The Optimal Indication for Testosterone Replacement Therapy in Late Onset Hypogonadism
Abstract
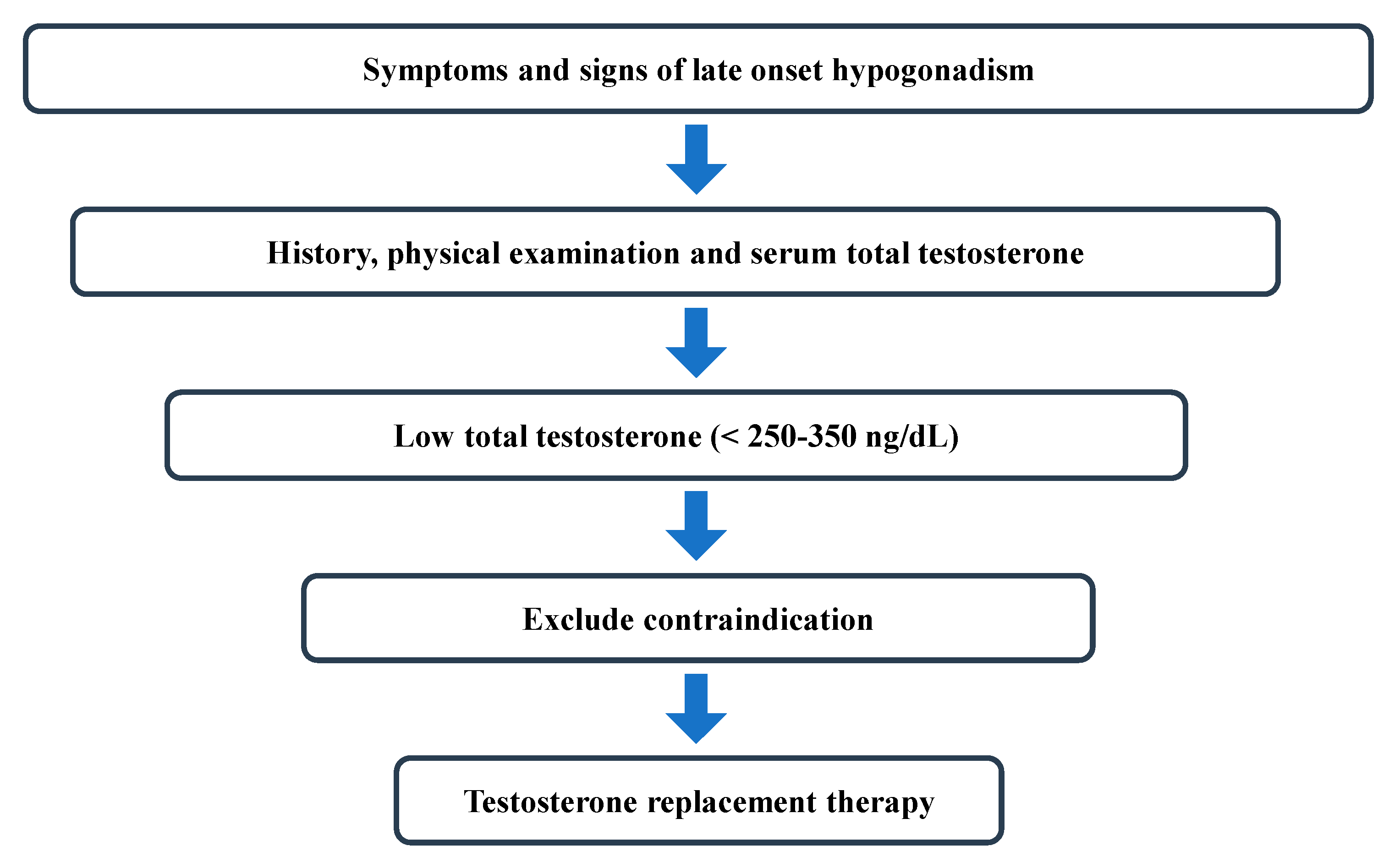
:1. Introduction
2. Diagnosis
2.1. ISSAM Guidelines
2.2. EAU Guidelines
2.3. ESE and EAA Guidelines
2.4. AUA Guidelines
2.5. Canadian Men’s Health Foundation Multidisciplinary Guidelines
3. Optimal Indication for TRT
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADAM | Androgen Deficiency in the Aging Male questionnaire |
| AMS | Aging Male’s Symptoms scale |
| AUA | American Association of Urology |
| BMD | bone mineral density |
| BMI | body mass index |
| BT | bioavailable testosterone |
| CVD | cardiovascular disease |
| EAA | European Academy of Andrology |
| EAU | European Association of Urology |
| ED | erectile dysfunction |
| ESE | European Society of Endocrinology |
| FT | free testosterone |
| Hb | hemoglobin |
| Hct | hematocrit |
| ISSAM | International Society for the Study of Aging Male |
| LOH | late-onset hypogonadism |
| MMAS | Massachusetts Male Aging Study questionnaire |
| PCa | prostate cancer |
| PSA | prostate-specific antigen |
| SHBG | sex hormone binding globulin |
| T | testosterone |
| TRT | testosterone replacement therapy |
| TT | total testosterone |
References
- Bassil, N.; Alkaade, S.; Morley, J.E. The benefits and risks of testosterone therapy: A review. Ther. Clin. Risk Manag. 2009, 5, 427–448. [Google Scholar] [PubMed]
- Choi, S.W.; Jeon, S.H.; Kwon, E.B.; Zhu, G.Q.; Lee, K.W.; Choi, J.B.; Jeong, H.C.; Kim, K.S.; Bae, S.R.; Bae, W.J.; et al. Effect of Korean Herbal Formula (Modified Ojayeonjonghwan) on Androgen Receptor Expression in an Aging Rat Model of Late Onset Hypogonadism. World J. Mens Health 2019, 37, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Traish, A.M.; Johansen, V. Impact of Testosterone Deficiency and Testosterone Therapy on Lower Urinary Tract Symptoms in Men with Metabolic Syndrome. World J. Mens Health 2018, 36, 199–222. [Google Scholar] [CrossRef]
- Hackett, G. Type 2 Diabetes and Testosterone Therapy. World J. Mens Health 2019, 37, 31–44. [Google Scholar] [CrossRef]
- Nam, Y.S.; Lee, G.; Yun, J.M.; Cho, B. Testosterone Replacement, Muscle Strength, and Physical Function. World J. Mens Health 2018, 36, 110–122. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, K.S.; Lee, E.K.; Park, N.C. Efficacy and Safety of a Mixed Extract of Trigonella foenum-graecum Seed and Lespedeza cuneata in the Treatment of Testosterone Deficiency Syndrome: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. World J. Mens Health 2018, 36, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.C.; Tajar, A.; Beynon, J.M.; Pye, S.R.; Silman, A.J.; Finn, J.D.; O’Neill, T.W.; Bartfai, G.; Casanueva, F.F.; Forti, G.; et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N. Engl. J. Med. 2010, 363, 123–135. [Google Scholar] [CrossRef]
- Samaras, N.; Papadopoulou, M.A.; Samaras, D.; Ongaro, F. Off-label use of hormones as an antiaging strategy: A review. Clin. Interv. Aging 2014, 9, 1175–1186. [Google Scholar] [CrossRef]
- Yialamas, M.A.; Hayes, F.J. Androgens and the ageing male and female. J. Clin. Endocrinol. Metab. 2003, 17, 223–236. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, N.; Thakur, D.S.; Patidar, A. Male hypogonadism: Symptoms and treatment. J. Adv. Pharm. Technol. Res. 2010, 1, 297–301. [Google Scholar] [CrossRef]
- Waite, L.J. The Demographic Faces of the Elderly. Popul. Dev. Rev. 2004, 30, 3–16. [Google Scholar]
- Wang, C.; Nieschlag, E.; Swerdloff, R.; Behre, H.M.; Hellstrom, W.J.; Gooren, L.J.; Kaufman, J.M.; Legros, J.J.; Lunenfeld, B.; Morales, A.; et al. Investigation, treatment and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA and ASA recommendations. Eur. J. Endocrinol. 2008, 159, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Lunenfeld, B.; Mskhalaya, G.; Zitzmann, M.; Arver, S.; Kalinchenko, S.; Tishova, Y.; Morgentaler, A. Recommendations on the diagnosis, treatment and monitoring of hypogonadismin men. Aging Male 2015, 18, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Lunenfeld, B.; Arver, S.; Moncada, I.; Rees, D.A.; Schulte, H.M. How to help the aging male? Current approaches to hypogonadism in primary care. Aging Male 2012, 15, 187–197. [Google Scholar] [CrossRef]
- Dohle, G.R.; Arver, S.; Bettocchi, C.; Jones, T.H.; Kliesch, S. EAU 2018 guideline on male hypogonadism. Available online: http://www.uroweb.org/guideline/male-hypogonadism/ (accessed on 16 June 2018).
- Morley, J.E.; Perry, H.M., III; Kevorkian, R.T.; Patrick, P. Comparison of screening questionnaires for the diagnosis of hypogonadism. Maturitas 2006, 53, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Brunton, S.A.; Sadovsky, R. Late-onset male hypogonadism and testosterone replacement therapy in primary care. J. Fam. Pract. 2010, 59, 1–8. [Google Scholar]
- Bhasin, S.; Brito, J.P.; Cunningham, G.R.; Hayes, F.J.; Hodis, H.N.; Matsumoto, A.M.; Snyder, P.J.; Swerdloff, R.S.; Wu, F.C.; Yialamas, M.A. Testosterone therapy in men with hypogonadism: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2018, 103, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Isidori, A.M.; Lenzi, A. Risk factors for androgen decline in older males: Lifestyle, chronic diseases and drugs. J. Endocrinol. Invest. 2005, 28, 14–22. [Google Scholar]
- Rosner, W.; Auchus, R.J.; Azziz, R.; Sluss, P.M.; Raff, H. Utility, limitations, and pitfalls in measuring testosterone: An Endocrine Society Position Statement. J. Clin. Endocrinol. Metab. 2007, 92, 405–413. [Google Scholar] [CrossRef]
- Diver, M.J.; Imtiaz, K.E.; Ahmad, A.M.; Vora, J.P.; Fraser, W.D. Diurnal rhythms of serum total, free and bioavailable testosterone and of SHBG in middleaged men compared with those in young men. Clin. Endocrinol. 2003, 58, 710–717. [Google Scholar] [CrossRef]
- Vermeulen, A.; Verdonck, L.; Kaufman, J.M. A critical evaluation of simple methods for the estimation of free testosterone in serum. J. Clin. Endocrinol. Metab. 1999, 84, 3666–3672. [Google Scholar] [CrossRef]
- Hall, S.A.; Esche, G.R.; Araujo, A.B.; Travison, T.G.; Clark, R.V.; Williams, R.E.; McKinlay, J.B. Correlates of low testosterone and symptomatic androgen deficiency in a population-based sample. J. Clin. Endocrinol. Metab. 2008, 93, 3870–3877. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, S.; Pencina, M.; Jasuja, G.K.; Travison, T.G.; Coviello, A.; Orwoll, E.; Wang, P.Y.; Nielson, C.; Wu, F.; Tajar, A.; et al. Reference ranges for testosterone in men generated using liquid chromatography tandem mass spectrometry in a community-based sample of healthy nonobese young men in the Framingham Heart Study and applied to three geographically distinct cohorts. J. Clin. Endocrinol. Metab. 2011, 96, 2430–2439. [Google Scholar] [CrossRef] [PubMed]
- Bremner, W.J.; Vitiello, M.V.; Prinz, P.N. Loss of circadian rhythmicity in blood testosterone levels with aging in normal men. J. Clin. Endocrinol. Metab. 1983, 56, 1278–1281. [Google Scholar] [CrossRef] [PubMed]
- Morales, A. Testosterone Deficiency Syndrome: An overview with emphasis on the diagnostic conundrum. Clin. Biochem. 2014, 47, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Buvat, J.; Lemaire, A. Endocrine screening in 1022 men with erectile dysfunction: clinical significance and cost-effective strategy. J. Urol. 1997, 158, 1764–1767. [Google Scholar] [CrossRef]
- Araujo, A.B.; Esche, G.R.; Kupelian, V.; O’Donnell, A.B.; Travison, T.G.; Williams, R.E.; Clark, R.V.; McKinlay, J.B. Prevalence of symptomatic androgen deficiency in men. J. Clin. Endocrinol. Metab. 2007, 92, 4241–4247. [Google Scholar] [CrossRef]
- Finkelstein, J.S.; Lee, H.; Burnett-Bowie, S.A.; Pallais, J.C.; Yu, E.W.; Borges, L.F.; Jones, B.F.; Barry, C.V.; Wulczyn, K.E.; Thomas, B.J.; et al. Gonadal steroids and body composition, strength, and sexual function in men. N. Engl. J. Med. 2013, 369, 1011–1022. [Google Scholar] [CrossRef]
- Mulhall, J.P.; Trost, L.W.; Brannigan, R.E.; Kurtz, E.G.; Redmon, J.B.; Chiles, K.A.; Lightner, D.J.; Miner, M.M.; Murad, M.H.; Nelson, C.J.; et al. Evaluation and Management of Testosterone Deficiency: AUA Guideline. J. Urol. 2018, 200, 423–432. [Google Scholar] [CrossRef]
- Morales, A.; Bebb, R.A.; Manjoo, P.; Assimakopoulos, P.; Axler, J.; Collier, C.; Elliott, S.; Goldenberg, L.; Gottesman, I.; Grober, E.D.; et al. Diagnosis and management of testosterone deficiency syndrome in men: clinical practice guideline. Can. Med. Assoc. J. 2015, 187, 1369–1377. [Google Scholar] [CrossRef]
- Malik, R.D.; Lapin, B.; Wang, C.E.; Lakeman, J.C.; Helfand, B.T. Are we testing appropriately for low testosterone?: Characterization of tested men and compliance with current guidelines. J. Sex. Med. 2015, 12, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Malik, R.D.; Lapin, B.; Wang, C.E.; Lakeman, J.C.; Helfand, B.T. Characteristics of men undergoing testosterone replacement therapy and adherence to follow-up recommendations in Metropolitan Multicenter Health Care System. Urology 2015, 85, 1382–1388. [Google Scholar] [CrossRef] [PubMed]
- Baillargeon, J.; Urban, R.J.; Kuo, Y.F.; Holmes, H.M.; Raji, M.A.; Morgentaler, A.; Howrey, B.T.; Lin, Y.L.; Ottenbacher, K.J. Screening and monitoring in men prescribed testosterone therapy in the US, 2001–2010. Public Health Rep. 2015, 130, 143–152. [Google Scholar] [CrossRef]
- Brand, J.S.; van der Tweel, I.; Grobbee, D.E.; Emmelot-Vonk, M.H.; van der Schouw, Y.T. Testosterone, sex hormone-binding globulin and the metabolic syndrome: A systematic review and meta-analysis of observational studies. Int. J. Epidemiol. 2011, 40, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, D.J.; Matsumoto, A.M.; Araujo, A.B.; McKinlay, J.B. The effect of diurnal variation on clinical measurement of serum testosterone and other sex hormone levels in men. J. Clin. Endocrinol. Metab. 2009, 94, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Trost, L.W.; Mulhall, J.P. Challenges in testosterone measurement, data interpretation, and methodological appraisal of interventional trials. J. Sex. Med. 2016, 13, 1029–1046. [Google Scholar] [CrossRef] [PubMed]
- Coviello, A.D.; Kaplan, B.; Lakshman, K.M.; Chen, T.; Singh, A.B.; Bhasin, S. Effects of graded doses of testosterone on erythropoiesis in healthy young and older men. J. Clin. Endocrinol. Metab. 2008, 93, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Balsells, M.M.; Murad, M.H.; Lane, M.; Lampropulos, J.F.; Albuquerque, F.; Mullan, R.J.; Agrwal, N.; Elamin, M.B.; Gallegos-Orozco, J.F.; Wang, A.T.; et al. Clinical review 1: Adverse effects of testosterone therapy in adult men: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2010, 95, 2560–2575. [Google Scholar] [CrossRef]
- Swerdloff, R.S.; Wang, C. Three-year follow-up of androgen treatment in hypogonadal men: preliminary report with testosterone gel. Aging Male 2003, 6, 207–211. [Google Scholar] [CrossRef]
- Carter, H.B.; Albertsen, P.C.; Barry, M.J.; Etzioni, R.; Freedland, S.J.; Greene, K.L.; Holmberg, L.; Kantoff, P.; Konety, B.R.; Murad, M.H.; et al. Early detection of prostate cancer: AUA Guideline. J. Urol. 2013, 190, 419–426. [Google Scholar] [CrossRef]
- Khera, M.; Bhattacharya, R.K.; Blick, G.; Kushner, H.; Nguyen, D.; Miner, M.M. Changes in prostate specific antigen in hypogonadal men after12 months of testosterone replacement therapy: support for the prostate saturation theory. J. Urol. 2011, 186, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Maggio, M.; Bandinelli, S.; Basaria, S.; Lauretani, F.; Ble, A.; Valenti, G.; Ershler, W.B.; Guralnik, J.M.; Longo, D.L. Low testosterone levels and the risk of anemia in older men and women. Arch. Intern. Med. 2006, 166, 1380–1388. [Google Scholar] [CrossRef] [PubMed]
- Shahani, S.; Braga-Basaria, M.; Maggio, M.; Basaria, S. Androgens and erythropoiesis: past and present. J. Endocrinol. Invest. 2009, 32, 704–716. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.S.; You, J.H.; Cha, J.S.; Park, J.K. The relationship between serum total testosterone and free testosterone levels with serum hemoglobin and hematocrit levels: A study in 1221 men. Aging Male 2016, 19, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Roy, C.N.; Snyder, P.J.; Stephens-Shields, A.J.; Artz, A.S.; Bhasin, S.; Cohen, H.J.; Farrar, J.T.; Gill, T.M.; Zeldow, B.; Cella, D.; et al. Association of Testosterone Levels with Anemia in Older Men: A Controlled Clinical Trial. JAMA Intern. Med. 2017, 177, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.T.; Shin, Y.S.; Kim, J.Y.; Park, J.K. Could testosterone replacement therapy in hypogonadal men ameliorate anemia, a cardiovascular risk factor? An observational, 54-week cumulative registry study. J. Urol. 2016, 195, 1057–1064. [Google Scholar] [CrossRef]
- Saad, F.; Doros, G.; Haider, K.S.; Haider, A. Hypogonadal men with moderate-to-severe lower urinary tract symptoms have a more severe cardiometabolic risk profile and benefit more from testosterone therapy than men with mild lower urinary tract symptoms. Investig. Clin. Urol. 2018, 59, 399–409. [Google Scholar] [CrossRef]
- Ferro, M.; Lucarelli, G.; Bruzzese, D.; Di Lorenzo, G.; Perdonà, S.; Autorino, R.; Cantiello, F.; La Rocca, R.; Busetto, G.M.; Cimmino, A.; et al. Low serum total testosterone level as a predictor of upstaging and upgrading in low-risk prostate cancer patients meeting the inclusion criteria for active surveillance. Oncotarget 2017, 8, 18424–18434. [Google Scholar] [CrossRef]
- Bhindi, B.; Locke, J.; Alibhai, S.M.; Kulkarni, G.S.; Margel, D.S.; Hamilton, R.J.; Finelli, A.; Trachtenberg, J.; Zlotta, A.R.; Toi, A.; et al. Dissecting the association between metabolic syndrome and prostate cancer risk: Analysis of a large clinical cohort. Eur. Urol. 2015, 67, 64–70. [Google Scholar] [CrossRef]
- de Cobelli, O.; Terracciano, D.; Tagliabue, E.; Raimondi, S.; Galasso, G.; Cioffi, A.; Cordima, G.; Musi, G.; Damiano, R.; Cantiello, F.; et al. Body mass index was associated with upstaging and upgrading in patients with low-risk prostate cancer who met the inclusion criteria for active surveillance. Urol. Oncol. 2015, 33, 201.e1–201.e8. [Google Scholar] [CrossRef]
| Physical | Cognitive | Sexual |
|---|---|---|
| Anemia Reduced energy | Depressive symptoms | Reduced sex drive |
| Reduced endurance | Cognitive dysfunction | Reduced erectile function |
| Diminished work performance | Reduced motivation | |
| Diminished physical performance | Poor concentration | |
| Loss of body hair | Poor memory | |
| Reduced beard growth | Irritability | |
| Fatigue | ||
| Reduced lean muscle mass | ||
| Obesity |
| Guideline | Threshold of TT | Threshold of FT | How Many Times T Needs to Measured | Questionnaire |
|---|---|---|---|---|
| ISSAM | 12.1 nmol/L | 225 pmol/L | Not suggested | Recommended |
| EAU | 12.1 nmol/L | 243 pmol/L | 2 times | Not recommended |
| ESE and EAA | 320 ng/dL | 220 pmol/L | 2 times | Not recommended |
| AUA | 300 ng/dL | Not suggested | 2 times | Not recommended |
| Canadian Men’s Health Foundation | Not suggested | Not suggested | Not suggested | Not recommended |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, Y.S.; Park, J.K. The Optimal Indication for Testosterone Replacement Therapy in Late Onset Hypogonadism. J. Clin. Med. 2019, 8, 209. https://doi.org/10.3390/jcm8020209
Shin YS, Park JK. The Optimal Indication for Testosterone Replacement Therapy in Late Onset Hypogonadism. Journal of Clinical Medicine. 2019; 8(2):209. https://doi.org/10.3390/jcm8020209
Chicago/Turabian StyleShin, Yu Seob, and Jong Kwan Park. 2019. "The Optimal Indication for Testosterone Replacement Therapy in Late Onset Hypogonadism" Journal of Clinical Medicine 8, no. 2: 209. https://doi.org/10.3390/jcm8020209
APA StyleShin, Y. S., & Park, J. K. (2019). The Optimal Indication for Testosterone Replacement Therapy in Late Onset Hypogonadism. Journal of Clinical Medicine, 8(2), 209. https://doi.org/10.3390/jcm8020209





