Predictive Factors of Efficacy Maintenance after Testosterone Treatment Cessation
Abstract
1. Introduction
2. Experimental Section
3. Results
3.1. Baseline Characteristics
3.2. Treatment-Related Factors
3.3. Predictors of Response Maintenance
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Mulhall, J.P.; Trost, L.W.; Brannigan, R.E.; Kurtz, E.G.; Redmon, J.B.; Chiles, K.A.; Lightner, D.J.; Miner, M.M.; Murad, M.H.; Nelson, C.J.; et al. Evaluation and Management of Testosterone Deficiency: AUA Guideline. J. Urol. 2018, 200, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Di Donato, M.; Bilancio, A.; D’Amato, L.; Claudiani, P.; Oliviero, M.A.; Barone, M.V.; Auricchio, A.; Appella, E.; Migliaccio, A.; Auricchio, F.; et al. Cross-talk between androgen receptor/filamin A and TrkA regulates neurite outgrowth in PC12 cells. Mol. Biol. Cell 2015, 26, 2858–2872. [Google Scholar] [CrossRef] [PubMed]
- Fargo, K.N.; Galbiati, M.; Foecking, E.M.; Poletti, A.; Jones, K.J. Androgen regulation of axon growth and neurite extension in motoneurons. Horm. Behav. 2008, 53, 716–728. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Sforza, A.; Maggi, M. Testosterone Replacement Therapy: Long-Term Safety and Efficacy. World J. Mens Health 2017, 35, 65–76. [Google Scholar] [CrossRef]
- Hackett, G.; Cole, N.; Mulay, A.; Strange, R.C.; Ramachandran, S. Long-Term Testosterone Therapy in Type 2 Diabetes Is Associated with Decreasing Waist Circumference and Improving Erectile Function. World J. Mens Health 2018. [Google Scholar] [CrossRef]
- Yassin, A.A.; Nettleship, J.; Almehmadi, Y.; Salman, M.; Saad, F. Effects of continuous long-term testosterone therapy (TTh) on anthropometric, endocrine and metabolic parameters for up to 10 years in 115 hypogonadal elderly men: Real-life experience from an observational registry study. Andrologia 2016, 48, 793–799. [Google Scholar] [CrossRef]
- Tsujimura, A.; Takada, S.; Matsuoka, Y.; Hirai, T.; Takao, T.; Miyagawa, Y.; Nonomura, N.; Okuyama, A. Is discontinuation of hormone replacement therapy possible for patients with late-onset hypogonadism? Int. J. Urol. 2008, 15, 625–629. [Google Scholar] [CrossRef]
- Cho, D.Y.; Yeo, J.K.; Cho, S.I.; Jung, J.E.; Yang, S.J.; Kong, D.H.; Ha, J.K.; Kim, J.G.; Park, M.G. Exercise improves the effects of testosterone replacement therapy and the durability of response after cessation of treatment: A pilot randomized controlled trial. Asian J. Androl. 2017, 19, 602–607. [Google Scholar]
- Kumagai, H.; Zempo-Miyaki, A.; Yoshikawa, T.; Tsujimoto, T.; Tanaka, K.; Maeda, S. Lifestyle modification increases serum testosterone level and decrease central blood pressure in overweight and obese men. Endocr. J. 2015, 62, 423–430. [Google Scholar] [CrossRef]
- Lunenfeld, B.; Mskhalaya, G.; Zitzmann, M.; Arver, S.; Kalinchenko, S.; Tishova, Y.; Morgentaler, A. Recommendations on the diagnosis, treatment and monitoring of hypogonadism in men. Aging Male 2015, 18, 5–15. [Google Scholar] [CrossRef]
- Heufelder, A.E.; Saad, F.; Bunck, M.C.; Gooren, L. Fifty-two-week treatment with diet and exercise plus transdermal testosterone reverses the metabolic syndrome and improves glycemic control in men with newly diagnosed type 2 diabetes and subnormal plasma testosterone. J. Androl. 2009, 30, 726–733. [Google Scholar] [CrossRef]
- Ng Tang Fui, M.; Hoermann, R.; Zajac, J.D.; Grossmann, M. The effects of testosterone on body composition in obese men are not sustained after cessation of testosterone treatment. Clin. Endocrinol. 2017, 87, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Finkle, W.D.; Greenland, S.; Ridgeway, G.K.; Adams, J.L.; Frasco, M.A.; Cook, M.B.; Fraumeni, J.F., Jr.; Hoover, R.N. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS ONE 2014, 9, e85805. [Google Scholar] [CrossRef] [PubMed]
- Vigen, R.; O’Donnell, C.I.; Baron, A.E.; Grunwald, G.K.; Maddox, T.M.; Bradley, S.M.; Barqawi, A.; Woning, G.; Wierman, M.E.; Plomondon, M.E.; et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA 2013, 310, 1829–1836. [Google Scholar] [CrossRef]
- Basaria, S.; Coviello, A.D.; Travison, T.G.; Storer, T.W.; Farwell, W.R.; Jette, A.M.; Eder, R.; Tennstedt, S.; Ulloor, J.; Zhang, A.; et al. Adverse events associated with testosterone administration. N. Engl. J. Med. 2010, 363, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Oni, O.A.; Gupta, K.; Chen, G.; Sharma, M.; Dawn, B.; Sharma, R.; Parashara, D.; Savin, V.J.; Ambrose, J.A.; et al. Normalization of testosterone level is associated with reduced incidence of myocardial infarction and mortality in men. Eur. Heart J. 2015, 36, 2706–2715. [Google Scholar] [CrossRef] [PubMed]
- Morgentaler, A.; Traish, A.M. Shifting the paradigm of testosterone and prostate cancer: The saturation model and the limits of androgen-dependent growth. Eur. Urol. 2009, 55, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Wallis, C.J.; Lo, K.; Lee, Y.; Krakowsky, Y.; Garbens, A.; Satkunasivam, R.; Herschorn, S.; Kodama, R.T.; Cheung, P.; Narod, S.A.; et al. Survival and cardiovascular events in men treated with testosterone replacement therapy: An intention-to-treat observational cohort study. Lancet Diabetes Endocrinol. 2016, 4, 498–506. [Google Scholar] [CrossRef]
- Cheetham, T.C.; An, J.; Jacobsen, S.J.; Niu, F.; Sidney, S.; Quesenberry, C.P.; Van Den Eeden, S.K. Association of Testosterone Replacement with Cardiovascular Outcomes among Men with Androgen Deficiency. JAMA Intern. Med. 2017, 177, 491–499. [Google Scholar] [CrossRef]
- Hackett, G.; Kirby, M.; Edwards, D.; Jones, T.H.; Wylie, K.; Ossei-Gerning, N.; David, J.; Muneer, A. British Society for Sexual Medicine Guidelines on Adult Testosterone Deficiency, with Statements for UK Practice. J. Sex. Med. 2017, 14, 1504–1523. [Google Scholar] [CrossRef]
- Bhasin, S.; Brito, J.P.; Cunningham, G.R.; Hayes, F.J.; Hodis, H.N.; Matsumoto, A.M.; Snyder, P.J.; Swerdloff, R.S.; Wu, F.C.; Yialamas, M.A. Testosterone Therapy in Men With Hypogonadism: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2018, 103, 1715–1744. [Google Scholar] [CrossRef] [PubMed]
- Dean, J.D.; McMahon, C.G.; Guay, A.T.; Morgentaler, A.; Althof, S.E.; Becher, E.F.; Bivalacqua, T.J.; Burnett, A.L.; Buvat, J.; El Meliegy, A.; et al. The International Society for Sexual Medicine’s Process of Care for the Assessment and Management of Testosterone Deficiency in Adult Men. J. Sex. Med. 2015, 12, 1660–1686. [Google Scholar] [CrossRef] [PubMed]
- Glueck, C.J.; Goldenberg, N.; Wang, P. Thromboembolism peaking 3 months after starting testosterone therapy: Testosterone-thrombophilia interactions. J. Investig. Med. 2018, 66, 733–738. [Google Scholar] [CrossRef]
- Wang, C.; Nieschlag, E.; Swerdloff, R.; Behre, H.M.; Hellstrom, W.J.; Gooren, L.J.; Kaufman, J.M.; Legros, J.J.; Lunenfeld, B.; Morales, A.; et al. ISA, ISSAM, EAU, EAA and ASA recommendations: Investigation, treatment and monitoring of late-onset hypogonadism in males. Int. J. Impot. Res. 2009, 21, 1–8. [Google Scholar] [CrossRef]
- Pearl, J.A.; Berhanu, D.; Francois, N.; Masson, P.; Zargaroff, S.; Cashy, J.; McVary, K.T. Testosterone supplementation does not worsen lower urinary tract symptoms. J. Urol. 2013, 190, 1828–1833. [Google Scholar] [CrossRef] [PubMed]
- Shigehara, K.; Sugimoto, K.; Konaka, H.; Iijima, M.; Fukushima, M.; Maeda, Y.; Mizokami, A.; Koh, E.; Origasa, H.; Iwamoto, T.; et al. Androgen replacement therapy contributes to improving lower urinary tract symptoms in patients with hypogonadism and benign prostate hypertrophy: A randomised controlled study. Aging Male 2011, 14, 53–58. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, M.D.; Roberts, S.A.; Srinivas-Shankar, U.; Tajar, A.; Connolly, M.J.; Adams, J.E.; Oldham, J.A.; Wu, F.C. Do the effects of testosterone on muscle strength, physical function, body composition, and quality of life persist six months after treatment in intermediate-frail and frail elderly men? J. Clin. Endocrinol. Metab. 2011, 96, 454–458. [Google Scholar] [CrossRef]
- Gooren, L.J. Advances in testosterone replacement therapy. Front. Horm. Res. 2009, 37, 32–51. [Google Scholar]
- Yassin, A.A.; Haffejee, M. Testosterone depot injection in male hypogonadism: A critical appraisal. Clin. Interv. Aging 2007, 2, 577–590. [Google Scholar]
- Chigurupati, S.; Son, T.G.; Hyun, D.H.; Lathia, J.D.; Mughal, M.R.; Savell, J.; Li, S.C.; Nagaraju, G.P.; Chan, S.L.; Arumugam, T.V.; et al. Lifelong running reduces oxidative stress and degenerative changes in the testes of mice. J. Endocrinol. 2008, 199, 333–341. [Google Scholar] [CrossRef]
- Lee, H.K.; Lee, J.K.; Cho, B. The role of androgen in the adipose tissue of males. World J. Mens Health 2013, 31, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, K.; Iemitsu, M.; Maeda, S.; Mesaki, N.; Ushida, T.; Akimoto, T. Endurance exercise training enhances local sex steroidogenesis in skeletal muscle. Med. Sci. Sports Exerc. 2011, 43, 2072–2080. [Google Scholar] [CrossRef]
- Yeo, J.K.; Cho, S.I.; Park, S.G.; Jo, S.; Ha, J.K.; Lee, J.W.; Cho, S.Y.; Park, M.G. Which Exercise Is Better for Increasing Serum Testosterone Levels in Patients with Erectile Dysfunction? World J. Mens Health 2018, 36, 147–152. [Google Scholar] [CrossRef]
- Dobs, A.S.; Meikle, A.W.; Arver, S.; Sanders, S.W.; Caramelli, K.E.; Mazer, N.A. Pharmacokinetics, efficacy, and safety of a permeation-enhanced testosterone transdermal system in comparison with bi-weekly injections of testosterone enanthate for the treatment of hypogonadal men. J. Clin. Endocrinol. Metab. 1999, 84, 3469–3478. [Google Scholar] [PubMed]
- Hohl, A.; Marques, M.O.; Coral, M.H.; Walz, R. Evaluation of late-onset hypogonadism (andropause) treatment using three different formulations of injectable testosterone. Arq. Bras. Endocrinol. Metabol. 2009, 53, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Saad, F.; Gooren, L.J.; Haider, A.; Yassin, A. A dose-response study of testosterone on sexual dysfunction and features of the metabolic syndrome using testosterone gel and parenteral testosterone undecanoate. J. Androl. 2008, 29, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Saad, F.; Gooren, L.; Haider, A.; Yassin, A. Effects of testosterone gel followed by parenteral testosterone undecanoate on sexual dysfunction and on features of the metabolic syndrome. Andrologia 2008, 40, 44–48. [Google Scholar] [CrossRef]
| Group I | Group II | p-Value | |
|---|---|---|---|
| N (%) | 92/151 (60.9) | 59/151 (30.1) | |
| Age (years) | 61.1 ± 9.3 | 60.4 ± 7.7 | 0.669 |
| BMI (kg/m2) | 25.0 ± 2.8 | 24.9 ± 2.6 | 0.745 |
| Waist (cm) | 86.5 ± 6.8 | 86.0 ± 6.2 | 0.640 |
| Comorbidities, N | 0.095 | ||
| None | 32/92 | 19/59 | |
| HTN | 28/92 | 18/59 | |
| DM | 18/92 | 13/59 | |
| Dyslipidemia | 8/92 | 7/59 | |
| Hepatobiliary disease | 8/92 | 4/59 | |
| Pulmonary disease | 9/92 | 5/59 | |
| Chronic kidney disease | 8/92 | 3/59 | |
| Alcohol, N | 0.753 | ||
| 65/92 | 41/59 | ||
| Smoking, N | 0.654 | ||
| 13/92 | 4/59 | ||
| Exercise, N | 0.000 | ||
| Yes | 9/92 | 32/59 | |
| No | 83/92 | 27/59 | |
| IIEF total | 27 ± 14.18 | 29.34 ± 14.1 | 0.323 |
| Erectile function | 11.02 ± 7.03 | 12.20 ± 7.08 | 0.316 |
| Orgasmic function | 3.71 ± 3.39 | 4.14 ± 3.35 | 0.447 |
| Sexual desire | 4.23 ± 1.80 | 4.46 ± 1.87 | 0.453 |
| Intercourse satisfaction | 4.32 ± 3.10 | 4.68 ± 3.03 | 0.480 |
| Overall satisfaction | 4.05 ± 1.78 | 4.20 ± 1.81 | 0.619 |
| AMS total | 38.52 ± 9.37 | 38.86 ± 9.69 | 0.829 |
| Psycho | 9.33 ± 3.63 | 9.46 ± 3.59 | 0.827 |
| Somato | 14.50 ± 4.27 | 14.46 ± 4.23 | 0.952 |
| Sexual | 14.70 ± 4.25 | 14.95 ± 4.32 | 0.723 |
| PSA (ug/dL) | 1.06 ± 0.71 | 1.07 ± 0.73 | 0.953 |
| Hb (g/dL) | 14.76 ± 1.09 | 14.82 ± 1.17 | 0.755 |
| Hct (%) | 42.92 ± 3.11 | 42.70 ± 2.85 | 0.662 |
| Glucose (mg/dL) | 106.86 ± 24.72 | 105.03 ± 20.54 | 0.638 |
| Total Cholesterol (mg/dL) | 176.12 ± 35.65 | 181.20 ± 40.24 | 0.418 |
| TG (mg/dL) | 220.01 ± 219.83 | 233.54 ± 235.79 | 0.726 |
| HDL (mg/dL) | 48.53 ± 10.27 | 46.96 ± 9.26 | 0.355 |
| LDL (mg/dL) | 114.44 ± 25.93 | 120.49 ± 30.79 | 0.206 |
| Group I | Group II | p-Value | |
|---|---|---|---|
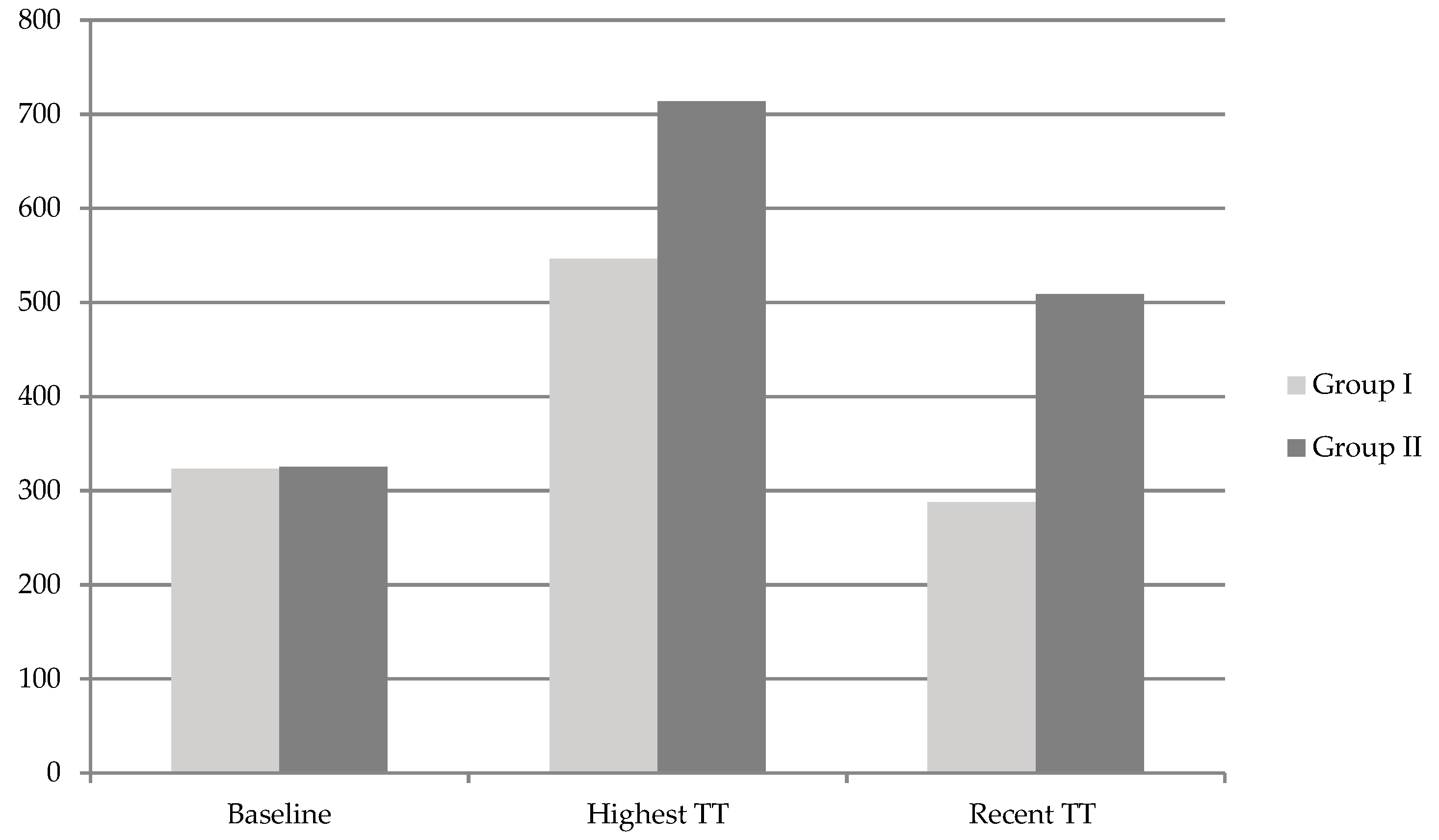
| Baseline TT (ng/dL) | 323.0 ± 15.4 | 325.2 ± 19.8 | 0.221 |
| Highest TT (ng/dL) | 546.1 ± 230.3 | 713.7 ± 139.2 | 0.000 |
| ∆ (Highest–Baseline)TT (ng/dL) | 223.0 ± 231.1 | 388.5 ± 244.0 | 0.000 |
| TRT duration (months) | 5.2 ± 4.4 | 10.7 ± 9.5 | 0.000 |
| Type of TRT, N (%) | 0.347 | ||
| Oral TU | 15/92 (16.3) | 9/59 (15.3) | |
| T-gel | 44/92 (47.8) | 31/59 (52.4) | |
| Injectable TE | 13/92 (14.1) | 5/59 (8.5) | |
| Injectable TU | 10/92 (10.9) | 9/59 (15.3) | |
| Mixed | 10/92 (10.9) | 5/59 (8.5) | |
| IIEF total | 35.01 ± 10.04 * | 34.92 ± 10.17 * | 0.955 |
| Erectile function | 14.27 ± 5.34 * | 14.14 ± 5.26 * | 0.878 |
| Orgasmic function | 5.08 ± 2.84 * | 5.10 ± 2.80 * | 0.957 |
| Sexual desire | 5.47 ± 1.84 * | 5.30 ± 1.81 * | 0.576 |
| Intercourse satisfaction | 5.79 ± 2.08 * | 5.71 ± 2.16 * | 0.817 |
| Overall satisfaction | 4.76 ± 1.73 * | 4.80 ± 1.76 * | 0.902 |
| AMS total | 32.332 ± 6.02 * | 32.49 ± 6.28 * | 0.871 |
| Psycho | 7.49 ± 2.11 * | 7.56 ± 2.08 * | 0.841 |
| Somato | 11.42 ± 2.90 * | 11.42 ± 2.75 * | 0.885 |
| Sexual | 13.38 ± 3.80 * | 13.47 ± 4.02 * | 0.885 |
| PSA (ug/dL) | 1.23 ± 0.91 ¥ | 1.31 ± 0.93 ¥ | 0.723 |
| Hb (g/dL) | 15.21 ± 1.21 * | 15.31 ± 1.05 * | 0.755 |
| Hct (%) | 45.21 ± 3.81 * | 45.70 ± 3.85 * | 0.760 |
| Odds Ratio | p-Value | |
|---|---|---|
| Exercise | 10.23 | 0.000 |
| Periods of TRT | 1.166 | 0.000 |
| Highest TT | 1.002 | 0.192 |
| ∆ (Highest–Baseline)TT | 1.000 | 0.978 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, M.G.; Yeo, J.K.; Park, S.G.; Na, W.; Moon, D.G. Predictive Factors of Efficacy Maintenance after Testosterone Treatment Cessation. J. Clin. Med. 2019, 8, 151. https://doi.org/10.3390/jcm8020151
Park MG, Yeo JK, Park SG, Na W, Moon DG. Predictive Factors of Efficacy Maintenance after Testosterone Treatment Cessation. Journal of Clinical Medicine. 2019; 8(2):151. https://doi.org/10.3390/jcm8020151
Chicago/Turabian StylePark, Min Gu, Jeong Kyun Yeo, Sun Gu Park, Woong Na, and Du Geon Moon. 2019. "Predictive Factors of Efficacy Maintenance after Testosterone Treatment Cessation" Journal of Clinical Medicine 8, no. 2: 151. https://doi.org/10.3390/jcm8020151
APA StylePark, M. G., Yeo, J. K., Park, S. G., Na, W., & Moon, D. G. (2019). Predictive Factors of Efficacy Maintenance after Testosterone Treatment Cessation. Journal of Clinical Medicine, 8(2), 151. https://doi.org/10.3390/jcm8020151





