Critical Evaluation of Sinonasal Disease in 64 Adults with Primary Ciliary Dyskinesia
Abstract
1. Introduction
2. Experimental Section
2.1. Patients and Methods
2.2. Study Population
2.3. Data Collection
2.4. Standardized ENT Evaluation
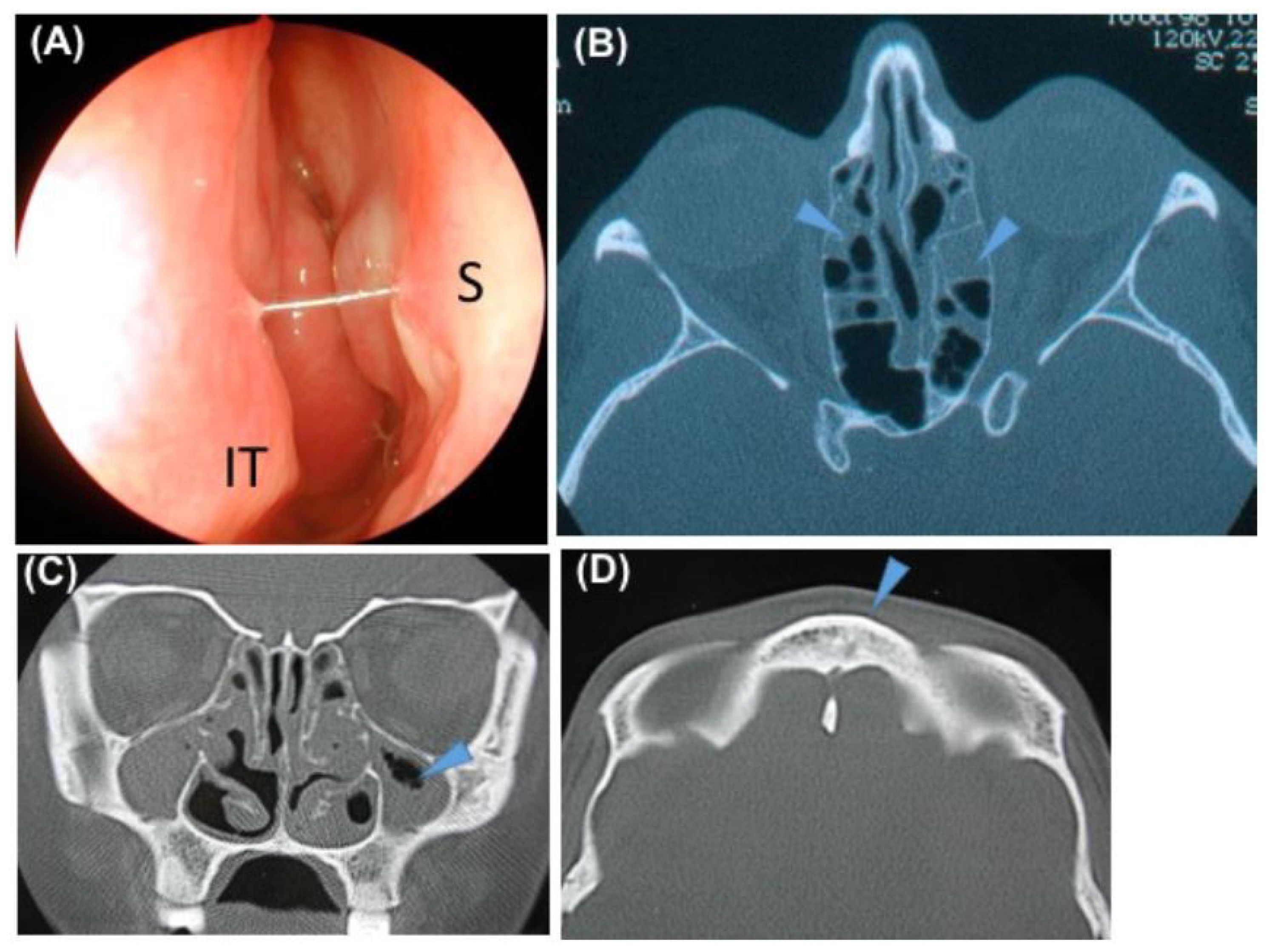
2.5. Sinonasal CT Evaluation
2.6. Statistical Analyses
3. Results
3.1. Study Population
3.2. Description of the Sinonasal Disease
3.3. Description of the Ear Disease
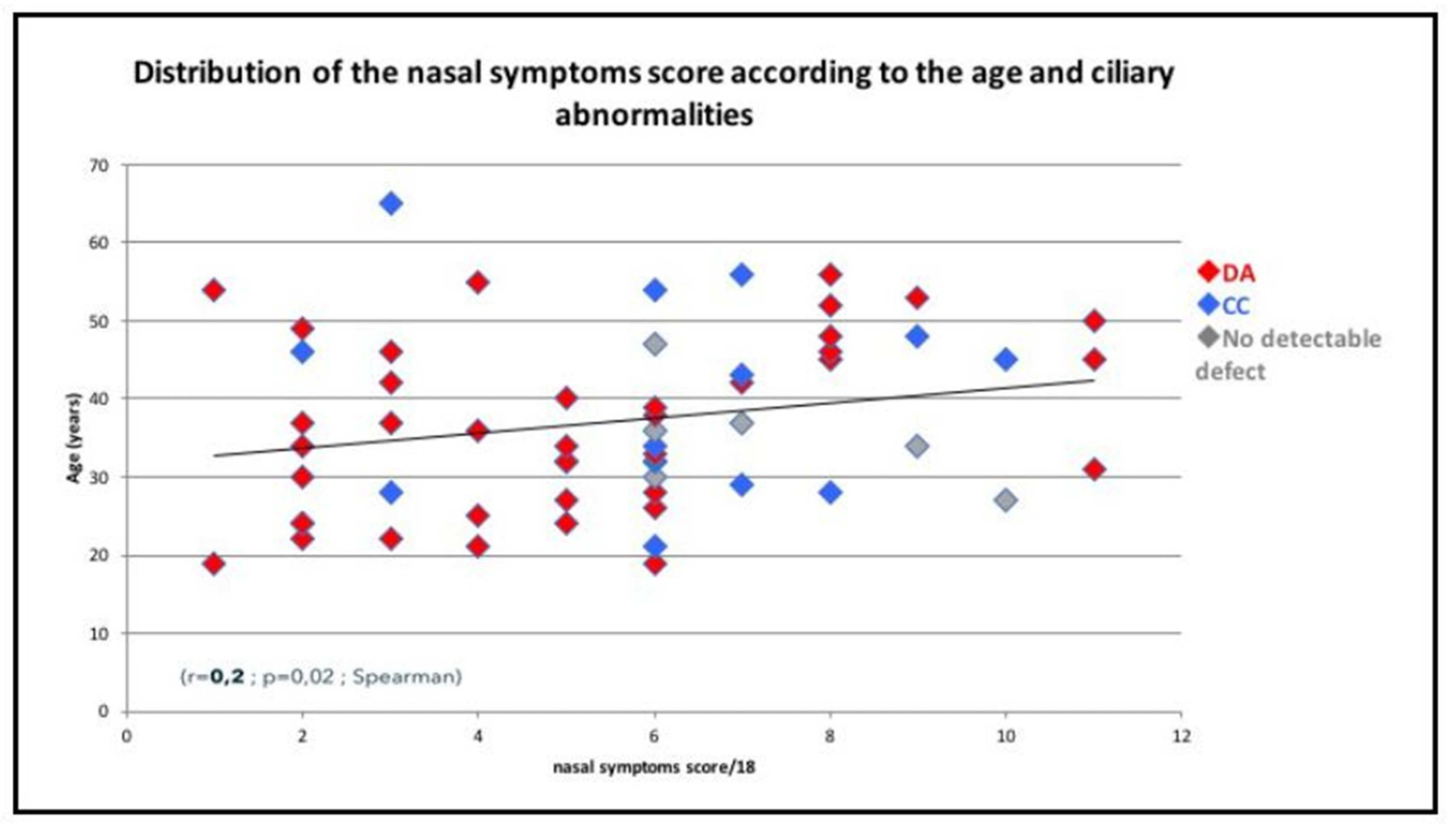
3.4. Correlation between Upper Airways Disease and Characteristics of PCD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| OME | otitis media with effusion |
| PCD | Primary Ciliary Dyskinesia |
| KS | Kartagener Syndrome |
| SI | situs inversus |
| CF | cystic fibrosis |
| n (NO) | Nasal Nitric Oxide |
| CBF | ciliary beat frequency |
| FEV1 | forced expiratory volume |
| TEM | Transmission electron microscope |
| CT | computed tomography |
References
- Bush, A.; Chodhari, R.; Collins, N.; Copeland, F.; Hall, P.; Harcourt, J.; Hariri, M.; Hogg, C.; Lucas, J.; Mitchison, H.M.; et al. Primary ciliary dyskinesia: Current state of the art. Arch. Dis. Child. 2007, 92, 1136–1140. [Google Scholar] [CrossRef]
- Armengot Carceller, M.; Mata Roig, M.; Milara Paya, X.; Cortijo Gimeno, J. Primary ciliary dyskinesia. Ciliopathies. Acta Otorrinolaringol. Esp. 2010, 61, 149–159. [Google Scholar] [CrossRef]
- Barbato, A.; Frischer, T.; Kuehni, C.E.; Snijders, D.; Azevedo, I.; Baktai, G.; Bartoloni, L.; Eber, E.; Escribano, A.; Haarman, E.; et al. Primary ciliary dyskinesia: A consensus statement on diagnostic and treatment approaches in children. Eur. Respir. J. 2009, 34, 1264–1276. [Google Scholar] [CrossRef]
- Pruliere-Escabasse, V.; Coste, A.; Chauvin, P.; Fauroux, B.; Tamalet, A.; Garabedian, E.N.; Escudier, E.; Roger, G. Otologic features in children with primary ciliary dyskinesia. Arch. Otolaryngol. Head Neck Surg. 2010, 136, 1121–1126. [Google Scholar] [CrossRef]
- Ceccaldi, P.F.; Carre-Pigeon, F.; Youinou, Y.; Delepine, B.; Bryckaert, P.E.; Harika, G.; Quereux, C.; Gaillard, D. [Kartagener’s syndrome and infertility: Observation, diagnosis and treatment]. J. Gynecol. Obstet. Biol. Reprod. 2004, 33, 192–194. [Google Scholar] [CrossRef]
- Majithia, A.; Fong, J.; Hariri, M.; Harcourt, J. Hearing outcomes in children with primary ciliary dyskinesia—A longitudinal study. Int. J. Pediatr. Otorhinolaryngol. 2005, 69, 1061–1064. [Google Scholar] [CrossRef]
- Campbell, R.G.; Birman, C.S.; Morgan, L. Management of otitis media with effusion in children with primary ciliary dyskinesia: A literature review. Int. J. Pediatr. Otorhinolaryngol. 2009, 73, 1630–1638. [Google Scholar] [CrossRef]
- Bush, A.; Cole, P.; Hariri, M.; Mackay, I.; Phillips, G.; O’Callaghan, C.; Wilson, R.; Warner, J.O. Primary ciliary dyskinesia: Diagnosis and standards of care. Eur. Respir. J. 1998, 12, 982–988. [Google Scholar] [CrossRef]
- Papon, J.F.; Bassinet, L.; Cariou-Patron, G.; Zerah-Lancner, F.; Vojtek, A.M.; Blanchon, S.; Crestani, B.; Amselem, S.; Coste, A.; Housset, B.; et al. Quantitative analysis of ciliary beating in primary ciliary dyskinesia: A pilot study. Orphanet J. Rare Dis. 2012, 7, 78. [Google Scholar] [CrossRef]
- Vallet, C.; Escudier, E.; Roudot-Thoraval, F.; Blanchon, S.; Fauroux, B.; Beydon, N.; Boule, M.; Vojtek, A.M.; Amselem, S.; Clement, A.; et al. Primary ciliary dyskinesia presentation in 60 children according to ciliary ultrastructure. Eur. J. Pediatr. 2013, 172, 1053–1060. [Google Scholar] [CrossRef]
- Tamalet, A.; Clement, A.; Roudot-Thoraval, F.; Desmarquest, P.; Roger, G.; Boule, M.; Millepied, M.C.; Baculard, T.A.; Escudier, E. Abnormal central complex is a marker of severity in the presence of partial ciliary defect. Pediatrics 2001, 108, E86. [Google Scholar] [CrossRef]
- Jackson, C.L.; Behan, L.; Collins, S.A.; Goggin, P.M.; Adam, E.C.; Coles, J.L.; Evans, H.J.; Harris, A.; Lackie, P.; Packham, S.; et al. Accuracy of diagnostic testing in primary ciliary dyskinesia. Eur. Respir. J. 2016, 47, 837–848. [Google Scholar] [CrossRef]
- Du Mayne, M.D.; Pruliere-Escabasse, V.; Zerah-Lancner, F.; Coste, A.; Papon, J.F. Polypectomy compared with ethmoidectomy in the treatment of nasal polyposis. Arch. Otolaryngol. Head Neck Surg. 2011, 137, 111–117. [Google Scholar] [CrossRef]
- Coste, A.; Yona, L.; Blumen, M.; Louis, B.; Zerah, F.; Rugina, M.; Peynègre, R.; Harf, A.; Escudier, E. Radiofrequency is a safe and effective treatment of turbinate hypertrophy. Laryngoscope 2001, 111, 894–899. [Google Scholar] [CrossRef]
- Hopkins, C.; Browne, J.P.; Slack, R.; Lund, V.; Brown, P. The Lund-Mackay staging system for chronic rhinosinusitis: How is it used and what does it predict? Otolaryngol. Head Neck Surg. 2007, 137, 555–561. [Google Scholar] [CrossRef]
- Orlandi, R.R.; Wiggins, R.H., III. Radiological sinonasal findings in adults with cystic fibrosis. Am. J. Rhinol. Allergy 2009, 23, 307–311. [Google Scholar] [CrossRef]
- Noone, P.G.; Leigh, M.W.; Sannuti, A.; Minnix, S.L.; Carson, J.L.; Hazucha, M.; Zariwala, M.A.; Knowles, M.R. Primary ciliary dyskinesia: Diagnostic and phenotypic features. Am. J. Respir. Crit. Care Med. 2004, 169, 459–467. [Google Scholar] [CrossRef]
- Sommer, J.U.; Schafer, K.; Omran, H.; Olbrich, H.; Wallmeier, J.; Blum, A.; Hormann, K.; Stuck, B.A. ENT manifestations in patients with primary ciliary dyskinesia: Prevalence and significance of otorhinolaryngologic co-morbidities. Eur. Arch. Otorhinolaryngol. 2011, 268, 383–388. [Google Scholar] [CrossRef]
- Frija-Masson, J.; Bassinet, L.; Honore, I.; Dufeu, N.; Housset, B.; Coste, A.; Papon, J.F.; Escudier, E.; Burgel, P.R.; Maitre, B. Clinical characteristics, functional respiratory decline and follow-up in adult patients with primary ciliary dyskinesia. Thorax 2017, 72, 154–160. [Google Scholar] [CrossRef]
- Weber, S.A.T.; Iyomasa, R.M.; Correa, C.C.; Florentino, W.N.M.; Ferrari, G.F. Nasal polyposis in cystic fibrosis: Follow-up of children and adolescents for a 3-year period. Braz. J. Otorhinolaryngol. 2017, 83, 677–682. [Google Scholar] [CrossRef]
- Henriksson, G.; Westrin, K.M.; Karpati, F.; Wikstrom, A.C.; Stierna, P.; Hjelte, L. Nasal polyps in cystic fibrosis: Clinical endoscopic study with nasal lavage fluid analysis. Chest 2002, 121, 40–47. [Google Scholar] [CrossRef]
- Schwitzguebel, A.J.; Jandus, P.; Lacroix, J.S.; Seebach, J.D.; Harr, T. Immunoglobulin deficiency in patients with chronic rhinosinusitis: Systematic review of the literature and meta-analysis. J. Allergy Clin. Immunol. 2015, 136, 1523–1531. [Google Scholar] [CrossRef]
- Coste, A.; Girodon, E.; Louis, S.; Pruliere-Escabasse, V.; Goossens, M.; Peynegre, R.; Escudier, E. Atypical sinusitis in adults must lead to looking for cystic fibrosis and primary ciliary dyskinesia. Laryngoscope 2004, 114, 839–843. [Google Scholar] [CrossRef]
- Pifferi, M.; Bush, A.; Caramella, D.; Di Cicco, M.; Zangani, M.; Chinellato, I.; Macchia, P.; Boner, A.L. Agenesis of paranasal sinuses and nasal nitric oxide in primary ciliary dyskinesia. Eur. Respir. J. 2011, 37, 566–571. [Google Scholar] [CrossRef]
- El-Sayed, Y.; al-Sarhani, A.; al-Essa, A.R. Otological manifestations of primary ciliary dyskinesia. Clin. Otolaryngol. Allied Sci. 1997, 22, 266–270. [Google Scholar] [CrossRef]
- Boon, M.; Jorissen, M.; Proesmans, M.; De Boeck, K. Primary ciliary dyskinesia, an orphan disease. Eur. J. Pediatr. 2013, 172, 151–162. [Google Scholar] [CrossRef]
- Razmpa, E.; Saedi, B.; Safavi, A. The effect of functional endoscopic sinus surgery on pulmonary improvement of controlled asthmatic patients with chronic sinusitis. Iran. J. Allergy Asthma Immunol. 2010, 9, 231–236. [Google Scholar]
- Andersen, T.N.; Alanin, M.C.; von Buchwald, C.; Nielsen, L.H. A longitudinal evaluation of hearing and ventilation tube insertion in patients with primary ciliary dyskinesia. Int. J. Pediatr. Otorhinolaryngol. 2016, 89, 164–168. [Google Scholar] [CrossRef]
- Da Costa, S.S.; Rosito, L.P.; Dornelles, C. Sensorineural hearing loss in patients with chronic otitis media. Eur. Arch. Otorhinolaryngol. 2009, 266, 221–224. [Google Scholar] [CrossRef]
- Lundman, L.; Santi, P.A.; Morizono, T.; Harada, T.; Juhn, S.K.; Bagger-Sjoback, D. Inner ear damage and passage through the round window membrane of Pseudomonas aeruginosa exotoxin A in a chinchilla model. Ann. Otol. Rhinol. Laryngol. 1992, 101, 437–444. [Google Scholar] [CrossRef]
- Roger, G.; Denoyelle, F.; Corré, A.; Escudier, E.; Tamalet, A.; Garabedian, E.N. Place de la chirurgie endonasale lors des dyskinésies ciliaires primitives. Ann. Otol. Rhinol. Laryngol. 2003, 120, 109–113. [Google Scholar]
- Behan, L.; Dimitrov, B.D.; Kuehni, C.E.; Hogg, C.; Carroll, M.; Evans, H.J.; Goutaki, M.; Harris, A.; Packham, S.; Walker, W.T.; et al. PICADAR: A diagnostic predictive tool for primary ciliary dyskinesia. Eur. Respir. J. 2016, 47, 1103–1112. [Google Scholar] [CrossRef]
- Boon, M.; Smits, A.; Cuppens, H.; Jaspers, M.; Proesmans, M.; Dupont, L.J.; Vermeulen, F.L.; Van Daele, S.; Malfroot, A.; Godding, V.; et al. Primary ciliary dyskinesia: Critical evaluation of clinical symptoms and diagnosis in patients with normal and abnormal ultrastructure. Orphanet J. Rare Dis. 2014, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Mullowney, T.; Manson, D.; Kim, R.; Stephens, D.; Shah, V.; Dell, S. Primary ciliary dyskinesia and neonatal respiratory distress. Pediatrics 2014, 134, 1160–1166. [Google Scholar] [CrossRef]
| Characteristics | ||||
|---|---|---|---|---|
| Mean | ±SD | |||
| Age at the first ENT visit in our center (years) | 32 | 11 | ||
| Patient (n) | % | |||
| Gender | ||||
| Female | 24 | 37.5 | ||
| Male | 40 | 62.5 | ||
| PCD related diseases | ||||
| Neonatal respiratory distress | 18 | 28.1 | ||
| Situs inversus | 22 | 34.3 | ||
| Heterotaxy | 4 | 6.2 | ||
| Fertility disorders | 36 | 56.2 | ||
| Retinitis pigmentosa | 3 | 4.7 | ||
| Polycystic kidney disease | 2 | 3.1 | ||
| Family history (n = 59 independent families) | Families (n) | |||
| Consanguinity | 18 | 30.5 | ||
| PCD * | 11 | 18.6 | ||
| Situs inversus | 4 | 6.8 | ||
| Rhinosinusitis | 15 | 25.4 | ||
| Otitis | 9 | 15.2 | ||
| Asthma | 9 | 15.2 | ||
| Bronchiectasis | 9 | 15.2 | ||
| Lung diseases | ||||
| Asthma | 14 | 22 | ||
| Bronchiectasis | 64 | 100 | ||
| Lung surgery | ||||
| Lobectomy | 15 | 23.4 | ||
| Transplantation | 1 | 1.6 | ||
| Lung function test n = 45 | ||||
| FEV1/FVC% mean ± SD (range) | 73.6 ± 13.8 (47–100) | |||
| FEV1% mean ± SD (range) | 72.7 ± 24.7 (18–128) | |||
| Patient (n) | % | ||
|---|---|---|---|
| History of Sinonasal Surgery before the First ENT Visit in our Reference Center | |||
| Unspecified surgery | 22 | 34.4 | |
| Middle meatotomy | 9 | 14.1 | |
| Ethmoidectomy | 8 | 12.5 | |
| Turbinate reduction | 8 | 12.5 | |
| Polypectomy | 1 | 1.6 | |
| Sinonasal symptoms | Score of severity *: mean ± SD (range) | ||
| Rhinorrhoea | 61 | 95.3 | 1.9 ± 0.8 (0–3) |
| Congestion | 42 | 65.6 | 1.3 ± 1.1 (0–3) |
| Facial pain | 35 | 54.7 | 1 ± 0.9 (0–3) |
| Dysosmia | 35 | 54.7 | 1 ± 1.19 (0–3) |
| Nasal hypereactivity | 9 | 14.1 | 0.2 ± 0.6 (0–3) |
| Epistaxis | 4 | 6.2 | 0 ± 0.2 (0–1) |
| Nasal endoscopy | |||
| Inferior turbinate | |||
| Normal | 33 | 51.6 | |
| Hypertrophy | 22 | 34.4 | |
| Atrophy/scar | 10 | 15.6 | |
| Nasal mucosa | |||
| Normal | 13 | 20.3 | |
| Congestion | 28 | 43.7 | |
| Œdema | 9 | 14 | |
| Polyps | 22 | 34.4 | |
| Nasal secretions | |||
| Normal | 8 | 12.5 | |
| Sticky | 35 | 54.7 | |
| Purulent | 21 | 32.8 | |
| n | % | |
|---|---|---|
| Symptoms (n = 64 patients) | ||
| Hearing loss | 34 | 53.1 |
| Ear pain | 9 | 14.1 |
| Tinnitus | 6 | 9.4 |
| Otorrhea | 5 | 7.8 |
| Otoscopy (n = 128 eardrums) | ||
| Normal | 53 | 41.4 |
| OME | 31 | 24.2 |
| Tympanic retraction | 13 | 10.1 |
| Perforation | 8 | 6.2 |
| Tympanosclerosis | 18 | 14 |
| Ventilation tubes | 5 | 3.9 |
| Audiogram (n = 90 ears) | ||
| Normal | 33 | 36.7 |
| Conductive hearing loss | 28 | 31.1 |
| Combined hearing loss | 16 | 17.8 |
| Sensorineural hearing loss | 13 | 14.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bequignon, E.; Dupuy, L.; Zerah-Lancner, F.; Bassinet, L.; Honoré, I.; Legendre, M.; Devars du Mayne, M.; Escabasse, V.; Crestani, B.; Maître, B.; et al. Critical Evaluation of Sinonasal Disease in 64 Adults with Primary Ciliary Dyskinesia. J. Clin. Med. 2019, 8, 619. https://doi.org/10.3390/jcm8050619
Bequignon E, Dupuy L, Zerah-Lancner F, Bassinet L, Honoré I, Legendre M, Devars du Mayne M, Escabasse V, Crestani B, Maître B, et al. Critical Evaluation of Sinonasal Disease in 64 Adults with Primary Ciliary Dyskinesia. Journal of Clinical Medicine. 2019; 8(5):619. https://doi.org/10.3390/jcm8050619
Chicago/Turabian StyleBequignon, Emilie, Laurence Dupuy, Francoise Zerah-Lancner, Laurence Bassinet, Isabelle Honoré, Marie Legendre, Marie Devars du Mayne, Virginie Escabasse, Bruno Crestani, Bernard Maître, and et al. 2019. "Critical Evaluation of Sinonasal Disease in 64 Adults with Primary Ciliary Dyskinesia" Journal of Clinical Medicine 8, no. 5: 619. https://doi.org/10.3390/jcm8050619
APA StyleBequignon, E., Dupuy, L., Zerah-Lancner, F., Bassinet, L., Honoré, I., Legendre, M., Devars du Mayne, M., Escabasse, V., Crestani, B., Maître, B., Escudier, E., Coste, A., & Papon, J.-F. (2019). Critical Evaluation of Sinonasal Disease in 64 Adults with Primary Ciliary Dyskinesia. Journal of Clinical Medicine, 8(5), 619. https://doi.org/10.3390/jcm8050619





