Erastin Inhibits Septic Shock and Inflammatory Gene Expression via Suppression of the NF-κB Pathway
Abstract
1. Introduction
2. Experimental Section
2.1. Animal Studies
2.2. Plasma Analysis
2.3. Reagents and Antibodies
2.4. Cells and Cell Culture
2.5. Measurement of NO Metabolites, PGE2, TNF-α, and IL-1β
2.6. Western Blot
2.7. Quantitative Real-Time PCR (qPCR)
2.8. Immunofluorescence Staining
2.9. Cell Viability Assay
2.10. Reactive Oxygen Species (ROS) Detection Assay
2.11. Statistical Analysis
3. Results
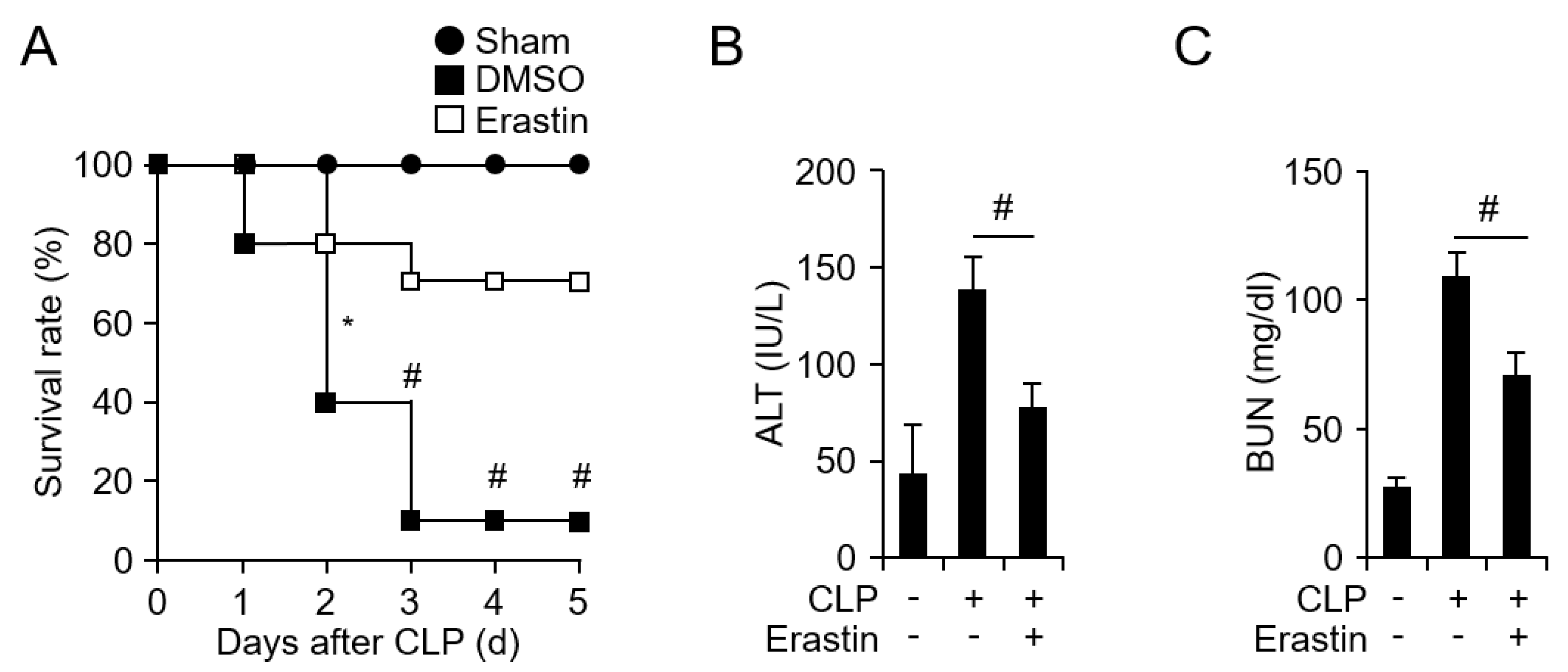
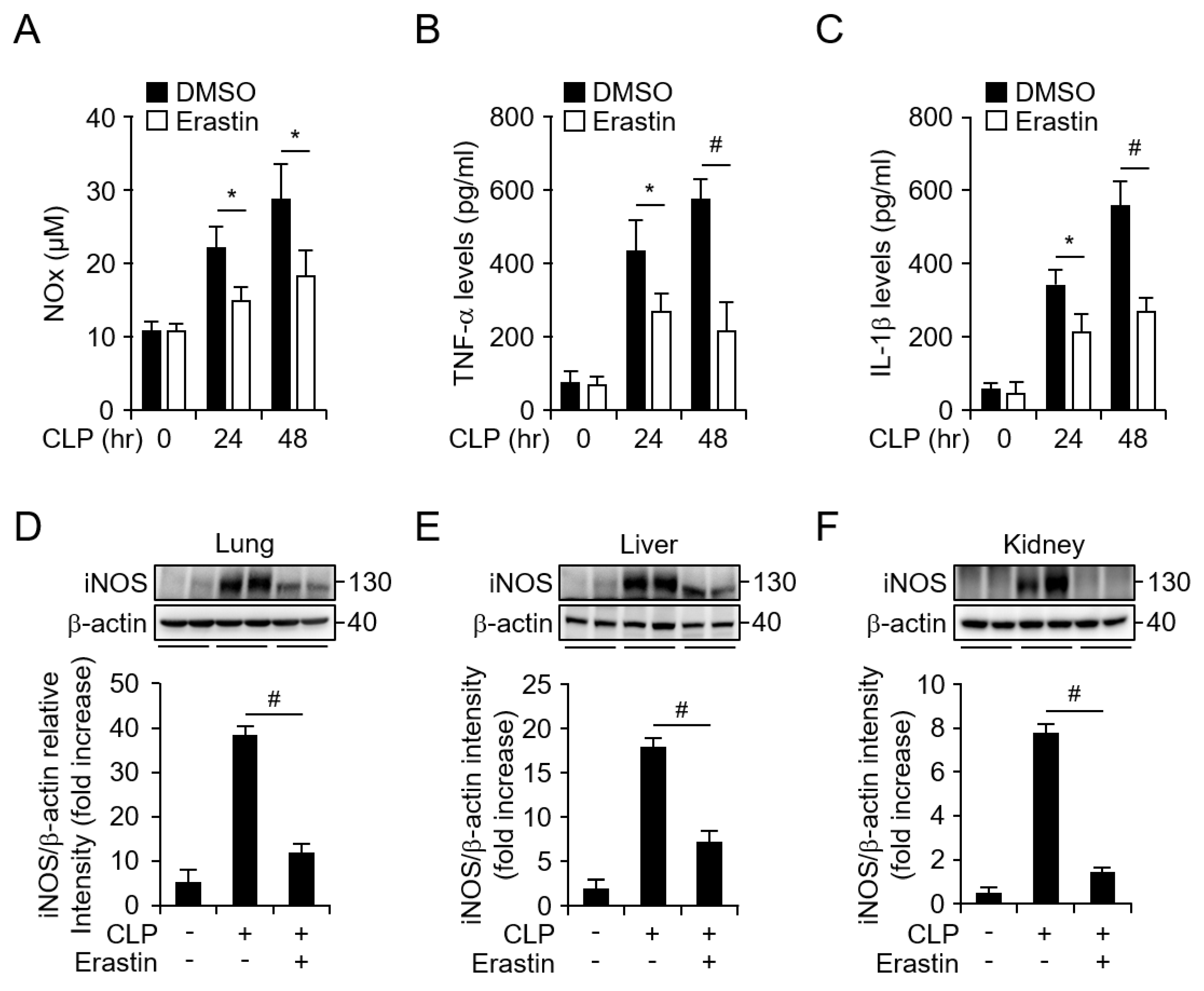
3.1. Erastin Prevents CLP-Induced Septic Shock Through Suppression of Inflammatory Responses
3.2. Erastin Inhibits Septic Death and the Inflammatory Response in an LPS-Induced Septic Model
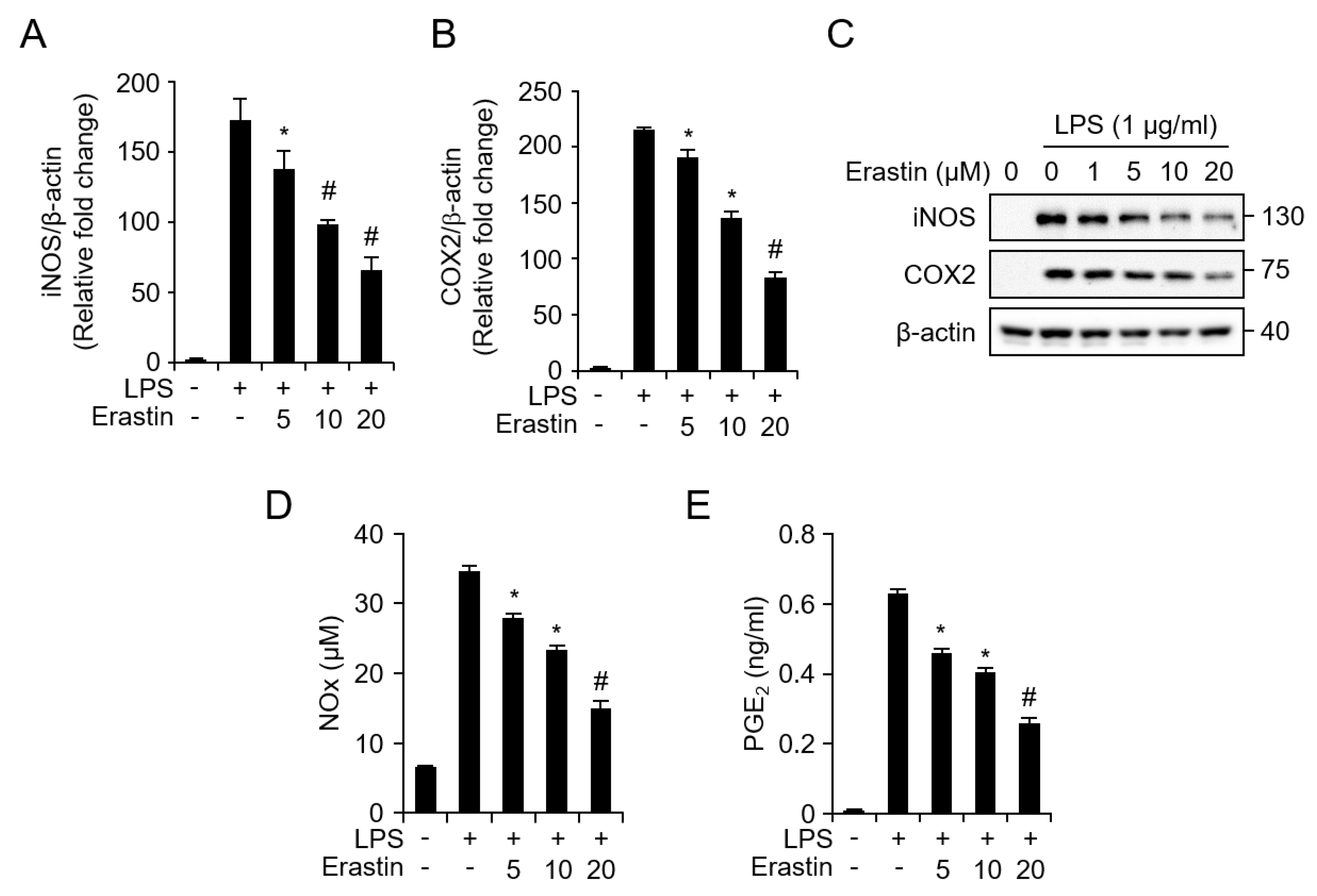
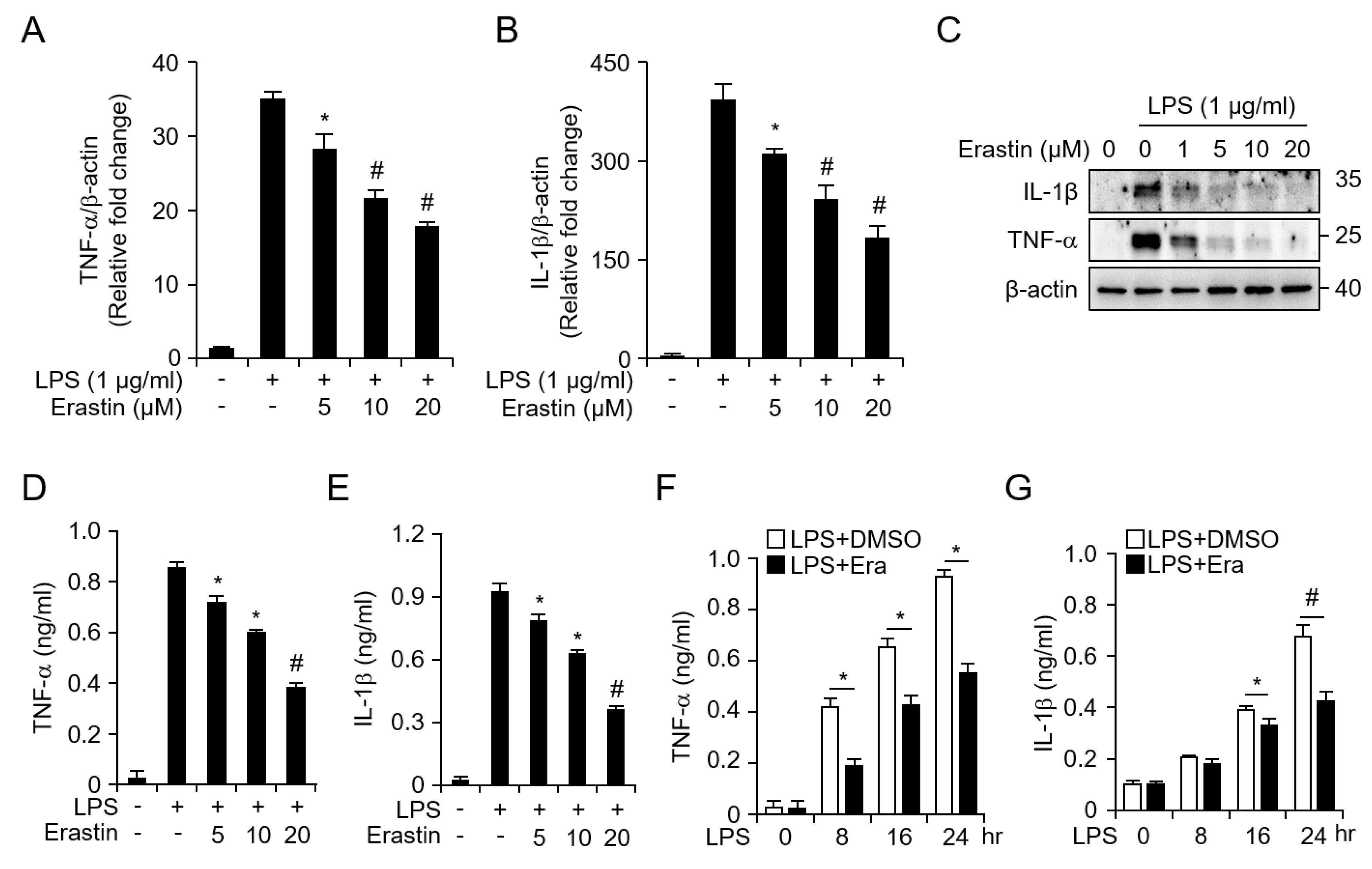
3.3. Erastin Inhibits LPS-Induced Inflammatory Responses in Bone Marrow-Derived Macrophages (BMDMs)
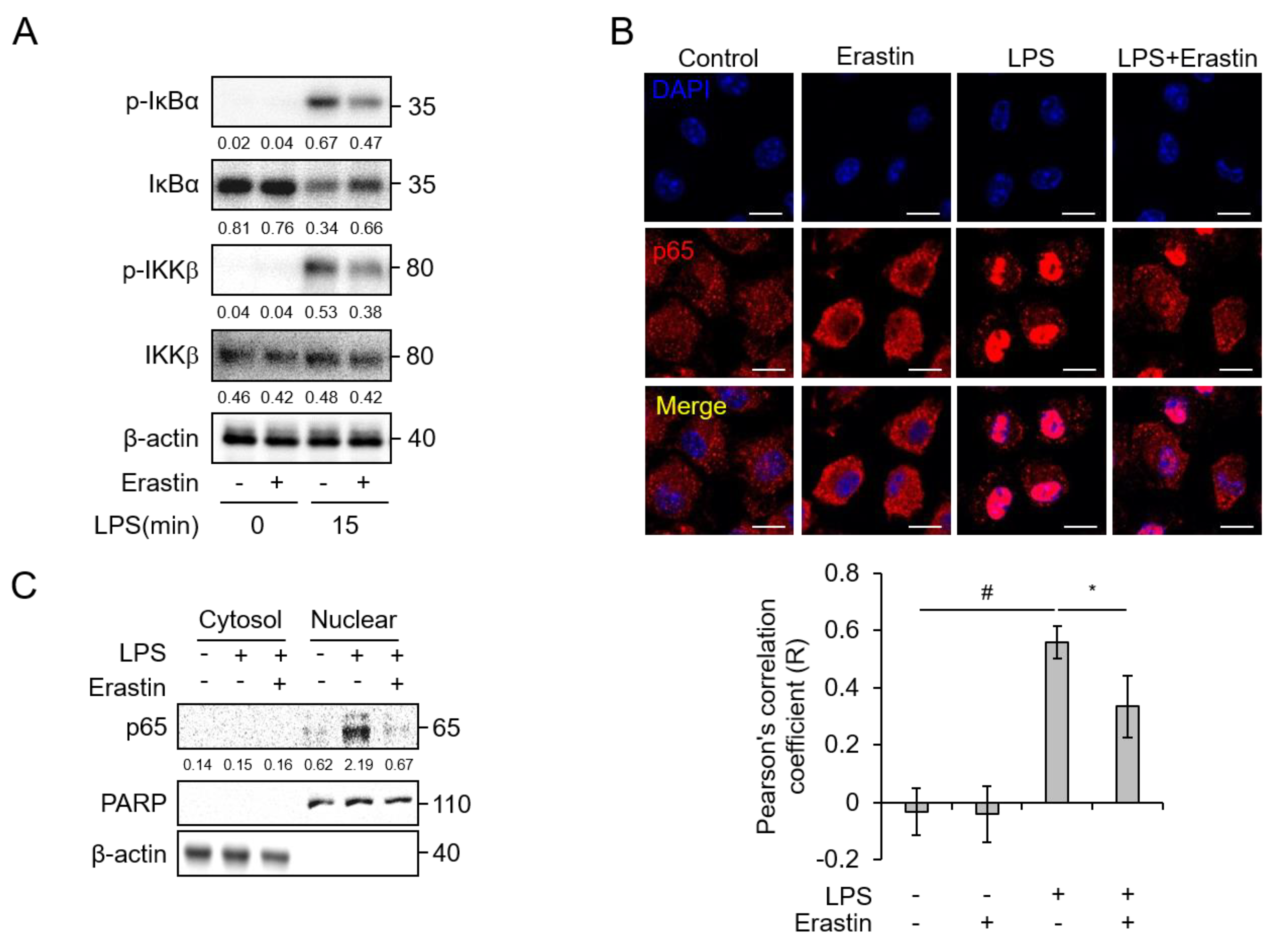
3.4. Erastin Suppresses NF-κB Activation by LPS Stimulation in BMDMs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Van der Poll, T.; van de Veerdonk, F.L.; Scicluna, B.P.; Netea, M.G. The immunopathology of sepsis and potential therapeutic targets. Nat. Rev. Immunol. 2017, 17, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Wyss-Coray, T.; Mucke, L. Inflammation in neurodegenerative disease—A double-edged sword. Neuron 2002, 35, 419–432. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, S. Organ dysfunction as a new standard for defining sepsis. Inflamm. Regen. 2016, 36, 24. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, N.; Duggal, A.K. Sepsis Associated Encephalopathy. Adv. Med. 2014, 2014, 762320. [Google Scholar] [CrossRef]
- Winkler, M.S.; Kluge, S.; Holzmann, M.; Moritz, E.; Robbe, L.; Bauer, A.; Zahrte, C.; Priefler, M.; Schwedhelm, E.; Boger, R.H.; et al. Markers of nitric oxide are associated with sepsis severity: An observational study. Crit. Care 2017, 21, 189. [Google Scholar] [CrossRef]
- Wiersinga, W.J.; Leopold, S.J.; Cranendonk, D.R.; van der Poll, T. Host innate immune responses to sepsis. Virulence 2014, 5, 36–44. [Google Scholar] [CrossRef]
- Titheradge, M.A. Nitric oxide in septic shock. Biochim. Biophys. Acta 1999, 1411, 437–455. [Google Scholar] [CrossRef]
- Mattson, M.P. Excitotoxic and excitoprotective mechanisms: Abundant targets for the prevention and treatment of neurodegenerative disorders. Neuromolecular Med. 2003, 3, 65–94. [Google Scholar] [CrossRef]
- Olney, J.W. New mechanisms of excitatory transmitter neurotoxicity. J. Neural Transm. Suppl. 1994, 43, 47–51. [Google Scholar] [PubMed]
- Piani, D.; Fontana, A. Involvement of the cystine transport system xc- in the macrophage-induced glutamate-dependent cytotoxicity to neurons. J. Immunol. 1994, 152, 3578–3585. [Google Scholar] [PubMed]
- Sato, H.; Fujiwara, K.; Sagara, J.; Bannai, S. Induction of cystine transport activity in mouse peritoneal macrophages by bacterial lipopolysaccharide. Biochem. J. 1995, 310 Pt 2, 547–551. [Google Scholar] [CrossRef]
- Chung, W.J.; Lyons, S.A.; Nelson, G.M.; Hamza, H.; Gladson, C.L.; Gillespie, G.Y.; Sontheimer, H. Inhibition of cystine uptake disrupts the growth of primary brain tumors. J. Neurosci. 2005, 25, 7101–7110. [Google Scholar] [CrossRef] [PubMed]
- Dolma, S.; Lessnick, S.L.; Hahn, W.C.; Stockwell, B.R. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell 2003, 3, 285–296. [Google Scholar] [CrossRef]
- Yagoda, N.; von Rechenberg, M.; Zaganjor, E.; Bauer, A.J.; Yang, W.S.; Fridman, D.J.; Wolpaw, A.J.; Smukste, I.; Peltier, J.M.; Boniface, J.J.; et al. RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature 2007, 447, 864–868. [Google Scholar] [CrossRef]
- Yang, W.S.; Stockwell, B.R. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem. Biol. 2008, 15, 234–245. [Google Scholar] [CrossRef]
- Bridges, R.J.; Natale, N.R.; Patel, S.A. System xc (-) cystine/glutamate antiporter: An update on molecular pharmacology and roles within the CNS. Br. J. Pharmacol. 2012, 165, 20–34. [Google Scholar] [CrossRef]
- Lewerenz, J.; Hewett, S.J.; Huang, Y.; Lambros, M.; Gout, P.W.; Kalivas, P.W.; Massie, A.; Smolders, I.; Methner, A.; Pergande, M.; et al. The cystine/glutamate antiporter system x(c) (-) in health and disease: From molecular mechanisms to novel therapeutic opportunities. Antioxid. Redox Signal. 2013, 18, 522–555. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Kim, S.J.; Yoon, S.J.; Kim, Y.M.; Hong, S.W.; Yeon, S.H.; Choe, K.I.; Lee, S.M. HS-23, Lonicera japonica extract, attenuates septic injury by suppressing toll-like receptor 4 signaling. J. Ethnopharmacol. 2014, 155, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Bosmann, M.; Ward, P.A. The inflammatory response in sepsis. Trends Immunol. 2013, 34, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef]
- Crouser, E.; Exline, M.; Knoell, D.; Wewers, M.D. Sepsis: Links between pathogen sensing and organ damage. Curr. Pharm. Des. 2008, 14, 1840–1852. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Xie, Y.; Hou, W.; Song, X.; Yu, Y.; Huang, J.; Sun, X.; Kang, R.; Tang, D. Ferroptosis: Process and function. Cell Death Differ. 2016, 23, 369–379. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, Y.; Wang, C.; Liu, Y. Glutathione depletion induces ferroptosis, autophagy, and premature cell senescence in retinal pigment epithelial cells. Cell Death Dis. 2018, 9, 753. [Google Scholar] [CrossRef]
- Wang, D.; Xie, N.; Gao, W.; Kang, R.; Tang, D. The ferroptosis inducer erastin promotes proliferation and differentiation in human peripheral blood mononuclear cells. Biochem. Biophys. Res. Commun. 2018, 503, 1689–1695. [Google Scholar] [CrossRef]
- Oeckinghaus, A.; Ghosh, S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal. Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Tak, P.P.; Firestein, G.S. NF-kappaB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.L.; Zhou, B.W.; Yan, Z.Z.; Zhao, J.; Zhao, B.C.; Liu, W.F.; Li, C.; Liu, K.X. 6-Gingerol attenuates macrophages pyroptosis via the inhibition of MAPK signaling pathways and predicts a good prognosis in sepsis. Cytokine 2019, 125, 154854. [Google Scholar] [CrossRef]
- Evonuk, K.S.; Baker, B.J.; Doyle, R.E.; Moseley, C.E.; Sestero, C.M.; Johnston, B.P.; De Sarno, P.; Tang, A.; Gembitsky, I.; Hewett, S.J.; et al. Inhibition of System Xc(-) Transporter Attenuates Autoimmune Inflammatory Demyelination. J. Immunol. 2015, 195, 450–463. [Google Scholar] [CrossRef]
- Chung, W.J.; Sontheimer, H. Sulfasalazine inhibits the growth of primary brain tumors independent of nuclear factor-kappaB. J. Neurochem. 2009, 110, 182–193. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, B.M.; Lee, S.-J.; Park, G.L.; Hwang, Y.S.; Lim, J.; Park, E.S.; Lee, K.H.; Kim, B.Y.; Kwon, Y.T.; Cho, H.J.; et al. Erastin Inhibits Septic Shock and Inflammatory Gene Expression via Suppression of the NF-κB Pathway. J. Clin. Med. 2019, 8, 2210. https://doi.org/10.3390/jcm8122210
Oh BM, Lee S-J, Park GL, Hwang YS, Lim J, Park ES, Lee KH, Kim BY, Kwon YT, Cho HJ, et al. Erastin Inhibits Septic Shock and Inflammatory Gene Expression via Suppression of the NF-κB Pathway. Journal of Clinical Medicine. 2019; 8(12):2210. https://doi.org/10.3390/jcm8122210
Chicago/Turabian StyleOh, Byung Moo, Seon-Jin Lee, Gyoung Lim Park, Yo Sep Hwang, Jeewon Lim, Eun Sun Park, Kyung Ho Lee, Bo Yeon Kim, Yong Tae Kwon, Hee Jun Cho, and et al. 2019. "Erastin Inhibits Septic Shock and Inflammatory Gene Expression via Suppression of the NF-κB Pathway" Journal of Clinical Medicine 8, no. 12: 2210. https://doi.org/10.3390/jcm8122210
APA StyleOh, B. M., Lee, S.-J., Park, G. L., Hwang, Y. S., Lim, J., Park, E. S., Lee, K. H., Kim, B. Y., Kwon, Y. T., Cho, H. J., & Lee, H. G. (2019). Erastin Inhibits Septic Shock and Inflammatory Gene Expression via Suppression of the NF-κB Pathway. Journal of Clinical Medicine, 8(12), 2210. https://doi.org/10.3390/jcm8122210



