Predictive Validity of the qSOFA Score for Sepsis in Adults with Community-Onset Staphylococcal Infection in Thailand
Abstract
1. Introduction
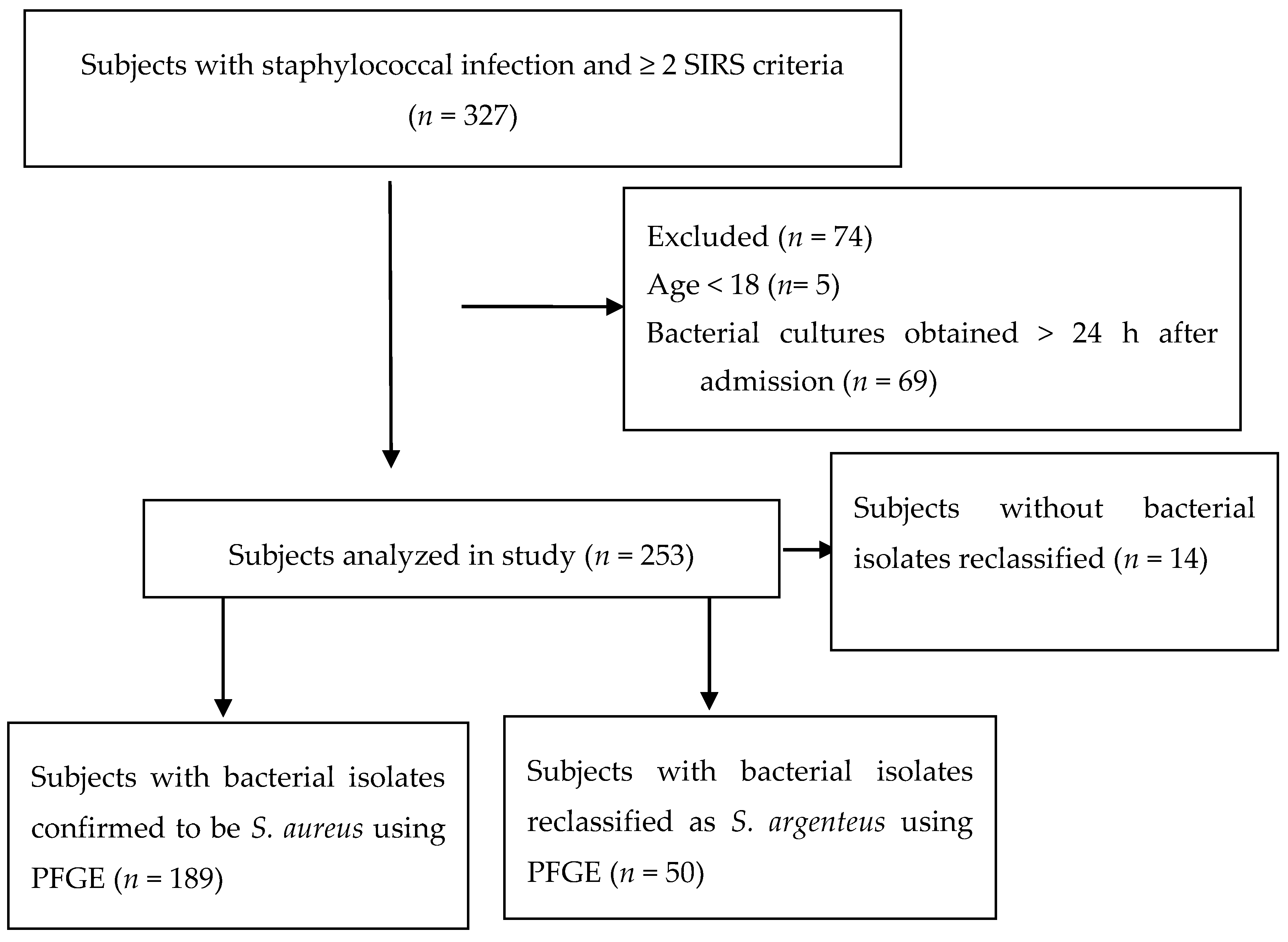
2. Experimental Section
2.1. Bacterial Isolates
2.2. Statistical Analysis
2.3. Ethics
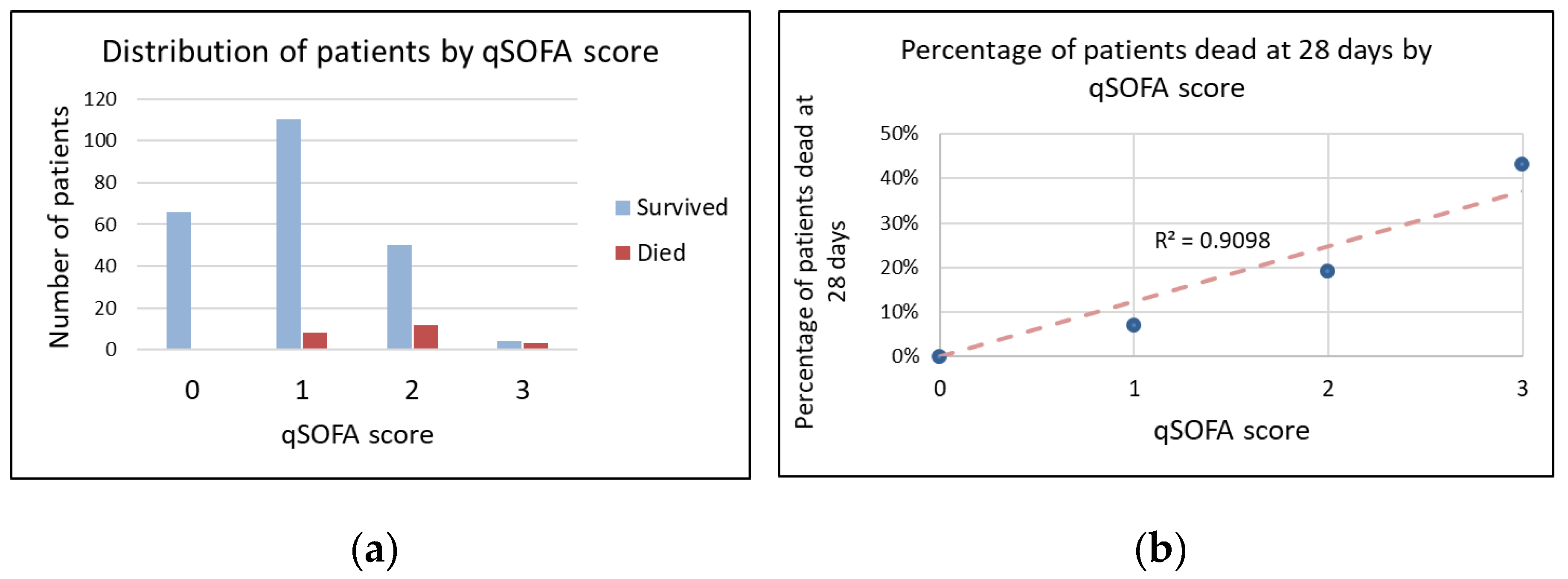
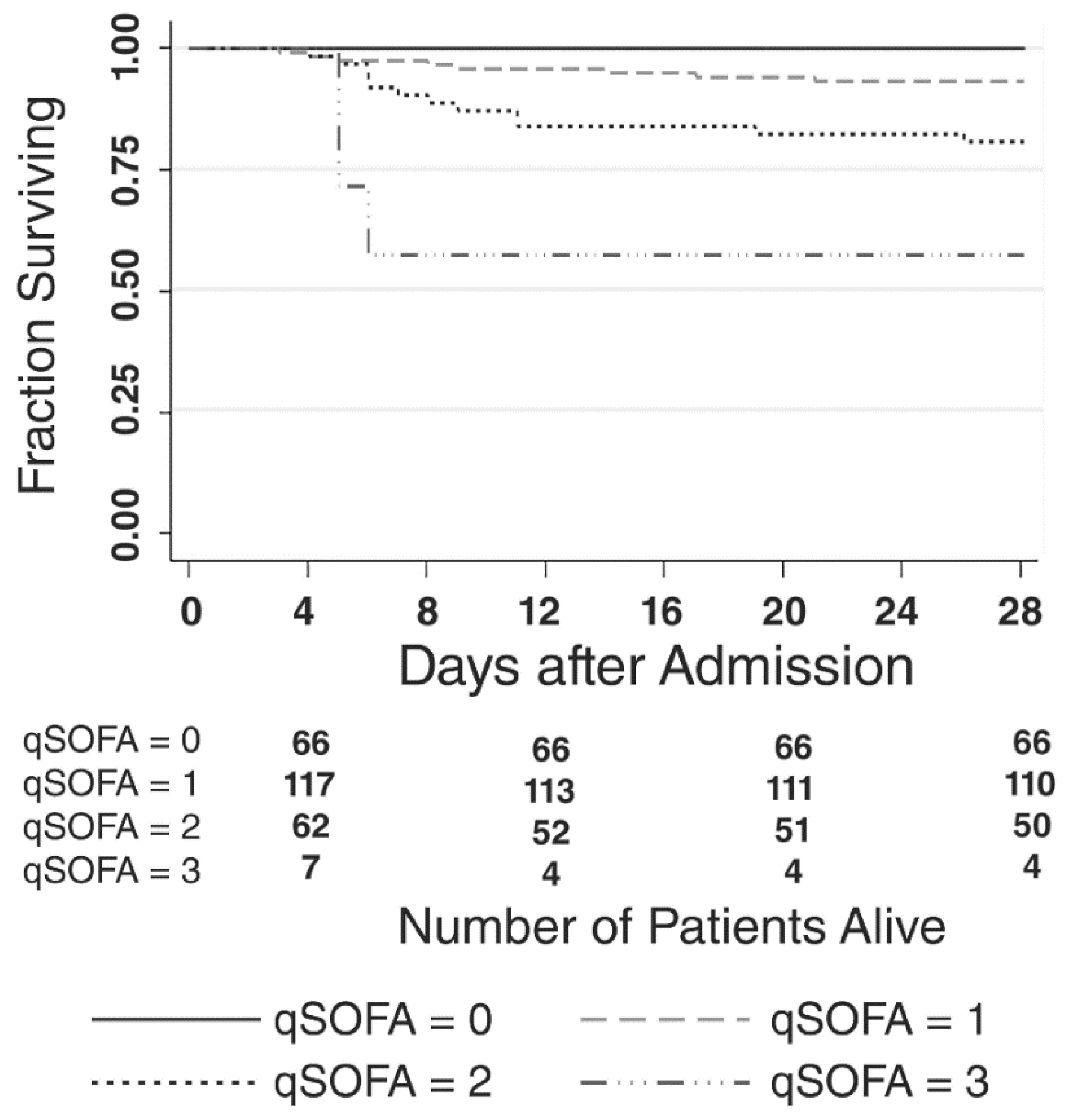
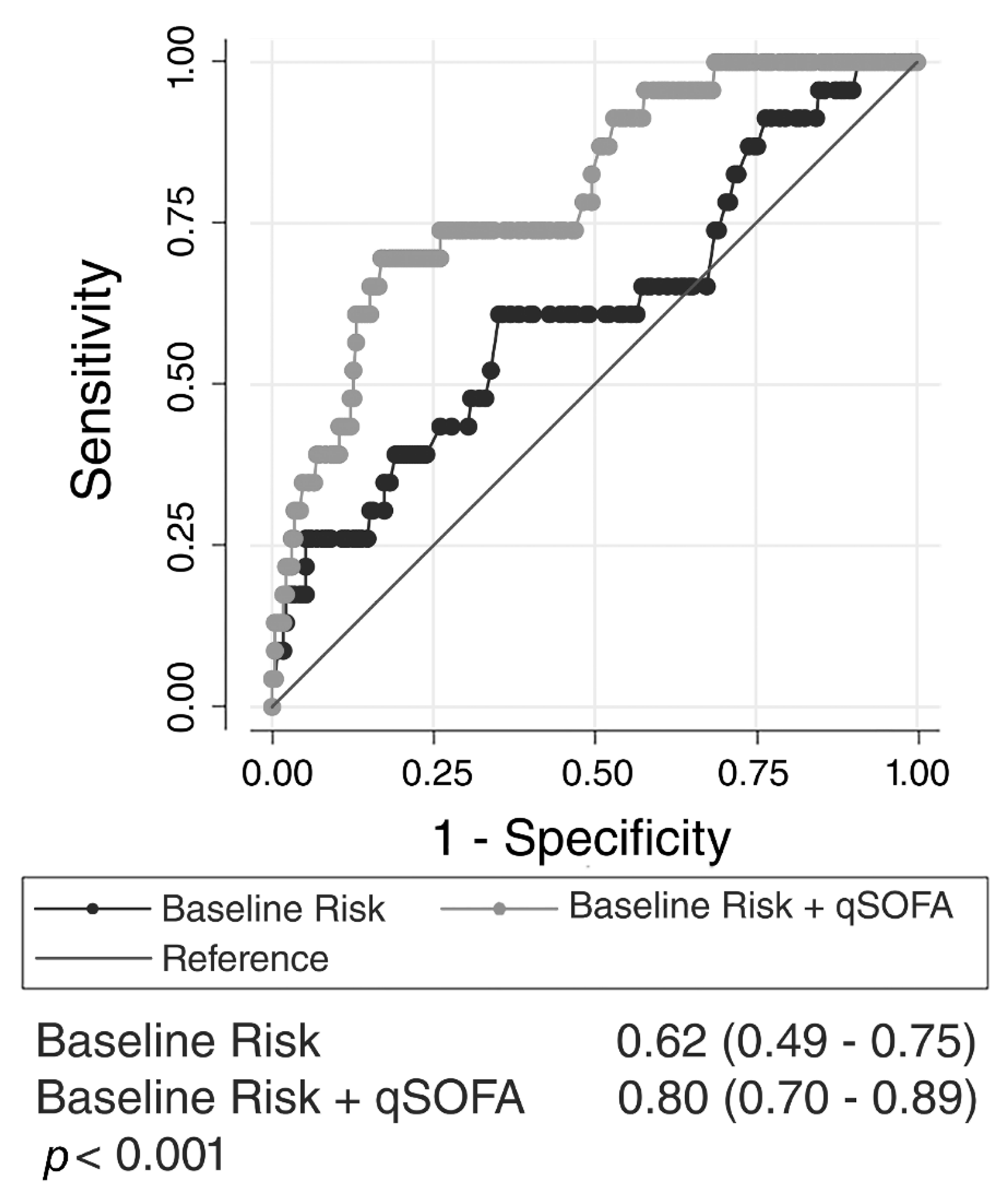
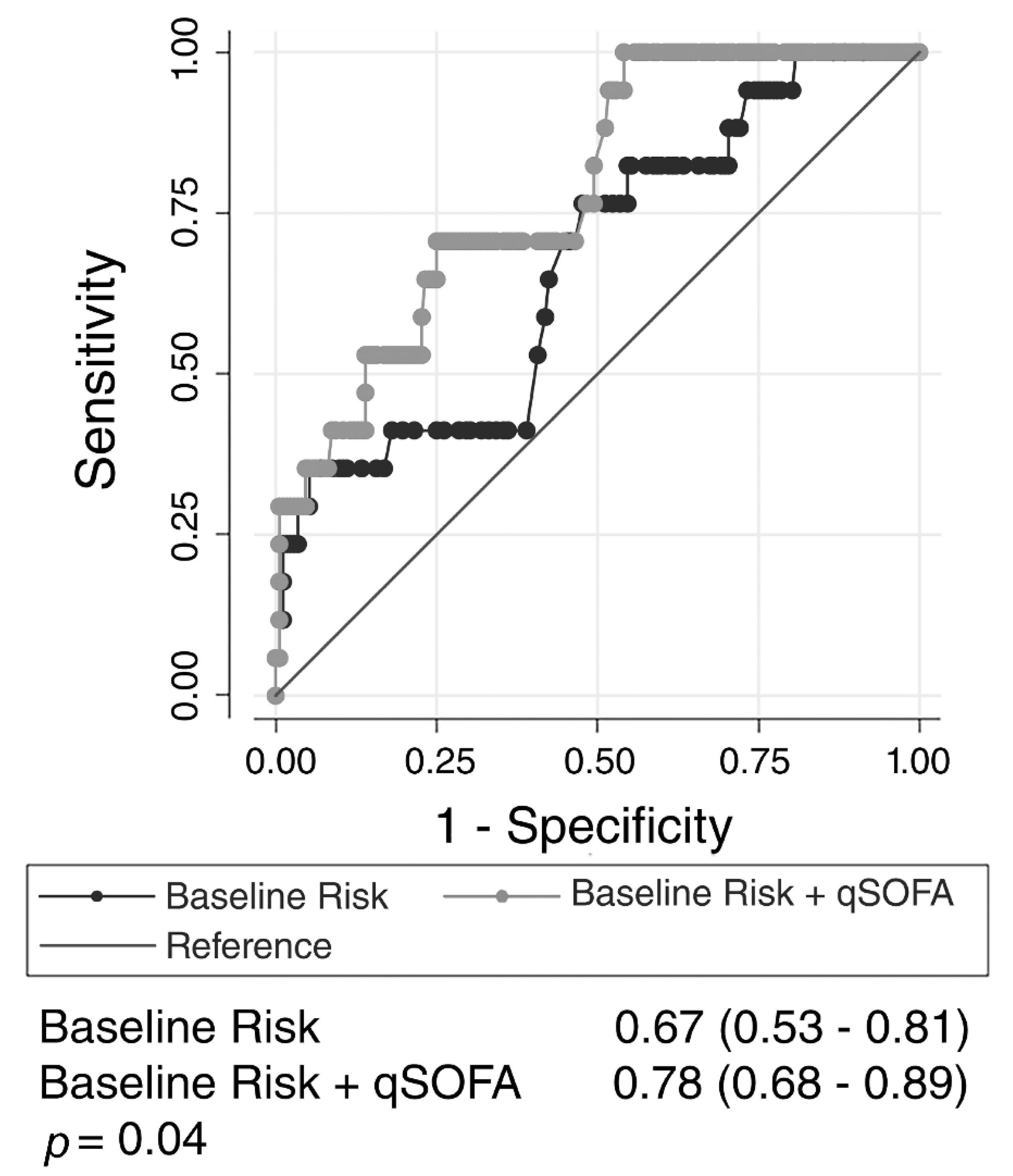
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vincent, J.L.; Marshall, J.C.; Namendys-Silva, S.A.; François, B.; Martin-Loeches, I.; Lipman, J.; Reinhart, K.; Antonelli, M.; Pickkers, P.; Njimi, H.; et al. Assessment of the worldwide burden of critical illness: The intensive care over nations (ICON) audit. Lancet Respir. Med. 2014, 2, 380–386. [Google Scholar] [CrossRef]
- Fleischmann, C.; Scherag, A.; Adhikari, N.K.; Hartog, C.S.; Tsaganos, T.; Schlattmann, P.; Angus, D.C.; Reinhart, K. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am. J. Respir. Crit. Care Med. 2016, 193, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Phetsouvanh, R.; Phongmany, S.; Soukaloun, D.; Rasachak, B.; Soukhaseum, V.; Soukhaseum, S.; Frichithavong, K.; Khounnorath, S.; Pengdee, B.; Phiasakha, K.; et al. Causes of community-acquired bacteremia and patterns of antimicrobial resistance in Vientiane, Laos. Am. J. Trop. Med. Hyg. 2006, 75, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Bunburaphong, P.; Chatrkaw, P.; Sriprachittichai, P.; Supleornsug, K.; Ultchaswadi, P.; Sumetha-aksorn, N. Risk factors for predicting mortality in a surgical intensive care unit in the year 2000. J. Med. Assoc. Thail. Chotmaihet Thangphaet 2003, 86, 8–15. [Google Scholar]
- Reechaipichitkul, W.; Tantiwong, P. Clinical features of community-acquired pneumonia treated at Srinagarind Hospital, Khon Kaen, Thailand. S. Asian J. Trop. Med. Public Health 2002, 33, 355–361. [Google Scholar]
- Nickerson, E.K.; Wuthiekanun, V.; Day, N.P.; Chaowagul, W.; Peacock, S.J. Methicillin-resistant Staphylococcus aureus in rural Asia. Lancet Infect. Dis. 2006, 6, 70–71. [Google Scholar] [CrossRef]
- Chen, S.Y.; Lee, H.; Wang, X.M.; Lee, T.F.; Liao, C.H.; Teng, L.J.; Hsueh, P.R. High mortality impact of Staphylococcus argenteus on patients with community-onset staphylococcal bacteraemia. Int. J. Antimicrob. Agents 2018, 52, 747–753. [Google Scholar] [CrossRef]
- Bone, R.C.; Balk, R.A.; Cerra, F.B.; Dellinger, R.P.; Fein, A.M.; Knaus, W.A.; Schein, R.M.; Sibbald, W.J. Definitions for Sepsis and Organ Failure and Guidelines for the Use of Innovative Therapies in Sepsis. Chest 1992, 101, 1644–1655. [Google Scholar] [CrossRef]
- Levy, M.M.; Fink, M.P.; Marshall, J.C.; Abraham, E.; Angus, D.C.; Cook, D.; Cohen, J.; Opal, S.M.; Vincent, J.L.; Ramsay, G. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003, 29, 530–538. [Google Scholar] [CrossRef]
- Angus, D.C.; Seymour, C.W.; Coopersmith, C.M.; Deutschman, C.S.; Klompas, M.; Levy, M.M.; Martin, G.S.; Osborn, T.M.; Rhee, C.; Watson, R.S. A Framework for the Development and Interpretation of Different Sepsis Definitions and Clinical Criteria. Crit. Care Med. 2016, 44, e113–e121. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Freund, Y.; Lemachatti, N.; Krastinova, E.; Van Laer, M.; Claessens, Y.E.; Avondo, A.; Occelli, C.; Feral-Pierssens, A.L.; Truchot, J.; Ortega, M.; et al. Prognostic Accuracy of Sepsis-3 Criteria for In-Hospital Mortality Among Patients with Suspected Infection Presenting to the Emergency Department. JAMA 2017, 317, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Raith, E.P.; Udy, A.A.; Bailey, M.; McGloughlin, S.; MacIsaac, C.; Bellomo, R.; Pilcher, D.V. Prognostic Accuracy of the SOFA Score, SIRS Criteria, and qSOFA Score for In-Hospital Mortality Among Adults with Suspected Infection Admitted to the Intensive Care Unit. JAMA 2017, 317, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Rudd, K.E.; Seymour, C.W.; Aluisio, A.R.; Augustin, M.E.; Bagenda, D.S.; Beane, A.; Byiringiro, J.C.; Chang, C.H.; Colas, L.N.; Day, N.P.J.; et al. Association of the Quick Sequential (Sepsis-Related) Organ Failure Assessment (qSOFA) Score with Excess Hospital Mortality in Adults with Suspected Infection in Low- and Middle-Income Countries. JAMA 2018, 319, 2202–2211. [Google Scholar] [CrossRef]
- West, T.E.; Wikraiphat, C.; Tandhavanant, S.; Ariyaprasert, P.; Suntornsut, P.; Okamoto, S.; Mahavanakul, W.; Srisamang, P.; Phiphitaporn, S.; Anukunananchai, J.; et al. Patient Characteristics, Management, and Predictors of Outcome from Severe Community-Onset Staphylococcal Sepsis in Northeast Thailand: A Prospective Multicenter Study. Am. J. Trop. Med. Hyg. 2017, 96, 1042–1049. [Google Scholar] [CrossRef]
- Chantratita, N.; Tandhavanant, S.; Seal, S.; Wikraiphat, C.; Wongsuvan, G.; Ariyaprasert, P.; Suntornsut, P.; Teerawattanasook, N.; Jutrakul, Y.; Srisurat, N.; et al. TLR4 genetic variation is associated with inflammatory responses in Gram-positive sepsis. Clin. Microbiol. Infect. 2017, 23, 47.e1–47.e10. [Google Scholar] [CrossRef]
- Chantratita, N.; Wikraiphat, C.; Tandhavanant, S.; Wongsuvan, G.; Ariyaprasert, P.; Suntornsut, P.; Thaipadungpanit, J.; Teerawattanasook, N.; Jutrakul, Y.; Srisurat, N.; et al. Comparison of community-onset Staphylococcus argenteus and Staphylococcus aureus sepsis in Thailand: A prospective multicentre observational study. Clin. Microbiol. Infect. 2016, 22, 458.e11–458.e19. [Google Scholar] [CrossRef]
- Moradigaravand, D.; Jamrozy, D.; Mostowy, R.; Anderson, A.; Nickerson, E.K.; Thaipadungpanit, J.; Wuthiekanun, V.; Limmathurotsakul, D.; Tandhavanant, S.; Wikraiphat, C.; et al. Evolution of the ST2250 Clone in Northeastern Thailand Is Linked with the Acquisition of Livestock-Associated Staphylococcal Genes. mBio 2017, 8, e00802-17. [Google Scholar] [CrossRef]
- Meredith, W.; Rutledge, R.; Fakhry, S.M.; Emery, S.; Kromhout-schiro, S. The conundrum of the Glasgow Coma Scale in intubated patients: A linear regression prediction of the Glasgow verbal score from the Glasgow eye and motor scores. J. Trauma 1998, 44, 839–844. [Google Scholar] [CrossRef]
- Rydenfelt, K.; Engerström, L.; Walther, S.; Sjöberg, F.; Strömberg, U.; Samuelsson, C. In-hospital vs. 30-day mortality in the critically ill—A 2-year Swedish intensive care cohort analysis. Acta Anaesthesiol. Scand. 2015, 59, 846–858. [Google Scholar] [CrossRef]
- Tong, S.Y.; Schaumburg, F.; Ellington, M.J.; Corander, J.; Pichon, B.; Leendertz, F.; Bentley, S.D.; Parkhill, J.; Holt, D.C.; Peters, G.; et al. Novel staphylococcal species that form part of a Staphylococcus aureus-related complex: The non-pigmented Staphylococcus argenteus sp. nov. and the non-human primate-associated Staphylococcus schweitzeri sp. Nov. Int. J. Syst. Evol. Microbiol. 2015, 65 Pt 1, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Aluisio, A.R.; Garbern, S.; Wiskel, T.; Mutabazi, Z.A.; Umuhire, O.; Ch’ng, C.C.; Rudd, K.E.; D’Arc Nyinawankusi, J.; Byiringiro, J.C.; Levine, A.C. Mortality outcomes based on ED qSOFA score and HIV status in a developing low income country. Am. J. Emerg. Med. 2018, 36, 2010–2019. [Google Scholar] [CrossRef] [PubMed]
- Rudd, K.E.; Kissoon, N.; Limmathurotsakul, D.; Bory, S.; Mutahunga, B.; Seymour, C.W.; Angus, D.C.; West, T.E. The global burden of sepsis: Barriers and potential solutions. Crit. Care 2018, 22, 232. [Google Scholar] [CrossRef] [PubMed]
- Minejima, E.; Delayo, V.; Lou, M.; Ny, P.; Nieberg, P.; She, R.C.; Wong-Beringer, A. Utility of qSOFA score in identifying patients at risk for poor outcome in Staphylococcus aureus bacteremia. BMC Infect. Dis. 2019, 19, 149. [Google Scholar] [CrossRef] [PubMed]
- Opal, S.M.; Cohen, J. Clinical gram-positive sepsis: Does it fundamentally differ from gram-negative bacterial sepsis? Crit. Care Med. 1999, 27, 1608–1616. [Google Scholar] [CrossRef] [PubMed]
- Abe, R.; Oda, S.; Sadahiro, T.; Nakamura, M.; Hirayama, Y.; Tateishi, Y.; Shinozaki, K.; Hirasawa, H. Gram-negative bacteremia induces greater magnitude of inflammatory response than Gram-positive bacteremia. Crit. Care 2010, 14, R27. [Google Scholar] [CrossRef]
- Weiss, S.L.; Fitzgerald, J.C.; Pappachan, J.; Wheeler, D.; Jaramillo-Bustamante, J.C.; Salloo, A.; Singhi, S.C.; Erickson, S.; Roy, J.A.; Bush, J.L.; et al. Global epidemiology of pediatric severe sepsis: The sepsis prevalence, outcomes, and therapies study. Am. J. Respir. Crit. Care Med. 2015, 191, 1147–1157. [Google Scholar] [CrossRef]
| Variables | All Patients | Alive at 28 Days | Dead at 28 Days |
|---|---|---|---|
| n = 253 | n = 230 | n = 23 | |
| Age (years), median (IQR) | 56 (43–66) | 56 (42–66) | 56 (46–65) |
| Male, n (%) | 168 (66%) | 152 (66%) | 16 (70%) |
| Systolic blood pressure (mmHg), median (IQR) | 102 (95–110) | 103 (96–110) | 93 (80–104) |
| Glasgow Coma Scale ≤ 14, n (%) | 14 (5%) | 8 (3%) | 6 (25%) |
| Respiratory rate > 20, n (%) | 130 (51%) | 111 (48%) | 19 (79%) |
| Liver disease present, n (%) | 9 (4%) | 5 (2%) | 4 (17%) |
| Cardiac disease present, n (%) | 12 (5%) | 11 (5%) | 1 (4%) |
| SIRS criteria, n (%) | |||
| 0 | 0 (0%) | 0 (0%) | 0 (0%) |
| 1 | 0 (0%) | 0 (0%) | 0 (0%) |
| 2 | 93 (37%) | 88 (38%) | 5 (22%) |
| 3 | 102 (40%) | 95 (41%) | 7 (30%) |
| 4 | 58 (23%) | 47 (20%) | 11 (48%) |
| qSOFA score, n (%) | |||
| 0 | 66 (26%) | 66 (29%) | 0 (0%) |
| 1 | 118 (47%) | 110 (48%) | 8 (38%) |
| 2 | 62 (25%) | 50 (22%) | 12 (50%) |
| 3 | 7 (3%) | 4 (2%) | 3 (13%) |
| qSOFA score ≥ 2, n (%) | 69 (27%) | 54 (23%) | 15 (65%) |
| Variables | All Patients | Alive at 28 Days | Dead at 28 Days |
|---|---|---|---|
| n = 189 | n = 172 | n = 17 | |
| SIRS criteria, n (%) | |||
| 0 | 0 (0%) | 0 (0%) | 0 (0%) |
| 1 | 0 (0%) | 0 (0%) | 0 (0%) |
| 2 | 66 (37%) | 63 (37%) | 3 (18%) |
| 3 | 72 (38%) | 66 (38%) | 6 (35%) |
| 4 | 51 (27%) | 43 (25%) | 8 (47%) |
| qSOFA score, n (%) | |||
| 0 | 47 (25%) | 47 (27%) | 0 (0%) |
| 1 | 82 (44%) | 76 (44%) | 6 (35%) |
| 2 | 54 (29%) | 45 (26%) | 9 (53%) |
| 3 | 6 (3%) | 4 (2%) | 2 (12%) |
| qSOFA score ≥ 2, n (%) | 60 (32%) | 49 (28%) | 11 (65%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, S.; Rudd, K.E.; Tandhavanant, S.; Suntornsut, P.; Chetchotisakd, P.; Angus, D.C.; Peacock, S.J.; Chantratita, N.; West, T.E. Predictive Validity of the qSOFA Score for Sepsis in Adults with Community-Onset Staphylococcal Infection in Thailand. J. Clin. Med. 2019, 8, 1908. https://doi.org/10.3390/jcm8111908
Gupta S, Rudd KE, Tandhavanant S, Suntornsut P, Chetchotisakd P, Angus DC, Peacock SJ, Chantratita N, West TE. Predictive Validity of the qSOFA Score for Sepsis in Adults with Community-Onset Staphylococcal Infection in Thailand. Journal of Clinical Medicine. 2019; 8(11):1908. https://doi.org/10.3390/jcm8111908
Chicago/Turabian StyleGupta, Supaksh, Kristina E. Rudd, Sarunporn Tandhavanant, Pornpan Suntornsut, Ploenchan Chetchotisakd, Derek C. Angus, Sharon J. Peacock, Narisara Chantratita, and Timothy Eoin West. 2019. "Predictive Validity of the qSOFA Score for Sepsis in Adults with Community-Onset Staphylococcal Infection in Thailand" Journal of Clinical Medicine 8, no. 11: 1908. https://doi.org/10.3390/jcm8111908
APA StyleGupta, S., Rudd, K. E., Tandhavanant, S., Suntornsut, P., Chetchotisakd, P., Angus, D. C., Peacock, S. J., Chantratita, N., & West, T. E. (2019). Predictive Validity of the qSOFA Score for Sepsis in Adults with Community-Onset Staphylococcal Infection in Thailand. Journal of Clinical Medicine, 8(11), 1908. https://doi.org/10.3390/jcm8111908





