yuan, K.; Buchsbaum, D.J.; Hashim, A.; Zayzafoon, M.; Hardy, R.W.; et al. Immunohistochemistry in the evaluation of neovascularization in tumor xenografts. Biotech. Histochem. 2008, 83, 179–189. [Google Scholar] [CrossRef]
- Achar, R.A.N.; Silva, T.C.; Achar, E.; Martines, R.B.; Machado, J.L. Use of insulin-like growth factor in the healing of open wounds in diabetic and non-diabetic rats. Acta Cir. Bras. 2014, 29, 125–131. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fourth Informational Supplement; CLSI document M100-SWayne; Clinical and Laboratory Standards Institute: Wayne, MI, USA, 2014. [Google Scholar]
- Sterling, C. Methods of wound assessment documentation: A study. Nurs. Stand. 1996, 11, 38–41. [Google Scholar] [CrossRef]
- Lázaro-Martínez, J.L.; Conde-Montero, E.; Alvarez-Vazquez, J.C.; Berenguer-Rodríguez, J.J.; Carlo, A.G.; Blasco-Gil, S.; Blasco-García, C.; Martínez-Cuervo, F. Preliminary experience of an expert panel using Triangle Wound Assessment for the evaluation of chronic wounds. J. Wound Care 2018, 27, 790–796. [Google Scholar] [CrossRef]
- World Medical Association. World medical association declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Maan, Z.N.; Januszyk, M.; Rennert, R.C.; Duscher, D.; Rodrigues, M.; Fujiwara, T.; Ho, N.; Whitmore, N.H.A.; Hu, M.S.; Longaker, M.T.; et al. Noncontact, Low-Frequency Ultrasound Therapy Enhances Neovascularization and Wound Healing in Diabetic Mice. Plast. Reconstr. Surg. 2014, 134, 402e–411e. [Google Scholar] [CrossRef]
- Roper, J.A.; Williamson, R.C.; Bally, B.; Cowell, C.A.; Brooks, R.; Stephens, P.; Harrison, A.J.; Bass, M.D. Ultrasonic Stimulation of Mouse Skin Reverses the Healing Delays in Diabetes and Aging by Activation of Rac1. J. Investig. Dermatol. 2015, 135, 2842–2851. [Google Scholar] [CrossRef]
- Butcher, G.; Pinnuck, L. Wound bed preparation: Ultrasonic-assisted debridement. Br. J. Nurs. 2013, 22, S36–S43. [Google Scholar] [CrossRef]
- Michailidis, L.; Bergin, S.M.; Haines, T.P.; Williams, C.M. A Systematic Review to Compare the Effect of Low-frequency Ultrasonic Versus Nonsurgical Sharp Debridement on the Healing Rate of Chronic Diabetes-related Foot Ulcers. Ostomy Wound Manag. 2018, 64, 39–46. [Google Scholar] [CrossRef]
- Michailidis, L.; Bergin, S.M.; Haines, T.; Williams, C.M. Healing rates in diabetes-related foot ulcers using low frequency ultrasonic debridement versus non-surgical sharps debridement: A randomised controlled trial. BMC Res. Notes 2018, 11, 732. [Google Scholar] [CrossRef]
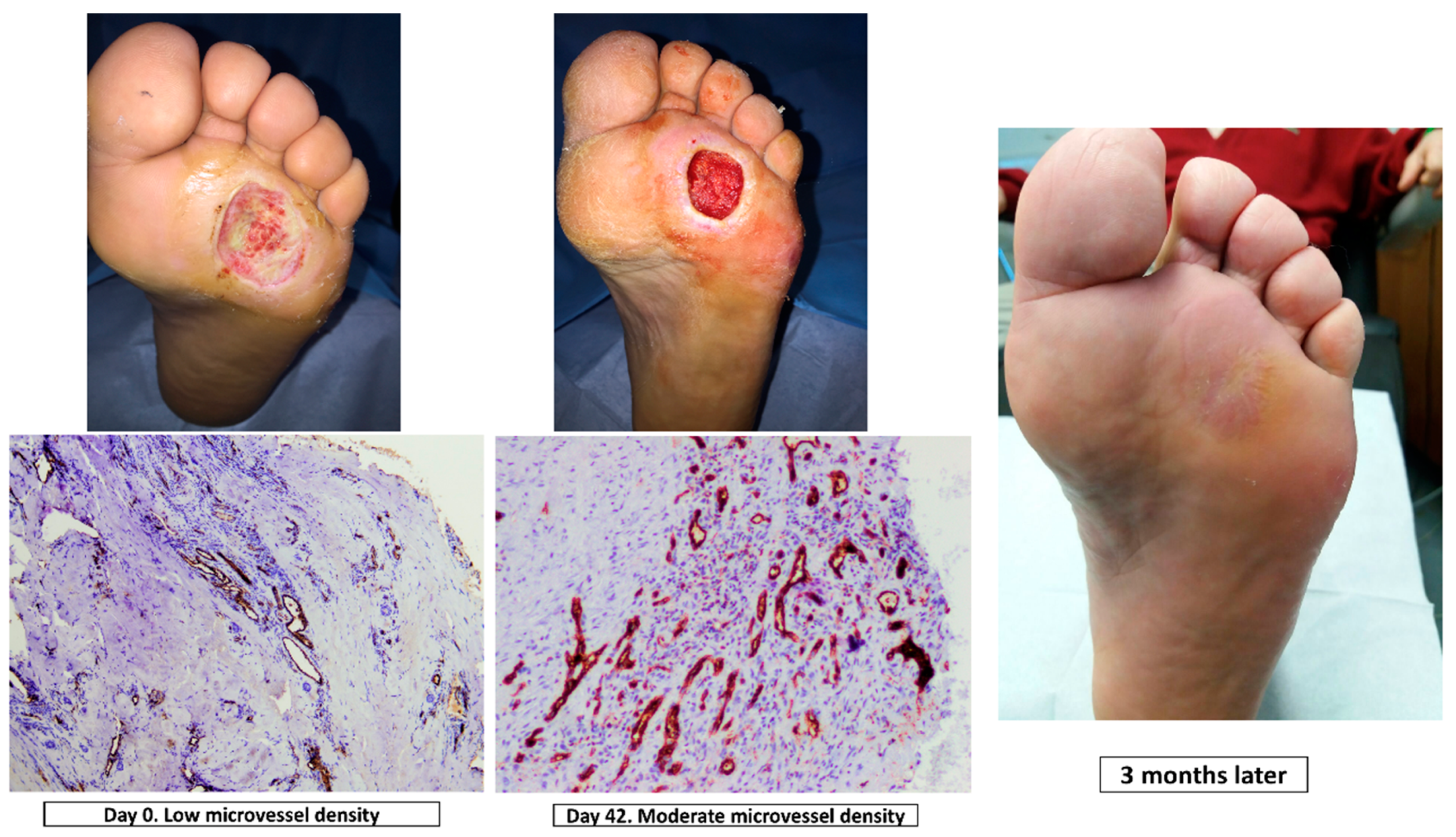
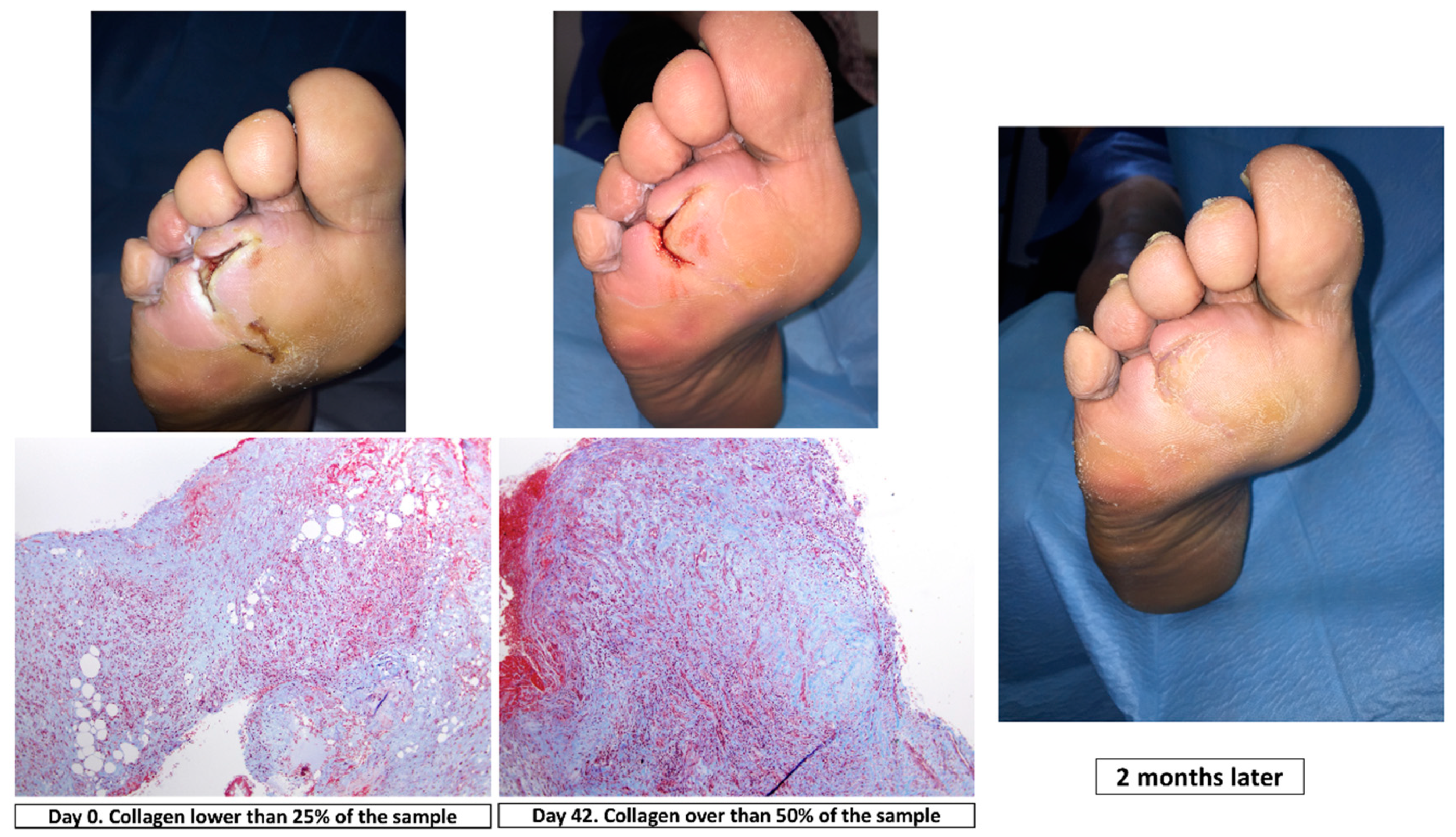

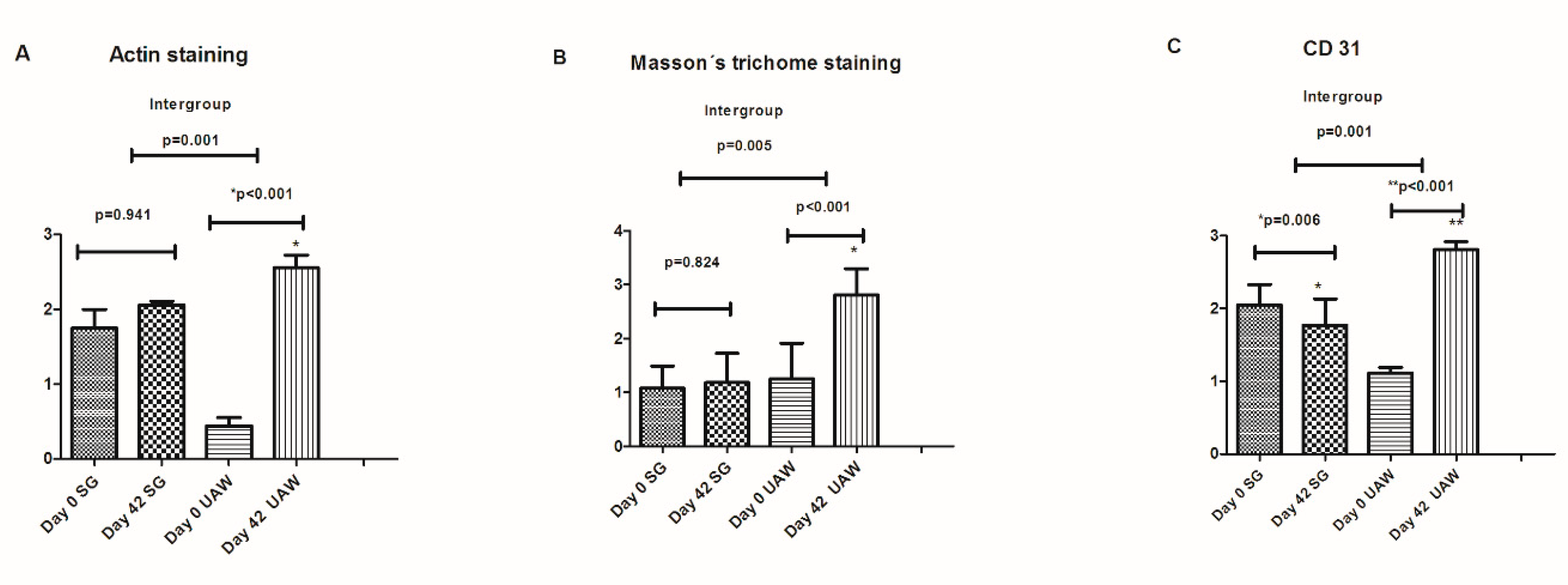
| Patients Included n = 51 | |||
|---|---|---|---|
| Surgical Group (n = 24) | UAW Group (n = 27) | p-Value | |
| Age (years) | 58 ± 5.4 | 64.1 ± 12.4 | 0.03 |
| Male/Female, n (%) | 24(100)/0 | 24 (88.8)/3 (11.2) | 0.09 |
| Type 1/Type 2 DM n (%) | 0/24 (100%) | 5 (18.5%)/22 (81.5%) | 0.02 |
| Duration of diabetes diagnosis, mean ± SD | 10.3 ± 5.0 | 22 ± 12.9 | 0.001 |
| Glycaemia (mmol/L), mean ± SD | 7.68 ± 2.62 | 8.79 ± 3.19 | 0.18 |
| Glycated hemoglobin mmol/mol, mean ± SD | 51 ± 4.5 | 57 ± 9.9 | 0.09 |
| Mean wound evolution (weeks) mean ± SD | 7.33 ± 8.95 | 8.63 ± 7.81 | 0.58 |
| Mean Ulcer area cm2, mean ± SD | 4.18 ± 3.32 | 7.47 ± 7.56 | 0.05 |
| Texas Classification IB IIB ID IID | 4 (16,7) 8 (33,3) 8 (33,3) 4 (16,7) | 4 (14.8) 3 (11.1) 12 (44.4) 8 (29.6) | 0.001 |
| Mild/Moderate infection, n (%) | 12 (50%)/12 (50%) | 22 (81.5%)/5 (18.5%) | 0.001 |
| Antibiotic treatment, n (%) | 12 (50%) | 2 (7.4%) | 0.001 |
| Variable | Surgical Group (n = 24) | p-Value | UAW Group (n = 27) | p-Value | p-Value Inter-Group | ||
|---|---|---|---|---|---|---|---|
| Day 0 | Day 42 | Day 0 | Day 42 | ||||
| Ulcer area (cm2), Mean (SD) | 4.18 ± 3.32 | 0.88 ± 1.04 | <0.001 | 7.47 ± 7.56 | 1.00 ± 1.22 | <0.001 | 0.711 |
| Periwound skin n (%) | |||||||
| Healthy | 4 (16.7%) | 8 (33.3%) | 8 (29.6%) | 20 (74.1%) | |||
| Macerated | 16 (66.7%) | 16 (66.7%) | 0.05 | 15 (55.5%) | 3 (11.1%) | <0.001 | 0.001 |
| Hyper-keratosis | 4 (16.7%) | 0 | 3 (11.1%) | 3 (11.1%) | |||
| Hyperemic | 0 | 0 | 1 (3.7%) | 1 (3.7%) | |||
| Exudate levels n (%) | |||||||
| Absent | 0 | 4 (16.7%) | 1 (3.7%) | 7 (25.9%) | |||
| Low | 8 (33.3%) | 8 (33.3%) | 0.22 | 5 (18.5%) | 16 (59.3%) | 0.009 | 0.05 |
| Medium | 16 (66.7%) | 12 (50%) | 20 (74.1%) | 3 (11.1%) | |||
| High | 0 | 0 | 1 (3.7%) | 0 | |||
| Tissue types on the wound bed | |||||||
| Granulated | 10 (41.6) | 18 (75%) | 4 (14.8%) | 23 (85.2%) | |||
| Hyper-granulated | 4 (16.7%) | 0 | <0.001 | 4 (14.8%) | 0 | <0.001 | 0.61 |
| Slough | 10 (41.6%) | 6 (25%) | 19 (70.4%) | 4 (14.8%) | |||
| Necrotic | 0 | 0 | 0 | 0 | |||
| Wollina score ± SD | 2.5 ± 1.2 | 5.6 ± 0.7 | <0.001 | 2.15 ± 1.4 | 5.4 ± 1.5 | <0.001 | 0.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lázaro-Martínez, J.L.; Álvaro-Afonso, F.J.; Sevillano-Fernández, D.; García-Álvarez, Y.; Sanz-Corbalan, I.; García-Morales, E. Cellular Proliferation, Dermal Repair, and Microbiological Effectiveness of Ultrasound-Assisted Wound Debridement (UAW) Versus Standard Wound Treatment in Complicated Diabetic Foot Ulcers (DFU): An Open-Label Randomized Controlled Trial. J. Clin. Med. 2020, 9, 4032. https://doi.org/10.3390/jcm9124032
Lázaro-Martínez JL, Álvaro-Afonso FJ, Sevillano-Fernández D, García-Álvarez Y, Sanz-Corbalan I, García-Morales E. Cellular Proliferation, Dermal Repair, and Microbiological Effectiveness of Ultrasound-Assisted Wound Debridement (UAW) Versus Standard Wound Treatment in Complicated Diabetic Foot Ulcers (DFU): An Open-Label Randomized Controlled Trial. Journal of Clinical Medicine. 2020; 9(12):4032. https://doi.org/10.3390/jcm9124032
Chicago/Turabian StyleLázaro-Martínez, José Luis, Francisco Javier Álvaro-Afonso, David Sevillano-Fernández, Yolanda García-Álvarez, Irene Sanz-Corbalan, and Esther García-Morales. 2020. "Cellular Proliferation, Dermal Repair, and Microbiological Effectiveness of Ultrasound-Assisted Wound Debridement (UAW) Versus Standard Wound Treatment in Complicated Diabetic Foot Ulcers (DFU): An Open-Label Randomized Controlled Trial" Journal of Clinical Medicine 9, no. 12: 4032. https://doi.org/10.3390/jcm9124032
APA StyleLázaro-Martínez, J. L., Álvaro-Afonso, F. J., Sevillano-Fernández, D., García-Álvarez, Y., Sanz-Corbalan, I., & García-Morales, E. (2020). Cellular Proliferation, Dermal Repair, and Microbiological Effectiveness of Ultrasound-Assisted Wound Debridement (UAW) Versus Standard Wound Treatment in Complicated Diabetic Foot Ulcers (DFU): An Open-Label Randomized Controlled Trial. Journal of Clinical Medicine, 9(12), 4032. https://doi.org/10.3390/jcm9124032










