Can the Macrogeometry of Dental Implants Influence Guided Bone Regeneration in Buccal Bone Defects? Histomorphometric and Biomechanical Analysis in Beagle Dogs
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Methods
2.1.1. Animal Preparation and Care
2.1.2. Materials and Group Formation
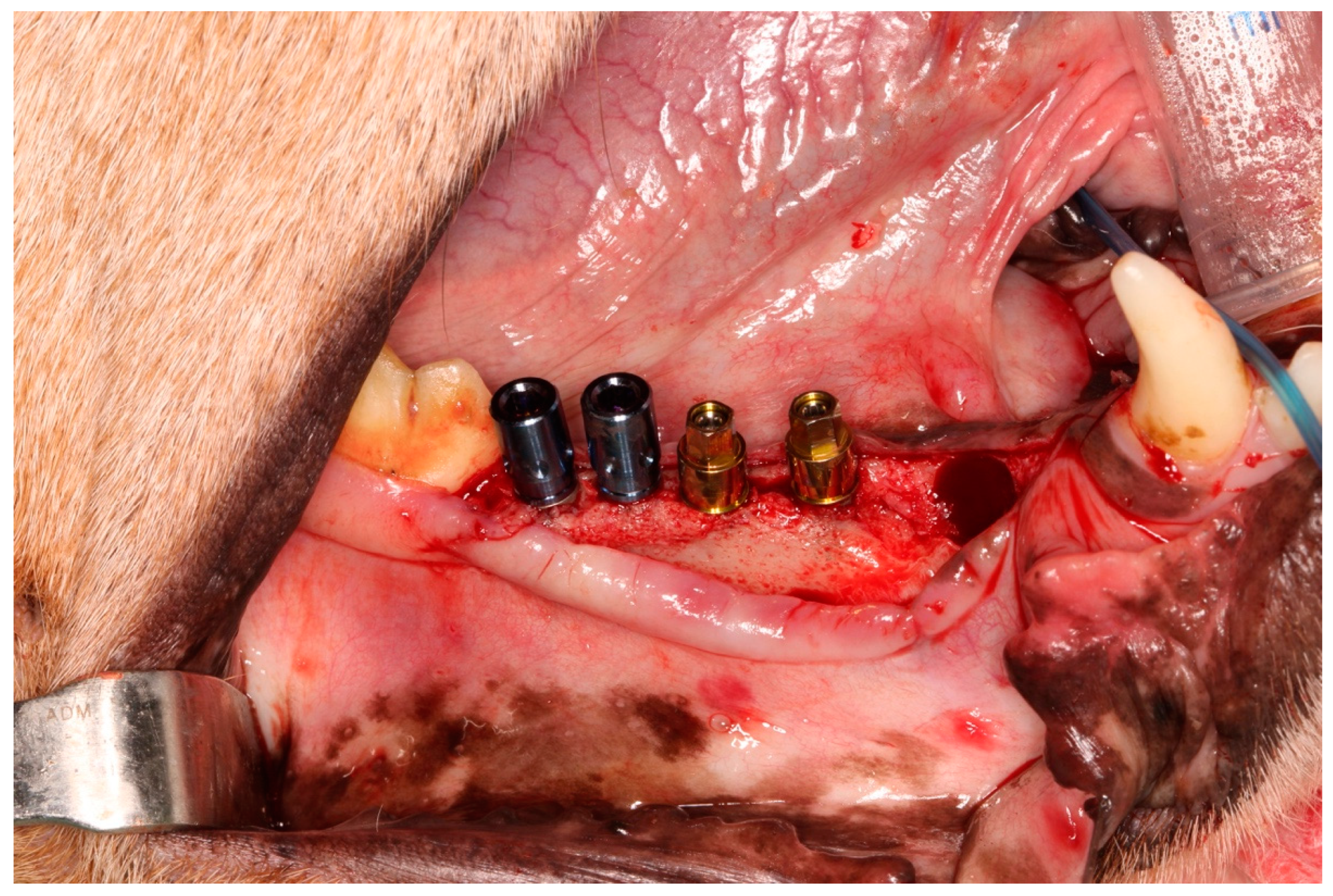
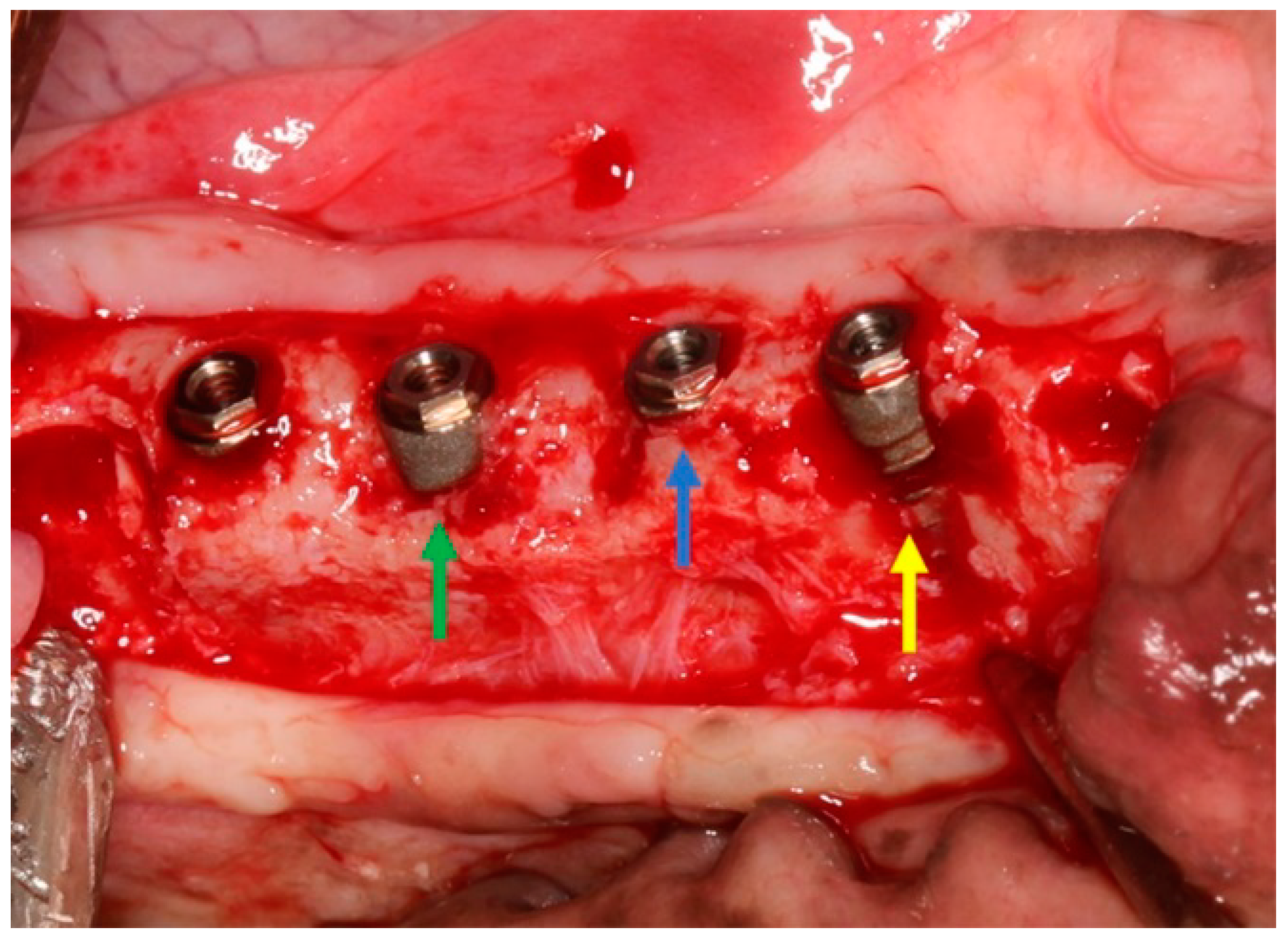
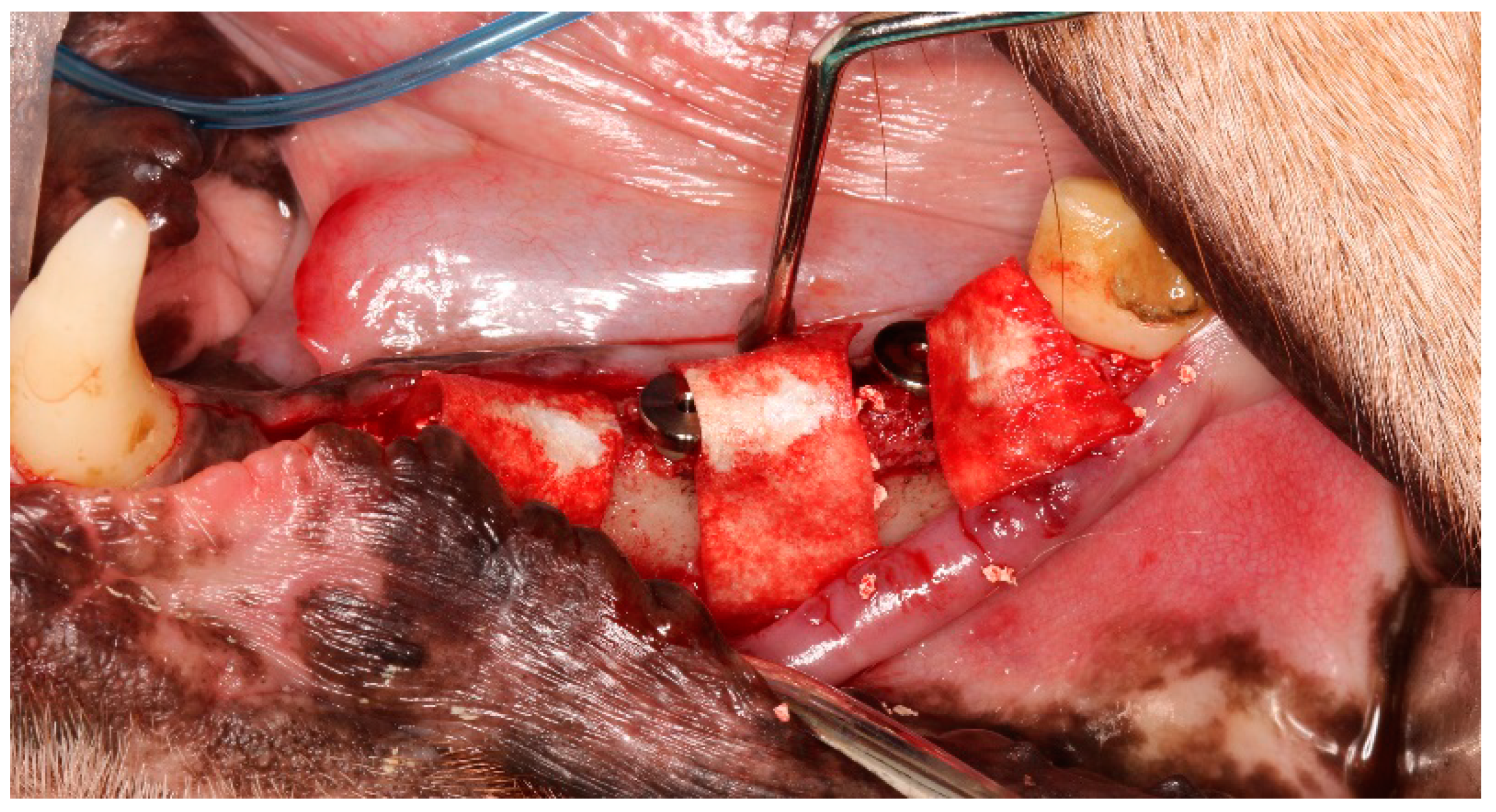
2.2. Surgical Procedures
2.3. Histological Procedures and Preparation
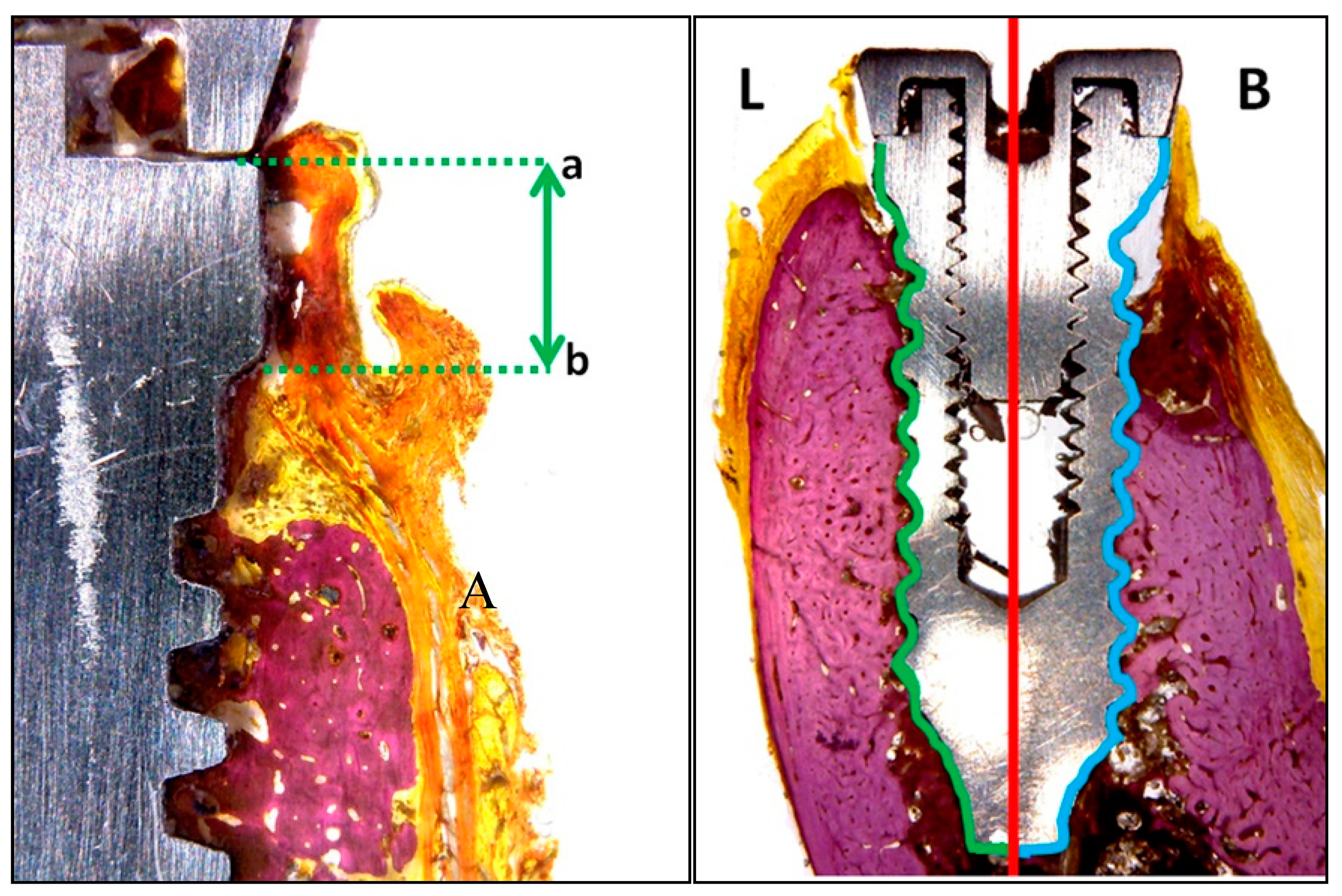
- Vestibular and lingual crestal bone loss (CBL) quantified the amount of bone loss (or bone that had failed to regenerate) around implants placed at sites with or without defects, measuring from the implant platform to the first point of bone-to-implant contact (Figure 5a).
- Bone-to-implant contact (BIC %) registered the percentage of bone in direct contact with the implant surface. To measure this parameter, the implant was divided at the center into two parts: the lingual portion and buccal portion, both extending from the implant shoulder to the apical portion (Figure 5b).
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Gehrke, S.A.; Bragança, L.K.; Velasco-Ortega, E.; Calvo-Guirado, J.L. Evaluation of dimensional behavior of peri-implant tissues in implants immediately exposed or submerged in fresh extraction and healed sites: A histological study in dogs. Int. J. Implant Dent. 2018, 4, 5. [Google Scholar] [CrossRef]
- Levin, L.; Frankenthal, S.; Zigdon, H.; Suzuki, M.; Coelho, P.G. Novel Implant Design for Initial Stability of Dental Implants Inserted in Fresh Extraction Sockets. Implant Dent. 2012, 21, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Jung, R.E.; Herzog, M.; Wolleb, K.; Ramel, C.F.; Thoma, D.S.; Hämmerle, C.H. A randomized controlled clinical trial comparing small buccal dehiscence defects around dental implants treated with guided bone regeneration or left for spontaneous healing. Clin. Oral Implant. Res. 2017, 28, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Wang, C.Y.; Wan, P.; Wang, S.G.; Wang, X.M. Comparison of bone regeneration in alveolar bone of dogs on mineralized collagen grafts with two composition ratios of nano-hydroxyapatite and collagen. Regen. Biomater. 2016, 3, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Valente, M.L.; de Castro, D.T.; Shimano, A.C.; Lepri, C.P.; dos Reis, A.C. Analyzing the Influence of a New Dental Implant Design on Primary Stability. Clin. Implant Dent. Relat. Res. 2011, 18, 168–173. [Google Scholar] [CrossRef]
- Tavakoli, J.; Khosroshahi, M.E. Surface morphology characterization of laser-induced titanium implants: Lesson to enhance osseointegration process. Biomed. Eng. Lett. 2018, 8, 249–257. [Google Scholar] [CrossRef]
- Fukuda, N.; Kanazawa, M.; Tsuru, K.; Tsuchiya, A.; Sunarso Toita, R.; Mori, Y.; Nakashima, Y.; Ishikawa, K. Synergistic effect of surface phosphorylation and micro-roughness on enhanced osseointegration ability of poly(ether ether ketone) in the rabbit tibia. Sci. Rep. 2018, 8, 16887. [Google Scholar] [CrossRef]
- Pellicer-Chover, H.; Peñarrocha-Diago, M.; Aloy-Prosper, A.; Canullo, L.; Peñarrocha-Diago, M.; Peñarrocha-Oltra, D. Does Apico-Coronal Implant Position Influence Peri-Implant Marginal Bone Loss? A 36-Month Follow-Up Randomized Clinical Trial. J. Oral Maxillofac. Surg. 2019, 77, 515–527. [Google Scholar] [CrossRef]
- Val, J.E.; Gómez-Moreno, G.; Ruiz-Linares, M.; Prados-Frutos, J.C.; Gehrke, S.A.; Calvo-Guirado, J.L. Effects of Surface Treatment Modification and Implant Design in Implants Placed Crestal and Subcrestally Applying Delayed Loading Protocol. J. Craniofac. Surg. 2017, 28, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Siles, M.; Muñoz-Cámara, D.; Salazar-Sánchez, N.; Camacho-Alonso, F.; Calvo-Guirado, J.L. Crestal bone loss around submerged and non-submerged implants during the osseointegration phase with different healing abutment designs: A randomized prospective clinical study. Clin. Oral Implant. Res. 2018, 29, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Guirado, J.L.; Gomez Moreno, G.; Aguilar-Salvatierra, A.; Mate Sanchez de Val, J.E.; Abboud, M.; Nemcovsky, C.E. Bone remodeling at implants with different configurations and placed immediately at different depth into extraction sockets. Experimental study in dogs. Clin. Oral Implant. Res. 2015, 26, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Meredith, N.; Shagaldi, F.; Alleyne, D.; Sennerby, L.; Cawley, P. The application of resonance frequency measurements to study the stability of titanium implants during healing in the rabbit tibia. Clin. Oral Implant. Res. 1997, 8, 234–243. [Google Scholar] [CrossRef]
- Shokri, M.; Daraeighadikolaei, A. Measurement of primary and secondary stability of dental implants by resonance frequency analysis method in mandible. Int. J. Dent. 2013, 2013, 506968. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Y.; Wang, Y.; Chen, J. Clinical research on a flapless surgical technique application of narrow implants. Medicine 2018, 97, e12646. [Google Scholar] [CrossRef] [PubMed]
- Ogle, O.E. Implant surface material, design, and osseointegration. Dent. Clin. N. Am. 2015, 59, 505–520. [Google Scholar] [CrossRef] [PubMed]
- Donath, K.; Breuner, G. A method for the study of undecalcified bones and teeth with attached soft tissues. The Säge-Schliff (sawing and grinding) technique. J. Oral Pathol. 1982, 11, 318–326. [Google Scholar] [CrossRef]
- Gehrke, S.A.; Prados-Frutos, J.C.; Prados-Privado, M.; Calvo-Guirado, J.L.; Aramburú Júnior, J.; Pérez-Díaz, L.; Mazón, P.; Aragoneses, J.M.; De Aza, P.N. Biomechanical and Histological Analysis of Titanium (Machined and Treated Surface) Versus Zirconia Implant Materials: An In Vivo Animal Study. Materials 2019, 12, 856. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, S.A.; Ramírez-Fernandez, M.P.; Granero Marín, J.M.; Barbosa Salles, M.; Del Fabbro, M.; Calvo Guirado, J.L. A comparative evaluation between aluminium and titanium dioxide microparticles for blasting the surface titanium dental implants: An experimental study in rabbits. Clin. Oral Implant. Res. 2018, 29, 802–807. [Google Scholar] [CrossRef]
- Jung, R.E.; Benic, G.I.; Scherrer, D.; Hämmerle, C.H. Cone beam computed tomography evaluation of regenerated buccal bone 5 years after simultaneous implant placement and guided bone regeneration procedures—A randomized, controlled clinical trial. Clin Oral Implant. Res. 2015, 26, 28–34. [Google Scholar] [CrossRef]
- Fu, J.H.; Oh, T.J.; Benavides, E.; Rudek, I.; Wang, H.L. A randomized clinical trial evaluating the efficacy of the sandwich bone augmentation technique in increasing buccal bone thickness during implant placement surgery. Clin. Oral Implant. Res. 2014, 25, 458–467. [Google Scholar] [CrossRef]
- Benic, G.I.; Hammerle, C.H. Horizontal bone augmentation by means of guided bone regeneration. Periodontology 2000 2014, 66, 13–40. [Google Scholar] [CrossRef]
- Jung, R.E.; Fenner, N.; Hammerle, C.H.; Zitzmann, N.U. Long-term outcome of implants placed with guided bone regeneration (GBR) using resorbable and non-resorbable membranes after 12–14 years. Clin. Oral Implant. Res. 2013, 24, 1065–1073. [Google Scholar] [CrossRef]
- Benic, G.I.; Jung, R.E.; Siegenthaler, D.W.; Hammerle, C.H. Clinical and radiographic comparison of implants in regenerated or native bone: 5-year results. Clin. Oral Implant. Res. 2009, 20, 507–513. [Google Scholar] [CrossRef]
- Zitzmann, N.U.; Scharer, P.; Marinello, C.P. Long-term results of implants treated with guided bone regeneration: A 5-year prospective study. Int. J. Oral Maxillofac. Implant. 2001, 16, 355–366. [Google Scholar]
- Zumstein, T.; Billstrom, C.; Sennerby, L. A 4- to 5-year retrospective clinical and radiographic study of Neoss implants placed with or without GBR procedures. Clin. Implant Dent. Relat. Res. 2012, 14, 480–490. [Google Scholar] [CrossRef]
- Randall, E.F.; Abou-Arraj, R.V.; Geurs, N.; Griffin, R.; Reddy, M.; Geisinger, M. The Effect of Dental Implant Collar Design on Crestal Bone Loss at 1 Year after Implant Placement. Int. J. Periodontics Restor. Dent. 2019, 39, 165–173. [Google Scholar] [CrossRef]
- Shimada, E.; Pilliar, R.M.; Deporter, D.A.; Schroering, R.; Atenafu, E. A pilot study to assess the performance of a partially threaded sintered porous-surfaced dental implant in the dog mandible. Int. J. Oral Maxillofac. Implant. 2007, 22, 948–954. [Google Scholar]
- Calvo-Guirado, J.L.; Jiménez-Soto, R.; Pérez Albacete-Martínez, C.; Fernández-Domínguez, M.; Gehrke, S.A.; Maté-Sánchez de Val, J.E. Influence of Implant Neck Design on Peri-Implant Tissue Dimensions: A Comparative Study in Dogs. Materials 2018, 11, 2007. [Google Scholar] [CrossRef] [PubMed]
- El Chaar, E.; Amin, S.; Cruz, S.; Gil-Fernandez, N.; Engebreston, S. Crestal Bone and Keratinized Tissue Around 3.0-mm Laser-Microtextured Dental Implants After 1 Year in Function: A Case Series. Int. J. Periodontics Restor. Dent. 2019, 39, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Valles, C.; Rodríguez-Ciurana, X.; Clementini, M.; Baglivo, M.; Paniagua, B.; Nart, J. Influence of subcrestal implant placement compared with equicrestal position on the peri-implant hard and soft tissues around platform-switched implants: A systematic review and meta-analysis. Clin. Oral Investig. 2018, 22, 555–570. [Google Scholar] [CrossRef]
- Lee, J.; Yoo, J.M.; Amara, H.B.; Lee, Y.M.; Lim, Y.J.; Kim, H.; Koo, K.T. Bone healing dynamics associated with 3 implants with different surfaces: Histologic and histomorphometric analyses in dogs. J. Periodontal Implant Sci. 2019, 49, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Abrahamson, I.; Welander, M.; Linder, E.; Berglundh, T. Healing at implants placed in an alveolar ridge whit a sloped configuration: An experimental study in dogs. Clin. Implant Dent. Relat. Res. 2014, 16, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Guirado, J.; Ortiz-Ruiz, A.; Lopez-Marin, L.; Delgado-Ruiz, R.; Mate-Sanchez, J.; Gonzalez, L. Immediate maxillary restoration of single-tooth implants using platform switching for crestal bone preservation: A 12-month study. Int. J. Oral Maxillofac. Implant. 2009, 24, 275–281. [Google Scholar]
- Al-Nsour, M.M.; Chan, H.L.; Wang, H.L. Effect of the platform switching technique on preservation of peri-implant marginal bone: A systematic review. Int. J. Oral Maxillofac. Implant. 2012, 27, 138–145. [Google Scholar]
- Kim, N.S.; Vang, M.S.; Yang, H.S.; Park, S.W.; Park, H.O.; Lim, H.P. Comparison of stability in titanium implants with different surface topographies in dogs. J. Adv. Prosthodont. 2009, 1, 47–55. [Google Scholar] [CrossRef][Green Version]
- Collins, J.R.; Berg, R.W.; Rodríguez, M.; Rodríguez, I.; Coelho, P.G.; Tovar, N. Evaluation of human periimplant soft tissues around nonsubmerged machined standard and platform-switched abutments. Implant Dent. 2015, 24, 57–66. [Google Scholar] [CrossRef]
- Gehrke, S.A.; Marin, G.W. Biomechanical evaluation of dental implants with three different designs: Removal torque and resonance frequency analysis in rabbits. Ann. Anat. 2015, 199, 30–35. [Google Scholar] [CrossRef]
- Palmer, R.M.; Floyd, P.D.; Palmer, P.J.; Smith, B.J.; Johansson, C.B.; Albrektsson, T. Healing of implant dehiscence defects with and without expanded polytetrafluoroethylene membranes: A controlled clinical and histological study. Clin. Oral Implant. Res. 1994, 5, 98–104. [Google Scholar] [CrossRef]
- Rasmusson, L.; Meredith, N.; Sennerby, L. Measurements of stability changes of titanium implants with exposed threads subjected to barrier membrane induced bone augmentation. An experimental study in the rabbit tibia. Clin. Oral Implant. Res. 1997, 8, 316–322. [Google Scholar] [CrossRef]
- Carmagnola, D.; Araujo, M.; Berglundh, T.; Albrektsson, T.; Lindhe, J. Bone tissue reaction around implants placed in a compromised jaw. J. Clin. Periodontol. 1999, 26, 629–635. [Google Scholar] [CrossRef]
- Janyaphadungpong, R.; Serichetaphongse, P.; Pimkhaokham, A. A Clinical Resonance Frequency Analysis of Implants Placed at Dehiscence-type Defects with Simultaneous Guided Bone Regeneration During Early Healing. Int. J. Oral Maxillofac. Implant. 2019. [CrossRef] [PubMed]

| Time 1 | Time 2 | |||||
|---|---|---|---|---|---|---|
| Group | c1 | c2 | c3 | c1 | c2 | c3 |
| G1 | 75.3 ± 5.10 a | 73.9 ± 4.35 | 71.6 ± 4.06 a,b | 81.2 ± 3.79 a | 78.5 ± 4.92 | 77.5 ± 5.32 a,b |
| G2 | 73.9 ± 2.38 a,b | 71.9 ± 4.38 | 67.8 ± 5.06 a,b | 78.8 ± 1.69 a,b | 75.7 ± 3.45 a | 72.6 ± 3.01 b |
| N | Mean | SD | Mean + Standard Error | ||
|---|---|---|---|---|---|
| Crestal Bone Loss (CBL) Buccal | MIS Biocom | 29 | 2.72293 | 1.905044 | 0.353758 |
| Klockner | 26 | 1.80527 | 1.193518 | 0.234068 | |
| CBL Lingual | MIS Biocom | 29 | 1.90383 | 1.72385 | 0.320111 |
| Klockner | 26 | 1.65369 | 1.631999 | 0.320061 | |
| BIC % | MIS Biocom | 29 | 79.559 | 12.99686 | 2.41346 |
| Klockner | 26 | 86.5354 | 7.9248 | 1.55418 |
| CBL | LINGUAL | BUCCAL | ||||
|---|---|---|---|---|---|---|
| Group | c1 | c2 | c3 | c1 | c2 | c3 |
| mean ± SD | mean ± SD | mean ± SD | mean ± SD | mean ± SD | mean ± SD | |
| G1 | 0.3 ± 0.21 | 0.5 ± 0.22 | 0.7 ± 0.16 | 0.3 ± 0.21 | 0.6 ± 0.18 | 1.4 ± 0.25 |
| G2 | 0.4 ± 0.19 | 0.7 ± 0.17 | 1.0 ± 0.25 | 0.9 ± 0.17 | 1.1 ± 0.23 | 2.3 ± 0.23 |
| p-value | 0.1971 | 0.0195 * | 0.0164 * | 0.0009 * | 0.0031 * | 0.0009 * |
| Group G1 | Group G2 | |||
|---|---|---|---|---|
| Condition | Mean ± SD | Median | Mean ± SD | Median |
| c1 | 73.25 ± 3.12 b | 73.32 | 70.52 ± 4.03 b | 70.75 |
| c2 | 72.97 ± 4.11 b | 73.05 | 69.62 ± 4.43 b | 69.88 |
| c3 | 71.43 ± 3.98 b | 71.42 | 68.32 ± 4.66 b | 68.55 |
| G1 Group | G2 Group | |||
|---|---|---|---|---|
| Condition | Mean ± SD | Median | Mean ± SD | Median |
| c1 | 73.98 ± 3.65 a,b | 74.03 | 70.43 ± 3.65 a,b | 70.50 |
| c2 | 71.04 ± 4.49 a,b | 71.10 | 65.23 ± 4.11 a,b | 65.37 |
| c3 | 68.40 ± 4.08 a,b | 69.00 | 60.72 ± 4.29 a,b | 60.85 |
| CBL Vestibular | Implant | Mean ± SD | p-Value |
|---|---|---|---|
| No treatment | MIS | 2.40 ± 0.11 | 0.671 |
| Klockner | 1.59 ± 0.22 * | 0.128 * | |
| Total | 1.99 ± 0.34 | 0.529 | |
| guided bone regeneration (GBR) 2 mm | MIS | 2.68 ± 0.28 | 0.762 |
| Klockner | 2.16 ± 0.17 | 0.821 | |
| Total | 2.42± 0.23 | 0.651 | |
| GBR 5 mm | MIS | 3.03± 0.53 | 0.566 |
| Klockner | 1.90± 0.38 * | 0.321 * | |
| Total | 2.47± 0.35 | 0.762 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Domínguez, M.; Ortega-Asensio, V.; Fuentes Numancia, E.; Aragoneses, J.M.; Barbu, H.M.; Ramírez-Fernández, M.P.; Delgado-Ruiz, R.A.; Calvo-Guirado, J.L.; Samet, N.; Gehrke, S.A. Can the Macrogeometry of Dental Implants Influence Guided Bone Regeneration in Buccal Bone Defects? Histomorphometric and Biomechanical Analysis in Beagle Dogs. J. Clin. Med. 2019, 8, 618. https://doi.org/10.3390/jcm8050618
Fernández-Domínguez M, Ortega-Asensio V, Fuentes Numancia E, Aragoneses JM, Barbu HM, Ramírez-Fernández MP, Delgado-Ruiz RA, Calvo-Guirado JL, Samet N, Gehrke SA. Can the Macrogeometry of Dental Implants Influence Guided Bone Regeneration in Buccal Bone Defects? Histomorphometric and Biomechanical Analysis in Beagle Dogs. Journal of Clinical Medicine. 2019; 8(5):618. https://doi.org/10.3390/jcm8050618
Chicago/Turabian StyleFernández-Domínguez, Manuel, Victor Ortega-Asensio, Elena Fuentes Numancia, Juan Manuel Aragoneses, Horia Mihail Barbu, María Piedad Ramírez-Fernández, Rafael Arcesio Delgado-Ruiz, José Luis Calvo-Guirado, Nahum Samet, and Sergio Alexandre Gehrke. 2019. "Can the Macrogeometry of Dental Implants Influence Guided Bone Regeneration in Buccal Bone Defects? Histomorphometric and Biomechanical Analysis in Beagle Dogs" Journal of Clinical Medicine 8, no. 5: 618. https://doi.org/10.3390/jcm8050618
APA StyleFernández-Domínguez, M., Ortega-Asensio, V., Fuentes Numancia, E., Aragoneses, J. M., Barbu, H. M., Ramírez-Fernández, M. P., Delgado-Ruiz, R. A., Calvo-Guirado, J. L., Samet, N., & Gehrke, S. A. (2019). Can the Macrogeometry of Dental Implants Influence Guided Bone Regeneration in Buccal Bone Defects? Histomorphometric and Biomechanical Analysis in Beagle Dogs. Journal of Clinical Medicine, 8(5), 618. https://doi.org/10.3390/jcm8050618










